Residual Effects of Transgenic Cotton on the Intestinal Microbiota of Dysdercus concinnus
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Specimen Dissection and Gut DNA Extraction
2.3. Amplification and Sequencing
2.4. Demultiplexing, Filtering, and Chimera Check
2.5. Statistical Analyses of Molecular Data
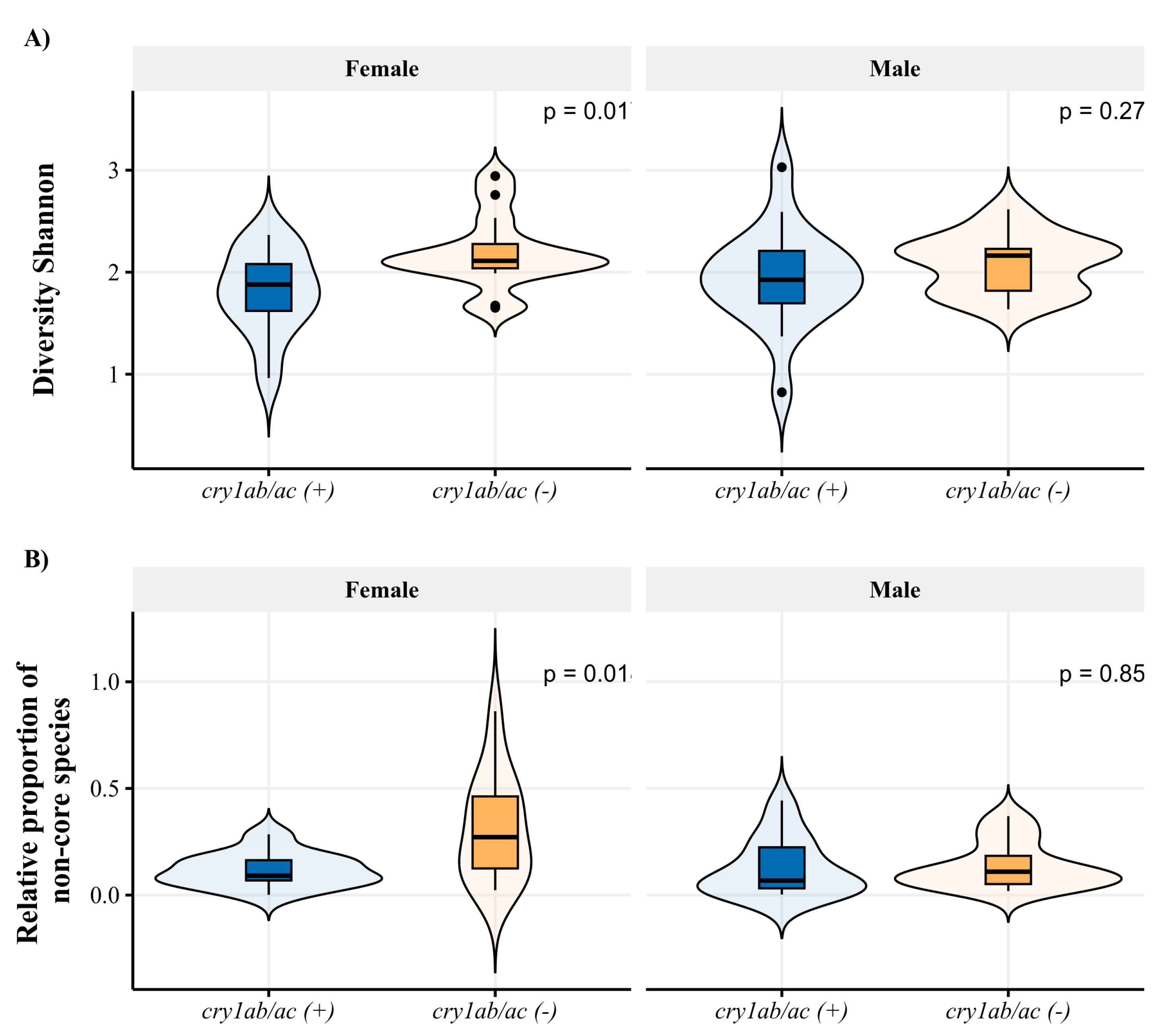
3. Results
3.1. Dysdercus Concinnus Gut Bacterial Communities
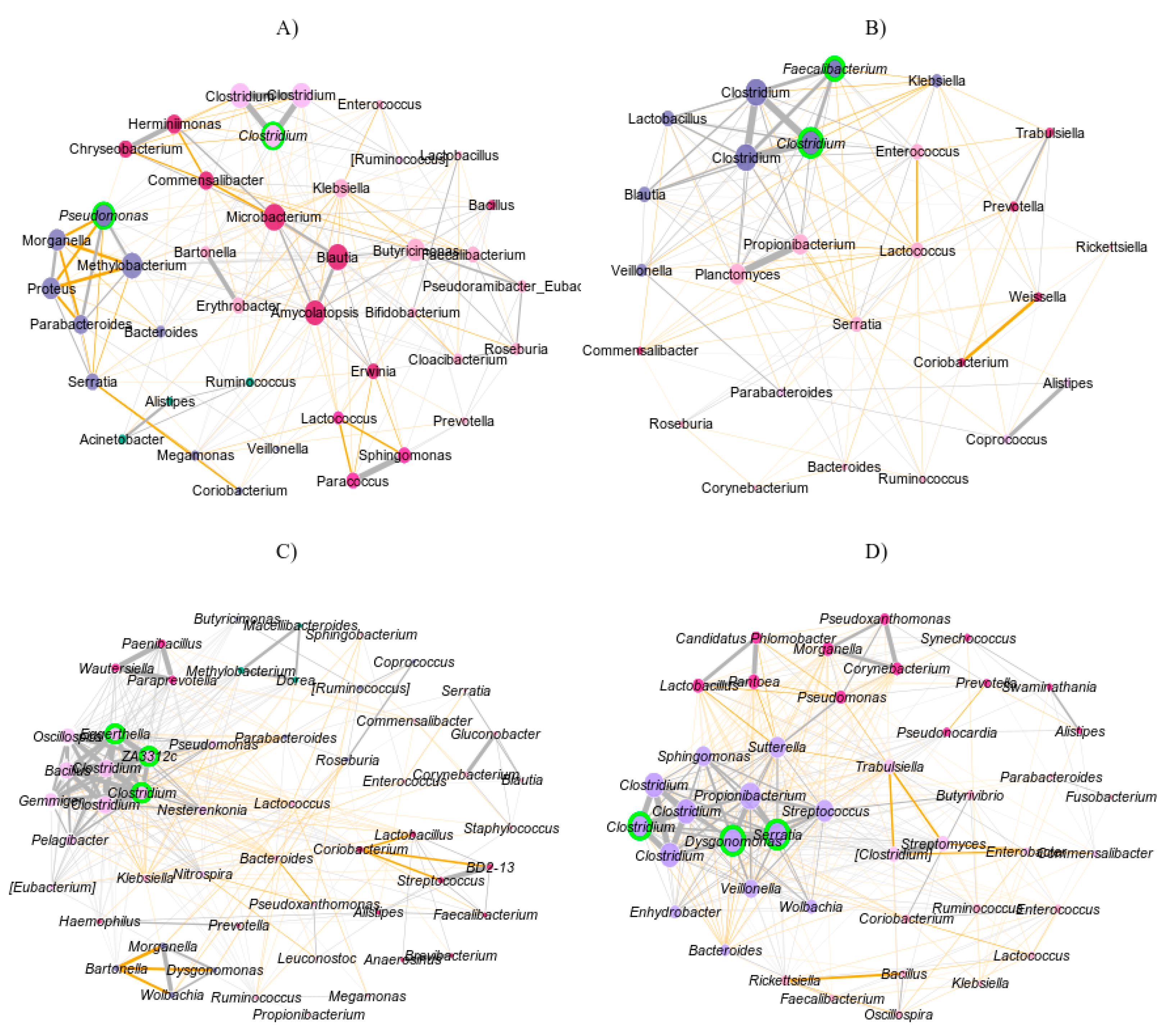
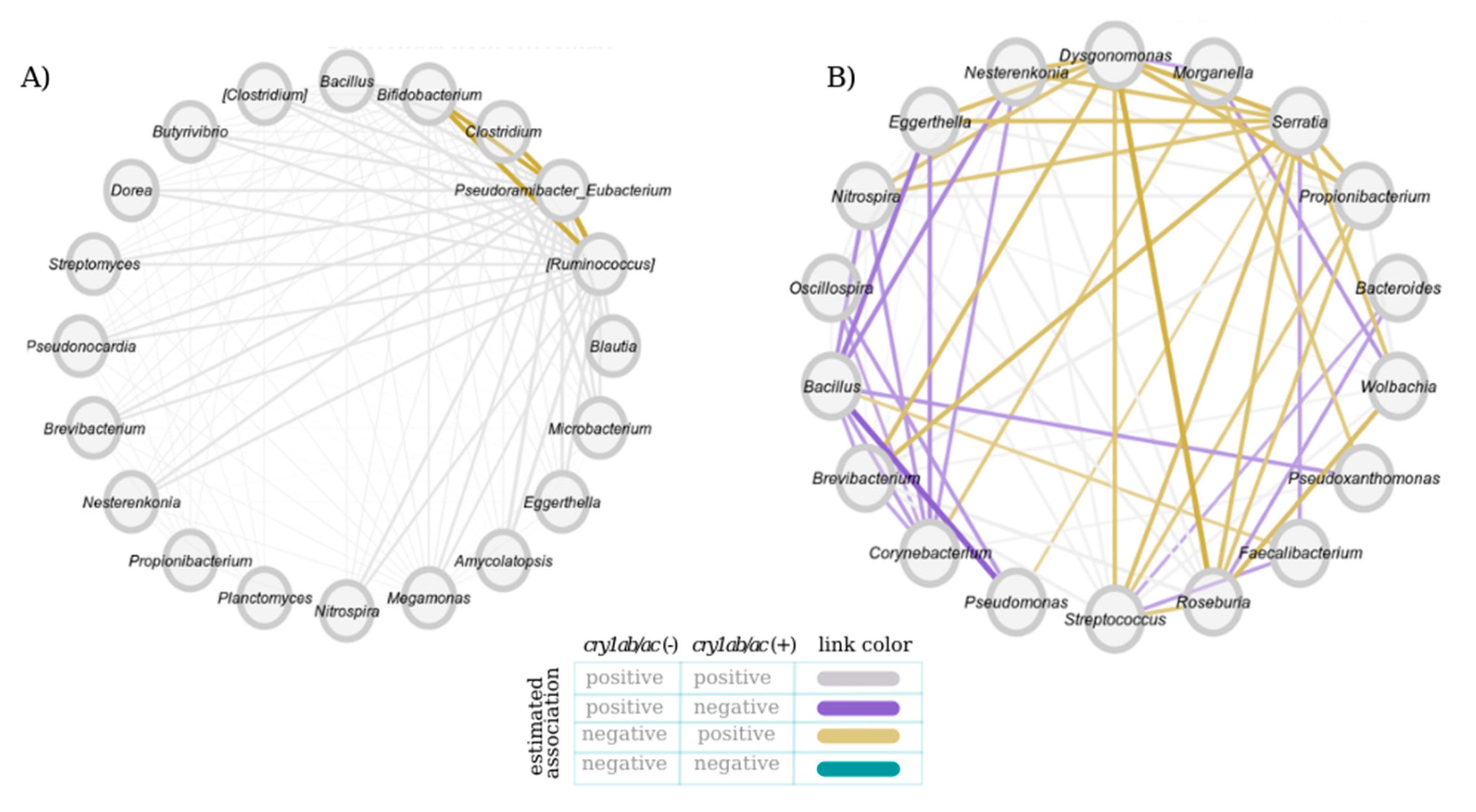
3.2. Bacterial Community Networks in the D. concinnus Gut
4. Discussion and Conclusions
4.1. Key Intestinal Microbial Taxa in Dysdercus Species and Their Influence on Fitness Traits
4.2. What Does Not Kill You Makes You Stronger (Inside and Outside the Gut Microbiota): Ecological and Evolutionary Considerations
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Noman, A.; Aqeel, M.; Qasim, M.; Haider, I.; Lou, Y. Plant-Insect-Microbe Interaction: A Love Triangle between Enemies in Ecosystem. Sci. Total. Environ. 2020, 699, 134181. [Google Scholar] [CrossRef] [PubMed]
- Itoh, H.; Tago, K.; Hayatsu, M.; Kikuchi, Y. Detoxifying Symbiosis: Microbe-Mediated Detoxification of Phytotoxins and Pesticides in Insects. Nat. Prod. Rep. 2018, 35, 434–454. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Nair, S. Dynamics of Insect-Microbiome Interaction Influence Host and Microbial Symbiont. Front. Microbiol. 2020, 11, 1357. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, J.A.; Khan, M.M.; Bamisile, B.S.; Hafeez, M.; Qasim, M.; Rasheed, M.T.; Rasheed, M.A.; Ahmad, S.; Shahid, M.I.; Xu, Y. Role of Insect Gut Microbiota in Pesticide Degradation: A Review. Front. Microbiol. 2022, 13, 870462. [Google Scholar] [CrossRef]
