Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS): Focus on the Pathophysiological and Diagnostic Role of Viruses
Abstract
1. Introduction
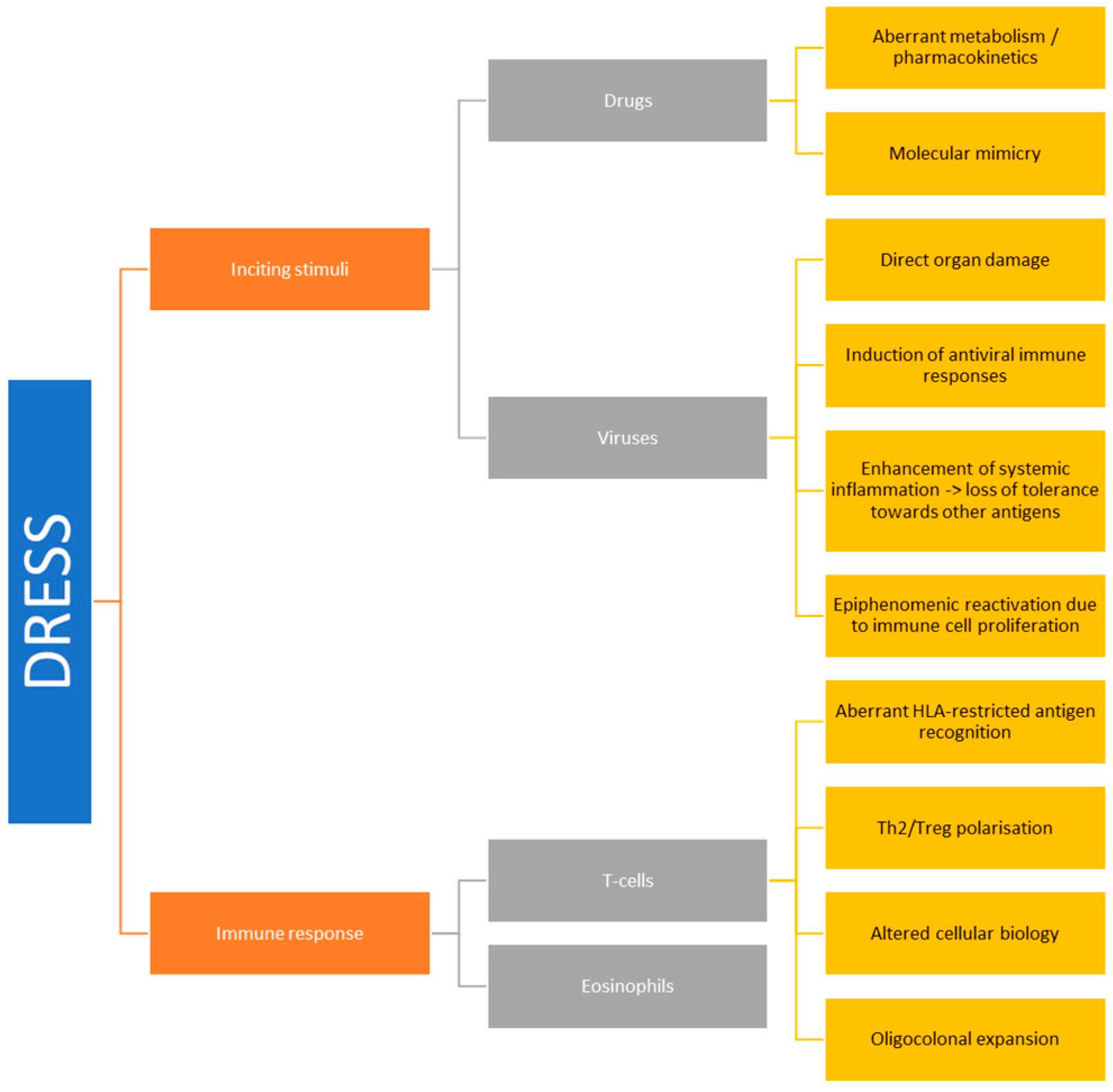
2. Aetiology and Pathogenesis
2.1. Drugs
| Drugs Categories | Drug | Ref. | |
|---|---|---|---|
| Urate lowering agents | Allopurinol | High Risk | [32] |
| Febuxostat | [33] | ||
| Aromatic antiepileptic agents | Carbamazepine | High Risk | [14] |
| Phenytoin | High Risk | ||
| Lamotrigine | High Risk | ||
| Oxcarbazepine | High Risk | ||
| Phenobarbital | High Risk | ||
| Sulphonamides | Sulfasalazine | High Risk | [14] |
| Dapsone | High Risk | ||
| Trimethoprim-Sulfamethoxazole | High Risk | ||
| Sulfadiazine | High Risk | ||
| Antibiotics | Vancomycin | High Risk | [23] |
| Minocycline | High Risk | ||
| Piperacillin-Tazobactam | |||
| Antituberculosis Agents | High Risk | ||
| Other Penicillins and Cephalosporins | |||
| Antiviral agents | Nevirapine | High Risk | [34] |
| Abacavir | High Risk | [35] | |
| Efavirenz | [36] | ||
| Boceprevir | [37] | ||
| Telaprevir | [38] | ||
| Anti-inflammatory drugs | Diclofenac | [23] | |
| Celecoxib | |||
| Ibuprofen | |||
| Anti-IL1 antibodies | Anakinra | [39] | |
| Canakinumab | |||
| Anti IL6 antibodies | Tocilizumab | [39] | |
| Targeted therapies | Imatinib | [21] | |
| Sorafenib | [40] | ||
| Vismodegib | [41] | ||
| Vemurafenib | [42] | ||
| Antipsychotic agents | Fluoxetine | [23] | |
| Olanzapine | |||
| Anti-coagulant | Rivaroxaban | [43] | |
| Immunomodulators | Hydroxychloroquine | [44] |
2.2. Viral Factors
- Viruses may cause direct tissue damage and contribute to the early manifestations of DRESS.
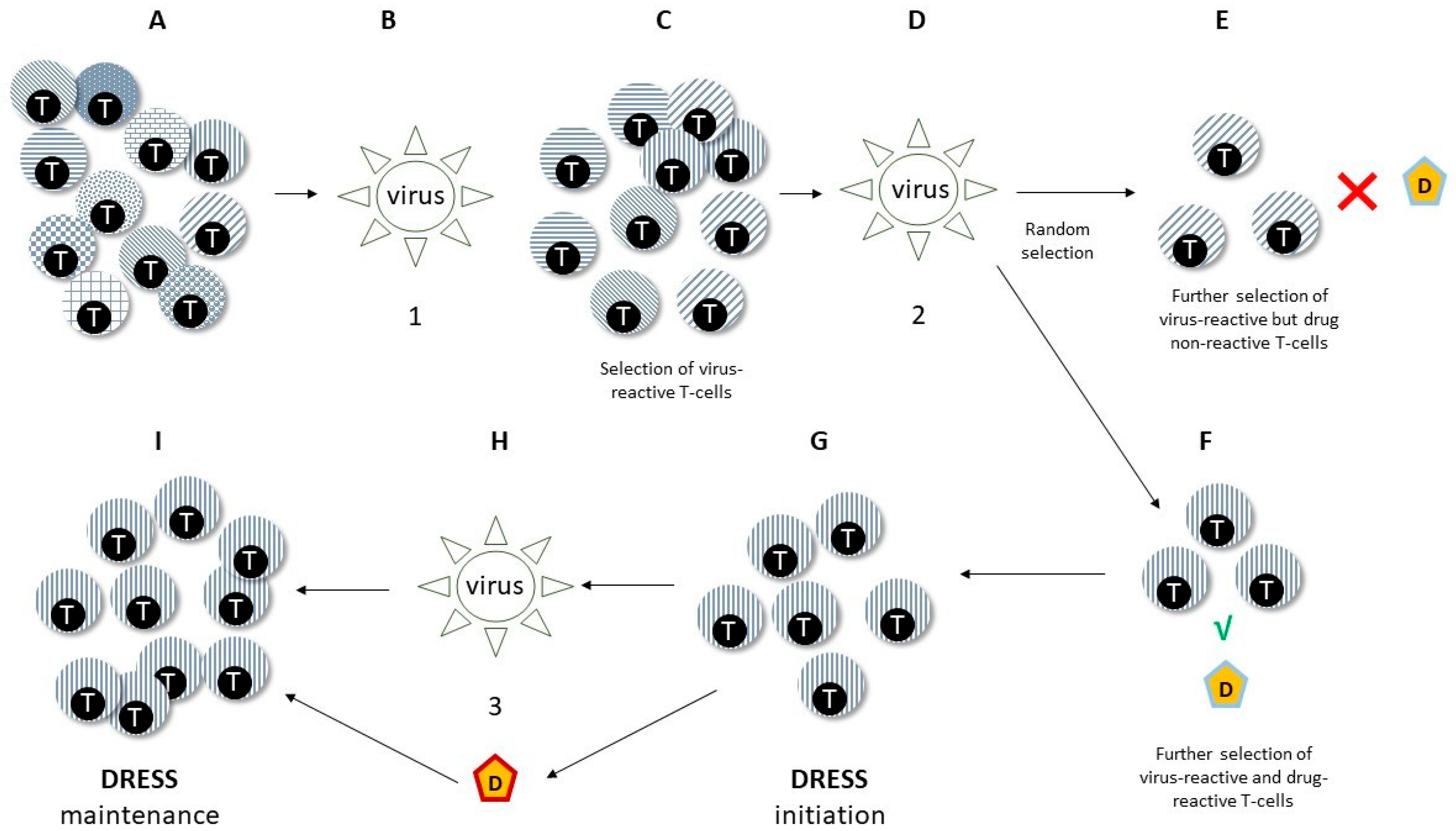
- Viral reactivation might constitute the epiphenomenon of a wider expansion of virus-harbouring immune cells in the setting of systemic inflammation. In fact, latent human herpesviruses (HHVs) chronically resides in cells of the immune system, including T-lymphocytes and cells of the monocyte/macrophage lineage. Thus, viral reactivation and release could represent an early marker of stimulation of these cell reservoirs following drug-driven expansion, rather than representing a trigger event of DRESS [52].
- Viruses might promote anti-drug responses and mis-differentiation of antigen-specific lymphocytes by molecular mimicry. In fact, T-lymphocytes previously selected and expanded by viral antigens might eventually be activated by drugs, inducing DRESS (see also below at Section 2.3.2) [53]. Furthermore, challenging EBV-immortalised B-lymphocytes from healthy subjects and from patients with DRESS with DRESS culprit drugs selectively prompts EBV production increases in DRESS subjects [54], suggesting generalised dysfunction of tolerance and pathogen control in both arms of the immune response during DRESS.
