Abstract
Sphingomonas morindae sp. NBD5, which we previously identified and tested, is a new bacterial strain for producing lutein. Here, based on the next-generation sequencing technology, we analyzed high throughput genomic sequences and compared related functional genes of Sphingomonas morindae sp. NBD5 and Sphingopyxis sp. USTB-05. The genome of Sphingomonas morindae sp. NBD5 has two sets of chromosomes, which is 4,239,716 bp and harbors 3882 protein coding genes. There are 59 protein-coding genes related to the macular pigment (MP) biosynthesis, of which four genes (ackA, pgm, gpmI and pckA) are unique. These genes, pckG, porB, meh, and fldA, are unique in Sphingopyxis sp. USTB-05. The analysis of Sphingomonas morindae sp. NBD5 and Sphingopyxis sp. USTB-05 genomes gives an insight into the new pathway for MP production. These genes for the transformation of glucose to MP were also found in Sphingomonas morindae sp. NBD5 and Sphingopyxis sp. USTB-05. This study expands the understanding of the pathway for complete biosynthesis of MP by Sphingomonas morindae sp. NBD5 and Sphingopyxis sp. USTB-05.
1. Introduction
Macular pigment (MP) is found in the macular region of the primate retina, and consists mainly of lutein and zeaxanthin that cannot be synthesized by the body itself [1,2]. Furthermore, the detection of lutein and zeaxanthin in the macular region of the retina can be used as the optical density of macular pigment (MPOD), which can be used as a biomarker to predict ophthalmic diseases and visual function [3]. Cataracts and age-related macular degeneration (AMD) are the leading causes of visual impairment and acquired blindness, affecting tens of millions of people worldwide to varying degrees. Although MP must be obtained from daily food, most people’s daily intake is seriously insufficient. The latest research shows that daily intake of 10 mg lutein and 2 mg zeaxanthin can improve visual function and delay the development of AMD [4,5]. Lutein can be provided to fetuses and infants through umbilical cord blood, breast milk, and diet. Lutein can accumulate in human oxidative stress and high metabolic organs, indicating that it plays a unique role in the development of human embryonic organs and the development of infant eyes and brain [6]. By investigating the changes in carotenoid content of pregnant women throughout pregnancy, it was determined that prenatal maternal lutein and zeaxanthin supplementation could offset maternal carotenoid consumption and improve maternal and infant physical fitness to a certain extent [7].
Lutein and zeaxanthin are two of the 600 carotenoids in nature, which are deposited in the retina (macula) of the eye. They can be biosynthesis, which widely exists in egg yolk, corn, vegetables and fruits [8]. At present, there are many reports on eukaryotic biosynthesis of carotenoids. Their synthesis pathway has been clarified in orange-yellow fruits such as marigold flowers, mangoes, broccoli, watercress, and other green vegetables [9,10,11]. As far as production is concerned, the cultivation of marigold is a means of obtaining natural lutein and lutein esters. As far as microorganisms are concerned, free lutein can be biosynthesized by microalgae [9]. However, the production of lutein and zeaxanthin by prokaryotes was reported rarely.
Sphingomonas morindae sp. NBD5 was a new species that was identified as this genus. Phylogenetic analysis showed that it belonged to the Sphingomonadaceae family [12]. In our previous research, Sphingomonas morindae sp. NBD5 had the function of producing lutein [13]. Sphingopyxis sp. USTB-05 is a well-known strain that can be used to completely biodegrade cyanobacterium hepatotoxin [14], but whether it can produce lutein has not been investigated. Genomic studies of MP producing bacterial strains are rarely reported, and numerous genes encoding MP synthases need to be elucidated. Therefore, in order to fully elucidate the MP biosynthesis pathway in Sphingomonas morindae sp. NBD5 and Sphingopyxis sp. USTB-05, it is very important to discover the encoding genes and enzymes through genome mining. Here, we firstly sequenced the genome of Sphingomonas morindae sp. NBD5, which was 4,239,716 bp and contained 3882 protein coding genes. Glucose converted to lutein or zeaxanthin pathways were discovered in Sphingomonas morindae sp. NBD5 and Sphingopyxis sp. USTB-05.
2. Materials and Methods
2.1. Bacterial Strains
Sphingomonas morindae sp. NBD5 was isolated and identified from Noni (Morinda citrifolia L.) branch [12]. Sphingopyxis sp. USTB-05, which we previously sequenced, was isolated and identified from the sediment of Dianchi Lake in Kunming, Yunnan, China [15]. Strain NBD5 and USTB-05 were grown in LB media (3.3 g/L peptone, 1.7 g/L yeast extract, 3.3 g/L NaCl), and incubated aerobically at 30 °C.
2.2. DNA Extraction and Sequencing
The colonies were obtained after 48 h of incubation on LB agar medium at 30 °C. A single colony was picked and cultured for 48 h at 30 °C, 200 rpm to logarithmic phase in LB liquid media. Cells were collected by centrifugation at 13,282× g for 10 min at 4 °C, and the supernatant was discarded. Genomic DNA was extracted using the Rapid Bacterial Genomic DNA Isolation Kit (CoWin Biosciences, Taizhou, Jiangsu, China) according to the manufacturer’s instructions. DNA quality, concentration and integrity were checked by NanoDrop (Thermo Fisher Scientific, Waltham, MA, USA) and gel electrophoresis. DNA damage repair and end repair, magnetic beads purification and linker connection were processed by using the official SQK-LSK ligation kit (BAIYITECH, Hangzhou, Zhejiang, China). The third-generation genomic library was constructed by the standard protocol of Oxford Nanopore Technologies (ONT, Oxford, UK). High quality data sets with corresponding sequencing depths of 100-fold were generated (Figure S1). Large fragments of DNA were recovered by using BluePippin (Notre Dame, IN, USA) automatic nucleic acid recovery system [16]. The second-generation genomic library of strain NBD5 was performed using the Illumina X10 platform (Madison, WI, USA) [17].
2.3. Genome Assembly and Quality Control
The subreads used second-generation sequencing technology were filtered by fastp software v0.23.2. These sequences with a quality value of Q < 25 were deleted. The original fastq format was obtained by base calling FAST5 file through Albacore software in MinKNOW software v4.0.4. The complete map of the strain NBD5 genome was assembled using SPAdes software v3.13.0. Meanwhile, Pilon software v1.5 was used to correct the assembly results.
2.4. Genome Annotation
The online NMPDR-rust server was used to predict coding sequences (CDSs) region of the assembly sequence. tRNA were predicted using tRNAscan-SE v2.0. All unigenes were functionally annotated using the Swiss-Prot and Pfam databases. Circos software was used to integrate the COG annotation results, methylation results, RNA annotation results, GC content, and GC-skew to map the entire genome of the bacterial strain. Function annotation of NBD5 and USTB-05 were searched for Kyoto Encyclopedia of Genes and Genomes (KEGG), Cluster of Orthologous Group (COG), Gene ontology (GO) to predict metabolic information using BLAST software v2.9.0 [18]. In addition, CRISPRFinder software v4.2.19 was used to predict the clustered regularly interspaced short palindromic repeats (CRISPR) structure of the strain NBD5 genome [19]. The coding sequences of the genome were aligned using MUMmer version 4.0+ and analyzed in conjunction with the results of the genome annotation [20].
2.5. Detection of Lutein of Sphingomonas morindae sp. NBD5
The crude extract of Sphingomonas morindae sp. NBD5 was obtained by 0.22 um organic filter membrane filtration. The crude extract of Sphingomonas morindae sp. NBD5 and standard lutein were detected on a Pillar Agilent Infinity Lab Poroshell 120 SB-C18 column (2.7 µm, 100 × 3.0 mm i.d.) (Agilent Technologies Inc., USA) in a liquid chromatography-mass spectrometry quadrupole time of flight (LC-Q-TOF/MS) (Agilent Technologies Inc., USA), connected to a UV-visible absorption spectrum detector. The crude extract of Sphingomonas morindae sp. NBD5 and standard lutein were dissolved in chemical reagent (ethanol/n-hexane, 1:6.7, v/v). The column was kept at 30 °C. The mobile phase was a mixed solution of methanol/water (97.5:2.5, v/v) at a flow rate of 1 mL/min. The injection volume was 5 µL. The MS parameters were set as follows: splitting voltage of 4000 V, collision energy of 15 eV, dry gas (N2) temperature of 325 °C at 10 L/min. MS spectra were acquired in the range of m/z 50 to 700 and MS/MS was obtained in automatic mode.
