Changes in Soil Phosphorus Availability and Microbial Community Structures in Rhizospheres of Oilseed Rapes Induced by Intercropping with White Lupins
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Conditions and Plant Materials
2.2. Soil Sampling Property Analysis
2.3. Analysis of Soil Available Phosphorus and Its Related Biological Properties
2.4. Analysis of Rhizosphere Microbial Diversity
2.5. Untargeted Metabolome Assays and Analysis
2.6. Untargeted Metabolome Assays and Analysis
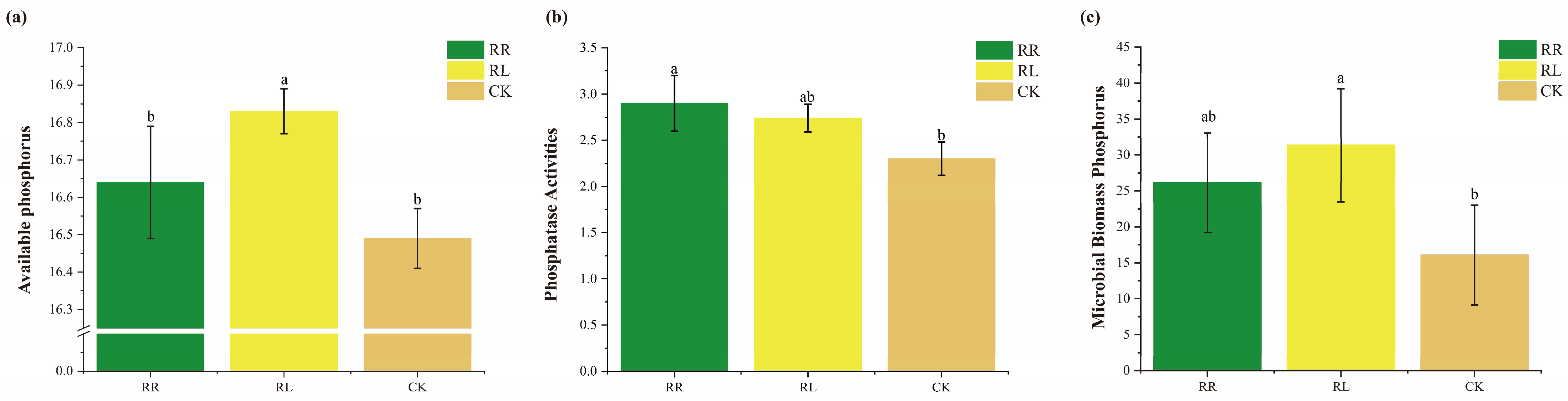
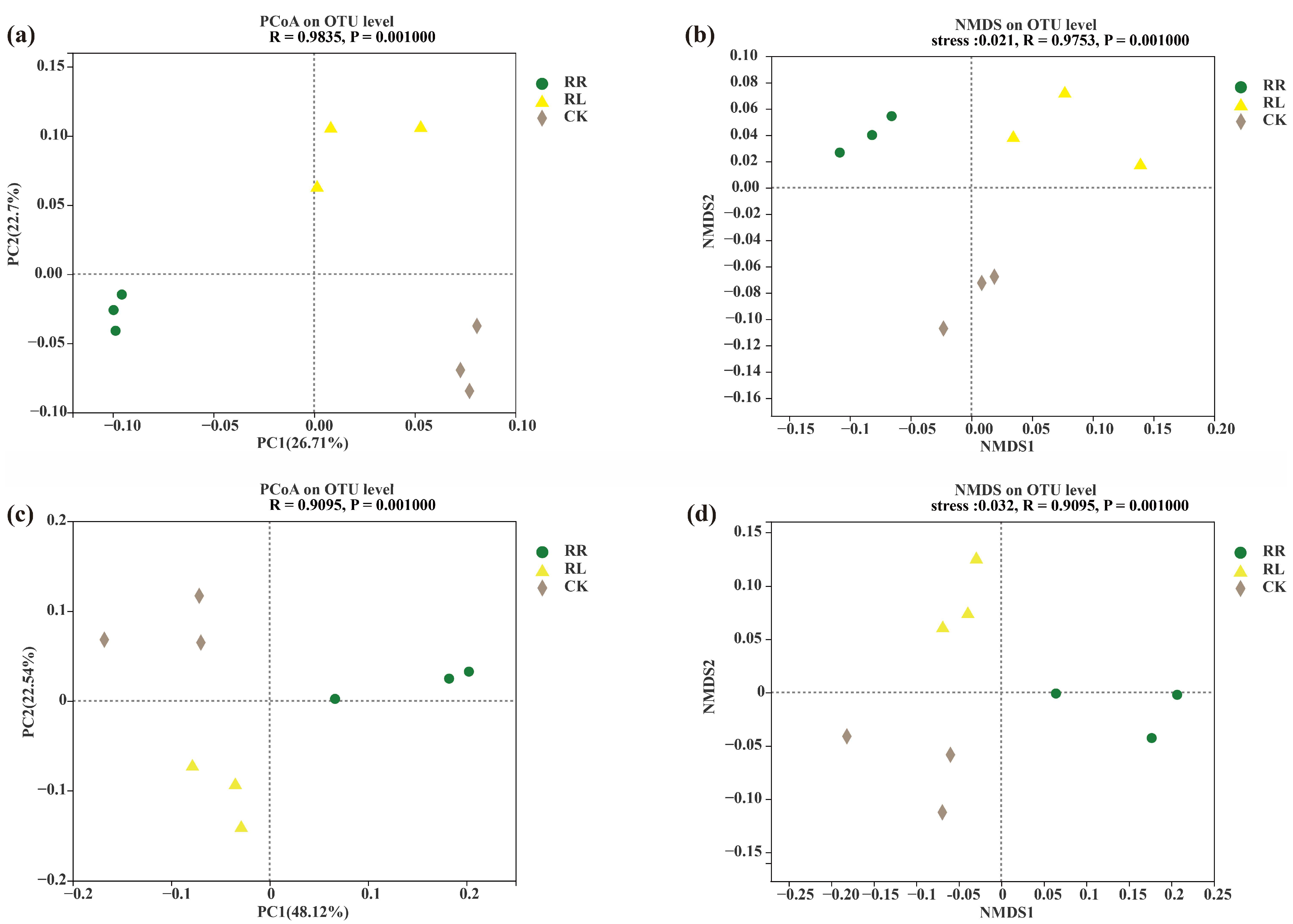
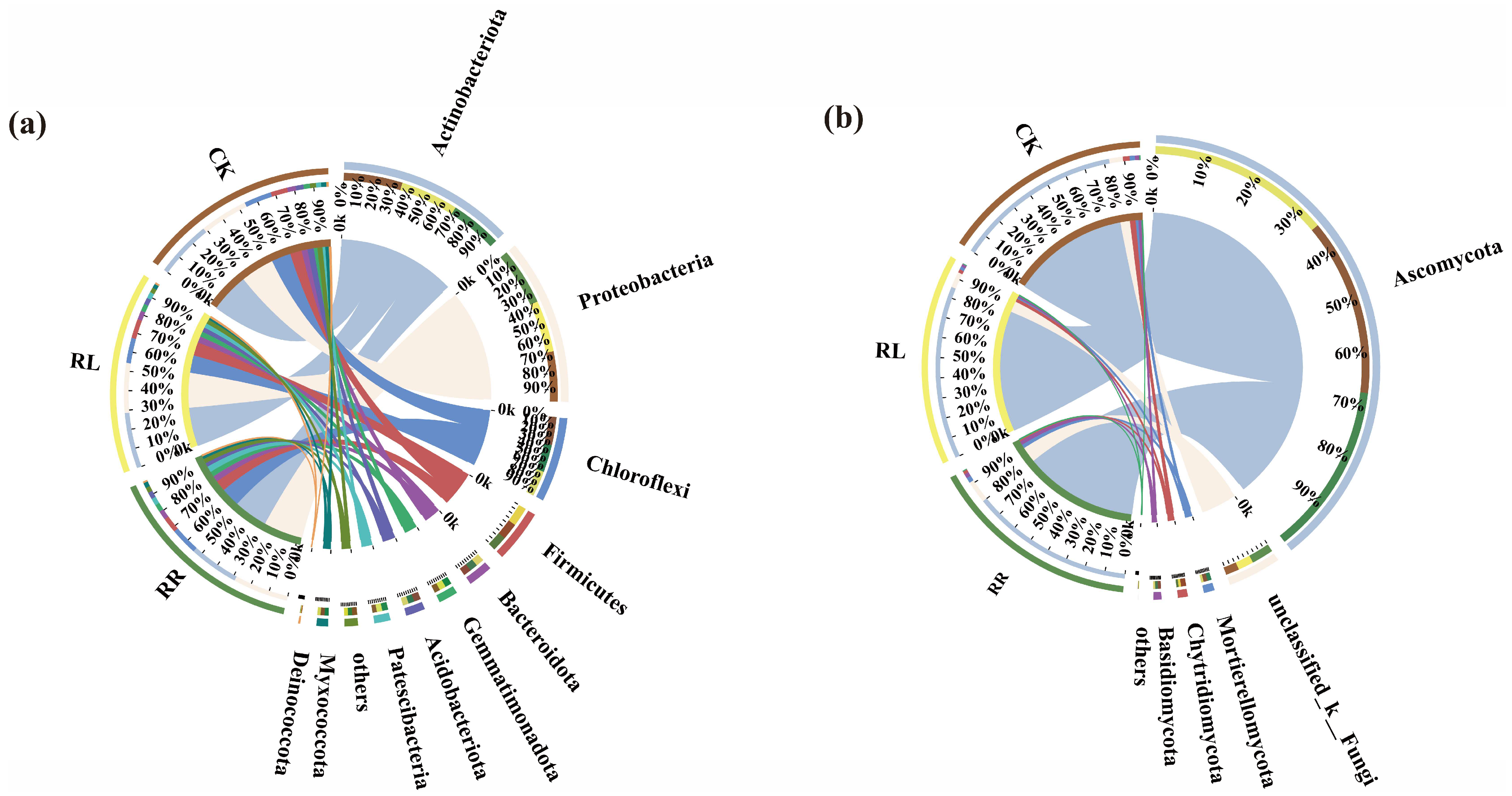
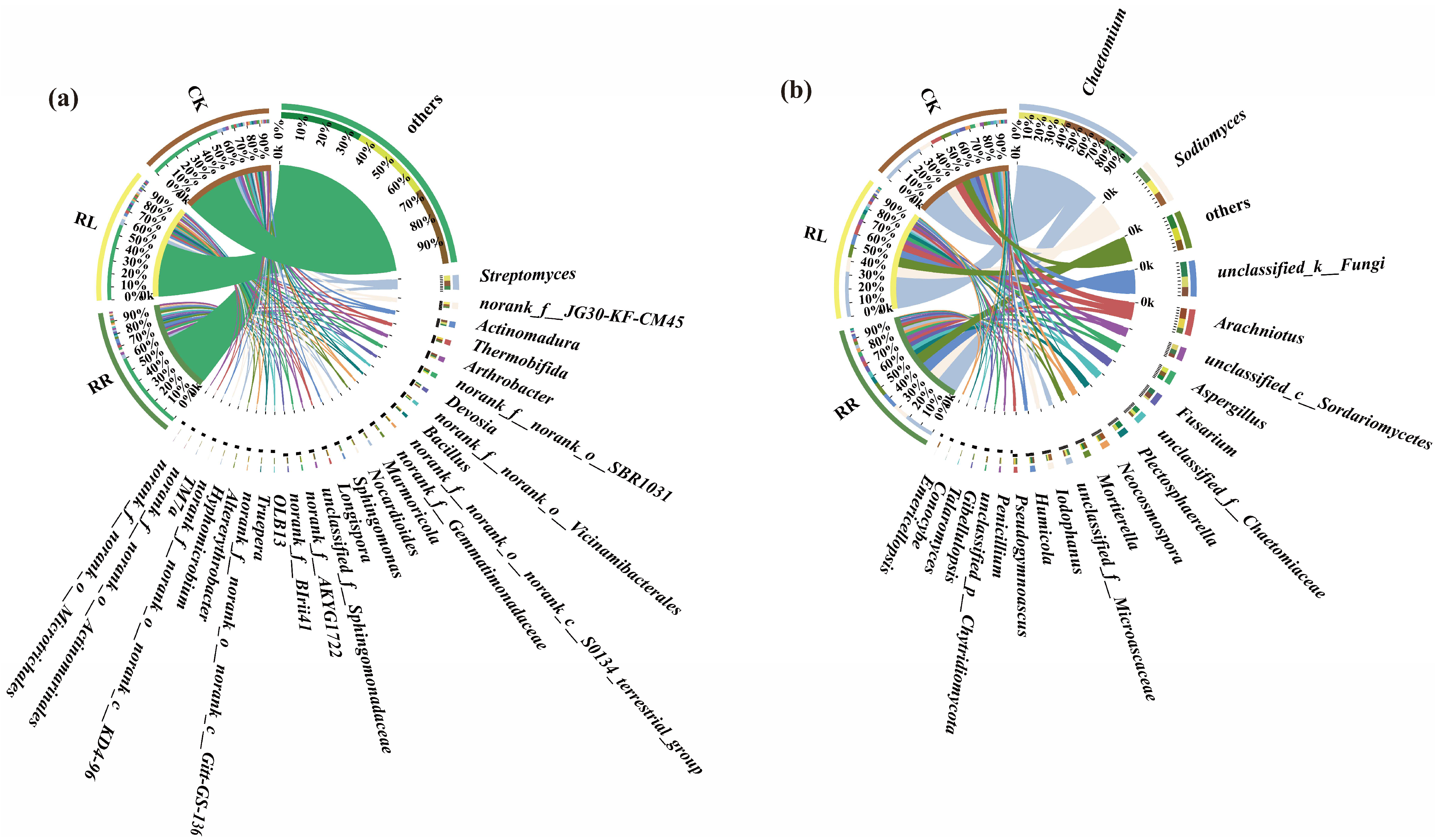
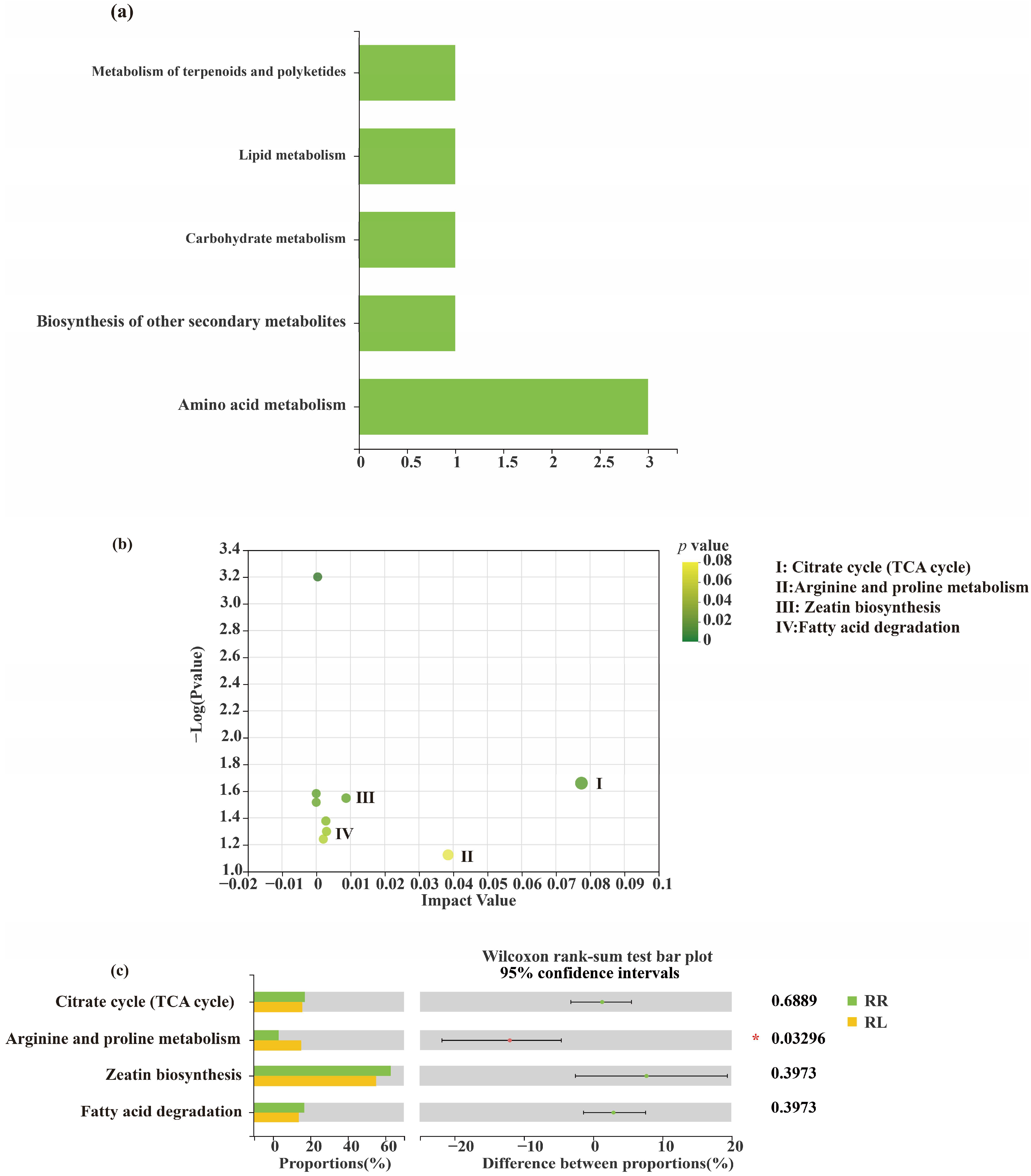
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aslam, M.M.; Karanja, J.K.; Yuan, W.; Zhang, Q.; Zhang, J.; Xu, W. Phosphorus uptake is associated with the rhizosheath formation of mature cluster roots in white lupin under soil drying and phosphorus deficiency. Plant Physiol. Biochem. 2021, 166, 531–539. [Google Scholar] [CrossRef]
- Batjes, N.H. Nitrous oxide emissions from grazed grassland. Soil Use Manag. 1997, 13, 9–16. [Google Scholar] [CrossRef]
- Vance, C.P.; Uhde-Stone, C.; Allan, D.L. Phosphorus acquisition and use: Critical adaptations by plants for securing a nonrenewable resource. New Phytol. 2003, 157, 423–447. [Google Scholar] [CrossRef]
- Alewell, C.; Ringeval, B.; Ballabio, C.; Robinson, D.A.; Panagos, P.; Borrelli, P. Global phosphorus shortage will be aggravated by soil erosion. Nat. Commun. 2020, 11, 4546. [Google Scholar] [CrossRef]
- Amadou, I.; Houben, D.; Faucon, M.-P. Unravelling the Role of Rhizosphere Microbiome and Root Traits in Organic Phosphorus Mobilization for Sustainable Phosphorus Fertilization. A Review. Agronomy 2021, 11, 2267. [Google Scholar] [CrossRef]
- Nuruzzaman, M.; Lambers, H.; Bolland, M.D.A.; Veneklaas, E.J. Phosphorus uptake by grain legumes and subsequently grown wheat at different levels of residual phosphorus fertiliser. Aust. J. Agric. Res. 2005, 56, 1041–1047. [Google Scholar] [CrossRef]
- Vu, D.T.; Armstrong, R.D.; Sale, P.W.G.; Tang, C. Phosphorus availability for three crop species as a function of soil type and fertilizer history. Plant Soil 2010, 337, 497–510. [Google Scholar] [CrossRef]
- Xiao, Y.; Cai, D.; Yang, W.; Ye, W.; Younas, M.; Wu, J.; Liu, K. Genetic structure and linkage disequilibrium pattern of a rapeseed (Brassica napus L.) association mapping panel revealed by microsatellites. Theor. Appl. Genet. 2012, 125, 437–447. [Google Scholar] [CrossRef]
- Lyu, Y.; Tang, H.; Li, H.; Zhang, F.; Rengel, Z.; Whalley, W.R.; Shen, J. Major Crop Species Show Differential Balance between Root Morphological and Physiological Responses to Variable Phosphorus Supply. Front. Plant Sci. 2016, 7, 1939. [Google Scholar] [CrossRef]
- Hocking, P.J.; Randall, P.J. Better growth and phosphorus nutrition of sorghum and wheat following organic acid secreting crops. In Plant Nutrition. Developments in Plant and Soil Sciences; Springer: Berlin/Heidelberg, Germany, 2001; pp. 548–549. [Google Scholar] [CrossRef]
