Giardia duodenalis Styles, 1902 Prevalence in Cattle (Bos taurus Linnaeus, 1758) in Europe: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy and Criteria Selection
2.2. Eligibility Criteria, Study Selection, and Data Extraction
2.3. Statistical Analysis
3. Results
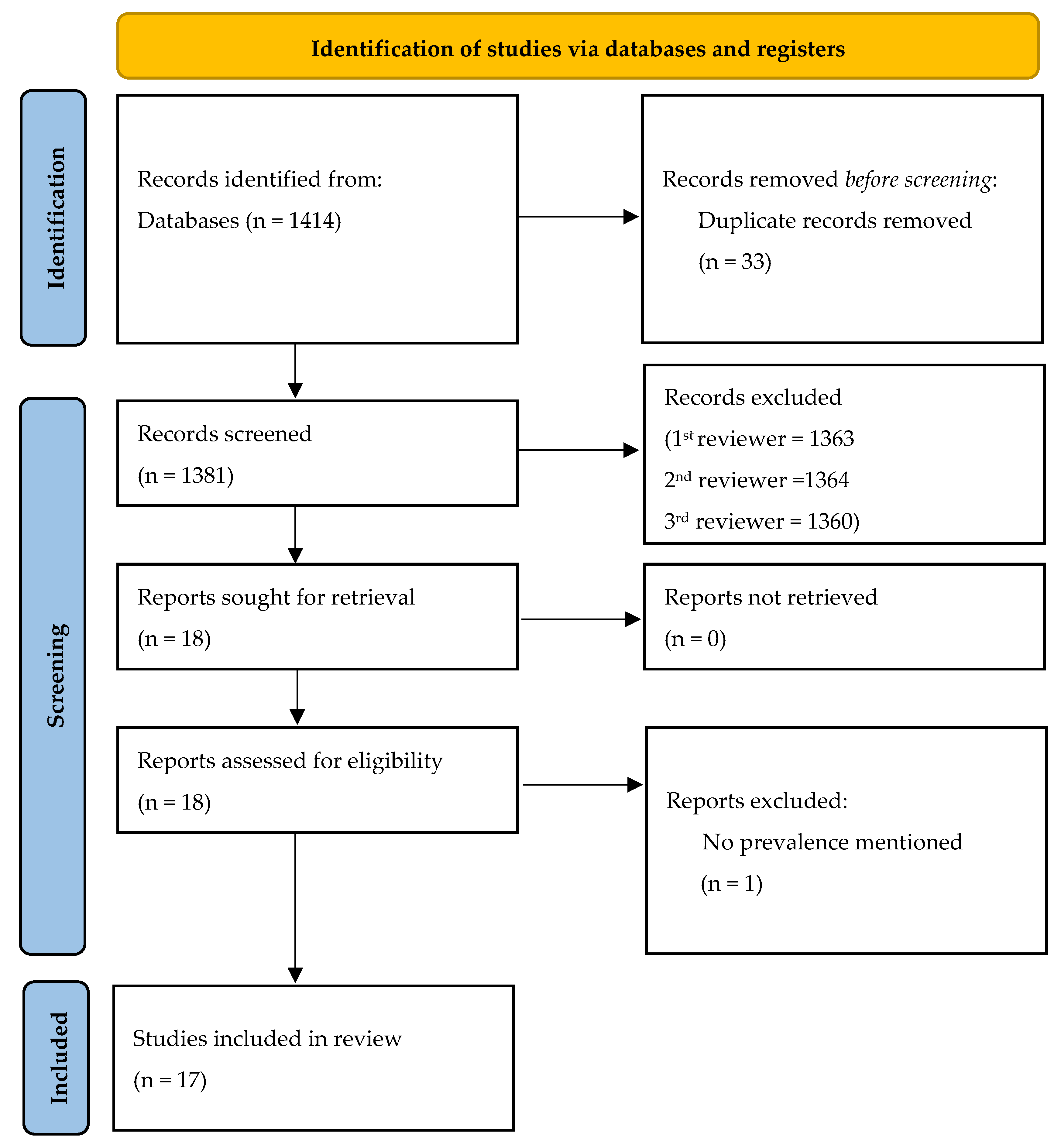
3.1. Search Results and Eligible Studies
3.2. Study Design
3.3. Giardia Duodenalis Detection in Faeces
3.3.1. Cyst Detection
3.3.2. Indirect Detection Methods
3.3.3. Molecular Methods
3.4. Prevalence of G. duodenalis in Europe
3.5. Risk Factors of G. duodenalis in Cattle Farms
3.5.1. Farm-Related Factors
3.5.2. Calf Husbandry-Related Factors
3.5.3. Seasonality
3.5.4. Age
3.5.5. Presence of Diarrhoea
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lyu, Z.; Shao, J.; Xue, M.; Ye, Q.; Chen, B.; Qin, Y.; Wen, J. A new species of Giardia Künstler, 1882 (Sarcomastigophora: Hexamitidae) in hamsters. Parasit. Vectors 2018, 11, 202. [Google Scholar] [CrossRef]
- Cacciò, S.M.; Lalle, M.; Svärd, S.G. Host specificity in the Giardia duodenalis species complex. Infect. Geneti. Evol. 2018, 66, 335–345. [Google Scholar] [CrossRef] [PubMed]
- O’Handley, R.M.; Cockwill, C.; McAllister, T.A.; Jelinski, M.; Morck, D.W.; Olson, M.E. Duration of naturally acquired giardiosis and cryptosporidiosis in dairy calves and their association with diarrhea. J. Am. Vet. Med. Assoc. 1999, 214, 391–396. [Google Scholar] [PubMed]
- Olson, M.E.; Ryan, M.; O’Handley, R.B.; McAllister, T.A.; Thompson, A. Update on Cryptosporidium and Giardia infections in cattle. Trends Parasitol. 2004, 20, 185–191. [Google Scholar] [CrossRef]
- Geurden, T.; Vercruysse, J.; Claerebout, E. Field testing of a fenbendazole treatment combined with hygienic and management measures against a natural Giardia infection in calves. Vet. Parasitol. 2006, 142, 367–371. [Google Scholar] [CrossRef]
- Geurden, T.; Vandenhoute, E.; Pohle, H.; Casaert, S.; Wilde, N.D.; Vercruysse, J.; Claerebout, C. The effect of a fenbendazole treatment on cyst excretion and weight gain in calves experimentally infected with Giardia duodenalis. Vet. Parasitol. 2010, 169, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Ankarklev, J.; Jerlström-Hultqvist, J.; Ringqvist, E.; Troell, K.; Svärd, S.G. Behind the smile: Cell biology and disease mechanisms of Giardia species. Nat. Rev. Microbiol. 2010, 8, 413–422. [Google Scholar] [CrossRef]
- O’Handley, R.M.; Buret, A.G.; McAllister, T.A.; Jelinski, M.; Olson, M.E. Giardiasis in dairy calves: Effects of fenbendazole treatment on intestinal structure and function. Int. J. Parasitol. 2001, 31, 73–79. [Google Scholar] [CrossRef]
- Ralston, B.J.; Cockwill, C.L.; Guselle, N.J.; Van Herk, F.H.; McAllister, T.A.; Olson, M.E. Prevalence of Giardia and Cryptosporidium andersoni and their effects on performance in feedlot beef cattle. Can. J. Anim. Sci. 2003, 83, 153–159. [Google Scholar] [CrossRef]