- Vázquez-Barrios, V.; Boege, K.; Sosa-Fuentes, T.G.; Rojas, P.; Wegier, A. Ongoing Ecological and Evolutionary Consequences by the Presence of Transgenes in a Wild Cotton Population. Sci. Rep. 2021, 11, 1959. [Google Scholar] [CrossRef]
- Yin, Y.; Cao, K.; Zhao, X.; Cao, C.; Dong, X.; Liang, J.; Shi, W. Bt Cry1Ab/2Ab Toxins Disrupt the Structure of the Gut Bacterial Community of Locusta Migratoria through Host Immune Responses. Ecotoxicol. Environ. Saf. 2022, 238, 113602. [Google Scholar] [CrossRef]
- Caccia, S.; Di Lelio, I.; La Storia, A.; Marinelli, A.; Varricchio, P.; Franzetti, E.; Banyuls, N.; Tettamanti, G.; Casartelli, M.; Giordana, B.; et al. Midgut Microbiota and Host Immunocompetence Underlie Bacillus Thuringiensis Killing Mechanism. Proc. Natl. Acad. Sci. USA 2016, 113, 9486–9491. [Google Scholar] [CrossRef]
- Dubovskiy, I.M.; Grizanova, E.V.; Whitten, M.M.A.; Mukherjee, K.; Greig, C.; Alikina, T.; Kabilov, M.; Vilcinskas, A.; Glupov, V.V.; Butt, T.M. Immuno-Physiological Adaptations Confer Wax Moth Galleria Mellonella Resistance to Bacillus Thuringiensis. Virulence 2016, 7, 860–870. [Google Scholar] [CrossRef]
- Mead, F.W.; Fasulo, T.R. Cotton Stainer Dysdercus Suturellus (Herrich-Schaeffer) (Insecta: Hemiptera: Pyrrhocoridae); EENY-330/IN606, Rev. 3/2005; UF/IFAS Extension: Gainesville, FL, USA, 2005; Volume 2005. [Google Scholar]