- Lambers, H.; Clements, J.C.; Nelson, M.N. How a phosphorus-acquisition strategy based on carboxylate exudation powers the success and agronomic potential of lupines (Lupinus, Fabaceae). Am. J. Bot. 2013, 100, 263–288. [Google Scholar] [CrossRef]
- Lelei, J.J.; Onwonga, R.N. White Lupin (Lupinus albus L. cv. Amiga) Increases Solubility of Minjingu Phosphate Rock, Phosphorus Balances and Maize Yields in Njoro Kenya. Sustain. Agric. Res. 2014, 3, 37. [Google Scholar] [CrossRef]
- Dissanayaka, D.M.S.B.; Maruyama, H.; Masuda, G.; Wasaki, J. Interspecific facilitation of P acquisition in intercropping of maize with white lupin in two contrasting soils as influenced by different rates and forms of P supply. Plant Soil 2015, 390, 223–236. [Google Scholar] [CrossRef]
- Chaiharn, M.; Lumyong, S. Phosphate solubilization potential and stress tolerance of rhizobacteria from rice soil in Northern Thailand. World J. Microbiol. Biotechnol. 2009, 25, 305–314. [Google Scholar] [CrossRef]
- Suleman, M.; Yasmin, S.; Rasul, M.; Yahya, M.; Atta, B.; Mirza, M.S. Phosphate solubilizing bacteria with glucose dehydrogenase gene for phosphorus uptake and beneficial effects on wheat. PLoS ONE 2018, 13, e0204408. [Google Scholar] [CrossRef]
- Reyes, I.; Valéry, A.; Valduz, Z. Phosphate-solubilizing microorganisms isolated from rhizospheric and bulk soils of colonizer plants at an abandoned rock phosphate mine. In Proceedings of the First International Meeting on Microbial Phosphate Solubilization, Salamanca, Spain, 16–19 July 2002; Springer Science + Business Media: Berlin, Germany, 2007; Volume 102, pp. 69–75. [Google Scholar] [CrossRef]
- Jiang, C.; Hu, J.; Huang, X.; Li, C.; Deng, J.; Zhang, J.; Liu, F. Phosphorus speciation in sediments of Lake Hongfeng, China. Chin. J. Oceanol. Limnol. 2011, 29, 53–62. [Google Scholar] [CrossRef]
- Rodríguez, H.; Fraga, R. Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnol. Adv. 1999, 17, 319–339. [Google Scholar] [CrossRef]
- Chen, Y.; Rekha, P.; Arun, A.; Shen, F.; Lai, W.-A.; Young, C. Phosphate solubilizing bacteria from subtropical soil and their tricalcium phosphate solubilizing abilities. Appl. Soil Ecol. 2006, 34, 33–41. [Google Scholar] [CrossRef]
- Naik, P.R.; Raman, G.; Narayanan, K.B.; Sakthivel, N. Assessment of genetic and functional diversity of phosphate solubilizing fluorescent pseudomonads isolated from rhizospheric soil. BMC Microbiol. 2008, 8, 230. [Google Scholar] [CrossRef]
- Shen, H.; Yan, X.; Zhao, M.; Zheng, S.; Wang, X. Exudation of organic acids in common bean as related to mobilization of aluminum- and iron-bound phosphates. Environ. Exp. Bot. 2002, 48, 1–9. [Google Scholar] [CrossRef]
- Feng, K.; Hai-Ming, L.; Sheng, H.; Wang, X.; Mao, J. Effect of Organic Ligands on Biological Availability of Inorganic Phosphorus in Soils. Pedosphere 2004, 14, 85–92. [Google Scholar] [CrossRef]
- Shen, H.; Yang, C.; Fan, X. Mobilization of sparingly soluble phosphates by root exudates and root cell wall of soybean seedlings. Ecol. Evol. 2004, 13, 633–635. [Google Scholar] [CrossRef]