- Valdez, J.R.; Gonzales-Avalos, R.; Avila-Cisneros, R.; Peña-Revuelta, B.; Reyes-Romero, A. Economic impact of mortality and morbidity from diseases in dairy calves. Abanico Vet. 2019, 9, 1–7. [Google Scholar] [CrossRef]
- Horton, B.; Bridle, H.; Alexander, C.L.; Katzer, F. Giardia duodenalis in the UK: Current knowledge of risk factors and public health implications. Parasitology 2018, 146, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Ralston, B.J.; McAllister, T.A.; Olson, M.E. Prevalence and infection pattern of naturally acquired giardiasis and cryptosporidiosis in range beef calves and their dams. Vet. Parasitol. 2003, 114, 113–122. [Google Scholar] [CrossRef]
- Grit, G.H.; Van Coppernolle, S.; Devriendt, B.; Geurden, T.; Dreesen, L.; Hope, J.; Vercruysse, J.; Cox, E.; Geldhof, P.; Claerebout, E. Evaluation of cellular and humoral systemic immune response against Giardia duodenalis infection in cattle. Vet. Parasitol. 2014, 202, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Geurden, T.; Vercruysse, J.; Claerebout, E. Is Giardia a significant pathogen in production animals? Exp. Parasitol. 2010, 124, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Dixon, B.R. Giardia duodenalis in humans and animals—Transmission and disease. Res. Vet. Sci. 2021, 135, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Olson, M.E.; Goh, J.; Phillips, M.; Guselle, N.; McAllister, T.A. Giardia cyst and Cryptosporidium oocyst survival in water, soil, and cattle feces. J. Environ. Qual. 1999, 28, 1991–1996. [Google Scholar] [CrossRef]
- Sprong, H.; Cacciò, S.M.; van der Giessen, J.W.; ZOOPNET network and partners. Identification of zoonotic genotypes of Giardia duodenalis. PLoS Negl. Trop. Dis. 2009, 3, e558. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Xiao, L. Zoonotic Potential and Molecular Epidemiology of Giardia Species and Giardiasis. Clin. Microbiol. Rev. 2011, 24, 110–140. [Google Scholar] [CrossRef]
- Grit, G.H.; Bénéré, E.; Ehsan, A.; De Wilde, N.; Claerebout, E.; Vercruysse, J.; Maes, L.; Geurden, T. Giardia duodenalis cyst survival in cattle slurry. Vet. Parasitol. 2012, 184, 330–334. [Google Scholar] [CrossRef]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, g7647. [Google Scholar] [CrossRef]
- Dean, A.G.; Sullivan, K.M.; Soe, M.M. OpenEpi: Open Source Epidemiologic Statistics for Public Health, Version. Available online: www.OpenEpi.com (accessed on 2 November 2022).
- Geurden, T.; Geldhof, P.; Levecke, B.; Martens, C.; Berkvens, D.; Casaert, S.; Vercruysse, J.; Claerebout, E. Mixed Giardia duodenalis assemblage A and E infections in calves. Int. J. Parasitol. 2008, 38, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Maddox-Hyttel, C.; Langkjaer, R.B.; Enemark, H.L.; Vigre, H. Cryptosporidium and Giardia in different age groups of Danish cattle and pigs-occurrence and management associated risk factors. Vet. Parasitol. 