- Jorge, A.; Lomonaco, C. Body Size, Symmetry and Couurtship Behavior of Dysdercus Maurus Distant (Hemiptera: Prrrhocoridae). Neotrop. Entomol. 2011, 40, 305–311. [Google Scholar] [CrossRef]
- Dingle, H.; Arora, G. Experimental Studies of Migration in Bugs of the Genus Dysdercus. Oecologia 1973, 12, 119–140. [Google Scholar] [CrossRef]
- Alavez, V.; Cuervo-Robayo, Á.P.; Martínez-Meyer, E.; Wegier, A. Eco-Geography of Feral Cotton: A Missing Piece in the Puzzle of Gene Flow Dynamics Among Members of Gossypium Hirsutum Primary Gene Pool. Front. Ecol. Evol. 2021, 9, 653271. [Google Scholar] [CrossRef]
- Wegier, A.; Piñeyro-Nelson, A.; Alarcón, J.; Gálvez-Mariscal, A.; Alvarez-Buylla, E.R.; Piñero, D. Recent Long-Distance Transgene Flow into Wild Populations Conforms to Historical Patterns of Gene Flow in Cotton (Gossypium Hirsutum) at Its Centre of Origin. Mol. Ecol. 2011, 20, 4182–4194. [Google Scholar] [CrossRef]
- Hernández-García, J.A.; Gonzalez-Escobedo, R.; Briones-Roblero, C.I.; Cano-Ramírez, C.; Rivera-Orduña, F.N.; Zúñiga, G. Gut Bacterial Communities of Dendroctonus Valens and D. Mexicanus (Curculionidae: Scolytinae): A Metagenomic Analysis across Different Geographical Locations in Mexico. Int. J. Mol. Sci. 2018, 19, 2578. [Google Scholar] [CrossRef] [PubMed]
- Rebollar, E.A.; Sandoval-Castellanos, E.; Roessler, K.; Gaut, B.S.; Alcaraz, L.D.; Benítez, M.; Escalante, A.E. Seasonal Changes in a Maize-Based Polyculture of Central Mexico Reshape the Co-Occurrence Networks of Soil Bacterial Communities. Front. Microbiol. 2017, 8, 2478. [Google Scholar] [CrossRef] [PubMed]