- Hinsinger, P. Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemical changes: A review. Plant Soil 2001, 237, 173–195. [Google Scholar] [CrossRef]
- Landi, L.; Valori, F.; Ascher, J.; Renella, G.; Falchini, L.; Nannipieri, P. Root exudate effects on the bacterial communities, CO2 evolution, nitrogen transformations and ATP content of rhizosphere and bulk soils. Soil Biol. Biochem. 2006, 38, 509–516. [Google Scholar] [CrossRef]
- Fisk, L.; Barton, L.; Jones, D.; Glanville, H.; Murphy, D. Root exudate carbon mitigates nitrogen loss in a semi-arid soil. Soil Biol. Biochem. 2015, 88, 380–389. [Google Scholar] [CrossRef]
- Aslam, M.M.; Karanja, J.K.; Zhang, Q.; Lin, H.; Xia, T.; Akhtar, K.; Liu, J.; Miao, R.; Xu, F.; Xu, W. In Vitro Regeneration Potential of White Lupin (Lupinus albus) from Cotyledonary Nodes. Plants 2020, 9, 318. [Google Scholar] [CrossRef]
- Hufnagel, B.; Marques, A.; Soriano, A.; Marquès, L.; Divol, F.; Doumas, P.; Sallet, E.; Mancinotti, D.; Carrere, S.; Marande, W.; et al. High-quality genome sequence of white lupin provides insight into soil exploration and seed quality. Nat. Commun. 2020, 11, 492. [Google Scholar] [CrossRef]
- Hama, J.R.; Strobel, B.W. Natural alkaloids from narrow-leaf and yellow lupins transfer to soil and soil solution in agricultural fields. Environ. Sci. Eur. 2020, 32, 126. [Google Scholar] [CrossRef]
- Cu, S.T.T.; Hutson, J.; Schuller, K.A. Mixed culture of wheat (Triticum aestivum L.) with white lupin (Lupinus albus L.) improves the growth and phosphorus nutrition of the wheat. Plant Soil 2005, 272, 143–151. [Google Scholar] [CrossRef]
- Pueyo, J.J.; Quiñones, M.A.; de la Peña, T.C.; Fedorova, E.E.; Lucas, M.M. Nitrogen and Phosphorus Interplay in Lupin Root Nodules and Cluster Roots. Front. Plant Sci. 2021, 12, 644218. [Google Scholar] [CrossRef]
- Li, L.; Li, S.-M.; Sun, J.-H.; Zhou, L.-L.; Bao, X.-G.; Zhang, H.-G.; Zhang, F.-S. Diversity enhances agricultural productivity via rhizosphere phosphorus facilitation on phosphorus-deficient soils. Proc. Natl. Acad. Sci. USA 2007, 104, 11192–11196. [Google Scholar] [CrossRef]
- Zhang, F.; Li, L. Using competitive and facilitative interactions in intercropping systems enhances crop productivity and nutrient-use efficiency. Plant Soil 2003, 248, 305–312. [Google Scholar] [CrossRef]
- Wang, D.; Marschner, P.; Solaiman, Z.; Rengel, Z. Growth, P uptake and rhizosphere properties of intercropped wheat and chickpea in soil amended with iron phosphate or phytate. Soil Biol. Biochem. 2007, 39, 249–256. [Google Scholar] [CrossRef]
- Coulis, M.; Bernard, L.; Gerard, F.; Hinsinger, P.; Plassard, C.; Villeneuve, M.; Blanchart, E. Endogeic earthworms modify soil phosphorus, plant growth and interactions in a legume-cereal intercrop. Plant Soil 2014, 379, 149–160. [Google Scholar] [CrossRef]
- Martin-Guay, M.-O.; Paquette, A.; Dupras, J.; Rivest, D. The new Green Revolution: Sustainable intensification of agriculture by intercropping. Sci. Total Environ. 2018, 615, 767–772. [Google Scholar] [CrossRef]