2.2.1. Human Herpesviruses (HHVs)
2.2.2. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2)
2.2.3. Other Viruses
2.2.4. Immunological Mechanisms of Virus Reactivation
2.3. T-Cell Responses
2.3.1. HLA
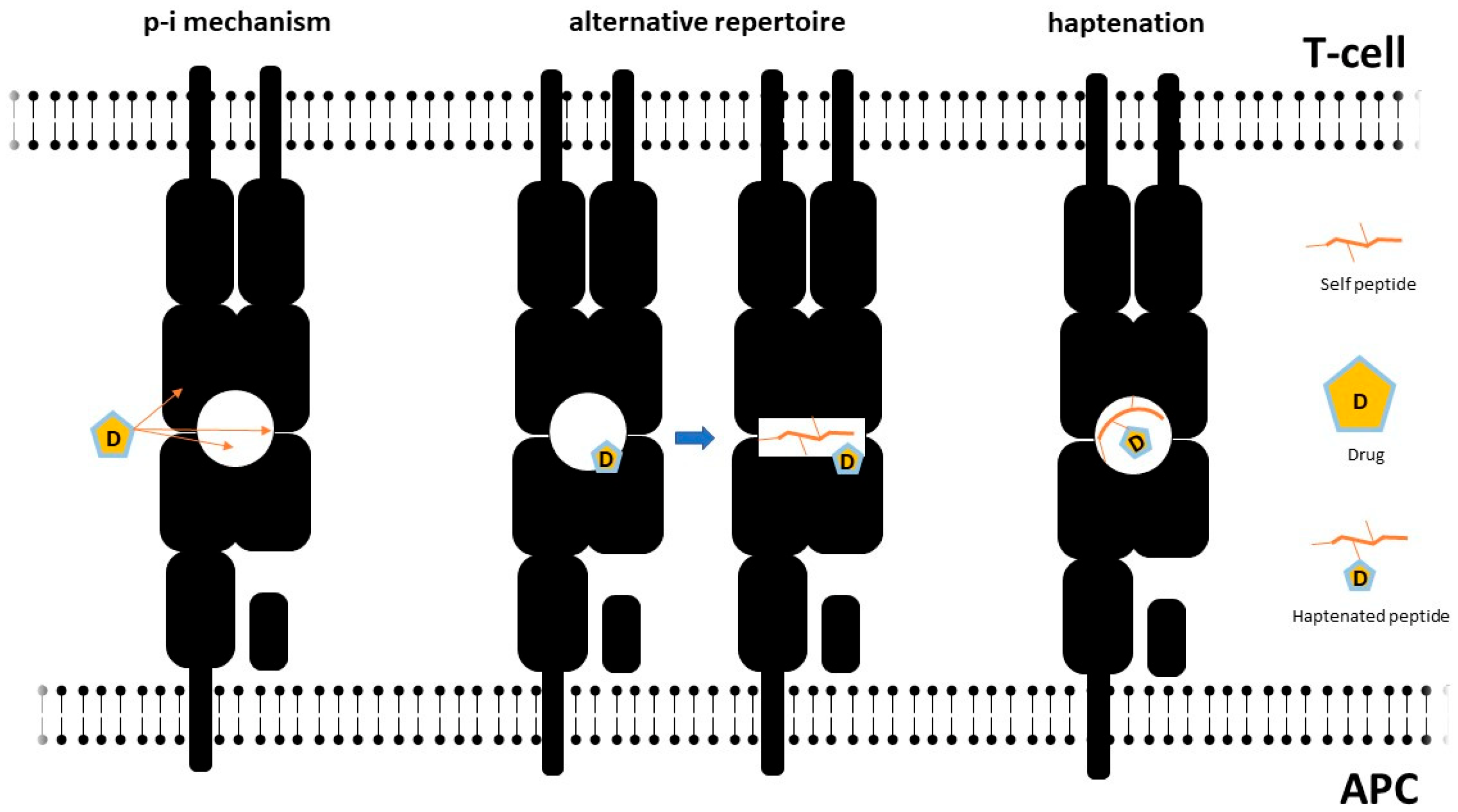
2.3.2. Molecular Mechanisms of T-Cell Activation and Aberrant HLA/TcR Interactions
2.3.3. T-Cell Polarisation and Functionality
2.4. Eosinophils
2.5. Pathophysiological Basis of DRESS Clinical Manifestations
3. Clinical Presentation and Laboratory Findings: When to Suspect DRESS
3.1. Systemic and Laboratory Findings
3.2. Cutaneous Manifestations
3.3. Internal Organ Involvement
3.3.1. Liver, Gastrointestinal, and Pancreatic Involvement
3.3.2. Kidney Involvement
3.3.3. Heart and Muscle Involvement
3.3.4. Lung Involvement
3.4. Nervous System Involvement
3.5. Other Manifestations
4. Diagnosis
4.1. Diagnostic Approach
4.2. Differential Diagnosis
4.3. Diagnostic and Prognostic Scoring Systems
5. Management
6. Final Remarks
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, H.; Du, W.; Gnjidic, D.; Chong, S.; Glasgow, N. Trends in Adverse Drug Reaction-Related Hospitalisations over 13 Years in New South Wales, Australia. Intern. Med. J. 2019, 49, 84–93. [Google Scholar] [CrossRef]
- Lee, S.; Hess, E.P.; Lohse, C.; Gilani, W.; Chamberlain, A.M.; Campbell, R.L. Trends, Characteristics, and Incidence of Anaphylaxis in 2001–2010: A Population-Based Study. J. Allergy Clin. Immunol. 2017, 139, 182–188.e2. [Google Scholar] [CrossRef]
- Zaidi, A.S.; Peterson, G.M.; Bereznicki, L.R.E.; Curtain, C.M.; Salahudeen, M.S. Ten-Year Trends in Adverse Drug Reaction-Related Hospitalizations among People with Dementia. Ther. Adv. Drug Saf. 2022, 13, 20420986221080796. [Google Scholar] [CrossRef]
- Classen, D.C.; Pestotnik, S.L.; Evans, R.S.; Lloyd, J.F.; Burke, J.P. Adverse Drug Events in Hospitalized Patients: Excess Length of Stay, Extra Costs, and Attributable Mortality. JAMA 1997, 277, 301–306. [Google Scholar] [CrossRef]
- Park, C.S.; Kim, T.B.; Kim, S.L.; Kim, J.Y.; Yang, K.A.; Bae, Y.J.; Cho, Y.S.; Moon, H.B. The Use of an Electronic Medical Record System for Mandatory Reporting of Drug Hypersensitivity Reactions Has Been Shown to Improve the Management of Patients in the University Hospital in Korea. Pharmacoepidemiol. Drug Saf. 2008, 17, 919–925. [Google Scholar] [CrossRef]
- Thong, B.Y.H.; Tan, T.C. Epidemiology and Risk Factors for Drug Allergy. Br. J. Clin. Pharmacol. 2011, 71, 684–700. [Google Scholar] [CrossRef]
- Chung, W.H.; Hung, S.I.; Hong, H.S.; Hsih, M.S.; Yang, L.C.; Ho, H.C.; Wu, J.Y.; Chen, Y.T. Medical Genetics: A Marker for Stevens-Johnson Syndrome. Nature 2004, 428, 486. [Google Scholar] [CrossRef]
- Lonjou, C.; Thomas, L.; Borot, N.; Ledger, N.; de Toma, C.; LeLouet, H.; Graf, E.; Schumacher, M.; Hovnanian, A.; Mockenhaupt, M.; et al. A Marker for Stevens-Johnson Syndrome …: Ethnicity Matters. Pharmacogenom. J. 2006, 6, 265–268. [Google Scholar] [CrossRef]
- Locharernkul, C.; Loplumlert, J.; Limotai, C.; Korkij, W.; Desudchit, T.; Tongkobpetch, S.; Kangwanshiratada, O.; Hirankarn, N.; Suphapeetiporn, K.; Shotelersuk, V. Carbamazepine and Phenytoin Induced Stevens-Johnson Syndrome Is Associated with HLA-B*1502 Allele in Thai Population. Epilepsia 2008, 49, 2087–2091. [Google Scholar] [CrossRef]
- Hung, S.L.; Chung, W.H.; Liou, L.B.; Chu, C.C.; Lin, M.; Huang, H.P.; Lin, Y.L.; Lan, J.L.; Yang, L.C.; Hong, H.S.; et al. HLA-B*5801 Allele as a Genetic Marker for Severe Cutaneous Adverse Reactions Caused by Allopurinol. Proc. Natl. Acad. Sci. USA 2005, 102, 4134–4139. [Google Scholar] [CrossRef]
- Ozeki, T.; Mushiroda, T.; Yowang, A.; Takahashi, A.; Kubo, M.; Shirakata, Y.; Ikezawa, Z.; Iijima, M.; Shiohara, T.; Hashimoto, K.; et al. Genome-Wide Association Study Identifies HLA-A*3101 Allele as a Genetic Risk Factor for Carbamazepine-Induced Cutaneous Adverse Drug Reactions in Japanese Population. Hum. Mol. Genet. 2011, 20, 1034–1041. [Google Scholar] [CrossRef]
- McCormack, M.; Alfirevic, A.; Bourgeois, S.; Farrell, J.J.; Kasperavičiūtė, D.; Carrington, M.; Sills, G.J.; Marson, T.; Jia, X.; de Bakker, P.I.W.; et al. HLA-A*3101 and Carbamazepine-Induced Hypersensitivity Reactions in Europeans. N. Engl. J. Med. 2011, 364, 1134–1143. [Google Scholar] [CrossRef]
- Pichler, W.J. Pharmacological Interaction of Drugs with Antigen-Specific Immune Receptors: The p-i Concept. Curr. Opin. Allergy Clin. Immunol. 2002, 2, 301–305. [Google Scholar] [CrossRef]
- Cacoub, P.; Musette, P.; Descamps, V.; Meyer, O.; Speirs, C.; Finzi, L.; Roujeau, J.C. The DRESS Syndrome: A Literature Review. Am. J. Med. 2011, 124, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Kardaun, S.H.; Sekula, P.; Valeyrie-Allanore, L.; Liss, Y.; Chu, C.Y.; Creamer, D.; Sidoroff, A.; Naldi, L.; Mockenhaupt, M.; Roujeau, J.C. Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS): An Original Multisystem Adverse Drug Reaction. Results from the Prospective RegiSCAR Study. Br. J. Dermatol. 2013, 169, 1071–1080. [Google Scholar] [CrossRef] [PubMed]
- Fiszenson-Albala, F.; Auzerie, V.; Make, E.; Farinotti, R.; Durand-Stocco, C.; Crickx, B.; Descamps, V. A 6-Month Prospective Survey of Cutaneous Drug Reactions in a Hospital Setting. Br. J. Dermatol. 2003, 149, 1018–1022. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, E.; Medrano-Casique, N.; Tong, H.Y.; Bellón, T.; Cabañas, R.; Fiandor, A.; González-Ramos, J.; Herranz, P.; Trigo, E.; Muñoz, M.; et al. Eosinophilic Drug Reactions Detected by a Prospective Pharmacovigilance Programme in a Tertiary Hospital. Br. J. Clin. Pharmacol. 2017, 83, 400–415. [Google Scholar] [CrossRef]
- Muller, P.; Dubreil, P.; Mahé, A.; Lamaury, I.; Salzer, B.; Deloumeaux, J.; Strobel, M. Drug Hypersensitivity Syndrome in a West-Indian Population. Eur. J. Dermatol. 2003, 13, 478–481. [Google Scholar]
- Bluestein, S.B.; Yu, R.; Stone, C.; Phillips, E.J. Reporting of Drug Reaction with Eosinophilia and Systemic Symptoms from 2002 to 2019 in the US Food and Drug Administration Adverse Event Reporting System. J. Allergy Clin. Immunol. Pract. 2021, 9, 3208–3211.e1. [Google Scholar] [CrossRef]
- Ramirez, G.A.; Della-Torre, E.; Tresoldi, M.; Scarpellini, P.; Ciceri, F.; Dagna, L.; Yacoub, M.R. Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) in Patients with COVID-19. Clin. Microbiol. Infect. 2021, 27, 1190–1192. [Google Scholar] [CrossRef]
- Schunkert, E.M.; Divito, S.J. Updates and Insights in the Diagnosis and Management of DRESS Syndrome. Curr. Dermatol. Rep. 2021, 10, 192–204. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.T.; Yang, C.W.; Chu, C.Y. Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS): An Interplay among Drugs, Viruses, and Immune System. Int. J. Mol. Sci. 2017, 18, 1243. [Google Scholar] [CrossRef] [PubMed]
- Cabañas, R.; Ramírez, E.; Sendagorta, E.; Alamar, R.; Barranco, R.; Blanca-López, N.; Doña, I.; Fernández, J.; Garcia-Nunez, I.; García-Samaniego, J.; et al. Spanish Guidelines for Diagnosis, Management, Treatment, and Prevention of DRESS Syndrome. J. Investig. Allergol. Clin. Immunol. 2020, 30, 229–253. [Google Scholar] [CrossRef] [PubMed]
- Soria, A.; Bernier, C.; Veyrac, G.; Barbaud, A.; Puymirat, E.; Milpied, B. Drug Reaction with Eosinophilia and Systemic Symptoms May Occur within 2 Weeks of Drug Exposure: A Retrospective Study. J. Am. Acad. Dermatol. 2020, 82, 606–611. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Lee, S.Y.; Hahm, J.E.; Ha, J.W.; Kim, C.W.; Kim, S.S. Clinical Features of Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) Syndrome: A Study of 25 Patients in Korea. Int. J. Dermatol. 2017, 56, 944–951. [Google Scholar] [CrossRef]
- Sharifzadeh, S.; Elyasi, S.; Mohammadpour, A.H. Antiviral Induced Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) Dyndrome: A Literature Review. J. Pharm. Care 2020, 8, 35–47. [Google Scholar] [CrossRef]
- Gangireddy, M.; Sarao, M.S.; Shrimanker, I.; Nookala, V.K. A Fatal Case of Vancomycin Associated Drug Reaction with Eosinophilia and Systemic Symptoms Syndrome in a Septuagenarian. Cureus 2019, 11, e5015. [Google Scholar] [CrossRef]
- Song, J.M.; Jung, Y.E.; Park, J.H.; Kim, M.D.; Cheon, M.S.; Lee, C.I. Neosensitization to Multiple Drugs Following Valproate-Induced Drug Reaction with Eosinophilia and Systemic Symptoms Syndrome. Psychiatry Investig. 2017, 14, 518–520. [Google Scholar] [CrossRef]
- Gentile, I.; Talamo, M.; Borgia, G. Is the Drug-Induced Hypersensitivity Syndrome (DIHS) Due to Human Herpesvirus 6 Infection or to Allergy-Mediated Viral Reactivation? Report of a Case and Literature Review. BMC Infect. Dis. 2010, 10, 49. [Google Scholar] [CrossRef]
- Chung, W.H.; Chang, W.C.; Lee, Y.S.; Wu, Y.Y.; Yang, C.H.; Ho, H.C.; Chen, M.J.; Lin, J.Y.; Hui, R.C.Y.; Ho, J.C.; et al. Genetic Variants Associated with Phenytoin-Related Severe Cutaneous Adverse Reactions. JAMA 2014, 312, 525–535. [Google Scholar] [CrossRef]
- Rieder, M.J.; Shear, N.H.; Kanee, A.; Tang, B.K.; Spielberg, S.P. Prominence of Slow Acetylator Phenotype among Patients with Sulfonamide Hypersensitivity Reactions. Clin. Pharmacol. Ther. 1991, 49, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Markel, A. Allopurinol-Induced DRESS Syndrome. Isr. Med. Assoc. J. 2005, 7, 656–660. [Google Scholar] [PubMed]
- Chou, H.Y.; Chen, C.B.; Cheng, C.Y.; Chen, Y.A.; Ng, C.Y.; Kuo, K.L.; Chen, W.L.; Chen, C.H. Febuxostat-Associated Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS). J. Clin. Pharm. Ther. 2015, 40, 689–692. [Google Scholar] [CrossRef] [PubMed]
- Pott Junior, H.; Gosuen, G.C.; Gales, A.C. DRESS Syndrome Due to Nevirapine Treated with Methylprednisolone. Case Rep. Med. 2013, 2013, 269501. [Google Scholar] [CrossRef]
- Mallal, S.; Phillips, E.; Carosi, G.; Molina, J.-M.; Workman, C.; Tomažič, J.; Jägel-Guedes, E.; Rugina, S.; Kozyrev, O.; Cid, J.F.; et al. HLA-B*5701 Screening for Hypersensitivity to Abacavir. N. Engl. J. Med. 2008, 358, 568–579. [Google Scholar] [CrossRef] [PubMed]
- Borrás-Blasco, J.; Navarro-Ruiz, A.; Borrás, C.; Casterá, E. Adverse Cutaneous Reactions Associated with the Newest Antiretroviral Drugs in Patients with Human Immunodeficiency Virus Infection. J. Antimicrob. Chemother. 2008, 62, 879–888. [Google Scholar] [CrossRef]