2006, 141, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Langkjaer, R.B.; Vigre, H.; Enemark, H.L.; Maddox-Hyttel, C. Molecular and phylogenetic characterization of Cryptosporidium and Giardia from pigs and cattle in Denmark. Parasitology 2007, 134, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Geurden, T.; Vanderstichel, R.; Pohle, H.; Ehsan, A.; von Samson-Himmelstjerna, G.; Morgan, E.R.; Camuset, P.; Capelli, G.; Vercruysse, J.; Claerebout, E. A multicentre prevalence study in Europe on Giardia duodenalis in calves, with molecular identification and risk factor analysis. Vet. Parasitol. 2012, 190, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Jäger, M.; Gauly, M.; Bauer, C.; Failing, K.; Erhardt, G.; Zagner, H. Endoparasites in calves of beef cattle herds: Management systems dependent and genetic influences. Vet. Parasitol. 2005, 131, 173–191. [Google Scholar] [CrossRef]
- Gillhuber, J.; Pallant, J.L.; Ash, A.; Thompson, R.C.A.; Pfister, K.; Scheuerle, M.C. Molecular identification of zoonotic and livestock-specific Giardia-species in faecal samples of calves in Southern Germany. Parasit. Vectors 2013, 6, 346. [Google Scholar] [CrossRef]
- Ligda, P.; Claerebout, E.; Kostopoulou, D.; Zdragas, A.; Casaert, S.; Robertson, L.J.; Sotiraki, S. Cryptosporidium and Giardia in surface water and drinking water: Animal sources and towards the use of a machine-learning approach as a tool for predicting contamination. Environ. Pollut. 2020, 264, 114766. [Google Scholar] [CrossRef]
- Gulliksen, S.M.; Evert, J.; Lie, K.I.; Hamnes, I.S.; Løken, T.; Åkerstedt, J.; Østerås, O. Enteropathogens and risk factors for diarrhea in Norwegian dairy calves. J. Dairy Sci. 2009, 92, 5057–5066. [Google Scholar] [CrossRef] [PubMed]
- Hamnes, I.S.; Gjerde, B.; Robertson, L.J. Prevalence of Giardia and Cryptosporidium in dairy calves in three areas of Norway. Vet. Parasitol. 2006, 140, 204–216. [Google Scholar] [CrossRef]
- Bartley, P.; Roehe, B.; Thomson, S.; Shaw, H.; Peto, F.; Innes, E.; Katzer, F. Detection of potentially human infectious assemblages of Giardia duodenalis in fecal samples from beef and dairy cattle in Scotland. Parasitology 2019, 146, 1123–1130. [Google Scholar] [CrossRef] [PubMed]
- Castro-Hermida, J.A.; Almeida, A.; González-Warleta, M.; Correia da Costa, J.M.; Rumbo-Lorenzo, C.; Mezo, M. Occurrence of Cryptosporidium parvum and Giardia duodenalis in healthy adult domestic ruminants. Parasitol. Res. 2007, 101, 1443–1448. [Google Scholar] [CrossRef] [PubMed]
- Quílez, J.; Sánchez-Acedo, C.; del Cacho, E.; Clavel, A.; Causapé, A.C. Prevalence of Cryptosporidium and Giardia infections in cattle in Aragón (northeastern Spain). Vet. Parasitol. 1996, 66, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Castro-Hermida, J.A.; García-Presedo, I.; Almeida, A.; González-Warleta, M.; Correia Da Costa, J.M.; Mezo, M. Cryptosporidium spp. and Giardia duodenalis in two areas of Galicia (NW Spain). Sci. Total Environ. 2011, 409, 2451–2459. [Google Scholar] [CrossRef]