2.6. Data and Drawing Tool
The genome circular map was generated by using the online software Circos (http://www.circos.ca, accessed on 4 June 2022). The heat map was generated by using the online software Hiplot (https://hiplot.com.cn, accessed on 1 May 2021). The sequence data were submitted to NCBI Sequence Read Archive (https://www.ncbi.nlm.nih.gov/sra/, accessed on 8 March 2020) with accession numbers CP084712, CP084930-CP084933. The sequence data will be released on 31 October 2023.
3. Results
3.1. General Features of the Genome
Sphingomonas morindae sp. NBD5 contains two chromosomes and two plasmids (Figure 1). The genome of 4,239,716 bases is, respectively, predicted guanine-cytosine (GC) content of 70%, 61 tRNA genes, 1 tmRNA gene and 3882 protein coding sequences (CDSs), accounting for 62.39% of the total coding sequences. The 16S rRNA of strain NBD5 has three complete copies (Table 1).
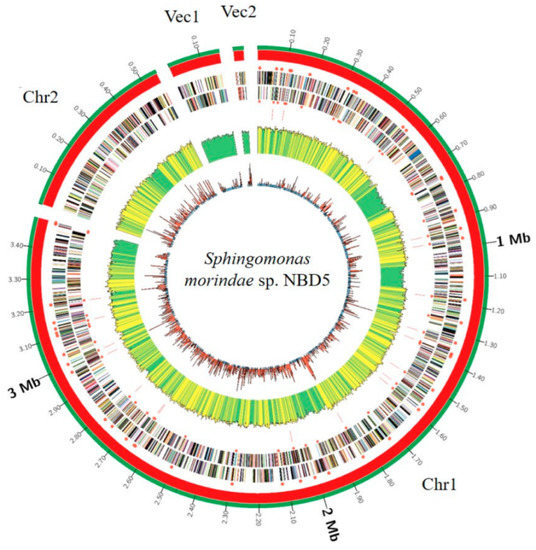
Figure 1.
Circular representation of two chromosomes and two plasmids of Sphingomonas morindae sp. NBD5. Marked information is illustrated from the inner to outer ring: GC skew, GC content, the distribution of rRNAs and tRNAs, CDSs on the reverse stand, CDSs on the forward stand, genomes size.

Table 1.
Genome features of Sphingomonas morindae sp. NBD5.
3.2. Gene Ontology Annotation
Gene ontology (GO) is a standardized gene functional classification system, which tenders a dynamic-updated controlled vocabulary. The GO analysis indicated that a total of 15,640 GO terms were associated with all unigenes (Figure 2, Supplementary Table S1). According to the secondary classification of the GO terms, all unigenes are sorted into 48 functional groups. Biological process is the main category of GO annotations (6771, 43.29%) unigenes, followed by cellular component (5074, 32.44%) and molecular function (3795, 24.26%). The metabolic process (25.88%) and cellular process (24.09%) represent most of the biological process category, indicating that the bacterium has high metabolic activity. There are also some subcategories including response to stimulus (8.83%), biological regulation (8.26%), regulation of biological process (6.96%), localization (6.94%), multi-organism process (4.31%) and cellular component organization or biogenesis (3.71%). Cell (29.96%), cell part (29.84%), membrane (17.80%), membrane part (11.69%) and protein containing complex (4.85%) are the cell gene clustering of five main components. The catalytic activity (42.77%) and binding (39.53%) represent most of the molecular function category, forecasting that the bacterium has a high degree of molecular catalysis (Figure 2).
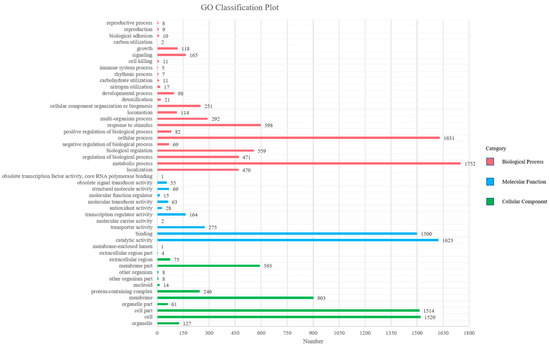
Figure 2.
Distributions of second level GO of Sphingomonas morindae sp. NBD5 genome sequence. The y-axis indicates the GO ontology; the x-axis represents the number of unigenes in a category.
3.3. Cluster of Orthologous Groups Annotation
Cluster of Orthologous Groups (COG) is a database for homologous classification of gene products. A total of 3668 classified unigenes were divided into 21 functional categories. Analysis of the assembled Sphingomonas morindae sp. NBD5 unigenes showed that 80.18% of unigenes were annotated in the COG database. Among these categories, the five main groups of transcription (281, 7.66%); cell wall, membrane, envelope biogenesis (259, 7.06%); carbohydrate transport and metabolism (250, 6.82%); amino acid transport and metabolism (247, 6.73%) and function unknown (727, 19.82%) are the most prevalent. The biosynthesis of lutein is completed by carbohydrate transport and metabolism (6.82%), this is also a significant group to be considered. Additionally, the biosynthesis of lutein depends on various biological enzymes, which are synthesized by cellular processes and signaling (25.00%) and metabolism (37.90%). Thus, posttranslational modification, protein turnover, chaperones (3.68%) is also considered an important functional group (Figure 3, Supplementary Table S2).
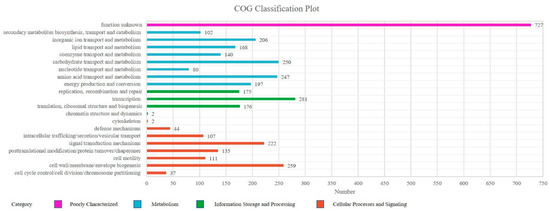
Figure 3.
COG functional classification of Sphingomonas morindae sp. NBD5. The columns represent the number of unigenes in each subcategory.
3.4. Gene Clusters and Pathway Associated with Lutein and Zeaxanthin Biosynthesis
Lutein and zeaxanthin contain two ionone rings in its chemical formula. They are carotenoids. Carbon fixation pathways in prokaryotes and glycolysis and gluconeogenesis are believed to be involved in the synthesis of lutein and zeaxanthin (Figure S2). These genes, ackA, pgm, gpmI, and pckA, are unique in Sphingomonas morindae sp. NBD5 (Table 2). gpmI encodes a cofactor independent phosphoglycerate mutase that catalyzes the conversion of glycerate-3-phosphate to glycerate-2-phosphate. It involves in glycolysis/gluconeogenesis to generate glyceraldehyde 3-phosphate and pyruvate, providing a carbon skeleton for MP biosynthesis. pckA encodes a phosphoenolpyruvate carboxykinase, which catalyzes the conversion of oxaloacetate to phosphoenol-pyruvate. It participates to generate pyruvate in the glycolysis/gluconeogenesis pathway, providing a carbon skeleton for MP biosynthesis. pgm encodes a phosphoglucomutase directly involved in the glycolysis/gluconeogenesis pathway. It catalyzes α-D-glucose 1-phosphate into α-D-glucose 6-phosphate, which provides a carbon skeleton for MP biosynthesis. ackA encodes an acetate kinase that catalyzes the conversion of acetate to acetyl phosphate. It involves in the glycolysis/gluconeogenesis pathway, and the negative feedback regulates the process of pyruvate to acetyl CoA (Figure S3). The genes pckG, porB, meh, and fldA are characteristic in Sphingopyxis sp. USTB-05 (Table 2). pckG encodes phosphoenolpyruvate carboxykinase, which catalyzes the conversion of oxaloacetate to phosphoenol-pyruvate. It directly participates in the glycolysis/gluconeogenesis pathway to generate pyruvate and provide a carbon skeleton for MP biosynthesis. porB encodes 2-oxoglutarate ferredoxin oxidoreductase, which catalyzes the conversion of succinyl-CoA to 2-oxoglutarate in carbon fixation pathways. meh encodes 3-methylfumaryl-CoA hydratase, which catalyzes the conversion of 3-methylfumaryl-CoA to (3S)-citramalyl-CoA in the carbon fixation pathways in prokaryotes. fldA encodes 2-methylfumaryl-CoA isomerase, which catalyzes the conversion of 2-methylfumaryl-CoA to 3-methylfumaryl-CoA in the carbon fixation pathways. pgm exists in the NBD5 strain and encodes phosphoglucomutase, which catalyzes α-D-glucose 1-phosphate into α-D-glucose 6-phosphate. No gene encoding phosphoglucomutase was found in USTB-05 strain.