- Walters, W.; Hyde, E.R.; Berg-Lyons, D.; Ackermann, G.; Humphrey, G.; Parada, A.; Gilbert, J.A.; Jansson, J.K.; Caporaso, J.G.; Fuhrman, J.A.; et al. Improved Bacterial 16S RRNA Gene (V4 and V4–5) and Fungal Internal Transcribed Spacer Marker Gene Primers for Microbial Community Surveys. mSystems 2016, 1, e00009-15. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME Allows Analysis of High-Throughput Community Sequencing Data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- DeSantis, T.Z.; Hugenholtz, P.; Larsen, N.; Rojas, M.; Brodie, E.L.; Keller, K.; Huber, T.; Dalevi, D.; Hu, P.; Andersen, G.L. Greengenes, a Chimera-Checked 16S RRNA Gene Database and Workbench Compatible with ARB. Appl. Environ. Microbiol. 2006, 72, 5069–5072. [Google Scholar] [CrossRef]
- Price, M.N.; Dehal, P.S.; Arkin, A.P. FastTree 2–Approximately Maximum-Likelihood Trees for Large Alignments. PLoS ONE 2010, 5, e9490. [Google Scholar] [CrossRef]
- Dixon, P. VEGAN, a Package of R Functions for Community Ecology. J. Veg. Sci. 2003, 14, 927–930. [Google Scholar] [CrossRef]
- Matchado, M.S.; Lauber, M.; Reitmeier, S.; Kacprowski, T.; Baumbach, J.; Haller, D.; List, M. Network Analysis Methods for Studying Microbial Communities: A Mini Review. Comput. Struct. Biotechnol. J. 2021, 19, 2687–2698. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. On the Adaptive Control of the False Discovery Rate in Multiple Testing With Independent Statistics. J. Educ. Behav. Stat. 2000, 25, 60–83. [Google Scholar] [CrossRef]
