Major Soilborne Pathogens of Field Processing Tomatoes and Management Strategies
Abstract
1. Introduction
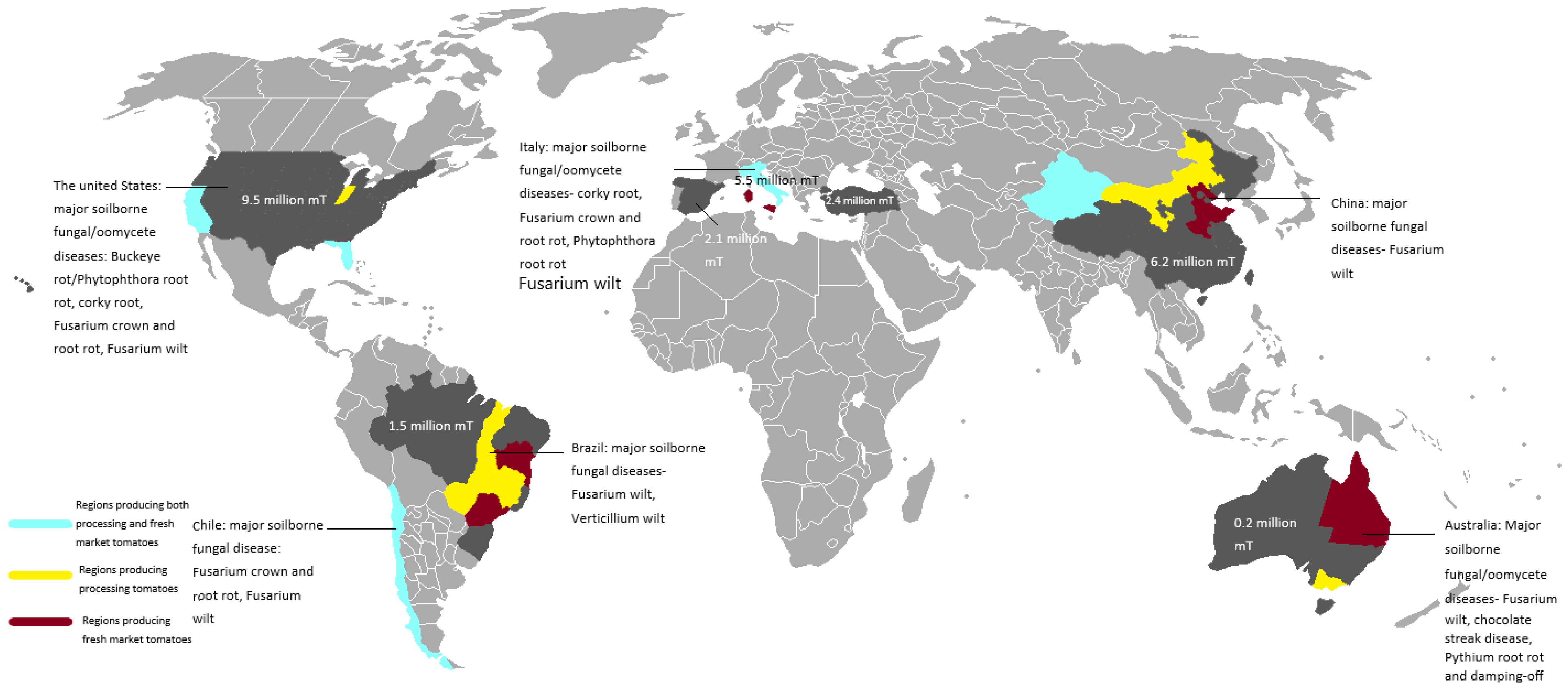
2. The Major Countries Producing Processing Tomatoes and Their Major Soilborne Fungal/Oomycete Diseases
2.1. Northern Hemisphere
2.1.1. The United States Tomato Industry and Major Soilborne/Fungal Diseases
2.1.2. The Chinese Tomato Industry and Major Soilborne Fungal/Oomycete Diseases
2.1.3. The Italian Tomato Industry and Major Soilborne Fungal/Oomycete Diseases
2.2. Southern Hemisphere
2.2.1. The Brazilian Tomato Industry and Major Soilborne Fungal/Oomycete Diseases
2.2.2. The Chilean Tomato Industry and Major Soilborne Fungal/Oomycete Diseases
2.2.3. The Australian Tomato Industry and Major Soilborne Fungal/Oomycete Diseases
3. Control Strategies of the Major Soilborne Fungal/Oomycete Diseases
3.1. Tomato Corky Root Rot
3.1.1. Conventional Control Methods
Cultural Control
Physical Control
Chemical Control
Resistance Breeding
3.1.2. Biological Control
3.2. Fusarium Crown and Root Rot (FCRR) of Tomato
3.2.1. Conventional Control Methods
Cultural Control
Physical Control
Chemical Control
Resistance Breeding
3.2.2. Biocontrol
3.3. Fusarium wilt Disease of Tomato
3.3.1. Conventional Control Methods
Cultural Control
Physical Control
Chemical Control
Resistance Breeding
3.3.2. Biological Control
3.4. Phytophthora Root Rot of Tomato
3.4.1. Conventional Control Methods
Cultural Control
Physical Control
Chemical Control
Resistance Breeding
3.4.2. Biocontrol
3.5. Pythium Root Rot and Damping-Off
3.5.1. Conventional Control Methods
Cultural Control
Physical Control
Chemical Control
Resistance Breeding
3.5.2. Biocontrol
3.6. Tomato Verticillium Wilt
3.6.1. Conventional Control Methods
Cultural Control
Physical Control
Chemical Control
Resistance Breeding
3.6.2. Biocontrol
4. Conclusions and Future Perspective
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rick, C.M. The tomato. Sci. Am. 1978, 239, 76–89. [Google Scholar] [CrossRef]
- FAOSTAT. Available online: http://www.fao.org/faostat/en/#home (accessed on 30 November 2022).
- Barringer, S. Canned tomatoes: Production and storage. In Handbook of Vegetable Preservation and Processing, 1st ed.; Hui, Y.H., Ghazala, S., Graham, D.M., Murrell, K.D., Nip, W., Eds.; CRC Press: New York, NY, USA, 2004; pp. 109–120. [Google Scholar]
- WPTC. WPTC Crop Update as of 25 October 2022. Available online: https://www.tomatonews.com/force_doc.php?file=c50d46545467b815c44e583ddcdd7731c1c4ae2f.pdf (accessed on 30 November 2022).
- Luster, C., III. A rapid and sensitive sterility monitoring technique for aseptically processed bulk tomato paste. J. Food Sci. 1978, 43, 1046–1048. [Google Scholar] [CrossRef]
- Barrett, D.M. Future innovations in tomato processing. In Proceedings of the XIII International Symposium on Processing Tomato, Sirmione, Italy, 8 June 2014; Volume 1081, pp. 49–55. [Google Scholar]
- Johnstone, P.R.; Hartz, T.K.; LeStrange, M.; Nunez, J.J.; Miyao, E.M. Managing fruit soluble solids with late-season deficit irrigation in drip-irrigated processing tomato production. HortScience 2005, 40, 1857–1861. [Google Scholar] [CrossRef]
- Singh, V.K.; Singh, A.K.; Kumar, A. Disease management of tomato through PGPB: Current trends and future perspective. 3 Biotech 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Bawa, I. Management strategies of Fusarium wilt disease of tomato incited by Fusarium oxysporum f. sp. lycopersici(Sacc.) A Review. Int. J. Adv. Acad. Res. 2016, 2, 32–42. [Google Scholar]
- Valenzuela-Lopez, N.; Cano-Lira, J.F.; Guarro, J.; Sutton, D.A.; Wiederhold, N.; Crous, P.W.; Stchigel, A.M. Coelomycetous Dothideomycetes with emphasis on the families Cucurbitariaceae and Didymellaceae. Stud. Mycol. 2018, 90, 1–69. [Google Scholar] [CrossRef]
- d’Errico, G.; Marra, R.; Crescenzi, A.; Davino, S.W.; Fanigliulo, A.; Woo, S.L.; Lorito, M. Integrated management strategies of Meloidogyne incognita and Pseudopyrenochaeta lycopersici on tomato using a Bacillus firmus-based product and two synthetic nematicides in two consecutive crop cycles in greenhouse. Crop. Prot. 2019, 122, 159–164. [Google Scholar] [CrossRef]
- Giotis, C.; Markelou, E.; Theodoropoulou, A.; Toufexi, E.; Hodson, R.; Shotton, P.; Leifert, C. Effect of soil amendments and biological control agents (BCAs) on soil-borne root diseases caused by Pyrenochaeta lycopersici and Verticillium albo-atrum in organic greenhouse tomato production systems. Eur. J. Plant Pathol. 2009, 123, 387–400. [Google Scholar] [CrossRef]
- Cheng, H.; Zhang, D.; Ren, L.; Song, Z.; Li, Q.; Wu, J.; Cao, A. Bio-activation of soil with beneficial microbes after soil fumigation reduces soil-borne pathogens and increases tomato yield. Environ. Pollut. 2021, 283, 117160. [Google Scholar] [CrossRef]
- Baysal-Gurel, F.; Gardener, B.M.; Miller, S.A. Soil Borne Disease Management in Organic Vegetable Production. Org. Agric. Available online: https://eorganic.org/node/7581. (accessed on 30 November 2022).