- Samain, A.; Duval-Modeste, A.B.; Joly, P.; Leblanc, C.; Massy, N.; Courville, P.; Goria, O.; Riachi, G. First Case of Drug Rash Eosinophilia and Systemic Symptoms Due to Boceprevir. J. Hepatol. 2014, 60, 891–893. [Google Scholar] [CrossRef]
- Roujeau, J.C.; Mockenhaupt, M.; Tahan, S.R.; Henshaw, J.; Martin, E.C.; Harding, M.; van Baelen, B.; Bengtsson, L.; Singhal, P.; Kauffman, R.S.; et al. Telaprevir-Related Dermatitis. JAMA Dermatol. 2013, 149, 152–158. [Google Scholar] [CrossRef]
- Saper, V.E.; Ombrello, M.J.; Tremoulet, A.H.; Montero-Martin, G.; Prahalad, S.; Canna, S.; Shimizu, C.; Deutsch, G.; Tan, S.Y.; Remmers, E.F.; et al. Severe Delayed Hypersensitivity Reactions to IL-1 and IL-6 Inhibitors Link to Common HLA-DRB1*15 Alleles. Ann. Rheum. Dis. 2022, 81, 406–415. [Google Scholar] [CrossRef]
- Kim, D.K.; Lee, S.W.; Nam, H.S.; Jeon, D.S.; Park, N.R.; Nam, Y.H.; Lee, S.K.; Baek, Y.H.; Han, S.Y.; Lee, S.W. A Case of Sorafenib-Induced DRESS Syndrome in Hepatocelluar Carcinoma. Korean J. Gastroenterol. 2016, 67, 337. [Google Scholar] [CrossRef]
- Thomas, C.L.; Arasaratnam, M.; Carlos, G.; Parasyn, A.; Baumgart, K.W.; Fernandez-Penas, P.; Marx, G. Drug Reaction with Eosinophilia and Systemic Symptoms in Metastatic Basal Cell Carcinoma Treated with Vismodegib. Australas. J. Dermatol. 2017, 58, 69–70. [Google Scholar] [CrossRef]
- Peuvrel, L.; Quéreux, G.; Saint-Jean, M.; Brocard, A.; Nguyen, J.M.; Khammari, A.; Knol, A.C.; Varey, E.; Dréno, B. Profile of Vemurafenib-Induced Severe Skin Toxicities. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 250–257. [Google Scholar] [CrossRef]
- Radu, C.; Barnig, C.; de Blay, F. Rivaroxaban-Induced Drug Reaction With Eosinophilia and Systemic Symptoms. J. Investig. Allergol. Clin. Immunol. 2016, 26, 124–126. [Google Scholar] [CrossRef] [PubMed]
- Volpe, A.; Marchetta, A.; Caramaschi, P.; Biasi, D.; Bambara, L.M.; Arcaro, G. Hydroxychloroquine-Induced DRESS Syndrome. Clin. Rheumatol. 2008, 27, 537–539. [Google Scholar] [CrossRef] [PubMed]
- Shiohara, T.; Iijima, M.; Ikezawa, Z.; Hashimoto, K. The Diagnosis of a DRESS Syndrome Has Been Sufficiently Established on the Basis of Typical Clinical Features and Viral Reactivations. Br. J. Dermatol. 2007, 156, 1083–1084. [Google Scholar] [CrossRef] [PubMed]
- Tohyama, M.; Hashimoto, K.; Oda, F.; Namba, C.; Sayama, K. Influence of Corticosteroid Therapy on Viral Reactivation in Drug-Induced Hypersensitivity Syndrome/Drug Reaction with Eosinophilia and Systemic Symptoms. J. Dermatol. 2020, 47, 476–482. [Google Scholar] [CrossRef]
- Descamps, V.; Brunet-Possenti, F. Monitoring of Human Herpesvirus 6 Infection in the Management of Drug Reaction with Eosinophilia and Systemic Symptoms. Clin. Exp. Dermatol. 2021, 46, 351–352. [Google Scholar] [CrossRef]
- Tohyama, M.; Hashimoto, K.; Yasukawa, M.; Kimura, H.; Horikawa, T.; Nakajima, K.; Urano, Y.; Matsumoto, K.; Iijima, M.; Shear, N.H. Association of Human Herpesvirus 6 Reactivation with the Flaring and Severity of Drug-Induced Hypersensitivity Syndrome. Br. J. Dermatol. 2007, 157, 934–940. [Google Scholar] [CrossRef]
- Miyagawa, F.; Asada, H. Current Perspective Regarding the Immunopathogenesis of Drug-Induced Hypersensitivity Syndrome/Drug Reaction with Eosinophilia and Systemic Symptoms (DIHS/DRESS). Int. J. Mol. Sci. 2021, 22, 2147. [Google Scholar] [CrossRef]
- Drago, F.; Cogorno, L.; Broccolo, F.; Ciccarese, G.; Parodi, A. A Fatal Case of DRESS Induced by Strontium Ranelate Associated with HHV-7 Reactivation. Osteoporos. Int. 2016, 27, 1261–1264. [Google Scholar] [CrossRef]
- Ahluwalia, J.; Abuabara, K.; Perman, M.J.; Yan, A.C. Human Herpesvirus 6 Involvement in Paediatric Drug Hypersensitivity Syndrome. Br. J. Dermatol. 2015, 172, 1090–1095. [Google Scholar] [CrossRef]
- Roujeau, J.C.; Dupin, N. Virus Reactivation in Drug Reaction with Eosinophilia and Systemic Symptoms (Dress) Results from a Strong Drug-Specific Immune Response. J. Allergy Clin. Immunol. Pract. 2017, 5, 811–812. [Google Scholar] [CrossRef]
- Stirton, H.; Shear, N.H.; Dodiuk-Gad, R.P. Drug Reaction with Eosinophilia and Systemic Symptoms (DReSS)/Drug-Induced Hypersensitivity Syndrome (DiHS)-Readdressing the DReSS. Biomedicines 2022, 10, 999. [Google Scholar] [CrossRef] [PubMed]
- Picard, D.; Janela, B.; Descamps, V.; D’Incan, M.; Courville, P.; Jacquot, S.; Rogez, S.; Mardivirin, L.; Moins-Teisserenc, H.; Toubert, A.; et al. Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS): A Multiorgan Antiviral T Cell Response. Sci. Transl. Med. 2010, 2, 46ra62. [Google Scholar] [CrossRef]
- Hama, N.; Abe, R.; Gibson, A.; Phillips, E.J. Drug-Induced Hypersensitivity Syndrome (DIHS)/Drug Reaction With Eosinophilia and Systemic Symptoms (DRESS): Clinical Features and Pathogenesis. J. Allergy Clin. Immunol. Pract. 2022, 10, 1155–1167.e5. [Google Scholar] [CrossRef] [PubMed]
- Shiohara, T.; Mizukawa, Y. Drug-Induced Hypersensitivity Syndrome (DiHS)/Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS): An Update in 2019. Allergol. Int. 2019, 68, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, H. Recent Advances in Drug-Induced Hypersensitivity Syndrome/Drug Reaction with Eosinophilia and Systemic Symptoms. J. Immunol. Res. 2018, 2018, 5163129. [Google Scholar] [CrossRef]
- Anci, E.; Braun, C.; Marinosci, A.; Rodieux, F.; Midun, E.; Torres, M.J.; Caubet, J.C. Viral Infections and Cutaneous Drug-Related Eruptions. Front. Pharmacol. 2021, 11, 586407. [Google Scholar] [CrossRef]
- Kagoyama, K.; Makino, T.; Ueda, C.; Takegami, Y.; Shimizu, T. Detection of Cytomegalovirus in the Gastric Ulcer of a Patient with Drug-Induced Hypersensitivity Syndrome. JAAD Case Rep. 2015, 1, 215–218. [Google Scholar] [CrossRef] [PubMed]
- Ganeshanandan, L.; Lucas, M. Drug Reaction with Eosinophilia and Systemic Symptoms: A Complex Interplay between Drug, T Cells, and Herpesviridae. Int. J. Mol. Sci. 2021, 22, 1127. [Google Scholar] [CrossRef]
- Kardaun, S.H.; Sidoroff, A.; Valeyrie-Allanore, L.; Halevy, S.; Davidovici, B.B.; Mockenhaupt, M.; Roujeau, J.C. Variability in the Clinical Pattern of Cutaneous Side-Effects of Drugs with Systemic Symptoms: Does a DRESS Syndrome Really Exist? Br. J. Dermatol. 2007, 156, 609–611. [Google Scholar] [CrossRef] [PubMed]
- Seishima, M.; Yamanaka, S.; Fujisawa, T.; Tohyama, M.; Hashimoto, K. Reactivation of Human Herpesvirus (HHV) Family Members Other than HHV-6 in Drug-Induced Hypersensitivity Syndrome. Br. J. Dermatol. 2006, 155, 344–349. [Google Scholar] [CrossRef]
- Kano, Y.; Hiraharas, K.; Sakuma, K.; Shiohara, T. Several Herpesviruses Can Reactivate in a Severe Drug-Induced Multiorgan Reaction in the Same Sequential Order as in Graft-versus-Host Disease. Br. J. Dermatol. 2006, 155, 301–306. [Google Scholar] [CrossRef]
- Suzuki, Y.; Inagi, R.; Aono, T.; Yamanishi, K.; Shiohara, T. Human Herpesvirus 6 Infection as a Risk Factor for the Development of Severe Drug-Induced Hypersensitivity Syndrome. Arch. Dermatol. 1998, 134, 1108–1112. [Google Scholar] [CrossRef]
- Ishida, T.; Kano, Y.; Mizukawa, Y.; Shiohara, T. The Dynamics of Herpesvirus Reactivations during and after Severe Drug Eruptions: Their Relation to the Clinical Phenotype and Therapeutic Outcome. Allergy 2014, 69, 798–805. [Google Scholar] [CrossRef]
- Chen, Y.C.; Chiang, H.H.; Cho, Y.T.; Chang, C.Y.; Chen, K.L.; Yang, C.W.; Lee, Y.H.; Chu, C.Y. Human Herpes Virus Reactivations and Dynamic Cytokine Profiles in Patients with Cutaneous Adverse Drug Reactions—A Prospective Comparative Study. Allergy 2015, 70, 568–575. [Google Scholar] [CrossRef]
- Pritchett, J.C.; Nanau, R.M.; Neuman, M.G. The Link between Hypersensitivity Syndrome Reaction Development and Human Herpes Virus-6 Reactivation. Int. J. Hepatol. 2012, 2012, 723062. [Google Scholar] [CrossRef]
- Shiohara, T.; Takahashi, R.; Kano, Y. Drug-Induced Hypersensitivity Syndrome and Viral Reactivation. Drug Hypersensit. 2007, 251–266. [Google Scholar] [CrossRef]
- Chiou, C.C.; Chung, W.H.; Hung, S.I.; Yang, L.C.; Hong, H.S. Fulminant Type 1 Diabetes Mellitus Caused by Drug Hypersensitivity Syndrome with Human Herpesvirus 6 Infection. J. Am. Acad. Dermatol. 2006, 54 (Suppl. S2), S14–S17. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.S.; Kiuchi, Y.; Inomata, N.; Sueki, H. Increased Expression of Human Herpes Virus 6 Receptor CD134/OX40 in Skin Lesions of Patients with Drug-Induced Hypersensitivity Syndrome/Drug Reaction with Eosinophilia and Systemic Symptoms. J. Dermatol. 2022. [Google Scholar] [CrossRef]
- Hall, C.; Caserta, M.T.; Schnabel, K.; Shelley, L.; Marino, A.S.; Carnahan, J.A.; Yoo, C.; Lofthus, G.K.; McDermott, M.P. Chromosomal Integration of Human Herpesvirus 6 Is the Major Mode of Congenital Human Herpesvirus 6 Infection. Pediatrics 2008, 122, 513–520. [Google Scholar] [CrossRef]
- Watanabe, H.; Daibata, M.; Tohyama, M.; Batchelor, J.; Hashimoto, K.; Iijima, M. Chromosomal Integration of Human Herpesvirus 6 DNA in Anticonvulsant Hypersensitivity Syndrome. Br. J. Dermatol. 2008, 158, 640–642. [Google Scholar] [CrossRef]
- Nanishi, E.; Hoshina, T.; Ohga, S.; Nishio, H.; Hara, T. Drug Reaction with Eosinophilia and Systemic Symptoms during Primary Epstein-Barr Virus Infection. J. Microbiol. Immunol. Infect. 2015, 48, 109–112. [Google Scholar] [CrossRef]
- Aouam, K.; Bel Hadj Ali, H.; Youssef, M.; Chaabane, A.; Amri, M.; Boughattas, N.A.; Zili, J.E. Carbamazepine-Induced DRESS and HHV6 Primary Infection: The Importance of Skin Tests. Epilepsia 2008, 49, 1630–1633. [Google Scholar] [CrossRef]
- Miyagawa, F.; Nakamura, Y.; Ommori, R.; Miyashita, K.; Iioka, H.; Miyashita, N.; Nishikawa, M.; Himuro, Y.; Ogawa, K.; Asada, H. Predominant Contribution of CD4 T Cells to Human Herpesvirus 6 (HHV-6) Load in the Peripheral Blood of Patients with Drug-Induced Hypersensitivity Syndrome and Persistent HHV-6 Infection. Acta Derm. Venereol. 2018, 98, 146–148. [Google Scholar] [CrossRef]
- Kanatani, Y.; Miyagawa, F.; Ogawa, K.; Arima, A.; Asada, H. Parallel Changes in Serum Thymus and Activation-Regulated Chemokine Levels in Response to Flare-Ups in Drug-Induced Hypersensitivity Syndrome. J. Dermatol. 2020, 47, e417–e419. [Google Scholar] [CrossRef]