- Cardona, G.A.; Carabin, H.; Goñi, P.; Arriola, L.; Robinson, G.; Fernández-Crespo, J.C.; Clavel, A.; Chalmers, R.M.; Carmena, D. Identification and molecular characterization of Cryptosporidium and Giardia in children and cattle populations from the province of Álava, North of Spain. Sci. Total Environ. 2011, 101, 412–413. [Google Scholar] [CrossRef] [PubMed]
- Castro-Hermida, J.A.; García-Presedo, I.; Almeida, A.; González-Warleta, M.; Correia Da Costa, J.M.; Mezo, M. Detection of Cryptosporidium spp. and Giardia duodenalis in surface water: A health risk for humans and animals. Water Res. 2009, 43, 4133–4142. [Google Scholar] [CrossRef] [PubMed]
- Huetink, R.E.; Giessen, J.W.; Noordhuizen, J.P.; Ploeger, H.W. Epidemiology of Cryptosporidium spp. and Giardia duodenalis on a dairy farm. Vet. Parasitol. 2001, 102, 53–67. [Google Scholar] [CrossRef]
- Minetti, C.; Taweenan, W.; Hogg, R.; Featherstone, C.; Randle, N.; Latham, S.M.; Wastling, J.M. Occurrence and diversity of Giardia duodenalis assemblages in livestock in the UK. Transbound. Emerg. Dis. 2014, 61, 60–67. [Google Scholar] [CrossRef]
- Bouwknegt, M.; Devleesschauwer, B.; Graham, H.; Robertson, L.J.; van der Giessen, J.W. Prioritisation of food-borne parasites in Europe, 2016. Eurosurveillance 2018, 23, 17–00161. [Google Scholar] [CrossRef]
- Alles, A.H.; Waldron, M.A.; Sierra, L.S.; Mattia, A.R. Prospective comparison of direct immunofluorescence and conventional staining methods for detection of Giardia and Cryptosporidium spp. in human fecal specimens. J. Clin. Microbiol. 1995, 33, 1632–1634. [Google Scholar] [CrossRef]
- Rimhanen-Finne, R.; Enemark, H.L.; Kolehmainen, J.; Toropainen, P.; Hänninen, M.L. Evaluation of immunofluorescence microscopy and enzyme-linked immunosorbent assay in detection of Cryptosporidium and Giardia infections in asymptomatic dogs. Vet. Parasitol. 2007, 145, 345–348. [Google Scholar] [CrossRef]
- Barbecho, J.M.; Bowman, D.D.; Liotta, J.L. Comparative performance of reference laboratory tests and in-clinic tests for Giardia in canine feces. Parasit. Vectors 2018, 11, 444. [Google Scholar] [CrossRef]
- Uchôa, F.F.M.; Sudré, A.P.; Campos, S.D.E.; Almosny, N.R.P. Assessment of the diagnostic performance of four methods for the detection of Giardia duodenalis in fecal samples from human, canine and feline carriers. J. Microbiol. Meth. 2018, 145, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Wilke, H.; Robertson, L.J. Preservation of Giardia cysts in stool samples for subsequent PCR analysis. J. Microbiol. Meth. 2009, 78, 292–296. [Google Scholar] [CrossRef] [PubMed]
- Cacciò, S.M.; Giacomo, M.D.; Pozio, E. Sequence analysis of the β-giardin gene and development of a polymerase chain reaction–restriction fragment length polymorphism assay to genotype Giardia duodenalis cysts from human faecal samples. Int. J. Parasitol. 2002, 32, 1023–1030. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.M.; Sterling, C.R. Sensitivity of nested PCR in the detection of low numbers of Giardia lamblia cysts. Appl. Environ. Microb. 2007, 73, 5949–5950. [Google Scholar] [CrossRef] [PubMed]