- Spadaro, D.; Gullino, M.L. Improving the efficacy of biocontrol agents against soilborne pathogens. Crop. Prot. 2005, 24, 601–613. [Google Scholar] [CrossRef]
- Gareau, B.J. Lessons from the Montreal Protocol delay in phasing out methyl bromide. J. Environ. Stud. Sci. 2015, 5, 163–168. [Google Scholar] [CrossRef]
- Piel, C.; Pouchieu, C.; Carles, C.; Beziat, B.; Boulanger, M.; Bureau, M.; Baldi, I. Agricultural exposures to carbamate herbicides and fungicides and central nervous system tumour incidence in the cohort AGRICAN. Environ. Int. 2019, 130, 104876. [Google Scholar] [CrossRef]
- Foolad, M.R.; Merk, H.L.; Ashrafi, H. Genetics, genomics and breeding of late blight and early blight resistance in tomato. Crit. Rev. Plant Sci. 2008, 27, 75–107. [Google Scholar] [CrossRef]
- Menda, N.; Strickler, S.R.; Edwards, J.D.; Bombarely, A.; Dunham, D.M.; Martin, G.B.; Mueller, L.A. Analysis of wild-species introgressions in tomato inbreds uncovers ancestral origins. BMC Plant Biol. 2014, 14, 1–16. [Google Scholar] [CrossRef] [PubMed]
- de Toledo Thomazella, D.P.; Brail, Q.; Dahlbeck, D.; Staskawicz, B. CRISPR-Cas9 mediated mutagenesis of a DMR6 ortholog in tomato confers broad-spectrum disease resistance. BioRxiv. Available online: https://www.biorxiv.org/content/10.1101/064824v3.full. (accessed on 30 November 2022).
- Panthee, D.R.; Brown, A.F.; Yousef, G.G.; Ibrahem, R.; Anderson, C. Novel molecular marker associated with Tm2a gene conferring resistance to tomato mosaic virus in tomato. Plant Breed. 2013, 132, 413–416. [Google Scholar] [CrossRef]
- Flint, M.L.; Dreistadt, S.H. Natural Enemies Handbook: The Illustrated Guide to Biological Pest Control; Univ of California Press: Berkely, CA, USA, 1998; Volume 3386, pp. 3–6. [Google Scholar]
- Landis, D.A.; Orr, D.B. Biological Control: Approaches and Applications. Electronic IPM Textbook. Available online: https://ipmworld.umn.edu/landis (accessed on 30 November 2022).
- Rechcigl, J.E.; Rechcigl, N.A. (Eds.) Biological and Biotechnological Control of Insect Pests; CRC Press: New York, NY, USA, 1999; pp. 3–5. [Google Scholar]
- van Lenteren, J.C.; Bolckmans, K.; Köhl, J.; Ravensberg, W.J.; Urbaneja, A. Biological control using invertebrates and microorganisms: Plenty of new opportunities. BioControl 2018, 63, 39–59. [Google Scholar] [CrossRef]
- Singh, S.; Bhatnagar, S.; Choudhary, S.; Nirwan, B.; Sharma, K. Fungi as biocontrol agent: An alternate to chemicals. In Fungi and Their Role in Sustainable Development: Current Perspectives, 1st ed.; Gehlot, P., Singh, J., Eds.; Springer: Singapore, 2018; pp. 23–33. [Google Scholar]
- Sood, M.; Kapoor, D.; Kumar, V.; Sheteiwy, M.S.; Ramakrishnan, M.; Landi, M.; Sharma, A. Trichoderma: The “secrets” of a multitalented biocontrol agent. Plants 2020, 9, 762. [Google Scholar] [CrossRef]
- Junaid, J.M.; Dar, N.A.; Bhat, T.A.; Bhat, A.H.; Bhat, M.A. Commercial biocontrol agents and their mechanism of action in the management of plant pathogens. Int. J. Mod. Plant Anim. Sci. 2013, 1, 39–57. [Google Scholar]
- Hort Innovation. Tomato Topics September 2022. Available online: https://www.aptrc.asn.au/wp-content/uploads/2022/10/Tomato-Topics-September-2022-1.pdf (accessed on 30 November 2022).
- Pathak, T.B.; Stoddard, C.S. Climate change effects on the processing tomato growing season in California using growing degree day model. Model. Earth Syst. Environ. 2018, 4, 765–775. [Google Scholar] [CrossRef]
- Jones, J.B., Jr. Tomato Plant Culture: In the Field, Greenhouse, and Home Garden; CRC Press: New York, NY, USA, 2007; pp. 55–80. [Google Scholar]
- Costa, J.M.; Heuvelink, E.P. The global tomato industry. In Tomatoes, 2nd ed.; Heuvelink, E., Ed.; CABI: Cambridge, MA, USA, 2018; pp. 1–26. [Google Scholar]
- Hartz, T.; Miyao, G.; Mickler, J.; Lestrange, M.; Stoddard, S.; Nuñez, J.; Aegerter, B. Processing Tomato Production in California. Available online: https://escholarship.org/content/qt4hc350c9/qt4hc350c9.pdf (accessed on 30 November 2022).
- The Top 10 Tomato Producing States in the United States. Available online: https://www.worldatlas.com/articles/the-top-10-tomato-producing-states-in-the-united-states.html (accessed on 30 November 2022).
- Guan, Z.; Biswas, T.; Wu, F. The US Tomato Industry: An Overview of Production and Trade. Available online: https://journals.flvc.org/edis/article/download/105009/118652/ (accessed on 30 November 2022).
- Baskins, S.; Bond, J.; Minor, T. Unpacking the Growth in per Capita Availability of Fresh Market Tomatoes. Available online: https://www.ers.usda.gov/webdocs/outlooks/92442/vgs-19c-01.pdf?v=5296.1 (accessed on 30 November 2022).
- Fernández-Pavía, S.P.; Rodríguez-Alvarado, G.; Sánchez-Yáñez, J.M. Buckeye rot of tomato caused by Phytophthora capsici in Michoacan, Mexico. Plant Dis. 2003, 87, 872. [Google Scholar] [CrossRef]
- Gupta, S.K.; Sachin, U.; Sharma, R.C. Biology, epidemiology and management of buckeye rot of tomato. In Challenging Problems in Horticultural and Forest Pathology, 1st ed.; Sharma, R.C., Sarma, G.N., Eds.; Indus Publishing Company: New Delhi, India, 2006; pp. 183–199. [Google Scholar]
- Hoy, M.W.; Ogawa, J.M.; Duniway, J.M. Effects of irrigation on buckeye rot of tomato fruit caused by Phytophthora parasitica. Phytopathology 1984, 74, 474–478. [Google Scholar] [CrossRef]
- Kendrick, J.B. Phytophthora rot of tomato, eggplant, and pepper. Proc. Indiana Acad. Sci. 1922, 32, 299–306. [Google Scholar]
- Tompkins, C.M.; Tucker, C.M. Buckeye rot of tomato in California. J. Agric. Res. 1941, 62, 467–474. [Google Scholar]
- Bolkan, H. A technique to evaluate tomatoes for resistance to Phytophthora root rot in the greenhouse. Plant Dis. 1985, 69, 708–709. [Google Scholar] [CrossRef]
- Filho, A.C.; Duniway, J.M. Dispersal of Phytophthora capsici and P. parasitica in furrow-irrigated rows of bell pepper, tomato and squash. Plant Pathol. 1995, 44, 1025–1032. [Google Scholar] [CrossRef]
- Babadoost, M. Important fungal diseases of tomato in the United States of America. In Proceedings of the III International Symposium on Tomato Diseases, Ischia, Italy, 25–30 July 2010; Volume 914, pp. 85–92. [Google Scholar]
- Ioannou, N.; Grogan, R.G. Control of Phytophthora root rot of processing tomato with ethazol and metalaxyl. Plant Dis. 1984, 68, 429–435. [Google Scholar] [CrossRef]
- Stamova, L. Resistance to Two Important Tomato Diseases in California. In Proceedings of the XV Meeting of the EUCARPIA Tomato Working Group, Bari, Italy, 20–23 September 2005; Volume 789, pp. 87–94. [Google Scholar]
- Campbell, R.N. Corky Root of Tomato in California Caused by Pyrenochaeta lycopersici and Control by Soil Fumigation. Plant Dis. 1981, 66, 657–661. [Google Scholar] [CrossRef]
- Workneh, F.; Van Bruggen AH, C.; Drinkwater, L.E.; Shennan, C. Variables associated with corky root and Phytophthora root rot of tomatoes in organic and conventional farms. Phytopathology 1993, 83, 581–589. [Google Scholar] [CrossRef]
- Vrisman, C.M.; Testen, A.L.; Elahi, F.; Miller, S.A. First report of tomato brown root rot complex caused by Colletotrichum coccodes and Pyrenochaeta lycopersici in Ohio. Plant Dis. 2017, 101, 247. [Google Scholar] [CrossRef]
- Srinivas, C.; Devi, D.N.; Murthy, K.N.; Mohan, C.D.; Lakshmeesha, T.R.; Singh, B.; Srivastava, R.K. Fusarium oxysporum f. sp. lycopersici causal agent of vascular wilt disease of tomato: Biology to diversity–A review. Saudi J. Biol. Sci. 2019, 26, 1315–1324. [Google Scholar] [CrossRef]
- Alexander, L. Physiologic specialization in the tomato wilt. J. Agric. Res. 1945, 70, 303. [Google Scholar]
- Cai, G.H.; Gale, L.R.; Schneider, R.W.; Kistler, H.C.; Davis, R.M.; Elias, K.S.; Miyao, E.M. Origin of race 3 of Fusarium oxysporum f. sp. lycopersici at a single site in California. Phytopathology 2003, 93, 1014–1022. [Google Scholar] [CrossRef] [PubMed]
- Volin, R.B.; Jones, J.P. A new race of Fusarium wilt of tomato in Florida and sources of resistance. Proc. Fla. State Hortic. Soc. 1982, 95, 268–269. [Google Scholar]
- Davis, R.M.; Kimble, K.A.; Farrar, J.J. A third race of Fusarium oxysporum f. sp. lycopersici identified in California. Plant Dis. 1988, 72, 453. [Google Scholar] [CrossRef]
- Benhamou, N.; Charest, P.M.; Jarvis, W.R. Biology and Host-Parasite Relations of Fusarium oxysporum f. sp. radicis-lycopersici. In Vascular Wilt Diseases of Plants, 1st ed.; Tjamos, E.C., Beckman, C.H., Eds.; Springer: Berlin, Germany, 1986; pp. 95–105. [Google Scholar]
- Ozbay, N.; Newman, S.E. Fusarium crown and root rot of tomato and control methods. Plant Pathol. J. 2004, 3, 9–18. [Google Scholar] [CrossRef]
- Rudolph, B.A. Verticillium wilt of tomatoes in California. sclerotia of Verticillium albo-atrum and its influence on infection. Phytopathology 1926, 16, 234. [Google Scholar]
- Alexander, L.J. Susceptibility of certain Verticillium-resistant Tomato varieties to an Ohio isolate of the pathogen. Phytopathology 1962, 52, 998–1000. [Google Scholar]