- Saraya, T.; Mikoshiba, M.; Kamiyama, H.; Yoshizumi, M.; Tsuchida, S.; Tsukagoshi, H.; Ishioka, T.; Terada, M.; Tanabe, E.; Tomioka, C.; et al. Evidence for Reactivation of Human Herpesvirus 6 in Generalized Lymphadenopathy in a Patient with Drug-Induced Hypersensitivity Syndrome. J. Clin. Microbiol. 2013, 51, 1979–1982. [Google Scholar] [CrossRef]
- Miyashita, K.; Shobatake, C.; Miyagawa, F.; Kobayashi, N.; Onmori, R.; Yonekawa, S.; Tanabe, K.; Kawate, K.; Morita, K.; Asada, H. Involvement of Human Herpesvirus 6 Infection in Renal Dysfunction Associated with DIHS/DRESS. Acta Derm. Venereol. 2016, 96, 114–115. [Google Scholar] [CrossRef]
- Miyashita, K.; Miyagawa, F.; Nakamura, Y.; Ommori, R.; Azukizawa, H.; Asada, H. Up-Regulation of Human Herpesvirus 6B-Derived MicroRNAs in the Serum of Patients with Drug-Induced Hypersensitivity Syndrome/Drug Reaction with Eosinophilia and Systemic Symptoms. Acta Derm. Venereol. 2018, 98, 612–613. [Google Scholar] [CrossRef]
- Shiohara, T.; Inaoka, M.; Kano, Y. Drug-Induced Hypersensitivity Syndrome(DIHS): A Reaction Induced by a Complex Interplay among Herpesviruses and Antiviral and Antidrug Immune Responses. Allergol. Int. 2006, 55, 1–8. [Google Scholar] [CrossRef]
- Kurata, M.; Shiohara, T. Herpes Simplex Virus Reactivation: Is It Common or Rare in Drug-Induced Hypersensitivity Syndrome/Drug Reaction with Eosinophilia and Systemic Symptoms? Eur. J. Dermatol. 2017, 27, 658–659. [Google Scholar] [CrossRef] [PubMed]
- Tsutsumi, R.; Adachi, K.; Yoshida, Y.; Yamamoto, O. Drug-Induced Hypersensitivity Syndrome in Association with Varicella. Acta Derm. Venereol. 2015, 95, 503–504. [Google Scholar] [CrossRef]
- Wong, Y.J.; Choo, K.J.L.; Soh, J.X.J.; Tan, C.K. Cytomegalovirus (CMV) Hepatitis: An Uncommon Complication of CMV Reactivation in Drug Reaction with Eosinophilia and Systemic Symptoms. Singap. Med. J. 2018, 59, 112–113. [Google Scholar] [CrossRef] [PubMed]
- Lachance, P.; Chen, J.; Featherstone, R.; Sligl, W.I. Association Between Cytomegalovirus Reactivation and Clinical Outcomes in Immunocompetent Critically Ill Patients: A Systematic Review and Meta-Analysis. Open Forum Infect. Dis. 2017, 4, ofx029. [Google Scholar] [CrossRef]
- Schildermans, J.; de Vlieger, G. Cytomegalovirus: A Troll in the ICU? Overview of the Literature and Perspectives for the Future. Front. Med. 2020, 7, 188. [Google Scholar] [CrossRef] [PubMed]
- Papazian, L.; Jaber, S.; Hraiech, S.; Baumstarck, K.; Cayot-Constantin, S.; Aissaoui, N.; Jung, B.; Leone, M.; Souweine, B.; Schwebel, C.; et al. Preemptive Ganciclovir for Mechanically Ventilated Patients with Cytomegalovirus Reactivation. Ann. Intensive Care 2021, 11, 33. [Google Scholar] [CrossRef]
- Limaye, A.P.; Stapleton, R.D.; Peng, L.; Gunn, S.R.; Kimball, L.E.; Hyzy, R.; Exline, M.C.; Files, D.C.; Morris, P.E.; Frankel, S.K.; et al. Effect of Ganciclovir on IL-6 Levels Among Cytomegalovirus-Seropositive Adults With Critical Illness: A Randomized Clinical Trial. JAMA 2017, 318, 731–740. [Google Scholar] [CrossRef]
- Cowley, N.J.; Owen, A.; Shiels, S.C.; Millar, J.; Woolley, R.; Ives, N.; Osman, H.; Moss, P.; Bion, J.F. Safety and Efficacy of Antiviral Therapy for Prevention of Cytomegalovirus Reactivation in Immunocompetent Critically Ill Patients: A Randomized Clinical Trial. JAMA Intern. Med. 2017, 177, 774–783. [Google Scholar] [CrossRef]
- Herman, A.; Matthews, M.; Mairlot, M.; Nobile, L.; Fameree, L.; Jacquet, L.M.; Baeck, M. Drug Reaction with Eosinophilia and Systemic Symptoms Syndrome in a Patient with COVID-19. J. Eur. Acad. Dermatol. Venereol. 2020, 34, e700–e768. [Google Scholar] [CrossRef]
- Grandolfo, M.; Romita, P.; Bonamonte, D.; Cazzato, G.; Hansel, K.; Stingeni, L.; Conforti, C.; Giuffrida, R.; Foti, C. Drug Reaction with Eosinophilia and Systemic Symptoms Syndrome to Hydroxychloroquine, an Old Drug in the Spotlight in the COVID-19 Era. Dermatol. Ther. 2020, 33, e13499. [Google Scholar] [CrossRef]
- Minenna, E.; Chaoul, N.; Rossi, M.P.; Giliberti, L.; Albanesi, M.; Nettis, E.; Foschino Barbaro, M.P.; di Bona, D.; Caiaffa, M.F.; Macchia, L. Sustained Drug-Related Reaction with Eosinophilia and Systemic Symptoms (DRESS) Triggered by Low Molecular Weight Heparins in COVID-19: Management and Precision Diagnosis. Postepy Dermatol. Alergol. 2022, 39, 816–818. [Google Scholar] [CrossRef] [PubMed]
- Brunasso, A.M.G.; Castellaneta, M.; Pontali, E.; Raggi, F.; Massone, C. Follow-up of Skin Lesions during COVID-19: A Description of a DRESS Case. Arch. Dermatol. Res. 2021, 313, 607–610. [Google Scholar] [CrossRef] [PubMed]
- Mata, L.; Torres-Zevallos, H.; Guerreros, A.G. Life-Threatening DRESS Syndrome with Kidney Damage Following Severe COVID-19 in a Patient with Down Syndrome. BMJ Case Rep. 2021, 14, e241418. [Google Scholar] [CrossRef]
- Castro Jiménez, A.; Navarrete Navarrete, N.; Gratacós Gómez, A.R.; Florido López, F.; García Rodríguez, R.; Gómez Torrijos, E. First Case of DRESS Syndrome Caused by Hydroxychloroquine with a Positive Patch Test. Contact Dermat. 2021, 84, 50–51. [Google Scholar] [CrossRef]
- Balconi, S.N.; Lopes, N.T.; Luzzatto, L.; Bonamigo, R.R. Detection of SARS-CoV-2 in a Case of DRESS by Sulfasalazine: Could There Be a Relationship with Clinical Importance? Int. J. Dermatol. 2021, 60, 125–126. [Google Scholar] [CrossRef] [PubMed]
- Schmid-Grendelmeier, P.; Steiger, P.; Naegeli, M.C.; Kolm, I.; Lang, C.C.V.; Maverakis, E.; Brüggen, M.C. Benralizumab for Severe DRESS in Two COVID-19 Patients. J. Allergy Clin. Immunol. Pract. 2021, 9, 481–483.e2. [Google Scholar] [CrossRef]
- Cruz, V.B.; Júnior, L.F.F.F.; Kobal, C.R.; da Silva, N.A. Does Sensitization by SARS-CoV-2 Immune Complexes Trigger DRESS Syndrome? Braz. J. Infect. Dis. 2022, 26. [Google Scholar] [CrossRef]
- Mesli, F.; Dumont, M.; Soria, A.; Groh, M.; Turpin, M.; Voiriot, G.; Rafat, C.; Sallé, D.S.; Gibelin, A.; Desnos, C. Benralizumab: A Potential Tailored Treatment for Life-Threatening DRESS in the COVID-19 Era. J. Allergy Clin. Immunol. Pract. 2021, 9, 3529–3531.e1. [Google Scholar] [CrossRef]
- Cucka, B.; Biglione, B.; Zhou, L.; Phillips, E.J.; Bassir, F.; Samarakoon, U.; Rrapi, R.; Chand, S.; Wang, L.; Alvarez-Arango, S.; et al. Drug Reaction with Eosinophilia and Systemic Symptoms in Patients Hospitalized with COVID-19: A Case Series from a Large US Healthcare System. Br. J. Dermatol. 2022, 187, 619–622. [Google Scholar] [CrossRef]
- Schmutz, J.L.; Barbaud, A.; Tréchot, P. Hydroxychloroquine and Dress. Ann. Dermatol. Venereol. 2008, 135, 903. [Google Scholar] [CrossRef]
- Mitamura, Y.; Schulz, D.; Oro, S.; Li, N.; Kolm, I.; Lang, C.; Ziadlou, R.; Tan, G.; Bodenmiller, B.; Steiger, P.; et al. Cutaneous and Systemic Hyperinflammation Drives Maculopapular Drug Exanthema in Severely Ill COVID-19 Patients. Allergy 2022, 77, 595–608. [Google Scholar] [CrossRef]
- Lang, C.C.V.; Schmid-Grendelmeier, P.; Maverakis, E.; Brüggen, M.C. Reply to “Benralizumab: A Potential Tailored Treatment for Life-Threatening DRESS in the COVID-19 Era”. J. Allergy Clin. Immunol. Pract. 2021, 9, 3531–3532. [Google Scholar] [CrossRef] [PubMed]
- Brooks, B.; Tancredi, C.; Song, Y.; Mogus, A.T.; Huang, M.L.W.; Zhu, H.; Phan, T.L.; Zhu, H.; Kadl, A.; Woodfolk, J.; et al. Epstein-Barr Virus and Human Herpesvirus-6 Reactivation in Acute COVID-19 Patients. Viruses 2022, 14, 1872. [Google Scholar] [CrossRef] [PubMed]
- Simonnet, A.; Engelmann, I.; Moreau, A.S.; Garcia, B.; Six, S.; el Kalioubie, A.; Robriquet, L.; Hober, D.; Jourdain, M. High Incidence of Epstein-Barr Virus, Cytomegalovirus, and Human-Herpes Virus-6 Reactivations in Critically Ill Patients with COVID-19. Infect. Dis. Now 2021, 51, 296–299. [Google Scholar] [CrossRef] [PubMed]
- Naendrup, J.H.; Garcia Borrega, J.; Eichenauer, D.A.; Shimabukuro-Vornhagen, A.; Kochanek, M.; Böll, B. Reactivation of EBV and CMV in Severe COVID-19-Epiphenomena or Trigger of Hyperinflammation in Need of Treatment? A Large Case Series of Critically Ill Patients. J. Intensive Care Med. 2022, 37, 1152–1158. [Google Scholar] [CrossRef]
- Takeno, A.; Kanazawa, I.; Morita, M.; Takedani, K.; Miyake, H.; Yamamoto, M.; Nogami, K.; Kaneko, S.; Sugimoto, T. A Case Report of Fulminant Type 1 Diabetes Mellitus Associated with Drug-Induced Hypersensitivity Syndrome in an Elderly Patient with Coxsackie B4 Virus Infection and Human Leukocyte Antigen-A24 Haplotype. Endocr. J. 2018, 65, 129–132. [Google Scholar] [CrossRef] [PubMed]
- Girijala, R.L.; Ramamurthi, A.; Wright, D.; Kwak, Y.; Goldberg, L.H. DRESS Syndrome Associated with Influenza Virus. Proc. (Bayl. Univ. Med. Cent.) 2019, 32, 277–278. [Google Scholar] [CrossRef]
- Sil, A.; Bhattacharjee, M.S.; Chandra, A.; Pramanik, J.D. Sulfasalazine-Induced Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) with Concomitant Acute Chikungunya Virus Infection: Possible Role of New Viral Trigger. BMJ Case Rep. 2021, 14, e244063. [Google Scholar] [CrossRef]
- Panigrahi, A.; Chakraborty, S.; Sil, A. Chik Sign in Chikungunya Fever. Infection 2021, 49, 1075–1076. [Google Scholar] [CrossRef] [PubMed]
- Sil, A.; Biswas, S.K.; Bhanja, D.B.; Das, S.; Panigrahi, A. Post-Chikungunya Hyperpigmentation. Postgrad. Med. J. 2021, 97, 59–60. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A. Hyperpigmentation and Chikungunya Fever. An. Bras. Dermatol. 2016, 91, 860–861. [Google Scholar] [CrossRef]
- Trubiano, J.A.; Gordon, C.L.; Castellucci, C.; Christo, S.N.; Park, S.L.; Mouhtouris, E.; Konvinse, K.; Rose, M.; Goh, M.; Boyd, A.S.; et al. Analysis of Skin-Resident Memory T Cells Following Drug Hypersensitivity Reactions. J. Investig. Dermatol. 2020, 140, 1442–1445.e4. [Google Scholar] [CrossRef]
- Chakraborty, U.; Biswas, P.; Chandra, A.; Pal, J.; Ray, A.K. Chik Sign: Post-Chikungunya Hyperpigmentation. QJM 2021, 114, 137–138. [Google Scholar] [CrossRef]
- Thomas, M.; Hopkins, C.; Duffy, E.; Lee, D.; Loulergue, P.; Ripamonti, D.; Ostrov, D.A.; Phillips, E. Association of the HLA-B*53:01 Allele With Drug Reaction With Eosinophilia and Systemic Symptoms (DRESS) Syndrome During Treatment of HIV Infection With Raltegravir. Clin. Infect. Dis. 2017, 64, 1198–1203. [Google Scholar] [CrossRef]
- Gill, S.; Sagar, A.; Shankar, S.; Nair, V. Nevirapine-Induced Rash with Eosinophilia and Systemic Symptoms (DRESS). Indian J. Pharmacol. 2013, 45, 401–402. [Google Scholar] [CrossRef]