- Gotfred-Rasmussen, H.; Lund, M.; Enemark, H.L.; Erlandsen, M.; Petersen, E. Comparison of sensitivity and specificity of 4 methods for detection of Giardia duodenalis in feces: Immunofluorescence and PCR are superior to microscopy of concentrated iodine-stained samples. Diagn. Micr. Infec. Dis. 2016, 84, 187–190. [Google Scholar] [CrossRef]
- Abdel-Moein, K.A.; Saeed, H. The zoonotic potential of Giardia intestinalis assemblage E in rural settings. Parasitol. Res. 2016, 115, 3197–3202. [Google Scholar] [CrossRef]
- Shirley, D.A.T.; Farr, A.; Watanabe, K.; Moonah, S. A Review of the Global Burden, New Diagnostics, and Current Therapeutics for Amebiasis. Open Forum Infect. Dis. 2018, 5, ofy161. [Google Scholar] [CrossRef]
- Fantinatti, M.; Bello, A.R.; Fernandes, O.; Da-Cruz, A.M. Identification of Giardia lamblia Assemblage E in Humans Points to a New Anthropozoonotic Cycle. J. Infect. Dis. 2016, 214, 1256–1259. [Google Scholar] [CrossRef]
- Zahedi, A.; Field, D.; Ryan, U. Molecular typing of Giardia duodenalis in humans in Queensland—First report of Assemblage E. Parasitology 2017, 144, 1154–1161. [Google Scholar] [CrossRef]
- Zajaczkowski, P.; Lee, R.; Fletcher-Lartey, S.M.; Alexander, K.; Mahimbo, A.; Stark, D.; Ellis, J.T. The controversies surrounding Giardia intestinalis assemblages A and B. Curr. Res. Parasitol. Vector Borne Dis. 2021, 1, 100055. [Google Scholar] [CrossRef]
- Oh, S.-I.; Jung, S.-H.; Lee, H.-K.; Choe, C.; Hur, T.-Y.; So, K.-M. Multilocus Genotyping of Giardia duodenalis Occurring in Korean Native Calves. Vet. Sci. 2021, 8, 118. [Google Scholar] [CrossRef] [PubMed]
- Uehlinger, F.D.; Greenwood, S.J.; O’Handley, R.; McClure, J.T.; Coklin, T.; Dixon, B.R.; de Boer, M.; Zwiers, H.; Barkema, H.W. Prevalence and genotypes of Giardia duodenalis in dairy and beef cattle in farms around Charlottetown, Prince Edward Island, Canada. Can. Vet. J. 2011, 52, 967–972. [Google Scholar] [PubMed]
- Trout, J.M.; Santín, M.; Greiner, E.; Fayer, R. Prevalence and genotypes of Giardia duodenalis in post-weaned dairy calves. Vet. Parasitol. 2005, 130, 177–183. [Google Scholar] [CrossRef]
- Gow, S.; Waldner, C. An examination of the prevalence of and risk factors for shedding of Cryptosporidium spp. and Giardia spp. in cows and calves from western Canadian cow–calf herds. Vet. Parasitol. 2006, 137, 50–61. [Google Scholar] [CrossRef]
- Smith, H.V.; Cacciò, S.M.; Tait, A.; McLauchlin, J.; Thompson, R.C.A. Tools for investigating the environmental transmission of Cryptosporidium and Giardia infections in humans. Trends Parasitol. 2006, 22, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Wade, S.E.; Mohammed, H.O.; Schaaf, S.L. Epidemiologic study of Giardia sp. infection in dairy cattle in southeastern New York State. Vet. Parasitol. 2000, 89, 11–21. [Google Scholar] [CrossRef]