- Grogan, R.G.; Ioannou, N.; Schneider, R.W.; Sall, M.A.; Kimble, K.A. Verticillium wilt on resistant tomato cultivars in California: Virulence of isolates from plants and soil and relationship of inoculum density to disease incidence. Phytopathology 1979, 69, 1176–1180. [Google Scholar] [CrossRef]
- Ashworth, L.J.; Huisman, O.C.; Harper, D.M.; Stromberg, L.K. Verticillium wilt disease of tomato: Influence of inoculum density and root extension upon disease severity. Phytopathology 1979, 69, 490–492. [Google Scholar] [CrossRef]
- Buller, S.; Inglis, D.; Miles, C. Plant growth, fruit yield and quality, and tolerance to verticillium wilt of grafted watermelon and tomato in field production in the Pacific Northwest. HortScience 2013, 48, 1003–1009. [Google Scholar] [CrossRef]
- Costa, J.M.; Heuvelink, E. Introduction: The tomato crop and industry. In Tomatoes, 1st ed.; Heuvelink, E., Ed.; CABI: Cambridge, MA, USA, 2005; pp. 1–20. [Google Scholar]
- Zhang, X.X.; Qiu, H.; Huang, Z. Farm structure of the apple and tomato production in the EU and China. In Apple and Tomato Chains in China and the EU. LEI 26, 1st ed.; Zhang, X.X., Qiu, H., Huang, Z., Eds.; LEI: The Hague, The Netherlands, 2010; pp. 21–27. [Google Scholar]
- Wang, Y.; Zhang, Y.; Gao, Z.; Yang, W. Breeding for resistance to tomato bacterial diseases in China: Challenges and prospects. Hortic. Plant J. 2018, 4, 193–207. [Google Scholar] [CrossRef]
- Chang, Y.D.; Du, B.; Wang, L.; Ji, P.; Xie, Y.J.; Li, X.F.; Wang, J.M. A study on the pathogen species and physiological races of tomato Fusarium wilt in Shanxi, China. J. Integr. Agric. 2018, 17, 1380–1390. [Google Scholar] [CrossRef]
- Xu, Z.; Huang, K.; Shou, W.; Zhou, S.; Li, G.; Chen, L.; Jin, B. The determination of physiological race of Fusarium oxysporum f. sp. lycopersici of tomato in Zhejiang, China. Acta Physiol. Plant. 2000, 22, 356–358. [Google Scholar] [CrossRef]
- Ye, Q.; Wang, R.; Ruan, M.; Yao, Z.; Cheng, Y.; Wan, H.; Zhou, G. Genetic diversity and identification of wilt and root rot pathogens of tomato in China. Plant Dis. 2020, 104, 1715–1724. [Google Scholar] [CrossRef]
- Perrotta, D. Processing tomatoes in the era of the retailing revolution: Mechanization and migrant labour in northern and southern Italy. In Migration and Agriculture, 1st ed.; Corrado, A., de Castro, C., Perrotta, D., Eds.; Routledge: Oxfordshire, UK, 2016; pp. 82–100. [Google Scholar]
- Vitale, A.; Castello, I.; Cascone, G.; D’Emilio, A.; Mazzarella, R.; Polizzi, G. Reduction of corky root infections on greenhouse tomato crops by soil solarization in South Italy. Plant Dis. 2011, 95, 195–201. [Google Scholar] [CrossRef]
- Garibaldi, A.; Gilardi, G.; Baudino, M.; Ortu, G.; Gullino, M.L. Phytophthora capsici: A soilborne pathogen dangerous on grafted tomato (Solanum lycopersicum × S. hirsutum) in Italy. Plant Dis. 2012, 96, 1830. [Google Scholar] [CrossRef]
- Stravato, V.M.; Buonaurio, R.; Cappelli, C. First Report of Fusarium oxysporum f. sp. lycopersici Race 2 on Tomato in Italy. Plant Dis. 1999, 83, 967. [Google Scholar] [CrossRef]
- Gullino, M.L.; Minuto, A.; Gilardi, G.; Garibaldi, A.; Ajwa, H.; Duafala, T. Efficacy of preplant soil fumigation with chloropicrin for tomato production in Italy. Crop Prot. 2002, 21, 741–749. [Google Scholar] [CrossRef]
- Rongai, D.; Pulcini, P.; Pesce, B.; Milano, F. Antifungal activity of pomegranate peel extract against fusarium wilt of tomato. Eur. J. Plant Pathol. 2017, 147, 229–238. [Google Scholar] [CrossRef]
- Srinivasan, K.; Gilardi, G.; Garibaldi, A.; Gullino, M.L. Bacterial antagonists from used rockwool soilless substrates suppress Fusarium wilt of tomato. J. Plant Pathol. 2009, 91, 147–154. [Google Scholar]
- Di Primo, P.; Cartia, G.; Katan, T. Vegetative compatibility and heterokaryon stability in Fusarium oxysporum f. sp. radicis-lycopersici from Italy. Plant Pathol. 2001, 50, 371–382. [Google Scholar] [CrossRef]
- Garibaldi, A.; Minuto, A.; Minuto, G. Influence of Recycled Substrates on the Severity of Crown Rot on Soilless Tomato. In Proceedings of the XV Meeting of the EUCARPIA Tomato Working Group, Bari, Italy, 20–23 September 2005; Volume 789, pp. 167–170. [Google Scholar]
- Cartia, G.; Asero, C. Investigation on spread and pathogenicity of Fusarium oxysporum f. sp. radicis lycopersici in Sicilian greenhouses. Colt. Protette 1994, 23, 75–78. [Google Scholar]
- Franceschini, A.; Maddau, L.; Corda, P.; Ionta, G. Observations on Fusarium crown and root rot of tomato and its control in Sardinia (Italy). Dif. Delle Piante 1996, 19, 125–138. [Google Scholar]
- Frenzel, B. Atlas of Paleoclimates and Paleoenvironments of the Northern Hemisphere. Available online: https://epic.awi.de/id/eprint/29922/1/Fre1992a.pdf (accessed on 30 November 2022).
- Elias, M.A.; Borges, F.J.; Bergamini, L.L.; Franceschinelli, E.V.; Sujii, E.R. Climate change threatens pollination services in tomato crops in Brazil. Agric. Ecosyst. Environ. 2017, 239, 257–264. [Google Scholar] [CrossRef]
- Marouelli, W.A.; Silva, W.L. Water tension thresholds for processing tomatoes under drip irrigation in Central Brazil. Irrig. Sci. 2007, 25, 411–418. [Google Scholar] [CrossRef]
- Inoue-Nagata, A.K.; Lima, M.F.; Gilbertson, R.L. A review of geminivirus diseases in vegetables and other crops in Brazil: Current status and approaches for management. Hortic. Bras. 2016, 34, 8–18. [Google Scholar] [CrossRef]
- Reis, A.; Costa, H.; Boiteux, L.S.; Lopes, C.A. First report of Fusarium oxysporum f. sp. lycopersici race 3 on tomato in Brazil. Fitopatol. Bras. 2005, 30, 426–428. [Google Scholar] [CrossRef]
- Reis, A.; Boiteux, L.S. Outbreak of Fusarium oxysporum f. sp. lycopersici race 3 in commercial fresh-market tomato fields in Rio de Janeiro State, Brazil. Hortic. Bras. 2007, 25, 451–454. [Google Scholar] [CrossRef]
- Gonçalves, A.M.; Costa, H.; Fonseca, M.E.N.; Boiteux, L.S.; Lopes, C.A.; Reis, A. Variability and geographical distribution of Fusarium oxysporum f. sp. lycopersici physiological races and field performance of resistant sources in Brazil. In Proceedings of the V International Symposium on Tomato Diseases: Perspectives and Future Directions in Tomato Protection, Málaga, Spain, 13–16 June 2016; Volume 1207, pp. 45–50. [Google Scholar]
- Cabral, C.S.; Gonçalves, A.M.; Fonseca ME, N.; Urben, A.F.; Costa, H.; Lourenço, V.; Reis, A. First detection of Fusarium oxysporum f. sp. radicis–lycopersici across major tomato–producing regions in Brazil. Phytoparasitica 2020, 48, 545–553. [Google Scholar] [CrossRef]
- Suaste-Dzul, A.P.; Costa, H.; Fonseca ME, N.; Boiteux, L.S.; Reis, A. Mating types and physiological races of Verticillium dahliae in Solanaceae crops in Brazil. Eur. J. Plant Pathol. 2022, 164, 1–14. [Google Scholar] [CrossRef]
- Suaste-Dzul, A.P.; Veloso, J.S.; Costa, H.; Boiteux, L.S.; Lourenço Jr, V.; Lopes, C.A.; Reis, A. Verticillium diseases of vegetable crops in Brazil: Host range, microsclerotia production, molecular haplotype network, and pathogen species determination. Plant Pathol. 2022, 71, 1417–1430. [Google Scholar] [CrossRef]
- Valdés, V. The tomato industry in Chile. In Proceedings of the IV International Symposium on Processing Tomatoes, Mendoza, Argentina, 18–21 February 1991; Volume 301, pp. 59–62. [Google Scholar]
- Valenzuela, M.; Fuentes, B.; Alfaro, J.F.; Galvez, E.; Salinas, A.; Besoain, X.A.; Seeger, M. First Report of bacterial speck caused by Pseudomonas syringae pv. tomato Race 1 affecting tomato in different Regions of Chile. Plant Dis. 2021, 106, 1979. [Google Scholar] [CrossRef] [PubMed]
- Saavedra Del, R.G.; Escaff, G.M.; Cortacáns, P.D.; Ruiz-Tagle, C. Recent developments in processing tomato production in Chile. In Proceedings of the IX International Symposium on the Processing Tomato, Melbourne, Australia, 15–18 November 2004; Volume 724, pp. 335–338. [Google Scholar]
- Sepúlveda-Chavera, G.; Huanca, W.; Salvatierra-Martínez, R.; Latorre, B.A. First report of Fusarium oxysporum f. sp. lycopersici race 3 and F. oxysporum f. sp. radicis-lycopersici in tomatoes in the Azapa Valley of Chile. Plant Dis. 2014, 98, 1432. [Google Scholar] [CrossRef] [PubMed]
- Elizondo-Pasten, E.; Boix-Ruiz, A.; Gómez-Tenorio, M.A.; Ruiz-Olmos, C.; Marín-Guirao, J.I.; Tello-Marquina, J.C.; Camacho-Ferre, F. Two complementary techniques allow detection of Fusarium oxysporum f. sp. radicis-lycopersici in soils from two different tomato-cultivated areas of Chile. In Proceedings of the V International Symposium on Tomato Diseases: Perspectives and Future Directions in Tomato Protection, Málaga, Spain, 13–16 June 2016; Volume 1207, pp. 315–318. [Google Scholar]
- Constable, F.; Chambers, G.; Penrose, L.; Daly, A.; Mackie, J.; Davis, K.; Gibbs, M. Viroid-infected tomato and capsicum seed shipments to Australia. Viruses 2019, 11, 98. [Google Scholar] [CrossRef]
- Australian Processing Tomato Grower. Tomatoes. Available online: https://www.accc.gov.au/system/files/public-registers/documents/D11%2B2315841.pdf (accessed on 30 November 2022).