- Lehloenya, R.J.; Dlamini, S.; Muloiwa, R.; Kakande, B.; Ngwanya, M.R.; Todd, G.; Dheda, K. Therapeutic Trial of Rifabutin After Rifampicin-Associated DRESS Syndrome in Tuberculosis-Human Immunodeficiency Virus Coinfected Patients. Open Forum Infect. Dis. 2016, 3, ofw130. [Google Scholar] [CrossRef]
- Walsh, S.A.; Creamer, D. Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS): A Clinical Update and Review of Current Thinking. Clin. Exp. Dermatol. 2011, 36, 6–11. [Google Scholar] [CrossRef]
- Aihara, Y.; Ito, S.I.; Kobayashi, Y.; Yamakawa, Y.; Aihara, M.; Yokota, S. Carbamazepine-Induced Hypersensitivity Syndrome Associated with Transient Hypogammaglobulinaemia and Reactivation of Human Herpesvirus 6 Infection Demonstrated by Real-Time Quantitative Polymerase Chain Reaction. Br. J. Dermatol. 2003, 149, 165–169. [Google Scholar] [CrossRef]
- Kano, Y.; Inaoka, M.; Shiohara, T. Association between Anticonvulsant Hypersensitivity Syndrome and Human Herpesvirus 6 Reactivation and Hypogammaglobulinemia. Arch. Dermatol. 2004, 140, 183–188. [Google Scholar] [CrossRef]
- Miyagawa, F.; Nakamura-Nishimura, Y.; Kanatani, Y.; Asada, H. Correlation Between Expression of CD134, a Human Herpesvirus 6 Cellular Receptor, on CD4+ T Cells and Th2-Type Immune Responses in Drug-Induced Hypersensitivity Syndrome/Drug Reaction with Eosinophilia and Systemic Symptoms. Acta Derm. Venereol. 2020, 100, adv00102. [Google Scholar] [CrossRef]
- Lu, J.; Thuraisingam, T.; Chergui, M.; Nguyen, K. Nivolumab-Associated DRESS Syndrome: A Case Report. JAAD Case Rep. 2019, 5, 216–218. [Google Scholar] [CrossRef]
- Ai, L.; Gao, J.; Zhao, S.; Li, Q.; Cui, Y.H.; Liu, Q.; Wu, D.; Wang, Y.; Jin, X.; Ji, Y.; et al. Nivolumab-Associated DRESS in a Genetic Susceptible Individual. J. Immunother. Cancer 2021, 9, e002879. [Google Scholar] [CrossRef]
- Sugita, K.; Tohyama, M.; Watanabe, H.; Otsuka, A.; Nakajima, S.; Iijima, M.; Hashimoto, K.; Tokura, Y.; Miyachi, Y.; Kabashima, K. Fluctuation of Blood and Skin Plasmacytoid Dendritic Cells in Drug-Induced Hypersensitivity Syndrome. J. Allergy Clin. Immunol. 2010, 126, 408–410. [Google Scholar] [CrossRef]
- Mizukawa, Y.; Kimishima, M.; Aoyama, Y.; Shiohara, T. Predictive Biomarkers for Cytomegalovirus Reactivation before and after Immunosuppressive Therapy: A Single-Institution Retrospective Long-Term Analysis of Patients with Drug-Induced Hypersensitivity Syndrome (DiHS)/Drug Reaction with Eosinophilia and Systemic Syndrome (DRESS). Int. J. Infect. Dis. 2020, 100, 239–246. [Google Scholar] [CrossRef]
- Farrell, J.; Lichtenfels, M.; Sullivan, A.; Elliott, E.C.; Alfirevic, A.; Stachulski, A.V.; Pirmohamed, M.; Naisbitt, D.J.; Park, B.K. Activation of Carbamazepine-Responsive T-Cell Clones with Metabolically Inert Halogenated Derivatives. J. Allergy Clin. Immunol. 2013, 132, 493–495. [Google Scholar] [CrossRef]
- Ye, Y.M.; Hur, G.Y.; Kim, S.H.; Ban, G.Y.; Jee, Y.K.; Naisbitt, D.J.; Park, H.S.; Kim, S.H. Drug-Specific CD4+ T-Cell Immune Responses Are Responsible for Antituberculosis Drug-Induced Maculopapular Exanthema and Drug Reaction with Eosinophilia and Systemic Symptoms Syndrome. Br. J. Dermatol. 2017, 176, 378–386. [Google Scholar] [CrossRef]
- Nicoletti, P.; Barrett, S.; McEvoy, L.; Daly, A.K.; Aithal, G.; Lucena, M.I.; Andrade, R.J.; Wadelius, M.; Hallberg, P.; Stephens, C.; et al. Shared Genetic Risk Factors Across Carbamazepine-Induced Hypersensitivity Reactions. Clin. Pharmacol. Ther. 2019, 106, 1028–1036. [Google Scholar] [CrossRef]
- Phillips, E.J.; Chung, W.H.; Mockenhaupt, M.; Roujeau, J.C.; Mallal, S.A. Drug Hypersensitivity: Pharmacogenetics and Clinical Syndromes. J. Allergy Clin. Immunol. 2011, 127 (Suppl. S3), S60–S66. [Google Scholar] [CrossRef]
- Konvinse, K.C.; Trubiano, J.A.; Pavlos, R.; James, I.; Shaffer, C.M.; Bejan, C.A.; Schutte, R.J.; Ostrov, D.A.; Pilkinton, M.A.; Rosenbach, M.; et al. HLA-A*32:01 Is Strongly Associated with Vancomycin-Induced Drug Reaction with Eosinophilia and Systemic Symptoms. J. Allergy Clin. Immunol. 2019, 144, 183–192. [Google Scholar] [CrossRef]
- Chen, C.B.; Abe, R.; Pan, R.Y.; Wang, C.W.; Hung, S.I.; Tsai, Y.G.; Chung, W.H. An Updated Review of the Molecular Mechanisms in Drug Hypersensitivity. J. Immunol. Res. 2018, 2018, 6431694. [Google Scholar] [CrossRef]
- Ramírez, E.; Bellón, T.; Tong, H.Y.; Borobia, A.M.; de Abajo, F.J.; Lerma, V.; Moreno Hidalgo, M.A.; Castañer, J.L.; Cabañas, R.; Fiandor, A.; et al. Significant HLA Class I Type Associations with Aromatic Antiepileptic Drug (AED)-Induced SJS/TEN Are Different from Those Found for the Same AED-Induced DRESS in the Spanish Population. Pharmacol. Res. 2017, 115, 168–178. [Google Scholar] [CrossRef]
- Hsiao, Y.H.; Hui, R.C.Y.; Wu, T.; Chang, W.C.; Hsih, M.S.; Yang, C.H.; Ho, H.C.; Chang, Y.G.; Chen, M.J.; Lin, J.Y.; et al. Genotype-Phenotype Association between HLA and Carbamazepine-Induced Hypersensitivity Reactions: Strength and Clinical Correlations. J. Dermatol. Sci. 2014, 73, 101–109. [Google Scholar] [CrossRef]
- Ksouda, K.; Affes, H.; Mahfoudh, N.; Chtourou, L.; Kammoun, A.; Charfi, A.; Chaabane, H.; Medhioub, M.; Sahnoun, Z.; Turki, H.; et al. HLA-A*31:01 and Carbamazepine-Induced DRESS Syndrom in a Sample of North African Population. Seizure 2017, 53, 42–46. [Google Scholar] [CrossRef]
- Genin, E.; Chen, D.P.; Hung, S.I.; Sekula, P.; Schumacher, M.; Chang, P.Y.; Tsai, S.H.; Wu, T.L.; Bellón, T.; Tamouza, R.; et al. HLA-A*31:01 and Different Types of Carbamazepine-Induced Severe Cutaneous Adverse Reactions: An International Study and Meta-Analysis. Pharm. J. 2014, 14, 281–288. [Google Scholar] [CrossRef]
- Kim, B.K.; Jung, J.W.; Kim, T.B.; Chang, Y.S.; Park, H.S.; Moon, J.; Lee, S.T.; Jung, K.H.; Jung, K.Y.; Chu, K.; et al. HLA-A*31:01 and Lamotrigine-Induced Severe Cutaneous Adverse Drug Reactions in a Korean Population. Ann. Allergy Asthma. Immunol. 2017, 118, 629–630. [Google Scholar] [CrossRef]
- Kang, H.R.; Jee, Y.K.; Kim, Y.S.; Lee, C.H.; Jung, J.W.; Kim, S.H.; Park, H.W.; Chang, Y.S.; Jang, I.J.; Cho, S.H.; et al. Positive and Negative Associations of HLA Class i Alleles with Allopurinol-Induced SCARs in Koreans. Pharm. Genom. 2011, 21, 303–307. [Google Scholar] [CrossRef]
- Chen, W.T.; Wang, C.W.; Lu, C.W.; Chen, C.B.; Lee, H.E.; Hung, S.I.; Choon, S.E.; Yang, C.H.; Liu, M.T.; Chen, T.J.; et al. The Function of HLA-B*13:01 Involved in the Pathomechanism of Dapsone-Induced Severe Cutaneous Adverse Reactions. J. Investig. Dermatol. 2018, 138, 1546–1554. [Google Scholar] [CrossRef]
- Zhang, F.-R.; Liu, H.; Irwanto, A.; Fu, X.-A.; Li, Y.; Yu, G.-Q.; Yu, Y.-X.; Chen, M.-F.; Low, H.-Q.; Li, J.-H.; et al. HLA-B*13:01 and the Dapsone Hypersensitivity Syndrome. N. Engl. J. Med. 2013, 369, 1620–1628. [Google Scholar] [CrossRef]
- Yang, F.; Gu, B.; Zhang, L.; Xuan, J.; Luo, H.; Zhou, P.; Zhu, Q.; Yan, S.; Chen, S.A.; Cao, Z.; et al. HLA-B∗13:01 Is Associated with Salazosulfapyridine-Induced Drug Rash with Eosinophilia and Systemic Symptoms in Chinese Han Population. Pharmacogenomics 2014, 15, 1461–1469. [Google Scholar] [CrossRef]
- Wang, C.W.; Tassaneeyakul, W.; Chen, C.B.; Chen, W.T.; Teng, Y.C.; Huang, C.Y.; Sukasem, C.; Lu, C.W.; Lee, Y.S.; Choon, S.E.; et al. Whole Genome Sequencing Identifies Genetic Variants Associated with Co-Trimoxazole Hypersensitivity in Asians. J. Allergy Clin. Immunol. 2021, 147, 1402–1412. [Google Scholar] [CrossRef]
- Tassaneeyakul, W.; Prabmeechai, N.; Sukasem, C.; Kongpan, T.; Konyoung, P.; Chumworathayi, P.; Tiamkao, S.; Khunarkornsiri, U.; Kulkantrakorn, K.; Saksit, N.; et al. Associations between HLA Class I and Cytochrome P450 2C9 Genetic Polymorphisms and Phenytoin-Related Severe Cutaneous Adverse Reactions in a Thai Population. Pharm. Genom. 2016, 26, 225–234. [Google Scholar] [CrossRef]
- Somogyi, A.A.; Barratt, D.T.; Phillips, E.J.; Moore, K.; Ilyas, F.; Gabb, G.M. High and Variable Population Prevalence of HLA-B*56:02 in Indigenous Australians and Relation to Phenytoin-Associated Drug Reaction with Eosinophilia and Systemic Symptoms. Br. J. Clin. Pharmacol. 2019, 85, 2163–2169. [Google Scholar] [CrossRef] [PubMed]
- Sukasem, C.; Chaichan, C.; Nakkrut, T.; Satapornpong, P.; Jaruthamsophon, K.; Jantararoungtong, T.; Koomdee, N.; Sririttha, S.; Medhasi, S.; Oo-Puthinan, S.; et al. Association between HLA-B Alleles and Carbamazepine-Induced Maculopapular Exanthema and Severe Cutaneous Reactions in Thai Patients. J. Immunol. Res. 2018, 2018, 2780272. [Google Scholar] [CrossRef]
- Chang, C.C.; Ng, C.C.; Too, C.L.; Choon, S.E.; Lee, C.K.; Chung, W.H.; Hussein, S.H.; Lim, K.S.; Murad, S. Association of HLA-B*15:13 and HLA-B*15:02 with Phenytoin-Induced Severe Cutaneous Adverse Reactions in a Malay Population. Pharm. J. 2017, 17, 170–173. [Google Scholar] [CrossRef]
- Carr, D.F.; Chaponda, M.; Jorgensen, A.L.; Castro, E.C.; van Oosterhout, J.J.; Khoo, S.H.; Lalloo, D.G.; Heyderman, R.S.; Alfirevic, A.; Pirmohamed, M. Association of Human Leukocyte Antigen Alleles and Nevirapine Hypersensitivity in a Malawian HIV-Infected Population. Clin. Infect. Dis. 2013, 56, 1330–1339. [Google Scholar] [CrossRef]
- Menegatti, J.; Schub, D.; Schäfer, M.; Grässer, F.A.; Ruprecht, K. HLA-DRB1*15:01 Is a Co-Receptor for Epstein–Barr Virus, Linking Genetic and Environmental Risk Factors for Multiple Sclerosis. Eur. J. Immunol. 2021, 51, 2348–2350. [Google Scholar] [CrossRef]
- Manson, L.E.N.; Swen, J.J.; Guchelaar, H.J. Diagnostic Test Criteria for HLA Genotyping to Prevent Drug Hypersensitivity Reactions: A Systematic Review of Actionable HLA Recommendations in CPIC and DPWG Guidelines. Front. Pharmacol. 2020, 11, 1450. [Google Scholar] [CrossRef]
- Hsu, Y.S.O.; Lu, K.L.; Fu, Y.; Wang, C.W.; Lu, C.W.; Lin, Y.F.; Chang, W.C.; Yeh, K.Y.; Hung, S.I.; Chung, W.H.; et al. The Roles of Immunoregulatory Networks in Severe Drug Hypersensitivity. Front. Immunol. 2021, 12, 597761. [Google Scholar] [CrossRef]
- Hospital Discharges and Length of Stay Statistics—Statistics Explained. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Hospital_discharges_and_length_of_stay_statistics&oldid=574861#Hospital_discharges (accessed on 16 December 2022).