- Westfall, P.H. The Benjamini-Hochberg Method with Infinitely Many Contrasts in Linear Models. Biometrika 2008, 95, 709–719. [Google Scholar] [CrossRef]
- Clauset, A.; Newman, M.E.J.; Moore, C. Finding Community Structure in Very Large Networks. Phys. Rev. E 2004, 70, 66111. [Google Scholar] [CrossRef]
- Hoffman, M.; Steinley, D.; Brusco, M.J. A Note on Using the Adjusted Rand Index for Link Prediction in Networks. Soc. Networks 2015, 42, 72–79. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. In Computer Science Review; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Lahti, L.; Shetty, S. Microbiome R Package. Bioconductor. 2017. Available online: https://doi.org/10.18129/B9.bioc.microbiome (accessed on 1 December 2022).
- Csardi, G.; Nepusz, T. The Igraph Software Package for Complex Network Research. InterJournal Complex Syst. 2006, 1695, 1–9. [Google Scholar]
- Bisanz, J.E. Qiime2R: Importing QIIME2 Artifacts and Associated Data into R Sessions. Version 0. 99. 2018. Available online: https://github.com/jbisanz/qiime2R (accessed on 1 December 2022).
- Peschel, S.; Müller, C.L.; von Mutius, E.; Boulesteix, A.-L.; Depner, M. NetCoMi: Network Construction and Comparison for Microbiome Data in R. Brief. Bioinform. 2021, 22, bbaa290. [Google Scholar] [CrossRef]
- Kassambara, A. Ggpubr: “ggplot2” Based Publication Ready Plots. 2020. Available online: https://github.com/kassambara/ggpubr (accessed on 1 December 2022).