- Li, H.; Shen, J.; Zhang, F.; Clairotte, M.; Drevon, J.J.; Le Cadre, E.; Hinsinger, P. Dynamics of phosphorus fractions in the rhizosphere of common bean (Phaseolus vulgaris L.) and durum wheat (Triticum turgidum durum L.) grown in monocropping and intercropping systems. Plant Soil 2008, 312, 139–150. [Google Scholar] [CrossRef]
- Ae, N.; Arihara, J.; Okada, K.; Yoshihara, T.; Johansen, C. Phosphorus Uptake by Pigeon Pea and Its Role in Cropping Systems of the Indian Subcontinent. Science 1990, 248, 477–480. [Google Scholar] [CrossRef]
- Betencourt, E.; Duputel, M.; Colomb, B.; Desclaux, D.; Hinsinger, P. Intercropping promotes the ability of durum wheat and chickpea to increase rhizosphere phosphorus availability in a low P soil. Soil Biol. Biochem. 2012, 46, 181–190. [Google Scholar] [CrossRef]
- Böttcher, U.; Rampin, E.; Hartmann, K.; Zanetti, F.; Flenet, F.; Morison, M.; Kage, H. A phenological model of winter oilseed rape according to the BBCH scale. Crop Pasture Sci. 2016, 67, 345–358. [Google Scholar] [CrossRef]
- Riley, D.; Barber, S.A. Bicarbonate Accumulation and pH Changes at the Soybean (Glycine max (L.) Merr.) Root-Soil Interface. Soil Sci. Soc. Am. J. 1969, 33, 905–908. [Google Scholar] [CrossRef]
- Zhalnina, K.; Louie, K.B.; Hao, Z.; Mansoori, N.; da Rocha, U.N.; Shi, S.; Cho, H.; Karaoz, U.; Loqué, D.; Bowen, B.P.; et al. Dynamic root exudate chemistry and microbial substrate preferences drive patterns in rhizosphere microbial community assembly. Nat. Microbiol. 2018, 3, 470–480. [Google Scholar] [CrossRef]
- Smith, F.W.; Ellis, B.G.; Grava, J. Use of Acid-Fluoride Solutions for the Extraction of Available Phosphorus in Calcareous Soils and in Soils to Which Rock Phosphate Has Been Added. Soil Sci. Soc. Am. J. 1957, 21, 400–404. [Google Scholar] [CrossRef]
- Powlson, D.; Prookes, P.; Christensen, B. Measurement of soil microbial biomass provides an early indication of changes in total soil organic matter due to straw incorporation. Soil Biol. Biochem. 1987, 19, 159–164. [Google Scholar] [CrossRef]
- Chen, S.; Qin, R.; Yang, D.; Liu, W.; Yang, S. A Comparison of Rhizospheric and Endophytic Bacteria in Early and Late-Maturing Pumpkin Varieties. Microorganisms 2022, 10, 1667. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Sun, Y.; Wei, Y.; Li, H.; Yang, S. Different rhizosphere soil microbes are recruited by tomatoes with different fruit color phenotypes. BMC Microbiol. 2022, 22, 210. [Google Scholar] [CrossRef] [PubMed]
- Yadav, R.; Tarafdar, J. Influence of organic and inorganic phosphorus supply on the maximum secretion of acid phosphatase by plants. Biol. Fertil. Soils 2001, 34, 140–143. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, J.; Zhou, X.; Ding, Y.; Wang, Y.; Zhu, X.; Xu, F.; Liu, J.; Wang, Z.; Zhang, J.; et al. Soilborne bacterium Klebsiella pneumoniae promotes cluster root formation in white lupin through ethylene mediation. New Phytol. 2023, 237, 1320–1332. [Google Scholar] [CrossRef] [PubMed]