Table 2.
Genes related to macular pigment biosynthesis in Spingomonas morindae sp. NBD5 and Sphingopyxis sp. USTB-05.
The lutein and zeaxanthin synthesis genes in Sphingomonas morindae sp. NBD5 and Sphingopyxis sp. USTB-05 genomes mainly exist in the terpenoid backbone biosynthesis pathway and the carotenoid biosynthesis pathway, which are roughly the same (Figures S4 and S5). Both strains are the presence of β-carotene 3-hydroxylase, which is involved in the last step of lutein and zeaxanthin synthesis, and its corresponding code gene is crtR. This is a fact that lycopene exists as a synthetic intermediate, which is synthesized through glycolysis pathway, terpenoid backbone biosynthesis pathway and carotenoid biosynthesis pathway (Figure 4).
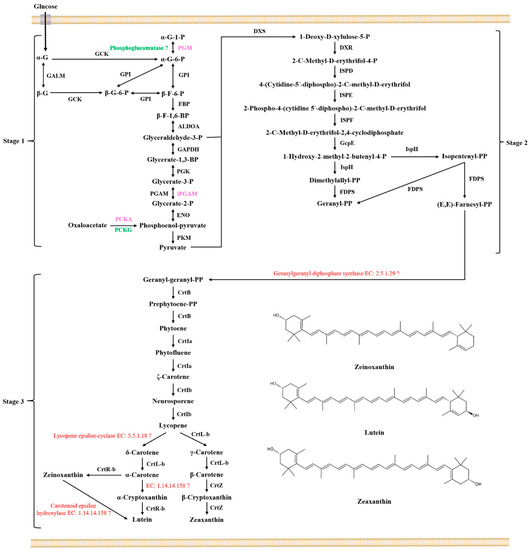
Figure 4.
The proposed biosynthetic pathway of MP of Sphingomonas morindae sp. NBD5 and Sphingopyxis sp. USTB-05. α-G-1-P: α-D-glucose 1-phosphate; PGM: phosphoglucomutase; α-G-6-P: α-D-glucose 6-phosphate; GCK: glucokinase; α-G: α-D-glucose; GALM: aldose 1-epimerase; β-G: β-D-glucose; β-G-6-P: β-D-glucose 6-phosphate; GPI: glucose-6-phosphate isomerase; β-F-6-P: β-D-fructose 6-phosphate; FBP: fructose bisphosphatase; β-F-1,6-BP: β-D-fructose 1,6-bisphosphate; ALDOA: fructose bisphosphate aldolase; Glyceraldehyde-3-P: Glyceraldehyde-3-phosphate; GAPDH: glyceraldehyde-3-phosphate dehydrogenase; Glycerate-1,3-BP: Glycerate-1,3-bisphosphate; PGK: phosphoglycerate kinase; Glycerate-3-P: Glycerate-3-phosphate; PGAM: cofactor dependent phosphoglycerate mutase; iPGAM: cofactor independent phosphoglycerate mutase; Glycerate-2-P: Glycerate-2-phosphate; ENO: phosphopyruvate hydratase; PCKA: phosphoenolpyruvate carboxykinase; PCKG: phosphoenolpyruvate carboxykinase; PKM: pyruvate kinase; DXS: 1-deoxy-D-xylulose-5-phosphate synthase; DXR: 1-deoxy-D-xylulose-5-phosphate reductoisomerase; ISPD: 2-C-methyl-D-erythritol 4-phosphate cytidylyltransferase; ISPE: 4-(cytidine 5′-diphospho)-2-C-methyl-D-erythritol kinase; ISPF: 2-C-methyl-D-erythritol 2,4-cyclodiphosphate synthase; GcpE: (E)-4-hydroxy-3-methylbut-2-enyl-diphosphate synthase; IspH: 4-hydroxy-3-methylbut-2-en-1-yl diphosphate reductase; FDPS: dimethylallyltranstransferase; Dimethylallyl-PP: Dimethylallyl-bisphosphate; Isopentenyl-PP: Isopentenyl-bisphosphate; Geranyl-PP: Geranyl-bisphosphate; (E,E)-Farnesyl-PP: (E,E)-Farnesyl-bisphosphate; Geranyl-geranyl-PP: Geranyl-geranyl-bisphosphate; Prephytoene-PP: Prephytoene-bisphosphate; CrtB: 15-cis-phytoene synthase; CrtIa: phytoene desaturase; CrtIb: phytoene desaturase; CrtL-b: lycopene beta-cyclase; CrtR-b: beta-carotene hydroxylase; CrtZ: beta-carotene 3-hydroxylase. The black font in the figure indicates that enzymes or genes coexist in two strains, the red font in the figure indicates that enzymes or genes not found in two strains, the pink font indicates that enzymes or genes exist in Sphingomonas morindae sp. NBD5, and the green font indicates that enzymes or genes exist in Sphingopyxis sp. USTB-05.
3.5. Identification of Lutein of Sphingomonas morindae sp. NBD5 and Sphingopyxis sp. USTB-05
Under the applied chromatographic conditions, the retention time of standard lutein was at 5.5 min on LC-Q-TOF/MS (Figure S6a). A peak in the extract of Sphingomonas morindae sp. NBD5 also arose at the same retention time (Figure S6b). Furthermore, the scanning profiles from 350 nm to 550 nm between lutein peak and a peak in the extract of Sphingomonas morindae sp. NBD5 at same retention time were almost same each other and the maximum absorbance were all at 446 nm or so (Figure S6). The mass spectral analysis of standard lutein (Figure S7a) and a peak at same retention time in the extract of Sphingomonas morindae sp. NBD5 (Figure S7b) revealed a major ion at m/z 568.4289, corresponding to the [M+H]+ protonated molecular ion, which all accorded with the lutein molecular weight [21,22,23]. The m/z 568.4289 was selected as the parent ion, and the secondary scan was performed. In the detection of standard lutein and the extract of Sphingomonas morindae sp. NBD5, the parent ion m/z 568.4283 was subjected to a collision cracking of 15 eV in the collision cell, where in the main ions were m/z 476.3656 [M+H-92]+, m/z 458.3542 [M+H-92-18]+ and m/z 430.3239 [M+H-92-18-28]+ (Figure S8). Among them, m/z 476.3656 is formed when the molecular ion peak loses the carotenoid characteristic group of m/z 92 [22]. Corresponding with the data in the report, the molecular formula of m/z 476.3656 characteristic ion fragment is C33H48O2, which is formed by the loss of C7H8 molecule by the parent ion m/z 568.4280 [21], which completely confirmed that lutein could be produced by Sphingomonas morindae sp. NBD5.
The streak plate colonies of Sphingomonas morindae sp. NBD5 grew on solid medium at five days. The yellow color of Sphingomonas morindae sp. NBD5 colonies is similar to that of lutein (Figure 5a). In conclusion, genomic annotation, mass spectrometry identification, and appearance comparison showed that strains of Sphingomonas morindae sp. NBD5 produced lutein. Similarly, the genomic annotation and appearance comparison of Sphingopyxis sp. USTB-05 showed that Sphingopyxis sp. USTB-05 produced lutein or zeaxanthin (Figure 4 and Figure 5b).
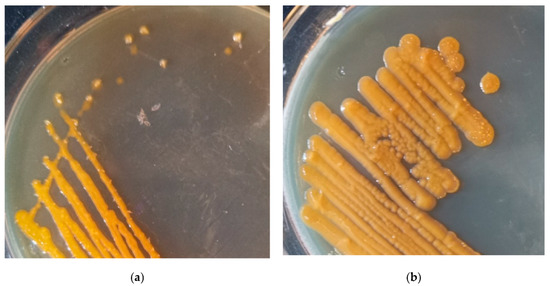
Figure 5.
(a) The streak plate colonies of Sphingomonas morindae sp. NBD5 grown on solid medium at five day; (b) the streak plate colonies of Sphingopyxis sp. USTB-05 grown on solid medium at five day.