- Lai, C.C.; Wang, C.Y.; Chu, C.C.; Tan, C.K.; Lu, C.L.; Lee, Y.L.; Huang, Y.T.; Lee, P.I.; Hsueh, P.R. Correlation between Antimicrobial Consumption and Resistance among Staphylococcus Aureus and Enterococci Causing Healthcare-Associated Infections at a University Hospital in Taiwan from 2000 to 2009. Eur. J. Clin. Microbiol. Infect. Dis. 2011, 30, 265–271. [Google Scholar] [CrossRef]
- Antimicrobial Resistance Surveillance in Europe 2022–2020 Data. Available online: https://www.ecdc.europa.eu/en/publications-data/antimicrobial-resistance-surveillance-europe-2022-2020-data (accessed on 16 December 2022).
- Junior, M.S.; Correa, L.; Marra, A.R.; Camargo, L.F.A.; Pereira, C.A.P. Analysis of Vancomycin Use and Associated Risk Factors in a University Teaching Hospital: A Prospective Cohort Study. BMC Infect. Dis. 2007, 7, 88. [Google Scholar] [CrossRef]
- Pichler, W.J.; Hausmann, O. Classification of Drug Hypersensitivity into Allergic, p-i, and Pseudo-Allergic Forms. Int. Arch. Allergy Immunol. 2016, 171, 166–179. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.; Adam, J.; Yerly, D.; Pichler, W.J. Human Leukocyte Antigens (HLA) Associated Drug Hypersensitivity: Consequences of Drug Binding to HLA. Allergy 2012, 67, 1338–1346. [Google Scholar] [CrossRef]
- Ostrov, D.A.; Grant, B.J.; Pompeu, Y.A.; Sidney, J.; Harndahl, M.; Southwood, S.; Oseroff, C.; Lu, S.; Jakoncic, J.; de Oliveira, C.A.F.; et al. Drug Hypersensitivity Caused by Alteration of the MHC-Presented Self-Peptide Repertoire. Proc. Natl. Acad. Sci. USA 2012, 109, 9959–9964. [Google Scholar] [CrossRef]
- Pichler, W.J.; Beeler, A.; Keller, M.; Lerch, M.; Posadas, S.; Schmid, D.; Spanou, Z.; Zawodniak, A.; Gerber, B. Pharmacological Interaction of Drugs with Immune Receptors: The p-i Concept. Allergol. Int. 2006, 55, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.Y.; Chung, W.H.; Huang, H.W.; Chen, Y.T.; Hung, S.I. Direct Interaction between HLA-B and Carbamazepine Activates T Cells in Patients with Stevens-Johnson Syndrome. J. Allergy Clin. Immunol. 2012, 129, 1562–1569.e5. [Google Scholar] [CrossRef]
- Pichler, W.J. Immune Pathomechanism and Classification of Drug Hypersensitivity. Allergy 2019, 74, 1457–1471. [Google Scholar] [CrossRef] [PubMed]
- Natkunarajah, J.; Watson, K.; Diaz-Cano, S.; Mufti, G.; du Vivier, A.; Creamer, D. Drug Rash with Eosinophilia and Systemic Symptoms and Graft-versus-Host Disease Developing Sequentially in a Patient. Clin. Exp. Dermatol. 2009, 34, 199–201. [Google Scholar] [CrossRef] [PubMed]
- Nakkam, N.; Gibson, A.; Mouhtouris, E.; Konvinse, K.C.; Holmes, N.E.; Chua, K.Y.; Deshpande, P.; Li, D.; Ostrov, D.A.; Trubiano, J.; et al. Cross-Reactivity between Vancomycin, Teicoplanin, and Telavancin in Patients with HLA-A∗32:01-Positive Vancomycin-Induced DRESS Sharing an HLA Class II Haplotype. J. Allergy Clin. Immunol. 2021, 147, 403–405. [Google Scholar] [CrossRef]
- Azoury, M.E.; Filì, L.; Bechara, R.; Scornet, N.; de Chaisemartin, L.; Weaver, R.J.; Claude, N.; Maillere, B.; Parronchi, P.; Joseph, D.; et al. Identification of T-Cell Epitopes from Benzylpenicillin Conjugated to Human Serum Albumin and Implication in Penicillin Allergy. Allergy 2018, 73, 1662–1672. [Google Scholar] [CrossRef] [PubMed]
- Nakkam, N.; Trubiano, J.; Gibson, A.; Phillips, E.J. Considerations for Cross-Reactivity between Vancomycin and Other Glycopeptides. J. Allergy Clin. Immunol. Pract. 2021, 9, 3233. [Google Scholar] [CrossRef]
- Monneaux, F.; Muller, S. Epitope Spreading in Systemic Lupus Erythematosus: Identification of Triggering Peptide Sequences. Arthr. Rheum 2002, 46, 1430–1438. [Google Scholar] [CrossRef] [PubMed]
- Lucas, A.; Lucas, M.; Strhyn, A.; Keane, N.M.; McKinnon, E.; Pavlos, R.; Moran, E.M.; Meyer-Pannwitt, V.; Gaudieri, S.; D’Orsogna, L.; et al. Abacavir-Reactive Memory T Cells Are Present in Drug Naïve Individuals. PLoS ONE 2015, 10, e0117160. [Google Scholar] [CrossRef]
- Sekiguchi, A.; Kashiwagi, T.; Ishida-Yamamoto, A.; Takahashi, H.; Hashimoto, Y.; Kimura, H.; Tohyama, M.; Hashimoto, K.; Iizuka, H. Drug-Induced Hypersensitivity Syndrome Due to Mexiletine Associated with Human Herpes Virus 6 and Cytomegalovirus Reactivation. J. Dermatol. 2005, 32, 278–281. [Google Scholar] [CrossRef]
- Yerly, D.; Pompeu, Y.A.; Schutte, R.J.; Eriksson, K.K.; Strhyn, A.; Bracey, A.W.; Buus, S.; Ostrov, D.A. Structural Elements Recognized by Abacavir-Induced T Cells. Int. J. Mol. Sci. 2017, 18, 1464. [Google Scholar] [CrossRef] [PubMed]
- Almeida, C.A.; van Miert, P.; O’Driscoll, K.; Zoet, Y.M.; Chopra, A.; Witt, C.; John, M.; Claas, F.H.J.; D’Orsogna, L.J. Virus-Specific T-Cell Clonotypes Might Contribute to Drug Hypersensitivity Reactions through Heterologous Immunity. J. Allergy Clin. Immunol. 2019, 144, 608–611.e4. [Google Scholar] [CrossRef]
- Niu, J.; Jia, Q.; Ni, Q.; Yang, Y.; Chen, G.; Yang, X.; Zhai, Z.; Yu, H.; Guan, P.; Lin, R.; et al. Association of CD8(+) T Lymphocyte Repertoire Spreading with the Severity of DRESS Syndrome. Sci. Rep. 2015, 5, 9913. [Google Scholar] [CrossRef] [PubMed]
- Chung, W.H.; Pan, R.Y.; Chu, M.T.; Chin, S.W.; Huang, Y.L.; Wang, W.C.; Chang, J.Y.; Hung, S.I. Oxypurinol-Specific T Cells Possess Preferential TCR Clonotypes and Express Granulysin in Allopurinol-Induced Severe Cutaneous Adverse Reactions. J. Investig. Dermatol. 2015, 135, 2237–2248. [Google Scholar] [CrossRef]
- Kurose, K.; Ohue, Y.; Sato, E.; Yamauchi, A.; Eikawa, S.; Isobe, M.; Nishio, Y.; Uenaka, A.; Oka, M.; Nakayama, E. Increase in Activated Treg in TIL in Lung Cancer and in Vitro Depletion of Treg by ADCC Using an Antihuman CCR4 MAb (KM2760). J. Thorac. Oncol. 2015, 10, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, K.; Morito, H.; Hasegawa, A.; Miyagawa, F.; Kobayashi, N.; Watanabe, H.; Sueki, H.; Tohyama, M.; Hashimoto, K.; Kano, Y.; et al. Elevated Serum Thymus and Activation-Regulated Chemokine (TARC/CCL17) Relates to Reactivation of Human Herpesvirus 6 in Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS)/Drug-Induced Hypersensitivity Syndrome (DIHS). Br. J. Dermatol. 2014, 171, 425–427. [Google Scholar] [CrossRef] [PubMed]
- Ushigome, Y.; Mizukawa, Y.; Kimishima, M.; Yamazaki, Y.; Takahashi, R.; Kano, Y.; Shiohara, T. Monocytes Are Involved in the Balance between Regulatory T Cells and Th17 Cells in Severe Drug Eruptions. Clin. Exp. Allergy 2018, 48, 1453–1463. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, R.; Kano, Y.; Yamazaki, Y.; Kimishima, M.; Mizukawa, Y.; Shiohara, T. Defective Regulatory T Cells in Patients with Severe Drug Eruptions: Timing of the Dysfunction Is Associated with the Pathological Phenotype and Outcome. J. Immunol. 2009, 182, 8071–8079. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, G.A.; Cariddi, A.; Noviello, S.; Campochiaro, C.; Canti, V.; Moroni, L.; Yacoub, M.-R.; Baldissera, E.M.; Bozzolo, E.P.; Dagna, L. Real-Life Efficacy and Safety of Mepolizumab for Eosinophilic Granulomatosis with Polyangiitis. Clin. Immunol. Commun. 2022, 2, 23–29. [Google Scholar] [CrossRef]
- Yamaya, M.; Sasaki, H. Rhinovirus and Asthma. Viral Immunol. 2003, 16, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Khatri, S.; Moore, W.; Gibson, P.G.; Leigh, R.; Bourdin, A.; Maspero, J.; Barros, M.; Buhl, R.; Howarth, P.; Albers, F.C.; et al. Assessment of the Long-Term Safety of Mepolizumab and Durability of Clinical Response in Patients with Severe Eosinophilic Asthma. J. Allergy Clin. Immunol. 2019, 143, 1742–1751.e7. [Google Scholar] [CrossRef]
- Sabogal Piñeros, Y.S.; Bal, S.M.; Dijkhuis, A.; Majoor, C.J.; Dierdorp, B.S.; Dekker, T.; Hoefsmit, E.P.; Bonta, P.I.; Picavet, D.; van der Wel, N.N.; et al. Eosinophils Capture Viruses, a Capacity That Is Defective in Asthma. Allergy 2019, 74, 1898–1909. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, G.A.; Yacoub, M.R.; Ripa, M.; Mannina, D.; Cariddi, A.; Saporiti, N.; Ciceri, F.; Castagna, A.; Colombo, G.; Dagna, L. Eosinophils from Physiology to Disease: A Comprehensive Review. BioMed Res. Int. 2018, 2018, 9095275. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.G.; Liou, J.H.; Hung, S.I.; Chen, C.B.; Chiu, T.M.; Wang, C.W.; Chung, W.H. Increased Type 2 Innate Lymphoid Cells in Patients with Drug Reaction with Eosinophilia and Systemic Symptoms Syndrome. J. Investig. Dermatol. 2019, 139, 1722–1731. [Google Scholar] [CrossRef] [PubMed]
- Stoeckle, C.; Simon, H.U. CD8(+) T Cells Producing IL-3 and IL-5 in Non-IgE-Mediated Eosinophilic Diseases. Allergy 2013, 68, 1622–1625. [Google Scholar] [CrossRef] [PubMed]
- Lyons, P.A.; Peters, J.E.; Alberici, F.; Liley, J.; Coulson, R.M.R.; Astle, W.; Baldini, C.; Bonatti, F.; Cid, M.C.; Elding, H.; et al. Genome-Wide Association Study of Eosinophilic Granulomatosis with Polyangiitis Reveals Genomic Loci Stratified by ANCA Status. Nat. Commun. 2019, 10, 5120. [Google Scholar] [CrossRef] [PubMed]
- Fulkerson, P.C.; Rothenberg, M.E. Targeting Eosinophils in Allergy, Inflammation and Beyond. Nat. Rev. Drug Discov. 2013, 12, 117–129. [Google Scholar] [CrossRef] [PubMed]
- James, J.; Sammou, Y.M.; Virata, A.R.; Nordin, T.A.; Dumic, I. Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) Syndrome Secondary to Furosemide: Case Report and Review of Literature. Am. J. Case Rep. 2018, 19, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Hiransuthikul, A.; Rattananupong, T.; Klaewsongkram, J.; Rerknimitr, P.; Pongprutthipan, M.; Ruxrungtham, K. Drug-Induced Hypersensitivity Syndrome/Drug Reaction with Eosinophilia and Systemic Symptoms (DIHS/DRESS): 11 Years Retrospective Study in Thailand. Allergol. Int. 2016, 65, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Behera, S.K.; Das, S.; Xavier, A.S.; Selvarajan, S. DRESS Syndrome: A Detailed Insight. Hosp. Pract. (1995) 2018, 46, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Dorrell BA, D.N.; Whitaker BS, L.F.; Anderson, K.L.; Strowd, L.C. Abnormal Erythrocyte Morphology in Drug Reaction with Eosinophilia and Systemic Symptoms. J. Am. Acad. Dermatol. 2018, 80, 1159–1160. [Google Scholar] [CrossRef]