- Aslam, M.M.; Waseem, M.; Xu, W.; Ying, L.; Zhang, J.; Yuan, W. Global Identification of White Lupin lncRNAs Reveals Their Role in Cluster Roots under Phosphorus Deficiency. Int. J. Mol. Sci. 2022, 23, 9012. [Google Scholar] [CrossRef]
- Boudsocq, S.; Cros, C.; Hinsinger, P.; Lambers, H. Changes in belowground interactions between wheat and white lupin along nitrogen and phosphorus gradients. Plant Soil 2022, 476, 97–115. [Google Scholar] [CrossRef]
- Schwerdtner, U.; Spohn, M. Plant Species Interactions in the Rhizosphere Increase Maize N and P Acquisition and Maize Yields in Intercropping. J. Soil Sci. Plant Nutr. 2022, 22, 3868–3884. [Google Scholar] [CrossRef]
- Jorquera, M.A.; Crowley, D.E.; Marschner, P.; Greiner, R.; Fernández, M.T.; Romero, D.; Menezes-Blackburn, D.; Mora, M.D.L.L. Identification of β-propeller phytase-encoding genes in culturable Paenibacillus and Bacillus spp. from the rhizosphere of pasture plants on volcanic soils. FEMS Microbiol. Ecol. 2011, 75, 163–172. [Google Scholar] [CrossRef]
- Tarafdar, J.; Claassen, N. Organic phosphorus compounds as a phosphorus source for higher plants through the activity of phosphatases produced by plant roots and microorganisms. Biol. Fertil. Soils 1988, 5, 308–312. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhu, L.; Wang, J.; Xie, H.; Wang, J.; Wang, F.; Sun, F. Effects of fomesafen on soil enzyme activity, microbial population, and bacterial community composition. Environ. Monit. Assess. 2014, 186, 2801–2812. [Google Scholar] [CrossRef] [PubMed]
- Bainard, L.D.; Koch, A.M.; Gordon, A.M.; Klironomos, J.N. Growth response of crops to soil microbial communities from conventional monocropping and tree-based intercropping systems. Plant Soil 2013, 363, 345–356. [Google Scholar] [CrossRef]
- Tang, X.; Placella, S.A.; Daydé, F.; Bernard, L.; Robin, A.; Journet, E.-P.; Justes, E.; Hinsinger, P. Phosphorus availability and microbial community in the rhizosphere of intercropped cereal and legume along a P-fertilizer gradient. Plant Soil 2016, 407, 119–134. [Google Scholar] [CrossRef]
- Yu, L.; Tang, Y.; Wang, Z.; Gou, Y.; Wang, J. Nitrogen-cycling genes and rhizosphere microbial community with reduced nitrogen application in maize/soybean strip intercropping. Nutr. Cycl. Agroecosyst. 2019, 113, 35–49. [Google Scholar] [CrossRef]
- Aslam, M.M.; Pueyo, J.J.; Pang, J.; Yang, J.; Chen, W.; Chen, H.; Waseem, M.; Li, Y.; Zhang, J.; Xu, W. Root acid phosphatases and rhizobacteria synergistically enhance white lupin and rice phosphorus acquisition. Plant Physiol. 2022, 190, 2449–2465. [Google Scholar] [CrossRef]
- Zaidi, A.; Khan, M.S.; Ahemad, M.; Oves, M.; Wani, P.A. Recent advances in plant growth promotion by phosphate-solubilizing microbes. In Microbial Strategies for Crop Improvement; Khan, M.S., Ed.; Springer: Berlin/Heidelberg, Germany, 2009; p. 23. ISBN 9783642019791. [Google Scholar]
- Islam, T.; Hossain, M. Plant Probiotics in Phosphorus Nutrition in Crops, with Special Reference to Rice. In Bacteria in Agrobiology: Plant Probiotics; Springer: Berlin/Heidelberg, Germany, 2012; pp. 325–363. [Google Scholar] [CrossRef]