- Goettsch, B.; Urquiza-Haas, T.; Koleff, P.; Acevedo Gasman, F.; Aguilar-Meléndez, A.; Alavez, V.; Alejandre-Iturbide, G.; Cuevas, F.A.; Pérez, C.A.; Carr, J.A.; et al. Extinction Risk of Mesoamerican Crop Wild Relatives. Plants People Planet 2021, 3, 775–795. [Google Scholar] [CrossRef]
- Stewart, C.N.J.; Halfhill, M.D.; Warwick, S.I. Transgene Introgression from Genetically Modified Crops to Their Wild Relatives. Nat. Rev. Genet. 2003, 4, 806–817. [Google Scholar] [CrossRef]
- Carey, H.V.; Duddleston, K.N. Animal-Microbial Symbioses in Changing Environments. J. Therm. Biol. 2014, 44, 78–84. [Google Scholar] [CrossRef]
- Bauer, E.; Salem, H.; Marz, M.; Vogel, H.; Kaltenpoth, M. Transcriptomic Immune Response of the Cotton Stainer Dysdercus Fasciatus to Experimental Elimination of Vitamin-Supplementing Intestinal Symbionts. PLoS ONE 2014, 9, e114865. [Google Scholar] [CrossRef]
- Onchuru, T.O.; Martinez, A.J.; Kaltenpoth, M. The Cotton Stainer’s Gut Microbiota Suppresses Infection of a Cotransmitted Trypanosomatid Parasite. Mol. Ecol. 2018, 27, 3408–3419. [Google Scholar] [CrossRef]
- Uzmi, S.; Sureshan, C.S.; Ghosh, S.; Habeeb, S.K.M. Identification of Microbial Community Colonizing the Gut of Dysdercus Cingulatus Fabricius (Hemiptera: Pyrrhocoridae). J. Microbiol. Biotechnol. Food Sci. 2019, 9, 496–501. [Google Scholar] [CrossRef]
- Li, R.; Li, M.; Yan, J.; Zhang, H. Composition and Function of the Microbiotas in the Different Parts of the Midgut of Pyrrhocoris Sibiricus (Hemiptera: Pyrrhocoridae) Revealed Using High-Throughput Sequencing of 16S RRNA. EJE 2020, 117, 352–371. [Google Scholar] [CrossRef]
- Biddle, A.; Stewart, L.; Blanchard, J.; Leschine, S. Untangling the Genetic Basis of Fibrolytic Specialization by Lachnospiraceae and Ruminococcaceae in Diverse Gut Communities. Diversity 2013, 5, 627–640. [Google Scholar] [CrossRef]
- Zheng, H.; Powell, J.E.; Steele, M.I.; Dietrich, C.; Moran, N.A. Honeybee Gut Microbiota Promotes Host Weight Gain via Bacterial Metabolism and Hormonal Signaling. Proc. Natl. Acad. Sci. USA 2017, 114, 4775–4780. [Google Scholar] [CrossRef]
- Douglas, A.E. The B Vitamin Nutrition of Insects: The Contributions of Diet, Microbiome and Horizontally Acquired Genes. Curr. Opin. Insect. Sci. 2017, 23, 65–69. [Google Scholar] [CrossRef]
- Liu, X.; Mao, B.; Gu, J.; Wu, J.; Cui, S.; Wang, G.; Zhao, J.; Zhang, H.; Chen, W. Blautia-a New Functional Genus with Potential Probiotic Properties? Gut Microbes 2021, 13, 1875796. [Google Scholar] [CrossRef]
- Flint, H.J.; Scott, K.P.; Louis, P.; Duncan, S.H. The Role of the Gut Microbiota in Nutrition and Health. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 577–589. [Google Scholar] [CrossRef]
- Salem, H.; Kreutzer, E.; Sudakaran, S.; Kaltenpoth, M. Actinobacteria as Essential Symbionts in Firebugs and Cotton Stainers (Hemiptera, Pyrrhocoridae). Environ. Microbiol. 2013, 15, 1956–1968. [Google Scholar] [CrossRef]