4. Discussion
Sphingomonas morindae sp. NBD5 had two genomes, which were relatively rare in Sphingomonas. Two genomes and two plasmids may have special significance in the biosynthesis and regulation of macular pigment of this strain. Both plasmids were stable in the strain, indicating that they have different replicons. Two genomes have been reported in eukaryotes, and this heterokaryosis plays a role in the adaptation of arbuscular mycorrhizal fungi to different plant hosts [24]. Sphingomonas morindae sp. NBD5 strain is an endophyte from Noni fruit, and the two genomes may play a key role in its adaptation in the host. Recent studies prove that genome-wide phylogeny can improve the phylogenetic accuracy and preferably delimit the species borderlines [25]. As of 7 May 2022, 127 strains of Sphingomonas have published genome data in the National Center for Biotechnology Information (NCBI) database. Sphingomonas morindae sp. NBD5 whole-genome sequencing enriches the whole genome data of MP-producing bacterium.
Lutein and zeaxanthin biosynthesis can be divided into three stages: basic components formation, components condensation, and skeleton structure assembly (Figure 4). Synthetase genes related to lutein and zeaxanthin are discovered in the glycolysis/gluconeogenesis pathway, terpenoid skeleton biosynthesis pathway and carotenoid biosynthesis pathway (Figures S3–S5). In the first stage, glyceraldehyde 3-phosphate (G3P) and pyruvate are synthesized via the glycolysis pathway (Figure S3). In the glycolysis pathway, the glucose was transferred into the bacteria through the receptors on the surface of the bacteria in two strains. All enzymes involved in the conversion of α-D-glucose 1-phosphate to pyruvate were found in two strains. PGM, iPGAM, PCKA are three unique enzymes in the genome of Sphingomonas morindae sp. NBD5. PCKG is a unique enzyme in the genome of Sphingopyxis sp. USTB-05. PGM is α-D-glucose-1,6-bisphosphate dependent phosphoglucomutases. iPGAM is 2,3-diphosphoglycerate independent phosphoglycerate mutase. In particularly, iPGAM shows the greatest activity in the absence of 2,3-diphosphoglycerate in Gram-positive bacteria and is rarely reported in Gram-negative bacteria [26].
In the second stage, the 2-C-methyl-D-erythritol 4-phosphate (MEP) pathway, also known as the 1-deoxy-D-xylulose 5-phosphate (DXP) or non-mevalonate pathway [27]. DXS, DXR, ISPD, ISPE, ISPF, GcpE, ISPH, and FDPS are annotated in the genome of Sphingomonas morindae sp. NBD5 and Sphingopyxis sp. USTB-05 (Figure S4). DXS catalyzes the condensation of pyruvate and glyceraldehyde 3-phosphate to form 1-deoxy-D-xylulose 5-phosphate. DXS is an open reading frame-encoding enzyme on the chromosomal map of E. coli. Cloning of dxs was overexpressed and purified to produce a specific activity of enzyme protein of 0.85 units per mg, and DXS may be widespread in bacteria and plant chloroplasts [28]. The dxs gene was cloned from Streptomyces sp. CL190 and E. coli, respectively. The overexpressed and purified experiments show that they have the same enzymatic properties although they have different origins [29]. DXR catalyses transform 1-deoxy-D-xylulose 5-phosphate into 2-C-methyl-D-erythritol 4-phosphate. DXR is an NADPH-dependent enzyme that also requires metal ions (Mn2+, Co2+ or Mg2+) with Mn2+ being the most efficient. The reaction mechanism of this enzyme is shown to be a retro-aldol/aldol reaction [30]. Earlier experiments, overexpressing the recombinant gene yaeM corresponding to DXR in E. coli is found that the enzyme can convert 1-deoxy-D-xylulose 5-phosphate to 2-C-methyl-D-erythritol 4-phosphate through one-step intramolecular rearrangement and reduction. This result suggests that DXR is responsible for terpenoid biosynthesis in E. coli [31]. ISPD catalyzes the conversion of 2-C-methyl-D-erythrifol 4-phosphate to 4-(cytidine-5′-diphosphate)-2-C-methyl-D-erythrifol. A new enzyme, ISPD, was discovered in E. coli [32]. These genes ygbP and ygbB corresponding to ISPD may be involved in this step of transformation [33]. ISPE catalyses transform 4-(cytidine-5′-diphosphate)-2-C-methyl-D-erythrifol into 2-phospho-4-(cytidine 5′-diphospho)-2-C-methyl-D-erythrifol. A new enzyme, 4-(cytidine 5′-diphospho)-2-C-methyl-D-erythritol kinase (ISPE), was discovered in E. coli [34]. Earlier experiments, overexpressing the gene ychB corresponding to ISPE in E. coli and combining with isotopic labeling, found the catalytic activity of ISPE. The predicted protein sequence similarity between YchB and tomato cDNA pTOM41 was 30%, and this phenomenon was related to the transformation of chloroplast to chromosome [35]. ISPF catalyzes the conversion of 2-phospho-4-(cytidine 5′-diphospho)-2-C-methyl-D-erythrifol to 2-C-methyl-D-erythritol 2,4-cyclodiphosphate. A new enzyme, ISPF, was discovered in E. coli [36]. Earlier experiments, overexpressing the gene ygbB corresponding to ISPF in E. coli and combining with isotopic labeling, found that the catalytic activity of ISPF [37]. GcpE catalyses transform 2-C-methyl-D-erythritol 2,4-cyclodiphosphate into 1-hydroxy-2-methyl-2-butenyl-4-diphosphate. Combining database information and heterologous expression of gcpE and petF genes indicated that PetF could transfer electrons to GcpE [38]. Experimental results indicate that GcpE is a ferredoxin-dependent enzyme with [4Fe-4S] cluster [39]. In bacteria, GcpE relies on NADPH/flavodoxin/flavodoxin reductase as a reductive shuttle system for electron transfer [40]. ISPH catalyzes the conversion of 1-hydroxy-2-methyl-2-butenyl-4-diphosphate to isopentenyl diphosphate (IPP) and dimethylallyl diphosphate (DMPP). Expression of the ispH (lytB) gene in E. coli converts 1-hydroxy-2-methyl-2-butenyl-4-diphosphate to IPP and DMPP [41]. Further studies reveal that the enzyme possesses molecular oxygen-sensitive [4Fe-4S] clusters [42]. Overexpression of the ispH gene in E. coli using the isc operon resulted in at least a 200-fold increase in the catalytic activity of the purified protein ISPH [43]. FDPS catalyses transform DMPP or IPP into geranyl diphosphate (GPP), and also catalyses transform IPP into farnesyl diphosphate (FPP). Previous studies have obtained isoprenyltransferase from Micrococcus lysodeikticus, which catalyzes the synthesis of all-trans GPP from FPP and IPP [44]. FPP synthase, obtained from Bacillus subtilis, catalyzes the conversion of IPP and DMPP or GPP to all-trans FPP. The metal ions Mg2+ or Mn2+ are crucial to the catalytic activity, but Mn2+ is less effective [45].
Geranylgeranyl diphosphate synthase is the most important to the initiation of the carotenoid synthesis pathway, producing the only product geranylgeranyl diphosphate (GGPP). This study has not annotated synthase (EC: 2.5.1.29) in the existing database. We predict that there are new synthases with lower homology in the genome of Sphingomonas morindae sp. NBD5 and Sphingopyxis sp. USTB-05. One molecule of FPP condenses with one molecule IPP to form GGPP under the catalysis of CrtE in Erwinia uredovora and Agrobacterium aurantiacum [46]. The GGPP synthase genes were screened from the genomic DNA libraries of archaebacterial Sulfolobus acidocaldarius and Streptomyces acidocaldarius. Heterologous expression showed that DMPP or GPP were used as substrates producing GGPP [46]. GGPP synthase (EC: 2.5.1.29) was purified from Methanobacterium thermoformicicum SF-4. It is a dimeric protein consisting of two identical subunits and is stable upon treatment at 65 °C for 30 min [47]. GGPP synthase from Erwinia uredovora is overexpressed in E. coli, reaction rates and Km values indicate that GPP and FPP are allyl substrates for GGPP synthase, but not DMPP [48]. Comparing the substrate specificity of GGPP synthases from Micrococcus lysodeikticus and pumpkin seedling, it is found that the enzyme in pumpkin seedling has the highest activity, while the lowest activity is in Micrococcus lysodeikticus [49]. It is consistent with the analysis results that GGPP synthases are derived from different organisms with widely different properties.