- Soumare, A.; Boubekri, K.; Lyamlouli, K.; Hafidi, M.; Ouhdouch, Y.; Kouisni, L. Efficacy of phosphate solubilizing Actinobacteria to improve rock phosphate agronomic effectiveness and plant growth promotion. Rhizosphere 2021, 17, 100284. [Google Scholar] [CrossRef]
- Guan, J.; Wang, J.; Zhang, X.; Xue, G.; Wang, M.; Zhou, X. Impact of Travel Disturbance on Soil Actinomycetes Communities Structure of Yaoquan Mountain in Wudalianchi. Sci. Geogr. Sin. 2016, 36, 772–779. [Google Scholar] [CrossRef]
- Guo, J.; Wang, G.; Wu, Y.; Shi, Y.; Feng, Y.; Cao, F. Ginkgo agroforestry practices alter the fungal community structures at different soil depths in Eastern China. Environ. Sci. Pollut. Res. 2019, 26, 21253–21263. [Google Scholar] [CrossRef] [PubMed]
- Midgley, D.J.; Letcher, P.M.; McGee, P.A. Access to organic and insoluble sources of phosphorus varies among soil Chytridiomycota. Arch. Microbiol. 2006, 186, 211–217. [Google Scholar] [CrossRef]
- Tarafdar, J.; Gharu, A. Mobilization of organic and poorly soluble phosphates by Chaetomium globosum. Appl. Soil Ecol. 2006, 32, 273–283. [Google Scholar] [CrossRef]
- Tarafdar, J.; Rao, A. Contribution of Aspergillus strains to acquisition of phosphorus by wheat (Triticum aestivum L.) and chick pea (Cicer arietinum Linn.) grown in a loamy sand soil. Appl. Soil Ecol. 1996, 3, 109–114. [Google Scholar] [CrossRef]
- Rashid, M.I.; Mujawar, L.H.; Shahzad, T.; Almeelbi, T.; Ismail, I.M.; Oves, M. Bacteria and fungi can contribute to nutrients bioavailability and aggregate formation in degraded soils. Microbiol. Res. 2016, 183, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Ström, L.; Owen, A.G.; Godbold, D.L.; Jones, D.L. Organic acid mediated P mobilization in the rhizosphere and uptake by maize roots. Soil Biol. Biochem. 2002, 34, 703–710. [Google Scholar] [CrossRef]
- Palomo, L.; Claassen, N.; Jones, D.L. Differential mobilization of P in the maize rhizosphere by citric acid and potassium citrate. Soil Biol. Biochem. 2006, 38, 683–692. [Google Scholar] [CrossRef]
- Onthong, J.; Osaki, M.; Nilnond, C.; Tadano, T. Phosphorus status of some highly weathered soils in peninsular Thailand and availability in relation to citrate and oxalate application. Soil Sci. Plant Nutr. 1999, 45, 627–637. [Google Scholar] [CrossRef]
- Asea, P.; Kucey, R.; Stewart, J. Inorganic phosphate solubilization by two Penicillium species in solution culture and soil. Soil Biol. Biochem. 1988, 20, 459–464. [Google Scholar] [CrossRef]
- Vives-Peris, V.; de Ollas, C.; Gómez-Cadenas, A.; Pérez-Clemente, R.M. Root exudates: From plant to rhizosphere and beyond. Plant Cell Rep. 2020, 39, 3–17. [Google Scholar] [CrossRef]
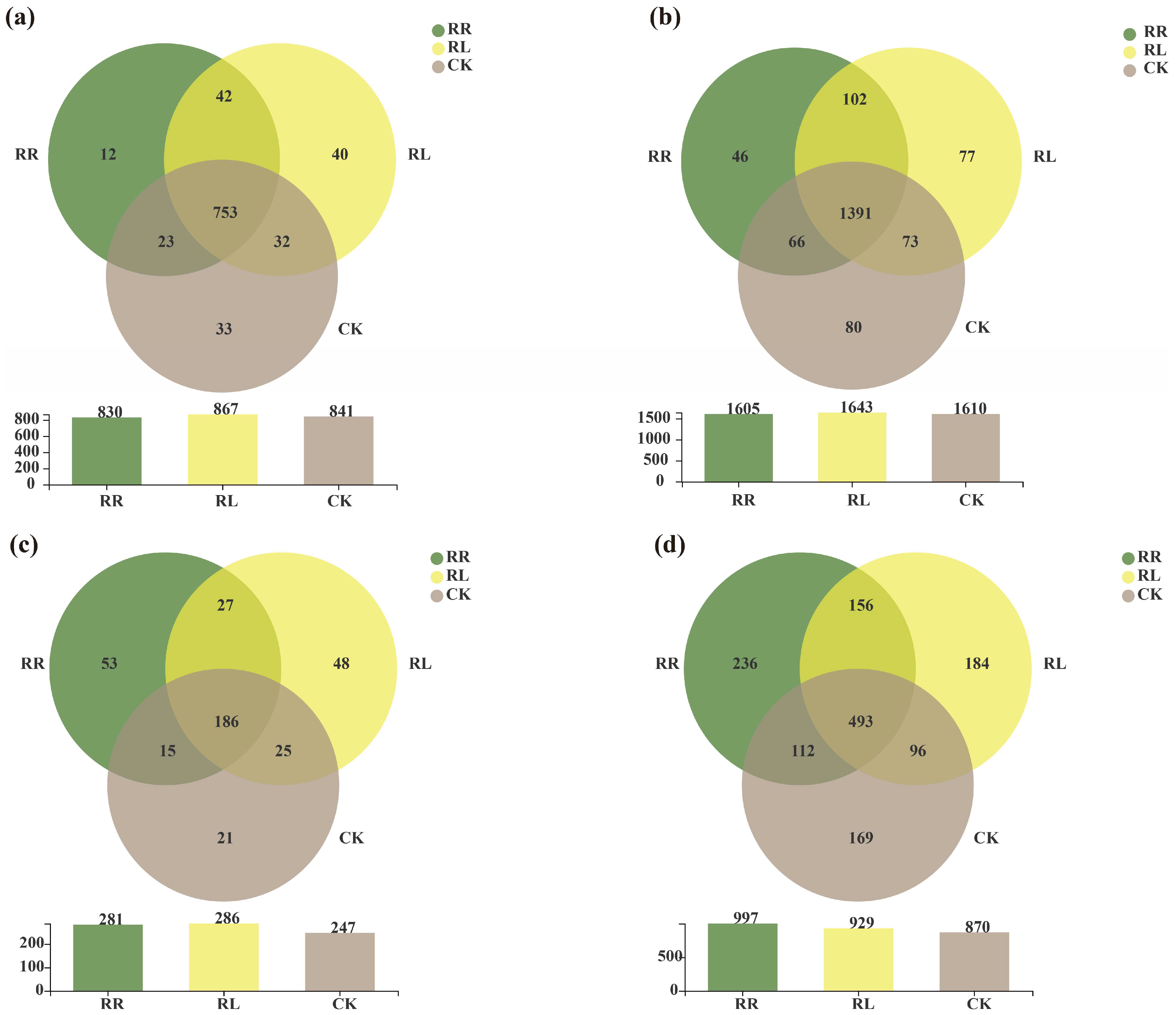

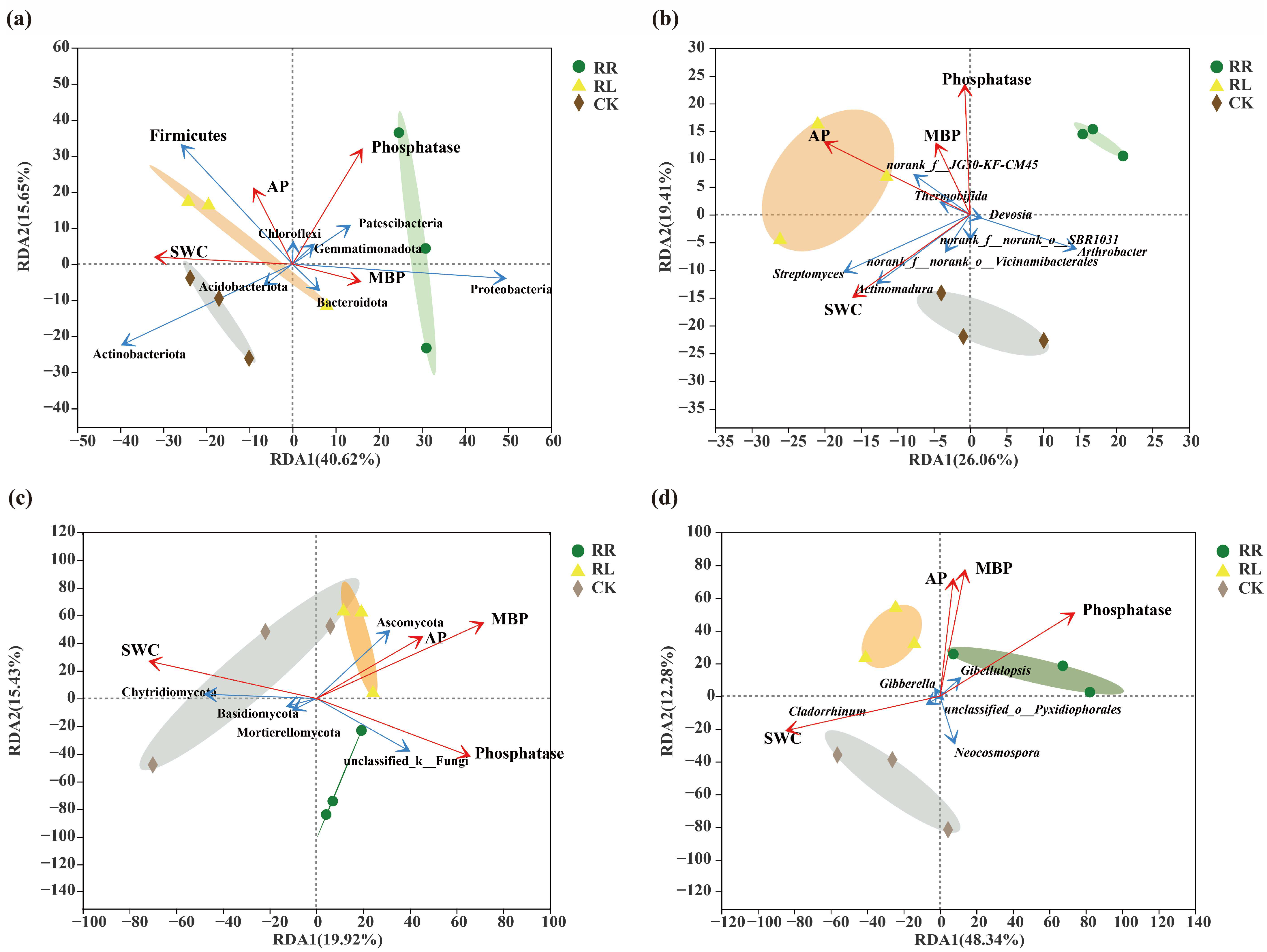
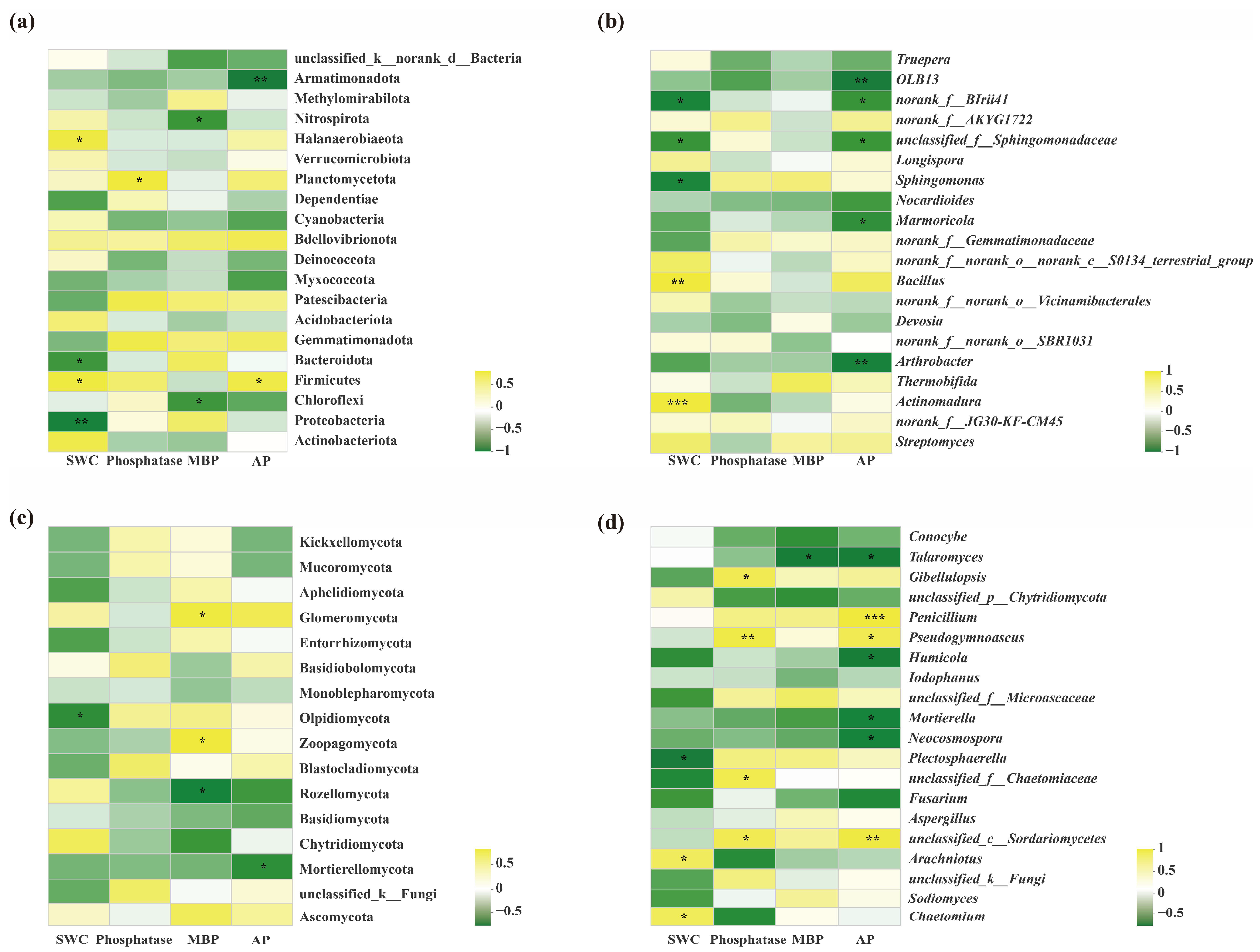
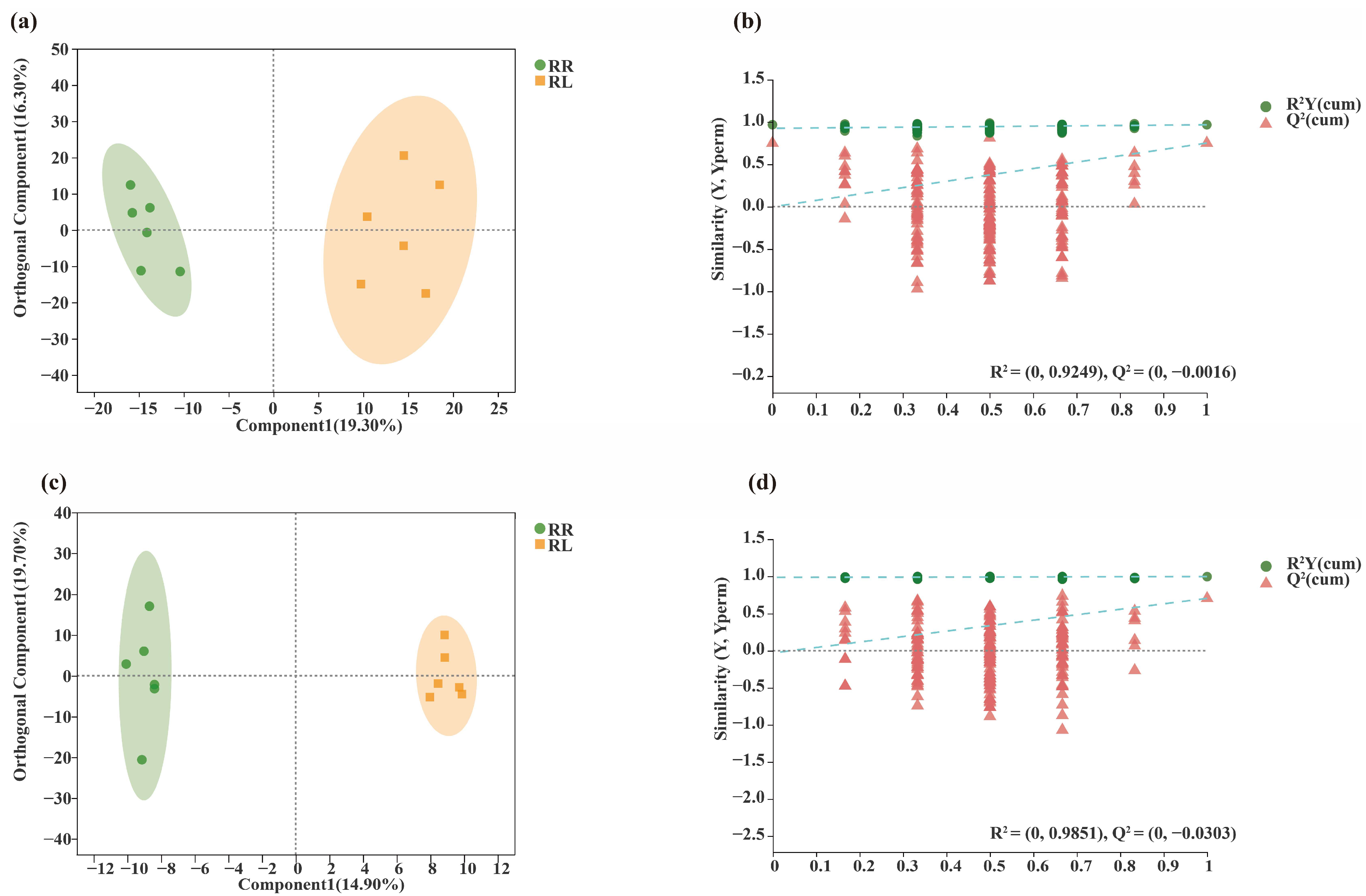

| Sequencing Type | Primer Name | Primer Sequence | Length | Sequencing Platform |
|---|---|---|---|---|