- Plant Health Australia. Processing Tomatoes. Available online: https://www.planthealthaustralia.com.au/industries/processing-tomatoes/ (accessed on 30 November 2022).
- Australian Processing Tomato Research Council. Annual Industry Survey 2020. Available online: https://www.aptrc.asn.au/wp-content/uploads/2020/12/Industry-Survey-2020-ed.pdf (accessed on 30 November 2022).
- Lim, G.T.T.; Wang, G.P.; Hemming, M.N.; Basuki, S.; McGrath, D.J.; Carroll, B.J.; Jones, D.A. Mapping the I-3 gene for resistance to Fusarium wilt in tomato: Application of an I-3 marker in tomato improvement and progress towards the cloning of I-3. Australas. Plant Pathol. 2006, 35, 671–680. [Google Scholar] [CrossRef]
- Grattidge, R.; O’Brien, R.G. Occurrence of a third race of Fusarium wilt of tomatoes in Queensland. Plant Dis. 1982, 66, 165–166. [Google Scholar] [CrossRef]
- Callaghan, S.E. Root and Collar Rot Pathogens Associated with Yield Decline of Processing Tomatoes in Victoria, Australia. Doctoral Dissertation, The University of Melbourne, Melbourne, Australia, 2020. [Google Scholar]
- Callaghan, S.E.; Burgess, L.W.; Ades, P.K.; Tesoriero, L.A.; Taylor, P.W.J. Diversity and pathogenicity of Pythium species associated with reduced yields of processing tomatoes (Solanum lycopersicum ) in Victoria, Australia. Plant Dis. 2022, 106, 1632–1638. [Google Scholar] [CrossRef]
- Watterson, J.C. Diseases. In The Tomato Crop, 1st ed.; Atherton, J.G., Rudich, J., Eds.; Springer: Dordrecht, Germany, 1986; pp. 443–484. [Google Scholar]
- Ekengren, S.K. Cutting the Gordian knot: Taking a stab at corky root rot of tomato. Plant Biotechnol. 2008, 25, 265–269. [Google Scholar] [CrossRef][Green Version]
- Aragona, M.; Minio, A.; Ferrarini, A.; Valente, M.T.; Bagnaresi, P.; Orrù, L.; Delledonne, M. De novo genome assembly of the soil-borne fungus and tomato pathogen Pyrenochaeta Lycopersici. BMC Genom. 2014, 15, 1–12. [Google Scholar] [CrossRef]
- Hasna, M.K.; Ögren, E.; Persson, P.; Mårtensson, A.; Rämert, B. Management of corky root disease of tomato in participation with organic tomato growers. Crop Prot. 2009, 28, 155–161. [Google Scholar] [CrossRef]
- Shankar, R.; Harsha, S.; Bhandary, R. A Practical Guide to Identification and Control of Tomato Diseases. Available online: https://www.researchgate.net/file.PostFileLoader.html?id=589eb904615e2793034a4db2&assetKey=AS%3A460474400677895%401486797060834 (accessed on 30 November 2022).
- Coelho, L.; Chellemi, D.O.; Mitchell, D.J. Efficacy of solarization and cabbage amendment for the control of Phytophthora spp. in North Florida. Plant Dis. 1999, 83, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Yücel, S.; Özarslandan, A.; Colak, A.; Ay, T.; Can, C. Effect of solarization and fumigant applications on soilborne pathogens and root-knot nematodes in greenhouse-grown tomato in Turkey. Phytoparasitica 2007, 35, 450–456. [Google Scholar] [CrossRef]
- Manö, S.; Andreae, M.O. Emission of methyl bromide from biomass burning. Science 1994, 263, 1255–1257. [Google Scholar] [CrossRef] [PubMed]
- Rosskopf, E.N.; Chellemi, D.O.; Kokalis-Burelle, N.; Church, G.T. Alternatives to methyl bromide: A Florida perspective. Plant Health Prog. 2005, 6, 19. [Google Scholar] [CrossRef]
- Locascio, S.J.; Gilreath, J.P.; Dickson, D.W.; Kucharek, T.A.; Jones, J.P.; Noling, J.W. Fumigant alternatives to methyl bromide for polyethylene-mulched. HortScience 1997, 32, 1208–1211. [Google Scholar] [CrossRef]
- Fiume, G.; Fiume, F. Biological control of corky root in tomato. Commun. Agric. Appl. Biol. Sci. 2008, 73, 233–248. [Google Scholar]
- Doganlar, S.; Dodson, J.; Gabor, B.; Beck-Bunn, T.; Crossman, C.; Tanksley, S.D. Molecular mapping of the py-1 gene for resistance to corky root rot (Pyrenochaeta lycopersici) in tomato. Theor. Appl. Genet. 1998, 97, 784–788. [Google Scholar] [CrossRef]
- Milc, J.; Bagnaresi, P.; Aragona, M.; Valente, M.T.; Biselli, C.; Infantino, A.; Pecchioni, N. Comparative transcriptome profiling of the response to Pyrenochaeta lycopersici in resistant tomato cultivar Mogeor and its background genotype—Susceptible Moneymaker. Funct. Integr. Genom. 2019, 19, 811–826. [Google Scholar] [CrossRef]
- Hasna, M.K.; Lagerlöf, J.; Rämert, B. Effects of fungivorous nematodes on corky root disease of tomato grown in compost-amended soil. Acta Agric. Scand. Sect. B-Soil Plant Sci. 2008, 58, 145–153. [Google Scholar] [CrossRef]
- Bubici, G.; Marsico, A.D.; D’Amico, M.; Amenduni, M.; Cirulli, M. Evaluation of Streptomyces spp. for the biological control of corky root of tomato and Verticillium wilt of eggplant. Appl. Soil Ecol. 2013, 72, 128–134. [Google Scholar] [CrossRef]
- Besoain, X.A.; Pérez, L.M.; Araya, A.; Lefever, L.; Montealegre, J.R. New strains obtained after UV treatment and protoplast fusion of native Trichoderma harzianum: Their biocontrol activity on Pyrenochaeta lycopersici. Electron. J. Biotechnol. 2007, 10, 604–617. [Google Scholar] [CrossRef][Green Version]
- Sánchez-Téllez, S.; Herrera-Cid, R.A.; Besoain-Canales, X.A.; Pérez-Roepke, L.M.; Montealegre-Andrade, J.R. In vitro and in vivo inhibitory effect of solid and liquid Trichoderma harzianum formulations on biocontrol of Pyrenochaeta lycopersici. Interciencia 2013, 38, 425–429. [Google Scholar]
- Pérez, L.; Besoaín, X.; Reyes, M.; Pardo, G.; Montealegre, J. The expression of extracellular fungal cell wall hydrolytic enzymes in different Trichoderma harzianum isolates correlates with their ability to control Pyrenochaeta Lycopersici. Biol. Res. 2002, 35, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Hasna, M.K.; Mårtensson, A.; Persson, P.; Rämert, B. Use of composts to manage corky root disease in organic tomato production. Ann. Appl. Biol. 2007, 151, 381–390. [Google Scholar] [CrossRef]
- Edel-Hermann, V.; Lecomte, C. Current status of Fusarium oxysporum formae speciales and races. Phytopathology 2019, 109, 512–530. [Google Scholar] [CrossRef] [PubMed]
- Mutlu, N.; Demirelli, A.; Ilbi, H.; Ikten, C. Development of co-dominant SCAR markers linked to resistant gene against the Fusarium oxysporum f. sp. radicis-lycopersici. Theor. Appl. Genet. 2015, 128, 1791–1798. [Google Scholar] [CrossRef]
- Baysal, Ö.; Çalışkan, M.; Yeşilova, Ö. An inhibitory effect of a new Bacillus subtilis strain (EU07) against Fusarium oxysporum f. sp. radicis-lycopersici. Physiol. Mol. Plant Pathol. 2008, 73, 25–32. [Google Scholar] [CrossRef]
- Gordon, T.R. Fusarium oxysporum and the Fusarium wilt syndrome. Annu. Rev. Phytopathol. 2017, 55, 23–39. [Google Scholar] [CrossRef]
- Gordon, T.R.; Okamoto, D. Population structure and the relationship between pathogenic and nonpathogenic strains of Fusarium oxysporum. Phytopathology 1992, 82, 73–77. [Google Scholar] [CrossRef]
- Olivain, C.; Humbert, C.; Nahalkova, J.; Fatehi, J.; l’Haridon, F.; Alabouvette, C. Colonization of tomato root by pathogenic and nonpathogenic Fusarium oxysporum strains inoculated together and separately into the soil. Appl. Environ. Microbiol. 2006, 72, 1523–1531. [Google Scholar] [CrossRef]
- Muslim, A.; Horinouchi, H.; Hyakumachi, M. Control of Fusarium crown and root rot of tomato with hypovirulent binucleate Rhizoctonia in soil and rock wool systems. Plant Dis. 2003, 87, 739–747. [Google Scholar] [CrossRef] [PubMed]
- Altinok, H.H.; Yüksel, G.; Altinok, M.A. Pathogenicity and phylogenetic analysis of Fusarium oxysporum f. sp. capsici isolates from pepper in Turkey. Can. J. Plant Pathol. 2020, 42, 279–291. [Google Scholar] [CrossRef]
- Zhang, S.; Roberts, P.D.; McGovern, R.J.; Datnoff, L.E. Fusarium Crown and Root Rot of Tomato in Florida. Institute of Food and Agricultural Sciences (IFAS), Publication PP52. Available online: https://edis.ifas.ufl.edu/publication/PG082 (accessed on 30 November 2022).