In the third stage, GGPP is transferred to lutein and zeaxanthin in carotenoid biosynthesis pathways (Figure 4). Six out of at least nine enzymes involved in the lutein and zeaxanthin biosynthesis are annotated into the genomes of two strains (Figure S5). CrtB catalyzes the conversion of GGPP to prephytoene diphosphate. Evidence for the presence of the crtB and the crtB operon was found in the purple photosynthetic bacterium Rhodobacter sphaeroides [50]. At the same time, ORF-A protein homologous to the amino acid sequence of CrtB was found in the Thernus thermophilus, heterologous expression showed that the strain containing ORF-A protein had three times the carotenoid production of the strain without containing ORF-A protein [51]. Further experiments showed that the CrtB enzyme in Erwinia uredovora requires Mn2+ and had an optimum pH of 8.2 to produce the only product phytoene. CrtB is a highly conserved enzyme that can be used to design new drugs or pesticides with specific targets [52].
Two desaturases are annotated in the genomic data of Sphingomonas morindae sp. NBD5 and Sphingopyxis sp. USTB-05. CrtIa catalyzes the conversion of phytoene in the cis conformation to ζ-carotene in two steps. CrtIb catalyzes the conversion of ζ-carotene in the trans conformation to lycopene. The complete crtI gene of Erwinia was heterologously expressed in E. coli, and the purified enzyme could catalyze the conversion of cis-phytoene into trans-lycopene as well as to bisdehydrolycopene [53]. The complete crtI gene was overexpressed in E. coli, Rhodobacter capsulatus, and Rhodobacter sphaeroides, which catalyzed the desaturation of phytoene to produce neurosporene. It is an ATP-dependent enzyme [50,54]. Further studies found that there were two different forms of CrtIa and CrtIb in Myxococcus xanthus. CrtIa catalyzes the dehydrogenation of carotene in the cis conformation, and CrtIb catalyzes the dehydrogenation of carotene in the trans conformation [55].
Two cyclases are found in the genomic data of Sphingomonas morindae sp. NBD5 and Sphingopyxis sp. USTB-05. CrtL-b is responsible for three step cyclization reaction. Unannotated enzymes catalyze the conversion of lycopene to δ-carotene (Figure 4). CrtL-b catalyzes the cyclization of lycopene to form different types of carotenes. Earlier reports showed that CrtL catalyzed the conversion of acyclic lycopene to bicyclic β-carotene [56]. The predicted lycopene cyclases in rice are CrtL-b and CrtL-e proteins. They are only about 34% and 50% similar to CrtL from Synechococcus sp. PCC 7942 [56]. Other types of lycopene cyclases are reported, lycopene cyclase genes from Erwinia (crtY) or the plant capsicum (lcy) can catalyze monocyclic β-carotene produce bicyclic carotenoids 7,8-dihydro-β-carotene [57]. A fourth class of lycopene cyclases are found in photosynthetic bacteria, CruA and CruP exhibit lycopene cyclase activity [58]. Two separate Lycopene epsilon-cyclases (EC: 5.5.1.18) are identified for the first time in the marine cyanobacterium Prochlorococcus marinus MED4. The enzyme crtL-e is responsible for the formation of cyclic carotenoids with β- or ε-terminal groups [59]. Although the cyclase crtL-e is not annotated in Sphingomonas morindae sp. NBD5 and Sphingopyxis sp. USTB-05, new genes distantly related to this cyclase is found based on the overall metabolic process.
Three hydroxylases are found in the genomic data of Sphingomonas morindae sp. NBD5 and Sphingopyxis sp. USTB-05. CrtR-b and CrtZ are responsible for four steps hydroxylation. Unannotated enzymes catalyze the conversion of zeinoxanthin to lutein and α-carotene to α-cryptoxanthin (Figure 4). CrtR-b catalyzes the conversion of α-Carotene to zeinoxanthin and α-Cryptoxanthin to lutein, respectively. Recently, this enzyme is found in the chloroplasts of tomato, tobacco and Haematococcus pluvialis [60]. It is an oxidoreductase, with reduced iron-sulfur protein as one donor, which can introduce hydroxyl groups to the donor molecule [60]. CrtZ catalyzes the conversion of β-carotene to zeaxanthin, β-carotene hydroxylase (CrtZ) acts as a hydroxylase. Meanwhile, β-carotene hydroxylase (CrtZ) is reported to be the rate-limiting enzyme in astaxanthin biosynthesis [61]. In eubacteria and cyanobacteria, multiple genes encoding β-carotene 3-hydroxylase, which are responsible for the conversion of β-carotene to zeaxanthin [61]. Furthermore, CrtZ adds a hydroxyl group to the 7,8-dihydro-β end group and the β end group in Erwinia [57]. There are also other types of tomato erythromycin cyclases have been reported to be CHXB and CHXE during the carotenoid biosynthesis in strawberry. The enzyme CHXB is responsible for the conversion of α-carotene to zeinoxanthin, β-carotene to β-cryptoxanthin and β-cryptoxanthin to zeaxanthin. The enzyme CHXE is responsible for catalyzing the conversion of zeinoxanthin to lutein [62]. The homology and other characteristics of CrtZ, CHXB and CHXE need be further clarified. The carotenoid epsilon-hydroxylase (EC: 1.14.14.158) is found in the cytochrome enzyme family in Arabidopsis thaliana, and it is named the LUT1 responsible for catalyzing the conversion of zeinoxanthin to lutein. Although the hydroxylase LUT1 is not annotated in these two strains in this study, a new carotenoid epsilon-hydroxylase or gene may be discovered based on the prediction of metabolic process [63].
The MEP pathway is normally present in most bacteria, green algae and plant plastids. However, IPP and FPP molecules are also produced via the mevalonate (MVA) pathway in archaea, fungi, higher plant cytoplasm and other eukaryotes [64,65]. In watercress and microalgae, the key steps are the conversion of three IPP molecules and DMPP by the enzyme (GGPPS/GGPS/FPPS) to generate GGPP by the condensation process [9,11,26]. In living organisms, the condensation of IPP with DMPP to form GPP is catalyzed by geranyl diphosphate synthase (GPS). Finally, GPP is condensed with one molecule of IPP to form FPP by farnesyl diphosphate synthase (FPS) [66]. In eukaryotes, especially higher plants, lutein and zeaxanthin are primarily synthesized from terpenoids. Two molecules of GGPP are used to synthesize phytoene. Phytoene is then converted to ζ-carotene, after which ζ-carotene is converted into lycopene. Lycopene is then converted to α-carotene by lycopene cyclase (εLCY/βLCY). Finally, the formation of lutein from α-carotene is catalyzed by α-carotene hydroxylase (βCHX/εCHX) [67].
Recently, the biosynthetic pathway from GGPP to zeaxanthin is also found in various organisms, such as: Echinicola marina, Acaryochloris, Prochlorococcus and Synechococcus PCC7942 [12,65,68,69]. Sphingomonas sp. SG73 is the same genus as Sphingomonas morindae sp. NBD5, the biosynthetic pathway from zeaxanthin to nostoxanthin is further discovered [70]. Based on Liquid Chromatography-UltraViolet-Mass Spectrometry (LC–UV-MS/MS) analysis, three biosynthetic pathways from GGPP to lutein, zeaxanthin, diketospirilloxanthin are discovered in Flavobacterium [13]. Further, we optimized the strain NBD5 fermentation culture by temperature, carbon source and nitrogen source, and obtained lutein yield of 1.6 mg/g, 1.8 mg/g and 1.9 mg/g, respectively [15]. In this article, the biosynthetic pathways of lutein and zeaxanthin are obtained from the genome annotation data of Sphingomonas morindae sp. NBD5 and Sphingopyxis sp. USTB-05. They are consistent with the above metabolic mass spectrometry data analysis results. We further clarified the MP biological enzymes involved in the reaction and the corresponding coding genes.