| Bacterial 16SrRNA | 338F | 5’-ACTCCTACGGGAGGCAGCAG-3’ | 311 bp | MiSeq PE250 |
| 806R | 5’-GGACTACHVGGGTWTCTAAT-3’ | |||
| Fungal ITS | ITS1F | 5’-CTTGGTCATTTAGAGAAGTAA-3’ | 350 bp | MiSeq PE300 |
| ITS2F | 5’-GCTGCGTTCTTCATCGATGC-3’ |
| Time (min) | Flow Rate (mL/min) | A (%) | B (%) |
|---|---|---|---|
| 0 | 0.4 | 100 | 0 |
| 0.1 | 0.4 | 95 | 0 |
| 2 | 0.4 | 75 | 25 |
| 9 | 0.4 | 0 | 100 |
| 13 | 0.4 | 0 | 100 |
| 13.1 | 0.4 | 100 | 0 |
| Shannon Index | Insimpson Index | Ace Index | Chao1 Index | Coverage | |
|---|---|---|---|---|---|
| RR | 6.58 ± 0.05 a | 270.42 ± 13.98 a | 3544.93 ± 25.42 a | 3562.24 ± 37.55 a | 0.97 |
| RL | 6.49 ± 0.04 ab | 234.76 ± 13.12 b | 3492.19 ± 92.62 a | 3490.77 ± 95.95 a | 0.97 |
| CK | 6.46 ± 0.05 b | 224.18 ± 20.39 b | 3581.83 ± 47.9 a | 3532.32 ± 111.15 a | 0.97 |
| Shannon Index | Insimpson Index | Ace Index | Chao1 Index | Coverage | |
|---|---|---|---|---|---|
| RR | 3.7 ± 0.07 a | 18.22 ± 2.45 a | 696.95 ± 11.3 a | 671.84 ± 8.13 a | 0.99 |
| RL | 3.52 ± 0.10 b | 13.18 ± 1.25 b | 660.93 ± 15.24 a | 669.31 ± 30.98 a | 0.99 |
| CK | 3.45 ± 0.07 b | 13.61 ± 2.08 b | 1656.28 ± 42.63 a | 634.92 ± 41.71 a | 0.99 |
| Metabolite | RR | RL | RR/RL | p-Value |
|---|---|---|---|---|
| Organic acids and their derivatives | ||||
| N-Undecanoylglycine | 5.86 ± 0.02 b | 5.97 ± 0.11 a | 0.98 | 0.0433 |
| Citric acid | 3.86 ± 0.15 a | 3.69 ± 0.07 b | 1.05 | 0.0299 |
| Creatine | 3.08 ± 0.12 b | 3.59 ± 0.33 a | 0.86 | 0.0051 |
| ADMA | 4.37 ± 0.12 a | 4.15 ± 0.21 b | 1.05 | 0.0471 |
| Tryptophyl-cysteine | 4.37 ± 0.14 a | 3.79 ± 0.50 b | 1.15 | 0.0208 |
| Isoleucylproline | 3.00 ± 0.14 b | 3.48 ± 0.49 a | 0.86 | 0.0436 |
| 2-Amino-4-[(2-hydroxy-1-oxopropyl)amino] butanoic acid | 3.99 ± 0.25 a | 3.58 ± 0.25 b | 1.11 | 0.0183 |
| L-trans-5-Hydroxy-2-piperidinecarboxylic acid | 4.37 ± 0.22 a | 4.03 ± 0.22 b | 1.09 | 0.0225 |
| Lysyl-valine | 4.33 ± 0.03 b | 4.42 ± 0.06 a | 0.98 | 0.0098 |
| Leucyl-threonine | 4.35 ± 0.05 b | 4.61 ± 0.03 a | 0.94 | 0.000001 |
| Organic oxygen compounds | ||||
| 5-Hydroxy-6-methoxyindole glucuronide | 3.86 ± 0.10 a | 3.63 ± 0.11 b | 1.25 | 0.0248 |
| Aminoacetone | 4.89 ± 0.03 b | 4.99 ± 0.04 a | 0.98 | 0.0008 |
| Organic nitrogen compounds | ||||
| L-Carnitine | 3.01 ± 0.1 b | 3.42 ± 0.33 a | 0.88 | 0.0163 |
| Memantine | 4.72 ± 0.02 b | 4.86 ± 0.12 a | 0.97 | 0.0229 |
| Tromethamine | 2.56 ± 0.69 b | 3.32 ± 0.38 a | 0.77 | 0.0410 |
| Organoheterocyclic compounds | ||||
| Cis-quinceoxepane | 4.95 ± 0.03 b | 5.09 ± 0.12 a | 0.97 | 0.0216 |
| 9-Azabicyclo [3.3.1] nonan-3-one | 3.01 ± 0.05 b | 3.66 ± 0.64 a | 0.82 | 0.0312 |
| 5-Oxo-2(5H)-isoxazolepropanenitrile | 2.98 ± 0.21 b | 3.29 ± 0.26 a | 0.91 | 0.0449 |
| Phenylpropanoids and polyketides | ||||
| 2-Pentyl-3-phenyl-2-propenal | 2.66 ± 0.32 b | 3.68 ± 0.99 a | 0.72 | 0.0363 |
| Nucleosides, nucleotides and their analogs | ||||
| Lamivudine sulfoxide | 4.95 ± 0.04 b | 5.04 ± 0.02 a | 0.98 | 0.0010 |
| 7-Methylinosine | 3.87 ± 0.15 a | 3.63 ± 0.17 b | 1.07 | 0.0288 |
| Lipids and lipid-like molecules | ||||