- McGovern, R.J. Management of tomato diseases caused by Fusarium oxysporum. Crop. Prot. 2015, 73, 78–92. [Google Scholar] [CrossRef]
- Jarvis, W.R. Epidemiology of Fusarium oxysporum f. sp. radicis-lycopersici. In Vascular Wilt Diseases of Plants, 1st ed.; Tjamos, E.C., Beckman, C.H., Eds.; Springer: Berlin, Germany, 1989; pp. 397–411. [Google Scholar]
- Cao, X.; Guan, Z.; Vallad, G.E.; Wu, F. Economics of fumigation in tomato production: The impact of methyl bromide phase-out on the Florida tomato industry. Int. Food Agribus. Manag. Rev. 2019, 22, 589–600. [Google Scholar] [CrossRef]
- Horinouchi, H.; Katsuyama, N.; Taguchi, Y.; Hyakumachi, M. Control of Fusarium crown and root rot of tomato in a soil system by combination of a plant growth-promoting fungus, Fusarium equiseti, and biodegradable pots. Crop. Prot. 2008, 27, 859–864. [Google Scholar] [CrossRef]
- McGovern, R.J.; Vavrina, C.S.; Noling, J.W.; Datnoff, L.A.; Yonce, H.D. Evaluation of application methods of metam sodium for management of Fusarium crown and root rot in tomato in southwest Florida. Plant Dis. 1998, 82, 919–923. [Google Scholar] [CrossRef]
- Minuto, A.; Gullino, M.L.; Lamberti, F.; D’addabbo, T.; Tescari, E.; Ajwa, H.; Garibaldi, A. Application of an emulsifiable mixture of 1, 3-dichloropropene and chloropicrin against root knot nematodes and soilborne fungi for greenhouse tomatoes in Italy. Crop Prot. 2006, 25, 1244–1252. [Google Scholar] [CrossRef]
- Fazio, G.; Stevens, M.R.; Scott, J.W. Identification of RAPD markers linked to fusarium crown and root rot resistance (Frl) in tomato. Euphytica 1999, 105, 205–210. [Google Scholar] [CrossRef]
- Sivan, A.; Ucko, O.; Chet, I. Biological control of Fusarium crown rot of tomato by Trichoderma harzianum under field conditions. Plant Dis. 1987, 71, 587–592. [Google Scholar] [CrossRef]
- Datnoff, L.E.; Nemec, S.; Pernezny, K. Biological control of Fusarium crown and root rot of tomato in Florida using Trichoderma harzianum and Glomus intraradices. Biol. Control 1995, 5, 427–431. [Google Scholar] [CrossRef]
- Malandrakis, A.; Daskalaki, E.R.; Skiada, V.; Papadopoulou, K.K.; Kavroulakis, N. A Fusarium solani endophyte vs. fungicides: Compatibility in a Fusarium oxysporum f. sp. radicis-lycopersici–tomato pathosystem. Fungal Biol. 2018, 122, 1215–1221. [Google Scholar] [CrossRef] [PubMed]
- Benhamou, N.; Rey, P.; Chérif, M.; Hockenhull, J.; Tirilly, Y. Treatment with the mycoparasite Pythium oligandrum triggers induction of defense-related reactions in tomato roots when challenged with Fusarium oxysporum f. sp. radicis-lycopersici. Phytopathology 1997, 87, 108–122. [Google Scholar] [CrossRef] [PubMed]
- Duffy, B.K.; Défago, G. Zinc improves biocontrol of Fusarium crown and root rot of tomato by Pseudomonas fluorescens and represses the production of pathogen metabolites inhibitory to bacterial antibiotic biosynthesis. Phytopathology 1997, 87, 1250–1257. [Google Scholar] [CrossRef]
- de Weert, S.; Kuiper, I.; Lagendijk, E.L.; Lamers, G.E.; Lugtenberg, B.J. Role of chemotaxis toward fusaric acid in colonization of hyphae of Fusarium oxysporum f. sp. radicis-lycopersici by Pseudomonas fluorescens WCS365. Mol. Plant-Microbe Interact. 2004, 17, 1185–1191. [Google Scholar] [CrossRef] [PubMed]
- Kamilova, F.; Validov, S.; Lugtenberg, B. Biological control of tomato foot and root rot caused by Fusarium oxysporum f. sp. radicis-lycopersici by Pseudomonas bacteria. In Proceedings of the II International Symposium on Tomato Diseases, Kusadasi, Turkey, 8–12 October 2007; Volume 808, pp. 317–320. [Google Scholar]
- Baysal, Ö.; Lai, D.; Xu, H.H.; Siragusa, M.; Çalışkan, M.; Carimi, F.; Tör, M. A proteomic approach provides new insights into the control of soil-borne plant pathogens by Bacillus species. PLoS ONE 2013, 8, e53182. [Google Scholar] [CrossRef]
- Kavroulakis, N.; Ehaliotis, C.; Ntougias, S.; Zervakis, G.I.; Papadopoulou, K.K. Local and systemic resistance against fungal pathogens of tomato plants elicited by a compost derived from agricultural residues. Physiol. Mol. Plant Pathol. 2005, 66, 163–174. [Google Scholar] [CrossRef]
- Ajilogba, C.F.; Babalola, O.O. Integrated management strategies for tomato Fusarium wilt. Biocontrol Sci. 2013, 18, 117–127. [Google Scholar] [CrossRef]
- Miller, S.A.; Rowe, R.C.; Riedel, R.M. Fusarium and Verticillium Wilts of Tomato, Potato, Pepper, and Eggplant. The Ohio State University Extension. Available online: https://www.cabdirect.org/cabdirect/abstract/20127800677 (accessed on 30 November 2022).