- Sudakaran, S.; Retz, F.; Kikuchi, Y.; Kost, C.; Kaltenpoth, M. Evolutionary Transition in Symbiotic Syndromes Enabled Diversification of Phytophagous Insects on an Imbalanced Diet. ISME J. 2015, 9, 2587–2604. [Google Scholar] [CrossRef] [PubMed]
- Sudakaran, S.; Salem, H.; Kost, C.; Kaltenpoth, M. Geographical and Ecological Stability of the Symbiotic Mid-Gut Microbiota in European Firebugs, Pyrrhocoris Apterus (Hemiptera, Pyrrhocoridae). Mol. Ecol. 2012, 21, 6134–6151. [Google Scholar] [CrossRef] [PubMed]
- Wexler, A.G.; Goodman, A.L. An Insider’s Perspective: Bacteroides as a Window into the Microbiome. Nat. Microbiol. 2017, 2, 17026. [Google Scholar] [CrossRef] [PubMed]
- Hosokawa, T.; Kikuchi, Y.; Nikoh, N.; Shimada, M.; Fukatsu, T. Strict Host-Symbiont Cospeciation and Reductive Genome Evolution in Insect Gut Bacteria. PLOS Biol. 2006, 4, e337. [Google Scholar] [CrossRef] [PubMed]
- Bordenstein, S.R.; Theis, K.R. Host Biology in Light of the Microbiome: Ten Principles of Holobionts and Hologenomes. PLOS Biol. 2015, 13, e1002226. [Google Scholar] [CrossRef]
- Shapira, M. Gut Microbiotas and Host Evolution: Scaling Up Symbiosis. Trends Ecol. Evol. 2016, 31, 539–549. [Google Scholar] [CrossRef]
- Itoh, H.; Jang, S.; Takeshita, K.; Ohbayashi, T.; Ohnishi, N.; Meng, X.Y.; Mitani, Y.; Kikuchi, Y. Host–Symbiont Specificity Determined by Microbe–Microbe Competition in an Insect Gut. Proc. Natl. Acad. Sci. USA 2019, 116, 22673–22682. [Google Scholar] [CrossRef]
- Fordyce, J.A. The Evolutionary Consequences of Ecological Interactions Mediated through Phenotypic Plasticity. J. Exp. Biol. 2006, 209, 2377–2383. [Google Scholar] [CrossRef]
- Padovani, R.J.; Salisbury, A.; Bostock, H.; Roy, D.B.; Thomas, C.D. Introduced Plants as Novel Anthropocene Habitats for Insects. Glob. Chang. Biol. 2020, 26, 971–988. [Google Scholar] [CrossRef]
- Carrière, Y.; Crowder, D.W.; Tabashnik, B.E. Evolutionary Ecology of Insect Adaptation to Bt Crops. Evol. Appl. 2010, 3, 561–573. [Google Scholar] [CrossRef]
- Tabashnik, B.E.; Carrière, Y. Global Patterns of Resistance to Bt Crops Highlighting Pink Bollworm in the United States, China, and India. J. Econ. Entomol. 2019, 112, 2513–2523. [Google Scholar] [CrossRef] [PubMed]
- Badran, A.H.; Guzov, V.M.; Huai, Q.; Kemp, M.M.; Vishwanath, P.; Kain, W.; Nance, A.M.; Evdokimov, A.; Moshiri, F.; Turner, K.H.; et al. Continuous Evolution of Bacillus Thuringiensis Toxins Overcomes Insect Resistance. Nature 2016, 533, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Terán, A.; Wegier, A.; Benítez, M.; Lira, R.; Escalante, A.E. Domesticated, Genetically Engineered, and Wild Plant Relatives Exhibit Unintended Phenotypic Differences: A Comparative Meta-Analysis Profiling Rice, Canola, Maize, Sunflower, and Pumpkin. Front. Plant Sci. 2017, 8, 2030. [Google Scholar] [CrossRef] [PubMed]
- Ramessar, K.; Peremarti, A.; Gómez-Galera, S.; Naqvi, S.; Moralejo, M.; Muñoz, P.; Capell, T.; Christou, P. Biosafety and Risk Assessment Framework for Selectable Marker Genes in Transgenic Crop Plants: A Case of the Science Not Supporting the Politics. Transgenic Res. 2007, 16, 261–280. [Google Scholar] [CrossRef]