- Peyrière, H.; Dereure, O.; Breton, H.; Demoly, P.; Cociglio, M.; Blayac, J.P.; Hillaire-Buys, D. Variability in the Clinical Pattern of Cutaneous Side-Effects of Drugs with Systemic Symptoms: Does a DRESS Syndrome Really Exist? Br. J. Dermatol. 2006, 155, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, S.; McLeod, M.; Torchia, D.; Romanelli, P. Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) Syndrome. J. Clin. Aesthet. Dermatol. 2013, 6, 31–37. [Google Scholar] [PubMed]
- Syn, W.K.; Naisbitt, D.J.; Holt, A.P.; Pirmohamed, M.; Mutimer, D.J. Carbamazepine-Induced Acute Liver Failure as Part of the DRESS Syndrome. Int. J. Clin. Pract. 2005, 59, 988–991. [Google Scholar] [CrossRef]
- de Campos, F.P.F.; de Lima, P.P.; Maragno, L.; Watanabe, F.T. Hepatic Necrosis Associated with Drug-Induced Hypersensitivity Syndrome. Autops. Case Rep. 2012, 2, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Subhani, M.; Dong, V.; Connolly, A.; Salisbury, J.; Miquel, R.; Walsh, S.; Pirani, T. Trimethoprim-Induced Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) Associated with Reactivation of Human Herpes Virus-6 (HHV-6) Leading to Acute Liver Failure. Clin. Case Rep. 2020, 8, 2568–2573. [Google Scholar] [CrossRef]
- Lee, T.; Lee, Y.S.; Yoon, S.Y.; Kim, S.; Bae, Y.J.; Kwon, H.S.; Cho, Y.S.; Moon, H.B.; Kim, T.B. Characteristics of Liver Injury in Drug-Induced Systemic Hypersensitivity Reactions. J. Am. Acad. Dermatol. 2013, 69, 407–415. [Google Scholar] [CrossRef]
- Miyasaka, A.; Kumagai, I.; Masda, T.; Takikawa, Y. A 51-Year-Old Woman with Drug-Induced Hypersensitivity Syndrome Associated with Carbamazepine, Reactivation of Human Herpesvirus 6, and Acute Liver Failure: A Case Report. Am. J. Case Rep. 2021, 22, e928587. [Google Scholar] [CrossRef] [PubMed]
- Husain, Z.; Reddy, B.Y.; Schwartz, R.A. DRESS Syndrome: Part I. Clinical Perspectives. J. Am. Acad. Dermatol. 2013, 68, 693.e1–693.e14. [Google Scholar] [CrossRef] [PubMed]
- Descamps, V.; Gautheret-Dejean, A.; Pelletier, A.L.; Bonnafous, P.; Deschamps, L.; Prusty, B.K. Chronic Persistent HHV-6B Infection after Sulfasalazine-Induced DRESS with Demonstration of HHV-6 Encoded Small Noncoding RNAs (SncRNAs) in Crohn’s-like Colitis: Case Report. Clin. Case Rep. 2020, 9, 841–844. [Google Scholar] [CrossRef] [PubMed]
- Asano, Y.; Kagawa, H.; Kano, Y.; Shiohara, T. Cytomegalovirus Disease during Severe Drug Eruptions: Report of 2 Cases and Retrospective Study of 18 Patients with Drug-Induced Hypersensitivity Syndrome. Arch. Dermatol. 2009, 145, 1030–1036. [Google Scholar] [CrossRef] [PubMed]
- Do-Pham, G.; Charachon, A.; Duong, T.A.; Thille, A.W.; Benhaiem, N.; Bagot, M.; Chosidow, O.; Roujeau, J.C.; Wolkenstein, P.; Valeyrie-Allanore, L. Drug Reaction with Eosinophilia and Systemic Symptoms and Severe Involvement of Digestive Tract: Description of Two Cases. Br. J. Dermatol. 2011, 165, 207–209. [Google Scholar] [CrossRef] [PubMed]
- Descamps, V.; Mahe, E.; Houhou, N.; Abramowitz, L.; Rozenberg, F.; Ranger-Rogez, S.; Crickx, B. Drug-Induced Hypersensitivity Syndrome Associated with Epstein–Barr Virus Infection. Br. J. Dermatol. 2003, 148, 1032–1034. [Google Scholar] [CrossRef] [PubMed]
- Sekine, N.; Motokura, T.; Oki, T.; Umeda, Y.; Sasaki, N.; Hayashi, M.; Sato, H.; Fujita, T.; Kaneko, T.; Asano, Y.; et al. Rapid Loss of Insulin Secretion in a Patient with Fulminant Type 1 Diabetes Mellitus and Carbamazepine Hypersensitivity Syndrome. JAMA 2001, 285, 1153–1154. [Google Scholar] [CrossRef]
- Kano, Y.; Ishida, T.; Hirahara, K.; Shiohara, T. Visceral Involvements and Long-Term Sequelae in Drug-Induced Hypersensitivity Syndrome. Med. Clin. N. Am. 2010, 94, 743–759. [Google Scholar] [CrossRef] [PubMed]
- Kano, Y.; Shiohara, T. The Variable Clinical Picture of Drug-Induced Hypersensitivity Syndrome/Drug Rash with Eosinophilia and Systemic Symptoms in Relation to the Eliciting Drug. Immunol. Allergy Clin. N. Am. 2009, 29, 481–501. [Google Scholar] [CrossRef] [PubMed]
- Esposito, A.J.; Murphy, R.C.; Toukatly, M.N.; Amro, O.W.; Kestenbaum, B.R.; Najafian, B. Acute Kidney Injury in Allopurinol-Induced DRESS Syndrome: A Case Report of Concurrent Tubulointerstitial Nephritis and Kidney-Limited Necrotizing Vasculitis. Clin. Nephrol. 2017, 87, 316–319. [Google Scholar] [CrossRef] [PubMed]
- Hagiya, H.; Iwamuro, M.; Tanaka, T.; Hasegawa, K.; Hanayama, Y.; Kimura, M.; Otsuka, F. Reactivation of Human Herpes Virus-6 in the Renal Tissue of a Patient with Drug-Induced Hypersensitivity Syndrome/Drug Rash with Eosinophilia and Systemic Symptoms (DIHS/DRESS). Intern. Med. 2016, 55, 1769–1774. [Google Scholar] [CrossRef] [PubMed]
- Bourgeois, G.P.; Cafardi, J.A.; Groysman, V.; Hughey, L.C. A Review of DRESS-Associated Myocarditis. J. Am. Acad. Dermatol. 2012, 66, e229–e236. [Google Scholar] [CrossRef]
- Taweesedt, P.T.; Nordstrom, C.W.; Stoeckel, J.; Dumic, I. Pulmonary Manifestations of Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) Syndrome: A Systematic Review. BioMed Res. Int. 2019, 2019, 7863815. [Google Scholar] [CrossRef] [PubMed]
- Masaki, T.; Fukunaga, A.; Tohyama, M.; Koda, Y.; Okuda, S.; Maeda, N.; Kanda, F.; Yasukawa, M.; Hashimoto, K.; Horikawa, T.; et al. Human Herpes Virus 6 Encephalitis in Allopurinol-Induced Hypersensitivity Syndrome. Acta Derm. Venereol. 2003, 83, 128–131. [Google Scholar] [CrossRef] [PubMed]
- Ozisik, L.; Tanriover, M.D.; Saka, E. Autoimmune Limbic Encephalitis and Syndrome of Inappropriate Antidiuretic Hormone Secretion Associated with Lamotrigine-Induced Drug Rash with Eosinophilia and Systemic Symptoms (DRESS) Syndrome. Intern. Med. 2016, 55, 1393–1396. [Google Scholar] [CrossRef]
- Yokote, A.; Tomita, S.; Sawada, H. Sensory Ganglionopathy Associated with Drug-Induced Hypersensitivity Syndrome Caused by Mexiletine. BMJ Case Rep. 2018, 2018, bcr-2017222540. [Google Scholar] [CrossRef]
- Zhu, B.; Wu, J.; Chen, G.; Yang, Y.; Yi, C. Fulminant Type 1 Diabetes Mellitus Caused by Drug Reaction With Eosinophilia and Systemic Symptoms (DRESS): A Case Report and Review of the Literature. Front. Endocrinol. 2019, 10, 474. [Google Scholar] [CrossRef]
- Mizukawa, Y.; Aoyama, Y.; Takahashi, H.; Takahashi, R.; Shiohara, T. Risk of Progression to Autoimmune Disease in Severe Drug Eruption: Risk Factors and the Factor-Guided Stratification. J. Investig. Dermatol. 2022, 142, 960–968.e9. [Google Scholar] [CrossRef]
- Owen, C.E.; Jones, J.M. Recognition and Management of Severe Cutaneous Adverse Drug Reactions (Including Drug Reaction with Eosinophilia and Systemic Symptoms, Stevens-Johnson Syndrome, and Toxic Epidermal Necrolysis). Med. Clin. N. Am. 2021, 105, 577–597. [Google Scholar] [CrossRef]
- Milani-Nejad, N.; Trinidad, J.; Kaffenberger, B.H. Viral Reactivation in Hospitalized Patients with Drug Reaction with Eosinophilia and Systemic Symptoms: A Retrospective Study from a Tertiary Medical Center in the United States. J. Am. Acad. Dermatol. 2020, 83, 278–279. [Google Scholar] [CrossRef]
- Abdelnabi, M.; Elmssary, M.; Sekhon, J.; Benjanuwattra, J. Acute Onset of Fever, Eosinophilia, Rash, Acute Kidney Injury, and a Positive Monospot Test in a Patient on Lamotrigine: DRESS Syndrome. Lancet 2022, 399, 1902. [Google Scholar] [CrossRef]
- Thongsri, T.; Chularojanamontri, L.; Pichler, W.J. Cardiac Involvement in DRESS Syndrome. Asian Pac. J. Allergy Immunol. 2017, 35, 3–10. [Google Scholar] [CrossRef]
- de Groot, A.C. Patch Testing in Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS): A Literature Review. Contact Dermat. 2022, 86, 443–479. [Google Scholar] [CrossRef] [PubMed]
- Pichler, W.J.; Tilch, J. The Lymphocyte Transformation Test in the Diagnosis of Drug Hypersensitivity. Allergy 2004, 59, 809–820. [Google Scholar] [CrossRef]
- Chi, M.H.; Hui, R.C.Y.; Yang, C.H.; Lin, J.Y.; Lin, Y.T.; Ho, H.C.; Chung, W.H.; Kuo, T.T. Histopathological Analysis and Clinical Correlation of Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS). Br. J. Dermatol. 2014, 170, 866–873. [Google Scholar] [CrossRef] [PubMed]
- Revuz, J.; Penso, D.; Roujeau, J.C.; Guillaume, J.C.; Payne, C.R.; Wechsler, J.; Touraine, R. Toxic Epidermal Necrolysis. Clinical Findings and Prognosis Factors in 87 Patients. Arch. Dermatol. 1987, 123, 1160–1165. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.-P.; Zhang, C.; Zhu, Q.-X. The Effect of Intravenous Immunoglobulin Combined with Corticosteroid on the Progression of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis: A Meta-Analysis. PloS ONE 2016, 11, e0167120. [Google Scholar] [CrossRef] [PubMed]
- Teraki, Y.; Shibuya, M.; Izaki, S. Stevens–Johnson Syndrome and Toxic Epidermal Necrolysis Due to Anticonvulsants Share Certain Clinical and Laboratory Features with Drug-Induced Hypersensitivity Syndrome, despite Differences in Cutaneous Presentations. Clin. Exp. Dermatol. 2010, 35, 723–728. [Google Scholar] [CrossRef]
- Zalewska-Janowska, A.; Spiewak, R.; Kowalski, M.L. Cutaneous Manifestation of Drug Allergy and Hypersensitivity. Immunol. Allergy Clin. N. Am. 2017, 37, 165–181. [Google Scholar] [CrossRef]
- Bircher, A.J.; Scherer, K. Delayed Cutaneous Manifestations of Drug Hypersensitivity. Med. Clin. N. Am. 2010, 94, 711–725. [Google Scholar] [CrossRef]