- Larkin, R.P.; Fravel, D.R. Effects of varying environmental conditions on biological control of Fusarium wilt of tomato by nonpathogenic Fusarium spp. Phytopathology 2002, 92, 1160–1166. [Google Scholar] [CrossRef]
- Yu, J.; Land, C.J.; Vallad, G.E.; Boyd, N.S. Tomato tolerance and pest control following fumigation with different ratios of dimethyl disulfide and chloropicrin. Pest Manag. Sci. 2019, 75, 1416–1424. [Google Scholar] [CrossRef]
- Bohn, G.W.; Tucker, C.M. Immunity to Fusarium wilt in the tomato. Science 1939, 89, 603–604. [Google Scholar] [CrossRef]
- Takken, F.; Rep, M. The arms race between tomato and Fusarium oxysporum. Mol. Plant Pathol. 2010, 11, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Sela-Buurlage, M.; Budai-Hadrian, O.; Pan, Q.; Carmel-Goren, L.; Vunsch, R.; Zamir, D.; Fluhr, R. Genome-wide dissection of Fusarium resistance in tomato reveals multiple complex loci. Mol. Genet. Genom. 2001, 265, 1104–1111. [Google Scholar] [CrossRef] [PubMed]
- Katan, T.; Shlevin, E.; Katan, J. Sporulation of Fusarium oxysporum f. sp. lycopersici on stem surfaces of tomato plants and aerial dissemination of inoculum. Phytopathology 1997, 87, 712–719. [Google Scholar] [CrossRef] [PubMed]
- Sarfatti, M.; Katan, J.; Fluhr, R.; Zamir, D. An RFLP marker in tomato linked to the Fusarium oxysporum resistance gene I2. Theor. Appl. Genet. 1989, 78, 755–759. [Google Scholar] [CrossRef] [PubMed]
- McGrath, D.J.; Gillespie, D.; Vawdrey, L. Inheritance of resistance to Fusarium oxysporum f. sp. lycopersici races 2 and 3 in Lycopersicon pennellii. Aust. J. Agric. Res. 1987, 38, 729–733. [Google Scholar] [CrossRef]
- Scott, J.W.; Jones, J.P. Monogenic resistance in tomato to Fusarium oxysporum f. sp. lycopersici race 3. Euphytica 1989, 40, 49–53. [Google Scholar] [CrossRef]
- Catanzariti, A.M.; Lim, G.T.; Jones, D.A. The tomato I-3 gene: A novel gene for resistance to Fusarium wilt disease. New Phytol. 2015, 207, 106–118. [Google Scholar] [CrossRef]
- Chitwood-Brown, J.; Vallad, G.E.; Lee, T.G.; Hutton, S.F. Breeding for resistance to Fusarium wilt of tomato: A review. Genes 2021, 12, 1673. [Google Scholar] [CrossRef]
- Raza, W.; Ling, N.; Zhang, R.; Huang, Q.; Xu, Y.; Shen, Q. Success evaluation of the biological control of Fusarium wilts of cucumber, banana, and tomato since 2000 and future research strategies. Crit. Rev. Biotechnol. 2017, 37, 202–212. [Google Scholar] [CrossRef]
- Alabouvette, C.; Lemanceau, P.; Steinberg, C. Recent advances in the biological control of Fusarium wilts. Pestic. Sci. 1993, 37, 365–373. [Google Scholar] [CrossRef]
- Aimé, S.; Cordier, C.; Alabouvette, C.; Olivain, C. Comparative analysis of PR gene expression in tomato inoculated with virulent Fusarium oxysporum f. sp. lycopersici and the biocontrol strain F. oxysporum Fo47. Physiol. Mol. Plant Pathol. 2008, 73, 9–15. [Google Scholar] [CrossRef]
- de Lamo, F.J.; Spijkers, S.B.; Takken, F.L. Protection to tomato wilt disease conferred by the nonpathogen Fusarium oxysporum Fo47 is more effective than that conferred by avirulent strains. Phytopathology 2021, 111, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Duijff, B.J.; Pouhair, D.; Olivain, C.; Alabouvette, C.; Lemanceau, P. Implication of systemic induced resistance in the suppression of fusarium wilt of tomato by Pseudomonas fluorescens WCS417r and by nonpathogenic Fusarium oxysporum Fo47. Eur. J. Plant Pathol. 1998, 104, 903–910. [Google Scholar] [CrossRef]
- Borrego-Benjumea, A.; Basallote-Ureba, M.J.; Abbasi, P.A.; Lazarovits, G.; Melero-Vara, J.M. Effects of incubation temperature on the organic amendment-mediated control of Fusarium wilt of tomato. Ann. Appl. Biol. 2014, 164, 453–463. [Google Scholar] [CrossRef]
- Zhao, F.; Zhang, Y.; Dong, W.; Zhang, Y.; Zhang, G.; Sun, Z.; Yang, L. Vermicompost can suppress Fusarium oxysporum f. sp. lycopersici via generation of beneficial bacteria in a long-term tomato monoculture soil. Plant Soil 2019, 440, 491–505. [Google Scholar] [CrossRef]
- Barakat, R.M.; Al-Masri, M.I. Trichoderma harzianum in combination with sheep manure amendment enhances soil suppressiveness of Fusarium wilt of tomato. Phytopathol. Mediterr. 2009, 48, 385–395. [Google Scholar]
- Mwangi, M.W.; Muiru, W.M.; Narla, R.D.; Kimenju, J.W.; Kariuki, G.M. Effect of soil sterilisation on biological control of Fusarium oxysporum f. sp. lycopersici and Meloidogyne javanica by antagonistic fungi and organic amendment in tomato crop. Acta Agric. Scand. Sect. B—Soil Plant Sci. 2018, 68, 656–661. [Google Scholar] [CrossRef]
- Lamour, K.H.; Stam, R.; Jupe, J.; Huitema, E. The oomycete broad-host-range pathogen Phytophthora capsici. Mol. Plant Pathol. 2012, 13, 329–337. [Google Scholar] [CrossRef]
- Wani, A.H. An overview of the fungal rot of tomato. Mycopath 2011, 9, 33–38. [Google Scholar]
- Hausbeck, M.K.; Lamour, K.H. Phytophthora capsici on vegetable crops: Research progress and management challenges. Plant Dis. 2004, 88, 1292–1303. [Google Scholar] [CrossRef]
- Ristaino, J.B.; Johnston, S.A. Ecologically based approaches to management of Phytophthora blight on bell pepper. Plant Dis. 1999, 83, 1080–1089. [Google Scholar] [CrossRef] [PubMed]
- Lamour, K.H.; Hausbeck, M.K. Effect of crop rotation on the survival of Phytophthora capsici in Michigan. Plant Dis. 2003, 87, 841–845. [Google Scholar] [CrossRef] [PubMed]
- Quesada-Ocampo, L.M.; Hausbeck, M.K. Resistance in tomato and wild relatives to crown and root rot caused by Phytophthora capsici. Phytopathology 2010, 100, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Bower, L.A.; Coffey, M.D. Development of laboratory tolerance to phosphorous acid, fosetyl-Al, and metalaxyl in Phytophthora capsici. Can. J. Plant Pathol. 1985, 7, 1–6. [Google Scholar] [CrossRef]
- Jackson, K.L.; Yin, J.; Ji, P. Sensitivity of Phytophthora capsici on vegetable crops in Georgia to mandipropamid, dimethomorph, and cyazofamid. Plant Dis. 2012, 96, 1337–1342. [Google Scholar] [CrossRef] [PubMed]
- Kousik, C.S.; Keinath, A.P. First report of insensitivity to cyazofamid among isolates of Phytophthora capsici from the southeastern United States. Plant Dis. 2008, 92, 979. [Google Scholar] [CrossRef] [PubMed]
- Siegenthaler, T.B.; Hansen, Z.R. Sensitivity of Phytophthora capsici from Tennessee to mefenoxam, fluopicolide, oxathiapiprolin, dimethomorph, mandipropamid, and cyazofamid. Plant Dis. 2021, 105, 3000–3007. [Google Scholar] [CrossRef]
- Quesada-Ocampo, L.M.; Vargas, A.M.; Naegele, R.P.; Francis, D.M.; Hausbeck, M.K. Resistance to crown and root rot caused by Phytophthora capsici in a tomato advanced backcross of Solanum habrochaites and Solanum lycopersicum. Plant Dis. 2016, 100, 829–835. [Google Scholar] [CrossRef]
- Moataza, M.S. Destruction of Rhizoctonia solani and Phytophthora capsici causing tomato root-rot by Pseudomonas fluorescences lytic enzymes. Res. J. Agric. Biol. Sci. 2006, 2, 274–281. [Google Scholar]
- Sharma, R.; Chauhan, A.; Shirkot, C.K. Characterization of plant growth promoting Bacillus strains and their potential as crop protectants against Phytophthora capsici in tomato. Biol. Agric. Hortic. 2015, 31, 230–244. [Google Scholar] [CrossRef]
- Syed-Ab-Rahman, S.F.; Xiao, Y.; Carvalhais, L.C.; Ferguson, B.J.; Schenk, P.M. Suppression of Phytophthora apsica infection and promotion of tomato growth by soil bacteria. Rhizosphere 2019, 9, 72–75. [Google Scholar] [CrossRef]
- Yang, K.; Dong, X.; Li, J.; Wang, Y.; Cheng, Y.; Zhai, Y.; Dou, D. Type 2 Nep1-like proteins from the biocontrol oomycete Pythium oligandrum suppress Phytophthora capsici infection in solanaceous plants. J. Fungi 2021, 7, 496. [Google Scholar] [CrossRef] [PubMed]
- Nicol, R.W.; Burlakoti, P. Effect of aerobic compost tea inputs and application methods on protecting tomato from Phytophthora capsici. In Proceedings of the IV International Symposium on Tomato Diseases, Orlando, FL, USA, 24–27 June 2013; Volume 1069, pp. 229–233. [Google Scholar]
- González-Hernández, A.I.; Suárez-Fernández, M.B.; Pérez-Sánchez, R.; Gómez-Sánchez M, Á.; Morales-Corts, M.R. Compost tea induces growth and resistance against Rhizoctonia solani and Phytophthora capsici in pepper. Agronomy 2021, 11, 781. [Google Scholar] [CrossRef]
- Jiang, Z.Q.; Guo, Y.H.; Li, S.M.; Qi, H.Y.; Guo, J.H. Evaluation of biocontrol efficiency of different Bacillus preparations and field application methods against Phytophthora blight of bell pepper. Biol. Control 2006, 36, 216–223. [Google Scholar] [CrossRef]
- Kim, K.D.; Nemec, S.; Musson, G. Control of Phytophthora root and crown rot of bell pepper with composts and soil amendments in the greenhouse. Appl. Soil Ecol. 1997, 5, 169–179. [Google Scholar] [CrossRef]
- Raaijmakers, J.M.; Paulitz, T.C.; Steinberg, C.; Alabouvette, C.; Moënne-Loccoz, Y. The rhizosphere: A playground and battlefield for soilborne pathogens and beneficial microorganisms. Plant Soil 2009, 321, 341–361. [Google Scholar] [CrossRef]
- Elshahawy, I.E.; El-Mohamedy, R.S. Biological control of Pythium damping-off and root-rot diseases of tomato using Trichoderma isolates employed alone or in combination. J. Plant Pathol. 2019, 101, 597–608. [Google Scholar] [CrossRef]
- Manjunath, M.; Prasanna, R.; Nain, L.; Dureja, P.; Singh, R.; Kumar, A.; Kaushik, B.D. Biocontrol potential of cyanobacterial metabolites against damping off disease caused by Pythium aphanidermatum in solanaceous vegetables. Arch. Phytopathol. Plant Prot. 2010, 43, 666–677. [Google Scholar] [CrossRef]
- Mdee, L.K.; Masoko, P.; Eloff, J.N. The activity of extracts of seven common invasive plant species on fungal phytopathogens. S. Afr. J. Bot. 2009, 75, 375–379. [Google Scholar] [CrossRef]
- Beckerman, J. Pythium Root Rot of Herbaceous Plants. Available online: https://bch.cbd.int/api/v2013/documents/7efc7c33-9c4d-4589-95b0-959650a561fa/attachments/20505/Pythium%20Root%20Rot%20of%20herbaceous%20plants.pdf (accessed on 30 November 2022).