| Aucubin | 4.43 ± 0.18 a | 3.84 ± 0.52 b | 1.15 | 0.0266 |
| Etonogestrel | 2.74 ± 0.04 a | 2.61 ± 0.08 b | 1.05 | 0.0034 |
| 4,5-Dihydrovomifoliol | 4.35 ± 0.02 b | 4.5 ± 0.14 a | 0.97 | 0.0346 |
| (2S,4R)-p-Mentha-1(7),8-dien-2-ol | 3.3 ± 0.06 b | 3.55 ± 0.22 a | 0.93 | 0.0233 |
| Dihydrozeatin-O-glucoside | 4.45 ± 0.19 a | 4.23 ± 0.13 b | 1.05 | 0.0419 |
| Benzenoids | ||||
| Benzaldehyde | 4.62 ± 0.03 b | 4.71 ± 0.05 a | 0.98 | 0.0061 |
| 4-Hydroxy-5-phenyltetrahydro-1,3-oxazin-2-one | 4.36 ± 0.06 a | 4.24 ± 0.05 b | 1.03 | 0.0023 |
| 2-Dodecylbenzenesulfonic acid | 4.82 ± 0.05 b | 4.99 ± 0.03 a | 0.97 | 0.000021 |
| Other | ||||
| Norethindrone Acetate | 4.99 ± 0.06 b | 5.12 ± 0.02 a | 0.97 | 0.0006 |
| 3-methyl-2-Quinoxalinone | 3.66 ± 0.1 b | 4.36 ± 0.43 a | 0.84 | 0.0030 |
| 13-Hydroxylupanine | 2.95 ± 0.14 a | 2.59 ± 0.32 b | 1.18 | 0.0323 |
| Allothreonine | 4.11 ± 0.05 b | 4.20 ± 0.05 a | 0.98 | 0.0048 |
| Eicosanoyl-EA | 3.5 ± 0.10 a | 3.10 ± 0.32 b | 1.13 | 0.0165 |
| 5-Hydroxyindol-2-carboxylic acid | 3.86 ± 0.10 a | 3.63 ± 0.11 b | 1.06 | 0.0035 |
| 3,5-dihydroxy-2-(3-hydroxyphenyl)-8,8-dimethyl-4H,8H-pyrano[2,3-f] chromen-4-one | 3.77 ± 0.41 a | 2.73 ± 0.66 b | 1.38 | 0.0085 |
| 3,4,5-trihydroxy-6-[(2-phenylacetyl)oxy] oxane-2-carboxylic acid | 3.9 ± 0.31 a | 3.52 ± 0.28 b | 1.11 | 0.0471 |
| 2-Deoxy-2-dimethylamino-alpha-D-Glucose | 3.67 ± 0.43 b | 4.39 ± 0.60 a | 0.84 | 0.0363 |
| 2-AI | 2.90 ± 0.19 b | 3.91 ± 0.30 a | 0.74 | 0.00004 |
| 3-(5,7-dihydroxy-2,2-dimethyl-3,4-dihydro-2H-1-benzopyran-6-yl)-7-hydroxy-4H-chromen-4-one | 4.21 ± 0.18 a | 3.66 ± 0.47 b | 1.15 | 0.0217 |
| 8-(1,2-dihydroxypropan-2-yl)-9-hydroxy-2H,8H,9H-furo[2,3-h] chromen-2-one | 3.74 ± 0.19 a | 3.19 ± 0.3 b | 1.17 | 0.0037 |
| Phenylenediamine | 4.63 ± 0.18 a | 4.40 ± 0.17 b | 1.05 | 0.0472 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Yang, D.; Wei, Y.; He, L.; Li, Z.; Yang, S. Changes in Soil Phosphorus Availability and Microbial Community Structures in Rhizospheres of Oilseed Rapes Induced by Intercropping with White Lupins. Microorganisms 2023, 11, 326. https://doi.org/10.3390/microorganisms11020326
Chen S, Yang D, Wei Y, He L, Li Z, Yang S. Changes in Soil Phosphorus Availability and Microbial Community Structures in Rhizospheres of Oilseed Rapes Induced by Intercropping with White Lupins. Microorganisms. 2023; 11(2):326. https://doi.org/10.3390/microorganisms11020326
Chicago/Turabian StyleChen, Siyu, Da Yang, Yufei Wei, Lizhen He, Zujian Li, and Shangdong Yang. 2023. "Changes in Soil Phosphorus Availability and Microbial Community Structures in Rhizospheres of Oilseed Rapes Induced by Intercropping with White Lupins" Microorganisms 11, no. 2: 326. https://doi.org/10.3390/microorganisms11020326
APA StyleChen, S., Yang, D., Wei, Y., He, L., Li, Z., & Yang, S. (2023). Changes in Soil Phosphorus Availability and Microbial Community Structures in Rhizospheres of Oilseed Rapes Induced by Intercropping with White Lupins. Microorganisms, 11(2), 326. https://doi.org/10.3390/microorganisms11020326