- Bagnasco, D.; Ferrando, M.; Caminati, M.; Bragantini, A.; Puggioni, F.; Varricchi, G.; Passalacqua, G.; Canonica, G.W. Targeting Interleukin-5 or Interleukin-5Rα: Safety Considerations. Drug Saf. 2017, 40, 559–570. [Google Scholar] [CrossRef] [PubMed]
- Ruiz Matta, J.M.; Domínguez Cherit, J.; Méndez Flores, S. Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) and Its Relation with Autoimmunity in a Reference Center in Mexico. An. Bras. Dermatol. 2017, 92, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Yee, B.E.; Nguyen, N.H.; Lee, D. Extensive Pulmonary Involvement with Raltegravir-Induced DRESS Syndrome in a Postpartum Woman with HIV. BMJ Case Rep. 2014, 2014, bcr2013201545. [Google Scholar] [CrossRef] [PubMed]
- Simmons, R.P.; Dudzinski, D.M.; Shepard, J.-A.O.; Hurtado, R.M.; Coffey, K.C. Case 16-2019: A 53-Year-Old Man with Cough and Eosinophilia. N. Engl. J. Med. 2019, 380, 2052–2059. [Google Scholar] [CrossRef]
- Lee, H.Y.; Walsh, S.; Creamer, D. Initial Presentation of DRESS: Often Misdiagnosed as Infections. Arch. Dermatol. 2012, 148, 1085–1087. [Google Scholar] [CrossRef] [PubMed]
- Bartal, C.; Sagy, I.; Barski, L. Drug-Induced Eosinophilic Pneumonia: A Review of 196 Case Reports. Medicine 2018, 97, e9688. [Google Scholar] [CrossRef]
- Kim, D.H.; Koh, Y.-I. Comparison of Diagnostic Criteria and Determination of Prognostic Factors for Drug Reaction with Eosinophilia and Systemic Symptoms Syndrome. Allergy Asthma. Immunol. Res. 2014, 6, 216–221. [Google Scholar] [CrossRef]
- Cardones, A.R. Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) Syndrome. Clin. Dermatol. 2020, 38, 702–711. [Google Scholar] [CrossRef]
- Duong, T.A.; Valeyrie-Allanore, L.; Wolkenstein, P.; Chosidow, O. Severe Cutaneous Adverse Reactions to Drugs. Lancet 2017, 390, 1996–2011. [Google Scholar] [CrossRef]
- Mizukawa, Y.; Hirahara, K.; Kano, Y.; Shiohara, T. Drug-Induced Hypersensitivity Syndrome/Drug Reaction with Eosinophilia and Systemic Symptoms Severity Score: A Useful Tool for Assessing Disease Severity and Predicting Fatal Cytomegalovirus Disease. J. Am. Acad. Dermatol. 2019, 80, 670–678.e2. [Google Scholar] [CrossRef]
- Natkunarajah, J.; Goolamali, S.; Craythorne, E.; Benton, E.; Smith, C.; Morris-Jones, R.; Wendon, J.; Higgins, E.; Creamer, D. Ten Cases of Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) Treated with Pulsed Intravenous Methylprednisolone. Eur. J. Dermatol. 2011, 21, 385–391. [Google Scholar] [CrossRef]
- Yacoub, M.R.; Berti, A.; Campochiaro, C.; Tombetti, E.; Ramirez, G.A.; Nico, A.; Leo, E.; Fantini, P.; Sabbadini, M.G.; Nettis, E.; et al. Drug Induced Exfoliative Dermatitis: State of the Art. Clin. Mol. Allergy 2016, 14, 9. [Google Scholar] [CrossRef]
- Gottlieb, M.; Figlewicz, M.R.; Rabah, W.; Buddan, D.; Long, B. Drug Reaction with Eosinophilia and Systemic Symptoms: An Emergency Medicine Focused Review. Am. J. Emerg. Med. 2022, 56, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, E.; Yanes, D.; Imadojemu, S.; Kroshinsky, D. Evaluation of Cyclosporine for the Treatment of DRESS Syndrome. JAMA Dermatol. 2020, 156, 704–706. [Google Scholar] [CrossRef]
- Della-Torre, E.; Yacoub, M.R.; Pignatti, P.; Della-Torre, F.; Sabbadini, M.G.; Colombo, G.; Tresoldi, M. Optimal Management of DRESS Syndrome in Course of Infectious Endocarditis. Ann. Allergy Asthma Immunol. 2013, 110, 303–305. [Google Scholar] [CrossRef] [PubMed]
- Husain, Z.; Reddy, B.Y.; Schwartz, R.A. DRESS Syndrome: Part II. Management and Therapeutics. J. Am. Acad. Dermatol. 2013, 68, 709.e1–709.e9. [Google Scholar] [CrossRef] [PubMed]
- Picard, D.; Vellar, M.; Janela, B.; Roussel, A.; Joly, P.; Musette, P. Recurrence of Drug-Induced Reactions in DRESS Patients. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 801–804. [Google Scholar] [CrossRef] [PubMed]
- Jörg, L.; Helbling, A.; Yerly, D.; Pichler, W.J. Drug-Related Relapses in Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS). Clin. Transl. Allergy 2020, 10, 52. [Google Scholar] [CrossRef] [PubMed]
| HLA | Viral Infection | Effects on Viral Infection | Drugs | Population | OR (95% C.I.) Ref. |
| HLA-A*24:02 | Lamotrigine | Spanish | 34.5 (2.03–209.71) [131] | ||
| HLA-A*31:01 | Carbamazepine | Han Chinese | 12.9 (3.7–45.3) [132] | ||
| Japanese | [133] | ||||
| European | 24.1 (9.6–60.3) [134] | ||||
| North African | 32.0 (2.6–389.2) [133] | ||||
| Lamotrigine | Korean | 11.43 (1.95–59.77) § [135] | |||
| HLA-A*32:01 | Vancomycin | European | [129] | ||
| HLA-A*33:03 | Allopurinol | Korean | 25.2 (5.2–121.8) [136] | ||
| HLA-B*13:01 | Dapsone | Han Chinese | [137] | ||
| Thai | 60.75 (7.44–496.18) [138] | ||||
| Taiwanese, Malaysian | 49.64 (5.89–418.13) [137] | ||||
| Sulfasalazine | Han Chinese | 11.16 (1.98–62.85) [139] | |||
| Sulphamethoxazole | Asian | 61 (21.5–175) [140] | |||
| HLA-B14:02 | Nevirapine | Caucasian | |||
| HLA-B* 51:01 | Carbamazepine | Han Chinese | 4.6 (2.0–10.5) [132] | ||
| Phenytoin | Thai | 5.2 (1.2–22.7) [141] | |||
| HLA-B*53:01 | HIV | Raltegravir | African | [114] | |
| HLA-B*56:02 | Phenytoin | Australian Aboriginal | [142] | ||
| HLA-B*58:01 | Allopurinol | Han Chinese, Thai, Japanese, Korean, European | 580.3 (34.4–9780.9) § [10] | ||
| Carbamazepine | Asian | 7.55 (1.20–47.58) [143] | |||
| CMV | Increased reactivation risk | ||||
| HLA-B*15:13 | Phenytoin | Malaysian | 59.0 (2.5–1395.7) [144] | ||
| HLA-C*03:02 | Allopurinol | Korean | 135.7 (15.6–1177.8) [136] | ||
| HLA-C*04:01 | Nevirapine | Malawian | 2.6 (1.1–2.6) § [145] | ||
| HLA-DRB1*15:01 | IL-1 and IL-6 inhibitors | European patients with AOSD or JIA | 40.8 (5.3–316) [39] | ||
| EBV | Coreceptor to EBV infection on B cells | [146] |
| Syndrome | Rash Features | Timing of Onset | Disease Extent | Systemic Manifestations | Other than Skin Involvement | Blood Analysis Findings | Histopathological Findings |
|---|---|---|---|---|---|---|---|
| DIHS/DRESS | Maculopapular exanthem | 2–8 weeks | Generalised | Fever | Hepatitis Lymphadenopathy Pneumonitis Nephritis | Eosinophilia, atypical lymphocytes, leucocytosis | Subtle, vacuolar interface dermatitis, with scattered, dyskeratotic keratinocytes along the dermo-epidermal junction zone |
| Erythroderma | Mucosal involvement Rare | Abnormal liver and renal function tests | |||||
| Facial oedema | |||||||
| AGEP | Generalised erythema | <3 days | Generalised usually with skin fold and facial localisation, | Higher fever (>38 °C) | Rare | Leucocytosis with neutrophilia (>7000/mm3) | Intraepidermal pustules with oedema of the papillary dermis and perivascular infiltrates of neutrophils and eosinophils |
| Pustules | Mucosal involvement rare | ||||||
| Erythroderma | |||||||
| SJS/TEN | Dusky red, coalescent macular exanthem | 4–21 days | Disseminated | Fever Photophobia Sore throat, Dysphagia | Pneumonitis | Lymphopenia | Necrosis of keratinocytes Epidermis shedding Absent inflammatory infiltrate |
| Atypical target lesions | |||||||
| Bullous lesions | Mucosal involvement rarely absent (stomatitis, conjunctivitis) | ||||||
| Epidermal necrosis | |||||||
| Nikolsky sign |
| Bocquet et al., 1996 | J-SCAR, 2006 | RegiSCAR, 2007 |
|---|---|---|
| Cutaneous drug eruption | Fever | Fever > 38.5 °C |
| Systemic involvement: lymphadenopathy ≥ 2 cm; liver involvement (transaminase twice the upper limit); kidney involvement (e.g., interstitial nephritis); lung and cardiac involvement (e.g., interstitial pneumonitis or myocarditis) | Latency time of 3 weeks from drug exposure to the onset of cutaneous manifestations | Enlarged lymph nodes in ≥2 lymph node stations |
| Hematologic alterations: eosinophilia ≥ 1.5 × 109/L; presence of atypical lymphocytes | Persistence of the eruption ≥ 2 weeks after drug interruption | Eosinophilia > 700/µL |
| Thrombocytopenia | ||
| HHV-6 reactivation at PCR or serology tests | Atypical lymphocytes | |
| Skin involvement (rash extended for > 50% of body surface area, biopsy) | ||
| Organ involvement (one or ≥ two organs involved) | ||
| Resolution in ≥15 days | ||
| ≥3 negative laboratory investigations including ANA screening, serological screening for HAV/HBV/HCV, blood cultures, tests for Chlamydia and Mycoplasma to exclude other diseases |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramirez, G.A.; Ripa, M.; Burastero, S.; Benanti, G.; Bagnasco, D.; Nannipieri, S.; Monardo, R.; Ponta, G.; Asperti, C.; Cilona, M.B.; et al. Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS): Focus on the Pathophysiological and Diagnostic Role of Viruses. Microorganisms 2023, 11, 346. https://doi.org/10.3390/microorganisms11020346
Ramirez GA, Ripa M, Burastero S, Benanti G, Bagnasco D, Nannipieri S, Monardo R, Ponta G, Asperti C, Cilona MB, et al. Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS): Focus on the Pathophysiological and Diagnostic Role of Viruses. Microorganisms. 2023; 11(2):346. https://doi.org/10.3390/microorganisms11020346
Chicago/Turabian StyleRamirez, Giuseppe A., Marco Ripa, Samuele Burastero, Giovanni Benanti, Diego Bagnasco, Serena Nannipieri, Roberta Monardo, Giacomo Ponta, Chiara Asperti, Maria Bernadette Cilona, and et al. 2023. "Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS): Focus on the Pathophysiological and Diagnostic Role of Viruses" Microorganisms 11, no. 2: 346. https://doi.org/10.3390/microorganisms11020346
APA StyleRamirez, G. A., Ripa, M., Burastero, S., Benanti, G., Bagnasco, D., Nannipieri, S., Monardo, R., Ponta, G., Asperti, C., Cilona, M. B., Castagna, A., Dagna, L., & Yacoub, M.-R. (2023). Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS): Focus on the Pathophysiological and Diagnostic Role of Viruses. Microorganisms, 11(2), 346. https://doi.org/10.3390/microorganisms11020346