- Arora, H.; Sharma, A.; Sharma, S.; Haron, F.F.; Gafur, A.; Sayyed, R.Z.; Datta, R. Pythium damping-off and root rot of capsicum annuum l.: Impacts, diagnosis, and management. Microorganisms 2021, 9, 823. [Google Scholar] [CrossRef]
- Male, M.F.; Vawdrey, L.L. Efficacy of fungicides against damping-off in papaya seedlings caused by Pythium Aphanidermatum. Australas. Plant Dis. Notes 2010, 5, 103–104. [Google Scholar] [CrossRef]
- Paulitz, T.C.; Zhou, T.; Rankin, L. Selection of rhizosphere bacteria for biological control of Pythium aphanidermatum on hydroponically grown cucumber. Biol. Control 1992, 2, 226–237. [Google Scholar] [CrossRef]
- Hassanisaadi, M.; Shahidi Bonjar, G.H.; Hosseinipour, A.; Abdolshahi, R.; Ait Barka, E.; Saadoun, I. Biological control of Pythium aphanidermatum, the causal agent of tomato root rot by two Streptomyces root symbionts. Agronomy 2021, 11, 846. [Google Scholar] [CrossRef]
- Harvey, P.; Lawrence, L. Managing Pythium root disease complexes to improve productivity of crop rotations. Outlooks Pest Manag. 2008, 19, 127. [Google Scholar] [CrossRef]
- Triki, M.A.; Priou, S.; El Mahjoub, M. Effects of soil solarization on soil-borne populations of Pythium aphanidermatum and Fusarium solani and on the potato crop in Tunisia. Potato Res. 2001, 44, 271–279. [Google Scholar] [CrossRef]
- Reddy, G.S.; Rao, V.K.; Sitaramaiah, K.; Chalam, T.V. Soil Solarization for Control of Soil-borne Pathogen Complex due to Meloidogyne incognita and Pythium aphanidermatum. Indian J. Nematol. 2001, 31, 136–138. [Google Scholar]
- Jayaraj, J.; Radhakrishnan, N.V. Enhanced activity of introduced biocontrol agents in solarized soils and its implications on the integrated control of tomato damping-off caused by Pythium spp. Plant Soil 2008, 304, 189–197. [Google Scholar] [CrossRef]
- Abbasi, P.A.; Lazarovits, G. Seed treatment with phosphonate (AG3) suppresses Pythium damping-off of cucumber seedlings. Plant Dis. 2006, 90, 459–464. [Google Scholar] [CrossRef]
- Al-Balushi, Z.M.; Agrama, H.; Al-Mahmooli, I.H.; Maharachchikumbura, S.S.; Al-Sadi, A.M. Development of resistance to hymexazol among Pythium species in cucumber greenhouses in Oman. Plant Dis. 2018, 102, 202–208. [Google Scholar] [CrossRef]
- Cuevas, V.C.; Sinohin, A.M. Performance of selected Philippine species of Trichoderma as biocontrol agents of damping off pathogens and as growth enhancer of vegetables in farmers’ field. Philipp. Agric. Sci. 2005, 88, 63–71. [Google Scholar]
- Garzón, C.D.; Molineros, J.E.; Yánez, J.M.; Flores, F.J.; del Mar Jiménez-Gasco, M.; Moorman, G.W. Sublethal doses of mefenoxam enhance Pythium damping-off of geranium. Plant Dis. 2011, 95, 1233–1238. [Google Scholar] [CrossRef] [PubMed]
- Samaras, A.; Roumeliotis, E.; Ntasiou, P.; Karaoglanidis, G. Bacillus subtilis MBI600 promotes growth of tomato plants and induces systemic resistance contributing to the control of soilborne pathogens. Plants 2021, 10, 1113. [Google Scholar] [CrossRef] [PubMed]
- Rajendraprasad, M.; Vidyasagar, B.; Devi, G.U.; Rao, S.K. Biological control of tomato damping off caused by Pythium debaryanum. Int. J. Chem. Stud. 2017, 5, 447–452. [Google Scholar]
- Salman, M.; Abuamsha, R. Potential for integrated biological and chemical control of damping-off disease caused by Pythium ultimum in tomato. BioControl 2012, 57, 711–718. [Google Scholar] [CrossRef]
- Dukare, A.S.; Prasanna, R.; Dubey, S.C.; Nain, L.; Chaudhary, V.; Singh, R.; Saxena, A.K. Evaluating novel microbe amended composts as biocontrol agents in tomato. Crop. Prot. 2011, 30, 436–442. [Google Scholar] [CrossRef]
- Porter, L.D.; Hamm, P.B.; David, N.L.; Gieck, S.L.; Miller, J.S.; Gundersen, B.; Inglis, D.A. Metalaxyl-M-resistant Pythium species in potato production areas of the Pacific Northwest of the USA. Am. J. Potato Res. 2009, 86, 315–326. [Google Scholar] [CrossRef]
- Del Castillo Múnera, J.; Hausbeck, M.K. Characterization of Pythium species associated with greenhouse floriculture crops in Michigan. Plant Dis. 2016, 100, 569–576. [Google Scholar] [CrossRef]
- Aegerter, B.J.; Greathead, A.S.; Pierce, L.E.; Davis, R.M. Mefenoxam-resistant isolates of Pythium irregulare in an ornamental greenhouse in California. Plant Dis. 2002, 86, 692. [Google Scholar] [CrossRef]
- Lee, S.; Garzón, C.D.; Moorman, G.W. Genetic structure and distribution of Pythium aphanidermatum populations in Pennsylvania greenhouses based on analysis of AFLP and SSR markers. Mycologia 2010, 102, 774–784. [Google Scholar] [CrossRef]
- Lookabaugh, E.C.; Kerns, J.P.; Shew, B.B. Evaluating Fungicide Selections to Manage Pythium Root Rot on Poinsettia Cultivars with Varying Levels of Partial Resistance. Plant Dis. 2021, 105, 1640–1647. [Google Scholar] [CrossRef]
- Niderman, T.; Genetet, I.; Bruyere, T.; Gees, R.; Stintzi, A.; Legrand, M.; Mosinger, E. Pathogenesis-related PR-1 proteins are antifungal (isolation and characterization of three 14-kilodalton proteins of tomato and of a basic PR-1 of tobacco with inhibitory activity against Phytophthora infestans). Plant Physiol. 1995, 108, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Tornero, P.; Gadea, J.; Conejero, V.; Vera, P. Two PR-1 genes from tomato are differentially regulated and reveal a novel mode of expression for a pathogenesis-related gene during the hypersensitive response and development. Mol. Plant-Microbe Interact. 1997, 10, 624–634. [Google Scholar] [CrossRef] [PubMed]
- Postma, J.; Clematis, F.; Nijhuis, E.H.; Someus, E. Efficacy of four phosphate-mobilizing bacteria applied with an animal bone charcoal formulation in controlling Pythium aphanidermatum and Fusarium oxysporum f. sp. radicis lycopersici in tomato. Biol. Control 2013, 67, 284–291. [Google Scholar] [CrossRef]
- Martinez, C.; Bourassa, A.; Roy, G.; Desbiens, M.C.; Bussières, P. Efficacy of PRO-MIX® with Biofungicide against Root Diseases caused by Pythium spp. and Rhizoctonia spp. In Proceedings of the XXVII International Horticultural Congress-IHC2006: International Symposium on Sustainability through Integrated and Organic, Seoul, Republic of Korea, 13–19 August 2006; Volume 767, pp. 185–192. [Google Scholar]
- Caron, J.; Laverdière, L.; Thibodeau, P.O.; Bélanger, R.R. Utilisation d’une souche indigène de Trichoderma harzianum contre cinq agents pathogènes chez le concombre et la tomate de serre au Québec. Phytoprotection 2002, 83, 73–87. [Google Scholar] [CrossRef][Green Version]
- Larsen, J.; Graham, J.H.; Cubero, J.; Ravnskov, S. Biocontrol traits of plant growth suppressive arbuscular mycorrhizal fungi against root rot in tomato caused by Pythium aphanidermatum. Eur. J. Plant Pathol. 2012, 133, 361–369. [Google Scholar] [CrossRef]
- St Martin, C.C.G.; Dorinvil, W.; Brathwaite, R.A.I.; Ramsubhag, A. Effects and relationships of compost type, aeration and brewing time on compost tea properties, efficacy against Pythium ultimum, phytotoxicity and potential as a nutrient amendment for seedling production. Biol. Agric. Hortic. 2012, 28, 185–205. [Google Scholar] [CrossRef]
- Jenana RK, B.; Haouala, R.; Triki, M.A.; Godon, J.J.; Hibar, K.; Khedher, M.B.; Henchi, B. Composts, compost extracts and bacterial suppressive action on Pythium aphanidermatum in tomato. Pak. J. Bot. 2009, 41, 315–327. [Google Scholar]
- Postma, J.; Nijhuis, E.H. Pseudomonas chlororaphis and organic amendments controlling Pythium infection in tomato. Eur. J. Plant Pathol. 2019, 154, 91–107. [Google Scholar] [CrossRef]
- Jayaraj, J.; Radhakrishnan, N.V.; Kannan, R.; Sakthivel, K.; Suganya, D.; Venkatesan, S.; Velazhahan, R. Development of new formulations of Bacillus subtilis for management of tomato damping-off caused by Pythium aphanidermatum. Biocontrol Sci. Technol. 2005, 15, 55–65. [Google Scholar] [CrossRef]
- Fradin, E.F.; Zhang, Z.; Juarez Ayala, J.C.; Castroverde, C.D.; Nazar, R.N.; Robb, J.; Thomma, B.P. Genetic dissection of Verticillium wilt resistance mediated by tomato Ve1. Plant Physiol. 2009, 150, 320–332. [Google Scholar] [CrossRef]
- Fradin, E.F.; Thomma, B.P. Physiology and molecular aspects of Verticillium wilt diseases caused by V. dahliae and V. albo-atrum. Mol. Plant Pathol. 2006, 7, 71–86. [Google Scholar] [CrossRef] [PubMed]
- Mandelc, S.; Timperman, I.; Radišek, S.; Devreese, B.; Samyn, B.; Javornik, B. Comparative proteomic profiling in compatible and incompatible interactions between hop roots and Verticillium albo-atrum. Plant Physiol. Biochem. 2013, 68, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Berlanger, I.; Powelson, M.L. Verticillium Wilt. Available online: https://www.apsnet.org/edcenter/disandpath/fungalasco/pdlessons/Pages/VerticilliumWilt.aspx (accessed on 30 November 2022).