5. Conclusions
The whole genome of Sphingomonas morindae sp. NBD5 consists of two circular chromosomes of 4,239,716 bp with 3882 protein coding genes including 59 protein-coding genes related to MP biosynthesis, of which four genes (ackA, pgm, gpmI and pckA) are unique. These genes, pckG, porB, meh, and fldA, are unique in Sphingopyxis sp. USTB-05. Lutein and zeaxanthin synthesis metabolic pathways and synthetic genes are discovered in Sphingomonas morindae sp. NBD5 and Sphingopyxis sp. USTB-05. Three new enzymes are found in whole metabolic pathway, they are GGPP synthase, lycopene epsilon-cyclase, carotenoid epsilon hydroxylase. This study expands the understanding of the pathway for complete biosynthesis of MP by Sphingomonas morindae sp. NBD5 and Sphingopyxis sp. USTB-05.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microorganisms11020266/s1, Figure S1: Sequence distribution of data lengths of nanopore sequencing; Figure S2: Comparison of genes related to lutein synthesis in the genomes of Sphingomonas morindae sp. NBD5 and Sphingopyxis sp. USTB-05; Figure S3: Comparison of genes related to glycolysis and gluconeogenesis pathway in the genomes of Sphingomonas morindae sp. NBD5 and Sphingopyxis sp. USTB-05; Figure S4: Comparison of genes related to terpenoid backbone biosynthesis in the genomes of Sphingomonas morindae sp. NBD5 and Sphingopyxis sp. USTB-05; Figure S5: Comparison of genes related to carotenoid biosynthesis in the genomes of Sphingomonas morindae sp. NBD5 and Sphingopyxis sp. USTB-05; Figure S6: (a) Peaks and scanning profiles of standard lutein; (b) Peaks and scanning profiles of the extract of S. morindae NBD5 on LC-Q-TOF/MS [15]; Figure S7: (a) First-order mass spectrometry of standard lutein on LC-Q-TOF/MS; (b) First-order mass spectrometry of the extract of S. morindae NBD5 on LC-Q-TOF/MS [15]; Figure S8: (a) Second-order mass spectrometry of standard lutein on LC-Q-TOF/MS; (b) Second-order mass spectrometry of the extract of S. morindae NBD5 on LC-Q-TOF/MS [15]; Table S1: Gene Ontology (GO) classifications of genes; Table S2: Clusters of Orthologous Group (COG) classifications of genes.
Author Contributions
Conceptualization, H.Y. and Y.L.; data curation, C.L.; investigation, C.L.; methodology, C.Y.; project administration, Q.X.; resources, Y.L.; software, C.L.; supervision, C.Y.; visualization, C.L.; writing—original draft, C.L.; writing—review and editing, Z.Z., Q.X., H.Z., X.L., H.Y. and Y.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (21677011) and the Fundamental Research Funds for the Central Universities (FRF-TP-20-044A2; FRF-MP-20-39).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in article https://doi.org/10.3390/toxins14050333.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Udayan, A.; Arumugam, M.; Pandey, A. Nutraceuticals From Algae and Cyanobacteria. Algal Green Chem. 2017, 65–89. [Google Scholar]
- Khachik, F.; Bernstein, P.S.; Garland, D.L. Identification of lutein and zeaxanthin oxidation products in human and monkey retinas. Investig. Ophthalmol. Vis. Sci. 1997, 38, 1802–1811. [Google Scholar]
- Wilson, L.M.; Tharmarajah, S.; Jia, Y.; Semba, R.D.; Schaumberg, D.A.; Robinson, K.A. The Effect of Lutein/Zeaxanthin Intake on Human Macular Pigment Optical Density: A Systematic Review and Meta-Analysis. Adv. Nutr. 2021, 12, 2244–2254. [Google Scholar] [CrossRef] [PubMed]
- Mrowicka, M.; Mrowicki, J.; Kucharska, E.; Majsterek, I. Lutein and Zeaxanthin and Their Roles in Age-Related Macular Degeneration-Neurodegenerative Disease. Nutrients 2022, 14, 827. [Google Scholar] [CrossRef] [PubMed]
- Scripsema, N.K.; Hu, D.N.; Rosen, R.B. Lutein, zeaxanthin, and meso-zeaxanthin in the clinical management of eye disease. J. Ophthalmol. 2015, 2015, 865179. [Google Scholar] [PubMed]
- Gazzolo, D.; Picone, S.; Gaiero, A.; Bellettato, M.; Montrone, G.; Riccobene, F.; Lista, G.; Pellegrini, G. Early Pediatric Benefit of Lutein for Maturing Eyes and Brain-An Overview. Nutrients 2021, 13, 3239. [Google Scholar] [CrossRef] [PubMed]
- Addo, E.K.; Gorusupudi, A.; Allman, S.; Bernstein, P.S. The Lutein and Zeaxanthin in Pregnancy (L-ZIP) study-carotenoid supplementation during pregnancy: Ocular and systemic effects-study protocol for a randomized controlled trial. Trials 2021, 22, 300. [Google Scholar] [CrossRef]
- Perrone, S.; Tei, M.; Longini, M.; Buonocore, G. The Multiple Facets of Lutein: A Call for Further Investigation in the Perinatal Period. Oxidative Med. Cell. Longev. 2016, 2016, 5381540. [Google Scholar] [CrossRef]
- Ren, Y.; Sun, H.; Deng, J.; Huang, J.; Chen, F. Carotenoid Production from Microalgae: Biosynthesis, Salinity Responses and Novel Biotechnologies. Mar. Drugs 2021, 19, 713. [Google Scholar] [CrossRef]
- Lin, J.H.; Lee, D.J.; Chang, J.S. Lutein production from biomass: Marigold flowers versus microalgae. Bioresour. Technol. 2014, 184, 421–428. [Google Scholar] [CrossRef]
- Sathasivam, R.; Bong, S.J.; Park, C.H.; Kim, J.H.; Kim, J.K.; Park, S.U. Identification, Characterization, and Expression Analysis of Carotenoid Biosynthesis Genes and Carotenoid Accumulation in Watercress (Nasturtium officinale R. Br.). ACS Omega 2022, 7, 430–442. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yao, S.; Lee, Y.J.; Cao, Y.; Zhai, L.; Zhang, X.; Su, J.; Ge, Y.; Kim, S.G.; Cheng, C. Sphingomonas morindae sp. nov., isolated from Noni (Morinda citrifolia L.) branch. Int. J. Syst. Evol. Microbiol. 2015, 65, 2817–2823. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, M.; Zhao, Z.; Liu, C.; Liu, Y.; Yan, H. A novel production of lutein by Sphingomonas morindae sp. NBD5. Fourrages 2020, 244, 99–109. [Google Scholar]
- Liu, C.; Xu, Q.; Zhao, Z.; Zhang, H.; Liu, X.; Yin, C.; Liu, Y.; Yan, H. Genomic Analysis of Sphingopyxis sp. USTB-05 for Biodegrading Cyanobacterial Hepatotoxins. Toxins 2022, 14, 333. [Google Scholar] [CrossRef]
- Wang, J.; Wu, P.; Chen, J.; Yan, H. Biodegradation of microcystin-RR by a new isolated Sphingopyxis sp. USTB-05. Chin. J. Chem. Eng. 2010, 18, 108–112. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef]
- Versluis, D.; McPherson, K.; Passel, M.W.J.; Smidt, H.; Sipkema, D. Recovery of previously uncultured bacterial genera from three mediterranean sponges. Mar. Biotechnol. 2017, 19, 454–468. [Google Scholar] [CrossRef]
- Haas, B.J.; Salzberg, S.L.; Zhu, W.; Pertea, M.; Allen, J.E.; Orvis, J.; White, O.; Robin, C.R.; Wortman, J.R. Automated eukaryotic gene structure annotation using EVidenceModeler and the Program to Assemble Spliced Alignments. Genome Biol. 2008, 9, R7. [Google Scholar] [CrossRef]
- Bland, C.; Ramsey, T.L.; Sabree, F.; Lowe, M.; Brown, K.; Kyrpides, N.C.; Hugenholtz, P. CRISPR Recognition Tool (CRT): A tool for automatic detection of clustered regularly interspaced palindromic repeats. BMC Bioinform. 2007, 8, 1–8. [Google Scholar] [CrossRef]