- Hoar, B.R.; Paul, R.R.; Siembieda, J.; Pereira, M.D.G.; Atwill, E.R. Giardia duodenalis in feedlot cattle from the central and western United States. BMC Vet. Res. 2009, 5, 37. [Google Scholar] [CrossRef]
- Svensson, C.; Lundborg, K.; Emanuelson, U.; Olsson, S. Morbidity in Swedish dairy calves from birth to 90 days of age and individual calf-level risk factors for infectious diseases. Prev. Vet. Med. 2003, 58, 179–197. [Google Scholar] [CrossRef]
- Xiao, L.; Herd, R.P.; Rings, D.M. Concurrent infections of Giardia and Cryptosporidium on two Ohio farms with calf diarrhea. Vet. Parasitol. 1993, 51, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Deksne, G.; Mateusa, M.; Cvetkova, S.; Derbakova, A.; Keidāne, D.; Troell, K.; Schares, G. Prevalence, risk factor and diversity of Cryptosporidium in cattle in Latvia. Vet. Parasitol. Reg. Stud. Rep. 2022, 28, 10067. [Google Scholar] [CrossRef] [PubMed]
- Onder, Z.; Simsek, E.; Duzlu, O.; Yetismis, G.; Ciloglu, A.; Okur, M.; Kokcu, N.D.; Inci, A.; Yildirim, A. Molecular prevalence and genotyping of Giardia duodenalis in cattle in Central Anatolia Region of Turkey. Parasitol. Res. 2020, 119, 2927–2934. [Google Scholar] [CrossRef] [PubMed]
- Mahato, M.K.; Singh, D.K.; Rana, H.B.; Acharya, K.P. Prevalence and risk factors associated with Giardia duodenalis infection in dairy cattle of Chitwan, Nepal. J. Parasit. Dis. 2018, 42, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Suman, M.S.H.; Alam, M.M.; Pun, S.B.; Khair, A.; Ahmed, S.; Uchida, R.Y. Prevalence of Giardia lamblia infection in children and calves in Bangladesh. Bangl. J. Vet. Med. 2011, 9, 177–182. [Google Scholar] [CrossRef]
- Heng, Z.J.; Yang, J.F.; Xie, X.Y.; Xu, C.R.; Chen, J.R.; Ma, J.; He, J.J.; Mao, H.M. Prevalence and multilocus genotyping of Giardia duodenalis in Holstein cattle in Yunnan, China. Front. Vet. Sci. 2022, 9, 949462. [Google Scholar] [CrossRef] [PubMed]
| Country | Study Year/-s | Microscopic Cyst Detection | PCR Confirmation | Assemblage Detected | Reference |
|---|---|---|---|---|---|
| Belgium | 2001–2005 | IMF | Yes | Yes | [22] |
| Denmark | 2003–2005 | IMF | No | N/A | [23] |
| Denmark | 2003–2004 | IMF | Yes | Yes | [24] |
| France | 2010 | ELISA b | Yes | Yes | [25] |
| Germany | n.r. | Light microscopy | No | N/A | [26] |
| Germany | 2011–2013 | Direct smear | Yes | Yes | [27] |
| Germany | 2010 | ELISA b | Yes | Yes | [25] |
| Greece | 2017 | IMF | Yes | Yes | [28] |
| Italy | 2010 | ELISA b | Yes | Yes | [25] |
| Norway | 2004–2007 | IMF | No | N/A | [29] |
| Norway | 2001–2003 | IMF | No | N/A | [30] |
| Scotland | n.r. | Not done | Yes | Yes | [31] |
| Spain | n.r. | IMF | Yes | Yes | [32] |
| Spain | 1990–1993 | Iodine staining | No | N/A | [33] |
| Spain | 2008–2010 | IMF | Yes | Yes | [34] |
| Spain | 2008–2009 | ELISA a, ICT | Yes | No | [35] |
| Spain | 2007 | IMF | No | N/A | [36] |
| The Netherlands | 1999–2000 | IMF | No | N/A | [37] |
| United Kingdom | 2007–2008 | Not done | Yes | Yes | [38] |
| United Kingdom | 2010 | ELISA b | Yes | Yes | [25] |