- Butterfield, E.J.; DeVay, J.E.; Garber, R.H. The influence of several crop sequences on the incidence of Verticillium wilt of cotton and on the populations of Verticillium dahliae in field soil. Phytopathology 1978, 68, 1217–1220. [Google Scholar] [CrossRef]
- Iott, M.C. Utility of Grafting and Evaluation of Rootstocks for the Management of Verticillium Wilt in Tomato Production in Western North Carolina. Master’s Thesis, North Carolina State University, Raleigh, NC, USA, 2013. [Google Scholar]
- Stapleton, J.J. Soil solarization in various agricultural production systems. Crop. Prot. 2000, 19, 837–841. [Google Scholar] [CrossRef]
- Goicoechea, N. To what extent are soil amendments useful to control Verticillium wilt. Pest Manag. Sci. Former. Pestic. Sci. 2009, 65, 831–839. [Google Scholar] [CrossRef]
- Rowe, R.C.; Powelson, M.L. Potato early dying: Management challenges in a changing production environment. Plant Dis. 2002, 86, 1184–1193. [Google Scholar] [CrossRef]
- Ordentlich, A.; Nachmias, A.; Chet, I. Integrated control of Verticillium dahliae in potato by Trichoderma harzianum and captan. Crop. Prot. 1990, 9, 363–366. [Google Scholar] [CrossRef]
- Baergen, K.D.; Hewitt, J.D.; Clair, D.S. Resistance of tomato genotypes to four isolates of Verticillium dahliae race 2. HortScience 1993, 28, 833–836. [Google Scholar] [CrossRef]
- Dhouib, H.; Zouari, I.; Abdallah, D.B.; Belbahri, L.; Taktak, W.; Triki, M.A.; Tounsi, S. Potential of a novel endophytic Bacillus velezensis in tomato growth promotion and protection against Verticillium wilt disease. Biol. Control 2019, 139, 104092. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Sakr, S.; Bufo, S.A.; Camele, I. An attempt of biocontrol the tomato-wilt disease caused by Verticillium dahliae using Burkholderia gladioli pv. agaricicola and its bioactive secondary metabolites. Int. J. Plant Biol. 2017, 8, 7263. [Google Scholar] [CrossRef]
- Jabnoun-Khiareddine, H.; Daami-Remadi, M.; Ayed, F.; El Mahjoub, M. Biocontrol of tomato Verticillium wilt by using indigenous Gliocladium spp. and Penicillium sp. isolates. Dyn. Soil Dyn. Plant 2009, 3, 70–79. [Google Scholar]
- Larena, I.; Sabuquillo, P.; Melgarejo, P.; De Cal, A. Biocontrol of Fusarium and Verticillium wilt of tomato by Penicillium oxalicum under greenhouse and field conditions. J. Phytopathol. 2003, 151, 507–512. [Google Scholar] [CrossRef]
- Jabnoun-Khiareddine, H.; Daami-Remadi, M.; Ayed, F.; El Mahjoub, M. Biological control of tomato Verticillium wilt by using indigenous Trichoderma spp. Afr. J. Plant Sci. Biotechnol. 2009, 3, 26–36. [Google Scholar]
- Naraghi, L.; Heydari, A.; Rezaee, S.; Razavi, M.; Jahanifar, H.; Khaledi, E. Biological control of tomato Verticillium wilt disease by Talaromyces flavus. J. Plant Prot. Res. 2010, 50, 360–365. [Google Scholar] [CrossRef]
- Puri, K.D.; Hu, X.; Gurung, S.; Short, D.P.; Sandoya, G.V.; Schild, M.; Subbarao, K.V. Verticillium klebahnii and V. isaacii Isolates Exhibit Host-dependent Biological Control of Verticillium Wilt Caused by V. dahliae. PhytoFrontiers 2021, 1, 276–290. [Google Scholar] [CrossRef]
- Acharya, B.; Ingram, T.W.; Oh, Y.; Adhikari, T.B.; Dean, R.A.; Louws, F.J. Opportunities and challenges in studies of host-pathogen interactions and management of Verticillium dahliae in tomatoes. Plants 2020, 9, 1622. [Google Scholar] [CrossRef]
- Kadoglidou, K.; Chatzopoulou, P.; Maloupa, E.; Kalaitzidis, A.; Ghoghoberidze, S.; Katsantonis, D. Mentha and oregano soil amendment induces enhancement of tomato tolerance against soilborne diseases, yield and quality. Agronomy 2020, 10, 406. [Google Scholar] [CrossRef]
- Ait Rahou, Y.; Ait-El-Mokhtar, M.; Anli, M.; Boutasknit, A.; Ben-Laouane, R.; Douira, A.; Meddich, A. Use of mycorrhizal fungi and compost for improving the growth and yield of tomato and its resistance to Verticillium dahliae. Arch. Phytopathol. Plant Prot. 2021, 54, 665–690. [Google Scholar] [CrossRef]
- Lazarovits, G.; Conn, K.; Tenuta, M. Control of Verticillium dahliae with soil amendments: Efficacy and mode of action. In Advances in Verticillium Research and Disease Management, 1st ed.; Tjamos, E.C., Rowe, R.C., Heale, J.B., Fravel, D.R., Eds.; APS Press: St Paul, MN, USA, 2000; pp. 274–291. [Google Scholar]
- Yuce, E.K.; Yigit, S.; Tosun, N. Efficacy of solarization combined with metam sodium and hydrogen peroxide in control of Fusarium oxysporum f. sp. radicis-lycopersici and Clavibacter michiganensis subsp. michiganensis in Tomato greenhouse. In Proceedings of the III International Symposium on Tomato Diseases, Ischia, Italy, 25–30 July 2010; Volume 914, pp. 385–391. [Google Scholar]
- Kulus, D. Genetic resources and selected conservation methods of tomato. J. Appl. Bot. Food Qual. 2018, 91, 135–144. [Google Scholar]
- Fones, H.N.; Bebber, D.P.; Chaloner, T.M.; Kay, W.T.; Steinberg, G.; Gurr, S.J. Threats to global food security from emerging fungal and oomycete crop pathogens. Nat. Food 2020, 1, 332–342. [Google Scholar] [CrossRef]
- Bebber, D.P.; Field, E.; Gui, H.; Mortimer, P.; Holmes, T.; Gurr, S.J. Many unreported crop pests and pathogens are probably already present. Glob. Chang. Biol. 2019, 25, 2703–2713. [Google Scholar] [CrossRef] [PubMed]
- Cammarano, D.; Jamshidi, S.; Hoogenboom, G.; Ruane, A.C.; Niyogi, D.; Ronga, D. Processing tomato production is expected to decrease by 2050 due to the projected increase in temperature. Nat. Food 2022, 3, 347–444. [Google Scholar] [CrossRef]
| Management Methods | Examples |
|---|---|
| Cultural control | Crop rotation, farrowing, hygiene |
| Physical control | Soil solarization, soil warming |
| Chemical control | Soil chemical fumigation, application of fungicide |
| Resistance breeding | Crossing the desired traits from wild relatives into cultivated tomato varieties |
| Biological control | Biocontrol agents, organic soil amendments |
| Management Methods | Advantages | Disadvantages |
|---|---|---|
| Cultural control | Basic, easy to be carried out | Limited controlling effects |
| Can be integrated into other management methods | Laborious | |
| Physical control | Effective against pathogens residing in soil | May not be economically feasible |
| Material highly accessible | Effectiveness depends on the local environment and the biology of the pathogen | |
| Less effective in deep soil | ||
| Chemical control | Highly effective-at least in the initial stages | High cost |
| Broad-spectrum effect of fumigation | Requiring registration | |
| Target-specificity of fungicides | Negative effects on the environment and human health | |
| Industrialized process | Decreasing public acceptance | |
| Resistance breeding | Target-specific resistance | Laborious |
| Sustainability | Time-consuming | |
| Environmentally friendly | Resistance traits against certain pathogens do not exist | |
| Biological control | Various mechanisms against specific pathogens | New, largely in in vitro trial stage |
| Sustainability | Impact on the indigenous microbial community | |
| Environmentally friendly | Requiring registration | |
| Highly levels of disease control effects | ||
| High public acceptance | ||
| Cost-efficiency | ||
| Turns waste into use |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, M.; Taylor, P.W.J.; Chen, D.; Vaghefi, N.; He, J.-Z. Major Soilborne Pathogens of Field Processing Tomatoes and Management Strategies. Microorganisms 2023, 11, 263. https://doi.org/10.3390/microorganisms11020263
Ma M, Taylor PWJ, Chen D, Vaghefi N, He J-Z. Major Soilborne Pathogens of Field Processing Tomatoes and Management Strategies. Microorganisms. 2023; 11(2):263. https://doi.org/10.3390/microorganisms11020263
Chicago/Turabian StyleMa, Minxiao, Paul W. J. Taylor, Deli Chen, Niloofar Vaghefi, and Ji-Zheng He. 2023. "Major Soilborne Pathogens of Field Processing Tomatoes and Management Strategies" Microorganisms 11, no. 2: 263. https://doi.org/10.3390/microorganisms11020263
APA StyleMa, M., Taylor, P. W. J., Chen, D., Vaghefi, N., & He, J.-Z. (2023). Major Soilborne Pathogens of Field Processing Tomatoes and Management Strategies. Microorganisms, 11(2), 263. https://doi.org/10.3390/microorganisms11020263