- Delcher, A.L.; Salzberg, S.L.; Phillippy, A.M. Using mummer to identify similar regions in large sequence sets. Curr. Protoc. Bioinform. 2003, 1, 10.3.1–10.3.18. [Google Scholar] [CrossRef]
- Qi, X.H.; Zhang, X.F.; Fan, X.L.; Zhang, F.; Wang, Y.F.; Xue, Q.; Zou, M.Q. Study on the ionization mechanism of lutein and lutein detected by Q-TOF under different ion sources. In Paper Presented at the Third China Third Party Testing Laboratory Development Forum; China Institute of Quarantine and Inspection Sciences: Beijing, China, 2011. (In Chinese) [Google Scholar]
- Rodrigues, D.B.; Mercadante, A.Z.; Mariutti, L.R.B. Marigold carotenoids: Much more than lutein esters. Food Res. Int. 2019, 119, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Nguyet, N.; Ha, V.; Binh, N.T.; Dang, N.M.; Bay, N.T. Quantification of lutein from Marigold flower (Tagetes erecta L.) petals by liquid chromatography-tandem mass spectrometry method. Vietnam. J. Chem. 2019, 57, 2. [Google Scholar] [CrossRef]
- Strom, N.B.; Bushley, K.E. Two genomes are better than one: History, genetics, and biotechnological applications of fungal heterokaryons. Fungal Biol. Biotechnol. 2016, 3, 4. [Google Scholar] [CrossRef]
- Zuo, G.H.; Hao, B.L. CVTree3 Web Server for Whole-genome-based and Alignment-free Prokaryotic Phylogeny and Taxonomy. Genom. Proteom. Bioinform. 2015, 13, 321–331. [Google Scholar] [CrossRef]
- Jedrzejas, M.J.; Chander, M.; Setlow, P.; Krishnasamy, G. Mechanism of Catalysis of the Cofactor-independent Phosphoglycerate Mutase from Bacillus stearothermophilus: Crystal structure of the complex with 2-phosphoglycerate*. J. Biol. Chem. 2000, 275, 23146–23153. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Chang, W.C.; Xiao, Y.; Liu, H.W.; Liu, P. Methylerythritol phosphate pathway of isoprenoid biosynthesis. Annu. Rev. Biochem. 2013, 82, 497–530. [Google Scholar] [CrossRef] [PubMed]
- Sprenger, G.A.; Schörken, U.; Wiegert, T.; Grolle, S.; de Graaf, A.A.; Taylor, S.V.; Begley, T.P.; Bringer-Meyer, S.; Sahm, H. Identification of a thiamin-dependent synthase in Escherichia coli required for the formation of the 1-deoxy-d-xylulose 5-phosphate precursor to isoprenoids, thiamin, and pyridoxol. Proc. Natl. Acad. Sci. USA 1997, 94, 12857–12862. [Google Scholar] [CrossRef]
- Kuzuyama, T.; Takagi, M.; Takahashi, S.; Seto, H. Cloning and characterization of 1-deoxy-D-xylulose 5-phosphate synthase from Streptomyces sp. Strain CL190, which uses both the mevalonate and nonmevalonate pathways for isopentenyl diphosphate biosynthesis. J. Bacteriol. 2000, 182, 891–897. [Google Scholar] [CrossRef]
- Munos, J.W.; Pu, X.T.; Mansoorabadi, S.O.; Kim, H.J.; Liu, H.W. A secondary kinetic isotope effect study of the 1-deoxy-D-xylulose-5-phosphate reductoisomerase-catalyzed reaction: Evidence for a retroaldol-aldol rearrangement. J. Am. Chem. Soc. 2009, 131, 2048–2049. [Google Scholar] [CrossRef]
- Takahashi, S.J.; Kuzuyama, T.; Watanabe, H.; Seto, H. A 1-deoxy-D-xylulose 5-phosphate reductoisomerase catalyzing the formation of 2-C-methyl-D-erythritol 4-phosphate in an alternative nonmevalonate pathway for terpenoid biosynthesis. Proc. Natl. Acad. Sci. USA 1998, 95, 9879–9884. [Google Scholar] [CrossRef]
- Kuzuyama, T.; Takagi, M.; Kaneda, K.; Dairi, T.; Seto, H. Formation of 4-(cytidine 5′-diphospho)-2-C-methyl-D-erythritol from 2-C-methyl-D-erythritol 4-phosphate by 2-C-methyl-D-erythritol 4-phosphate cytidylyltransferase, a new enzyme in the nonmevalonate pathway. Tetrahedron Lett. 2000, 41, 703–706. [Google Scholar] [CrossRef]
- Rohdich, F.; Wungsintaweekul, J.; Fellermeier, M.; Sagner, S.; Herz, S.; Kis, K.; Eisenreich, W.; Bacher, A.; Zenk, M.H. Cytidine 5′-triphosphate-dependent biosynthesis of isoprenoids: YgbP protein of Escherichia coli catalyzes the formation of 4-diphosphocytidyl-2-C-methylerythritol. Proc. Natl. Acad. Sci. USA 1999, 96, 11758–11763. [Google Scholar] [CrossRef] [PubMed]
- Kuzuyama, T.; Takagi, M.; Kaneda, K.; Watanabe, H.; Dairi, T.; Seto, H. Studies on the nonmevalonate pathway: Conversion of 4-(cytidine 5′-diphospho)-2-C-methyl-D-erythritol to its 2-phospho derivative by 4-(cytidine 5′-diphospho)-2-C-methyl-D-erythritol kinase. Tetrahedron Lett. 2000, 41, 2925–2928. [Google Scholar] [CrossRef]
- Lüttgen, H.; Rohdich, F.; Herz, S.; Wungsintaweekul, J.; Hecht, S.; Schuhr, C.A.; Fellermeier, M.; Sagner, S.; Zenk, M.H.; Bacher, A.; et al. Biosynthesis of terpenoids: YchB protein of Escherichia coli phosphorylates the 2-hydroxy group of 4-diphosphocytidyl-2C-methyl-D-erythritol. Proc. Natl. Acad. Sci. USA 2000, 97, 1062–1067. [Google Scholar] [CrossRef]
- Takagi, M.; Kuzuyama, T.; Kaneda, K.; Watanabe, H.; Dairi, T.; Seto, H. Studies on the nonmevalonate pathway: Formation of 2-C-methyl-D-erythritol 2,4-cyclodiphosphate from 2-phospho-4-(cytidine 5′-diphospho)-2-C-methyl-D-erythritol. Tetrahedron Lett. 2000, 41, 3395–3398. [Google Scholar] [CrossRef]
- Herz, S.; Wungsintaweekul, J.; Schuhr, C.A.; Hecht, S.; Lüttgen, H.; Sagner, S.; Fellermeier, M.; Eisenreich, W.; Zenk, M.H.; Bacher, A.; et al. Biosynthesis of terpenoids: YgbB protein converts 4-diphosphocytidyl-2C-methyl-D-erythritol 2-phosphate to 2C-methyl-D-erythritol 2,4-cyclodiphosphate. Proc. Natl. Acad. Sci. USA 2000, 97, 2486–2490. [Google Scholar] [CrossRef]
- Okada, K.; Hase, T. Cyanobacterial non-mevalonate pathway: (E)-4-hydroxy-3-methylbut-2-enyl diphosphate synthase interacts with ferredoxin in Thermosynechococcus elongatus BP-1. J. Biol. Chem. 2005, 280, 20672–20679. [Google Scholar] [CrossRef]
- Seemann, M.; Bui, B.T.S.; Wolff, M.; Tritsch, D.; Campos, N.; Boronat, A.; Marquet, A.; Rohmer, M. Isoprenoid biosynthesis through the methylerythritol phosphate pathway: The (E)-4-hydroxy-3-methylbut-2-enyl diphosphate synthase (GcpE) is a [4Fe-4S] protein. Angew. Chem. 2002, 41, 4337–4339. [Google Scholar] [CrossRef]
- Seemann, M.; Wegner, P.; Schünemann, V.; Bui, B.T.S.; Wolff, M.; Marquet, A.; Trautwein, A.X.; Rohmer, M. Isoprenoid biosynthesis in chloroplasts via the methylerythritol phosphate pathway: The (E)-4-hydroxy-3-methylbut-2-enyl diphosphate synthase (GcpE) from Arabidopsis thaliana is a [4Fe-4S] protein. J. Biol. Inorg. Chem. 2005, 10, 131–137. [Google Scholar] [CrossRef]
- Rohdich, F.; Hecht, S.; Gärtner, K.; Adam, P.; Krieger, C.; Amslinger, S.; Arigoni, D.; Bacher, A.; Eisenreich, W. Studies on the nonmevalonate terpene biosynthetic pathway: Metabolic role of IspH (LytB) protein. Proc. Natl. Acad. Sci. USA 2002, 99, 1158–1163. [Google Scholar] [CrossRef]
- Wolff, M.; Seemann, M.; Bui, B.T.S.; Frapart, Y.; Tritsch, D.; Estrabot, A.G.; Rodríguez-Concepción, M.; Boronat, A.; Marquet, A.; Rohmer, M. Isoprenoid biosynthesis via the methylerythritol phosphate pathway: The (E)-4-hydroxy-3-methylbut-2-enyl diphosphate reductase (LytB/IspH) from Escherichia coli is a [4Fe-4S] protein. FEBS Lett. 2003, 541, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Gräwert, T.; Kaiser, J.; Zepeck, F.; Laupitz, R.; Hecht, S.; Amslinger, S.; Schramek, N.; Schleicher, E.; Weber, S.; Haslbeck, M.; et al. IspH protein of Escherichia coli: Studies on iron-sulfur cluster implementation and catalysis. J. Am. Chem. Soc. 2004, 126, 12847–12855. [Google Scholar] [CrossRef] [PubMed]