| Country | No. of Studies Included | Age Group | No. Positive Animals | Total No. of Animals | Pooled Prevalence, % (95% CI) | Assemblage | References |
|---|---|---|---|---|---|---|---|
| Belgium | 1 | Total | 139 | 832 | 16.7 (14.3–19.4) | A, E | [22] |
| Denmark | 1 | Neonatal | 229 | 377 | 60.7 (55.7–65.5) | N/A * | [23] |
| Calves | 221 | 518 | 43.7 (38.5–47.0) | N/A * | |||
| Adults | 51 | 255 | 20.0 (15.5–25.4) | N/A * | |||
| 2 | Total | 1002 | 5300 | 18.9 (17.9–20.0) | E | [23,24] | |
| France | 1 | Total | 477 | 190 | 39.8 (35.6–44.3) | A, E | [25] |
| Germany | 2 | Total | 384 | 688 | 55.8 (51.2–59.5) | A, E | [25,27] |
| Greece | 1 | Total | 105 | 256 | 41.3 (35.1–47.1) | A, E | [28] |
| Italy | 1 | Total | 503 | 162 | 32.2 (28.3–36.4) | A, E | [25] |
| Norway | 2 | Total | 1187 | 2145 | 55.3 (53.2–57.4) | N/A * | [29,30] |
| Scotland | 1 | Neonatal | 54 | 179 | 49.0 (46.4–37.3) | A, B, E | [31] |
| Calves | 54 | 167 | 32.3 (25.7–39.7) | B, E | |||
| Heifers | 18 | 42 | 42.9 (27.7–59.0) | B, E | |||
| Total | 126 | 388 | 32.5 (28.0–37.3) | A, B, E | |||
| Spain | 2 | Neonatal | 40 | 217 | 18.3 (13.8–25.1) | A, E | [33,36] |
| 1 | Calves | 46 | 121 | 38.0 (29.8–46.9) | N/A * | [33] | |
| 2 | Heifers | 48 | 610 | 7.8 (6.0–10.3) | A, E | [33,36] | |
| 3 | Adults | 155 | 1283 | 12.1 (10.4–14.0) | A, E | [32,33,36] | |
| 5 | Total | 306 | 2645 | 11.6 (10.4–12.8) | A, E | [32,33,34,35,36] | |
| The Netherlands | 1 | Neonatal | 29 | 112 | 25.9 (18.6–34.7) | N/A * | [37] |
| 1 | Calves | 10 | 71 | 14.1 (7.6–24.2) | A | ||
| 1 | Total | 39 | 183 | 21.3 (16.0–27.8) | A | ||
| United Kingdom | 1 | Neonatal | 7 | 22 | 31.8 (16.2–52.8) | A, E | [38] |
| 1 | Calves | 34 | 78 | 43.6 (33.1–54.6) | A, E | ||
| 1 | Heifers | 6 | 23 | 26.1 (12.7–46.8) | A, E | ||
| 1 | Adults | 32 | 139 | 23.0 (16.8–30.7) | A, E | ||
| 2 | Total | 384 | 818 | 44.9 (43.5–50.4) | A, E | [25,38] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mateusa, M.; Ozoliņa, Z.; Terentjeva, M.; Deksne, G. Giardia duodenalis Styles, 1902 Prevalence in Cattle (Bos taurus Linnaeus, 1758) in Europe: A Systematic Review. Microorganisms 2023, 11, 309. https://doi.org/10.3390/microorganisms11020309
Mateusa M, Ozoliņa Z, Terentjeva M, Deksne G. Giardia duodenalis Styles, 1902 Prevalence in Cattle (Bos taurus Linnaeus, 1758) in Europe: A Systematic Review. Microorganisms. 2023; 11(2):309. https://doi.org/10.3390/microorganisms11020309
Chicago/Turabian StyleMateusa, Maira, Zanda Ozoliņa, Margarita Terentjeva, and Gunita Deksne. 2023. "Giardia duodenalis Styles, 1902 Prevalence in Cattle (Bos taurus Linnaeus, 1758) in Europe: A Systematic Review" Microorganisms 11, no. 2: 309. https://doi.org/10.3390/microorganisms11020309
APA StyleMateusa, M., Ozoliņa, Z., Terentjeva, M., & Deksne, G. (2023). Giardia duodenalis Styles, 1902 Prevalence in Cattle (Bos taurus Linnaeus, 1758) in Europe: A Systematic Review. Microorganisms, 11(2), 309. https://doi.org/10.3390/microorganisms11020309