- Sagami, H.; Ogura, K.; Seto, S.C.; Kurokawa, T. A new prenyltransferase from Micrococcus lysodeikticus. Biochem. Biophys. Res. Commun. 1978, 85, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, I.; Ogura, K. Farnesyl pyrophosphate synthetase from Bacillus subtilis. J. Biochem. 1981, 89, 1581–1587. [Google Scholar] [CrossRef] [PubMed]
- Ohnuma, S.I.; Suzuki, M.; Nishino, T. Archaebacterial ether-linked lipid biosynthetic gene. Expression cloning, sequencing, and characterization of geranylgeranyl-diphosphate synthase. J. Biol. Chem. 1994, 269, 14792–14797. [Google Scholar] [CrossRef]
- Tachibana, A.; Tanaka, T.; Taniguchi, M.; Susumu, O. Purification and Characterization of Geranylgeranyl Diphosphate Synthase from Methanobacterium thermoformicicum SF-4. Biosci. Biotechnol. Biochem. 1993, 57, 1129–1133. [Google Scholar] [CrossRef] [PubMed]
- Wiedemann, M.; Misawa, N.; Sandmann, G. Purification and Enzymatic Characterization of the Geranylgeranyl Pyrophosphate Synthase from Erwinia uredovora after Expression in Escherichia coli. Arch. Biochem. Biophys. 1993, 306, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Shinka, T.; Ogura, K.; Seto, S. Comparative Specificity of Geranylgeranyl Pyrophosphate Synthetase of Micrococcus lysodeikticus and Pumpkin. J. Biochem. 1975, 78, 1177–1181. [Google Scholar] [CrossRef]
- Lang, H.P.; Cogdell, R.J.; Gardiner, A.T.; Hunter, C.N. Early steps in carotenoid biosynthesis: Sequences and transcriptional analysis of the crtI and crtB genes of Rhodobacter sphaeroides and overexpression and reactivation of crtI in Escherichia coli and R. sphaeroides. J. Bacteriol. 1994, 176, 3859–3869. [Google Scholar] [CrossRef]
- Hoshino, T.; Fujii, R.; Nakahara, T. Molecular cloning and sequence analysis of the crtB gene of Thermus thermophilus HB27, an extreme thermophile producing carotenoid pigments. Appl. Environ. Microbiol. 1993, 59, 3150–3153. [Google Scholar] [CrossRef]
- Vandermoten, S.; Haubruge, É.; Cusson, M. New insights into short-chain prenyltransferases: Structural features, evolutionary history and potential for selective inhibition. Cell. Mol. Life Sci. 2009, 66, 3685–3695. [Google Scholar] [CrossRef] [PubMed]
- Fraser, P.; Misawa, N.; Linden, H.; Yamano, S.; Kobayashi, K.; Sandmann, G. Expression in Escherichia coli, purification, and reactivation of the recombinant Erwinia uredovora phytoene desaturase. J. Biol. Chem. 1992, 267, 19891–19895. [Google Scholar] [CrossRef] [PubMed]
- Raisig, A.; Bartley, G.; Scolnik, P.; Sandmann, G. Purification in an Active State and Properties of the 3-Step Phytoene Desaturase from Rhodobacter capsulatus Overexpressed in Escherichia coli. J. Biochem. 1996, 119, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Iniesta, A.A.; Cervantes, M.; Murillo, F.J. Cooperation of two carotene desaturases in the production of lycopene in Myxococcus xanthus. FEBS J. 2007, 274, 4306–4314. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, F.X.; Sun, J.Z.; Chamovitz, D.; Hirschberg, J.; Gantt, E. Molecular structure and enzymatic function of lycopene cyclase from the cyanobacterium Synechococcus sp. strain PCC7942. Plant Cell 1994, 6, 1107–1121. [Google Scholar]
- Takaichi, S.; Sandmann, G.; Schnurr, G.; Satomi, Y.; Suzuki, A.; Misawa, N. The carotenoid 7,8-dihydro-ψ end group can be cyclized by the lycopene cyclases from the bacterium Erwinia uredovora and the higher plant Capsicum annuum. Eur. J. Biochem. 1996, 241, 291–296. [Google Scholar] [CrossRef]
- Maresca, J.; Graham, J.E.; Wu, M.; Eisen, J.A.; Bryant, D. Identification of a fourth family of lycopene cyclases in photosynthetic bacteria. Proc. Natl. Acad. Sci. USA 2007, 104, 11784–11789. [Google Scholar] [CrossRef]
- Stickforth, P.; Steiger, S.; Hess, W.R.; Sandmann, G. A novel type of lycopene ε-cyclase in the marine cyanobacterium Prochlorococcus marinus MED4. Arch. Microbiol. 2003, 179, 409–415. [Google Scholar] [CrossRef]
- Tan, C.P.; Zhao, F.Q.; Su, Z.L.; Liang, C.W.; Qin, S. Expression of β-carotene hydroxylase gene (crtR-B) from the green alga Haematococcus pluvialis in chloroplasts of Chlamydomonas reinhardtii. J. Appl. Phycol. 2007, 19, 347–355. [Google Scholar] [CrossRef]
- Choi, S.K.; Matsuda, S.; Hoshino, T.; Peng, X.; Misawa, N. Characterization of bacterial β-carotene 3,3´-hydroxylases, CrtZ, and P450 in astaxanthin biosynthetic pathway and adonirubin production by gene combination in Escherichia coli. Appl. Microbiol. Biotechnol. 2006, 72, 1238–1246. [Google Scholar] [CrossRef]
- Zhu, H.; Chen, M.; Wen, Q.; Li, Y. Isolation and characterization of the carotenoid biosynthetic genes LCYB, LCYE and CHXB from strawberry and their relation to carotenoid accumulation. Sci. Hortic. 2015, 182, 134–144. [Google Scholar] [CrossRef]
- Tian, L.; Musetti, V.; Kim, J.; Magallanes-Lundback, M.; DellaPenna, D. The Arabidopsis LUT1 locus encodes a member of the cytochrome P450 family that is required for carotenoid ε-ring hydroxylation activity. Proc. Natl. Acad. Sci. USA 2004, 101, 402–407. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Zhang, Y.; Wang, M. Discovery and Engineering of Cytochrome P450s for Terpenoid Biosynthesis. Trends Biotechnol. 2019, 37, 618–631. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, Z.; Sun, J.; Xue, C.; Mao, X. Biotechnological production of zeaxanthin by microorganisms. Trends Food Sci. Technol. 2018, 71, 225–234. [Google Scholar] [CrossRef]
- Buhaescu, I.; Izzedine, H. Mevalonate pathway: A review of clinical and therapeutical implications. Clin. Biochem. 2007, 40, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Lado, J.; Zacarías, L.; Rodrigo, M.J. Regulation of Carotenoid Biosynthesis during Fruit Development. Sub-Cell. Biochem. 2016, 79, 161–198. [Google Scholar]
- Takaichi, S.; Mochimaru, M.; Uchida, H.; Murakami, A.; Hirose, E.; Maoka, T.; Tsuchiya, T.; Mimuro, M. Opposite Chilarity of α-Carotene in Unusual Cyanobacteria with Unique Chlorophylls, Acaryochloris and Prochlorococcus. Plant Cell Physiol. 2012, 53, 1881–1888. [Google Scholar] [CrossRef]
- SchÄFer, L.; Sandmann, M.; Woitsch, S.; Sandmann, G. Coordinate up-regulation of carotenoid biosynthesis as a response to light stress in Synechococcus PCC7942. Plant Cell Environ. 2006, 29, 1349–1356. [Google Scholar] [CrossRef]
- Kikukawa, H.; Okaya, T.; Maoka, T.; Miyazaki, M.; Murofushi, K.; Kato, T.; Hirono-Hara, Y.; Katsumata, M.; Miyahara, S.; Hara, K.Y. Carotenoid Nostoxanthin Production by Sphingomonas sp. SG73 Isolated from Deep Sea Sediment. Mar. Drugs 2021, 19, 274. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).