- Bennett, P.M.; Livesey, C.T.; Nathwani, D.; Reeves, D.S.; Saunders, J.R.; Wise, R. An Assessment of the Risks Associated with the Use of Antibiotic Resistance Genes in Genetically Modified Plants: Report of the Working Party of the British Society for Antimicrobial Chemotherapy. J. Antimicrob. Chemother. 2004, 53, 418–431. [Google Scholar] [CrossRef]

| Sex | Cotton Genotype | n |
|---|---|---|
| Female | cry1ab/ac(+) | 11 |
| cry1ab/ac(−) | 15 | |
| Male | cry1ab/ac(+) | 18 |
| cry1ab/ac(−) | 21 | |
| Total | - | 65 |
| Variable | Factor | df | Mean Sq | F Value | p-Value |
|---|---|---|---|---|---|
| Diversity | Diet | 1 | 0.89 | 6.15 | 0.01 |
| sex | 1 | 0.001 | 0.01 | 0.92 | |
| Interaction | 1 | 0.23 | 1.61 | 0.2 | |
| Non-core | Diet | 1 | 0.11 | 4.93 | 0.03 |
| sex | 1 | 0.14 | 5.9 | 0.01 | |
| Interaction | 1 | 0.13 | 5.73 | 0.01 |
| Sex | Phylum | Class | Order | Family | Genus | Species | p Value |
|---|---|---|---|---|---|---|---|
| Female | Bacteroidetes | Bacteroidia | Bacteroidales | Bacteroidaceae | Bacteroides | ovatus | 0.04 |
| Bacteroidetes | Bacteroidia | Bacteroidales | Bacteroidaceae | Bacteroides | ovatus | 0.05 | |
| Firmicutes | Bacilli | Lactobacillales | Enterococcaceae | Enterococcus | - | 0.04 | |
| Firmicutes | Clostridia | Clostridiales | Lachnospiraceae | Blautia | - | 0.05 | |
| Firmicutes | Clostridia | Clostridiales | - | - | - | 0.04 | |
| Lentisphaerae | [Lentisphaeria] | Lentisphaerales | Lentisphaeraceae | - | - | 0.04 | |
| Planctomycetes | Planctomycetia | Gemmatales | Isosphaeraceae | - | - | 0.04 | |
| Planctomycetes | Planctomycetia | Planctomycetales | Planctomycetaceae | Planctomyces | - | 0.04 | |
| Proteobacteria | Gammaproteobacteria | Alteromonadales | OM60 | - | - | 0.04 | |
| Proteobacteria | Gammaproteobacteria | Enterobacteriales | Enterobacteriaceae | Klebsiella | oxytoca | 0.04 | |
| Proteobacteria | Gammaproteobacteria | Enterobacteriales | Enterobacteriaceae | - | - | 0.04 | |
| Proteobacteria | Gammaproteobacteria | Enterobacteriales | Enterobacteriaceae | - | - | 0.06 | |
| Male | Actinobacteria | Coriobacteriia | Coriobacteriales | Coriobacteriaceae | Coriobacterium | - | 0.03 |
| Bacteroidetes | Bacteroidia | Bacteroidales | Bacteroidaceae | Bacteroides | ovatus | 0.05 | |
| Cyanobacteria | Synechococcophycideae | Synechococcales | Synechococcaceae | Synechococcus | - | 0.05 | |
| Firmicutes | Bacilli | Lactobacillales | Enterococcaceae | Enterococcus | - | 0.01 | |
| Firmicutes | Clostridia | Clostridiales | Lachnospiraceae | Clostridium | hathewayi | 0.05 | |
| Proteobacteria | Gammaproteobacteria | Enterobacteriales | Enterobacteriaceae | - | - | 0.02 | |
| Proteobacteria | Gammaproteobacteria | Enterobacteriales | Enterobacteriaceae | - | - | 0.05 | |
| Proteobacteria | Gammaproteobacteria | Enterobacteriales | Enterobacteriaceae | - | - | 0.05 | |
| Proteobacteria | Gammaproteobacteria | Enterobacteriales | Enterobacteriaceae | Pantoea | - | 0.05 | |
| Proteobacteria | Gammaproteobacteria | Enterobacteriales | Enterobacteriaceae | Serratia | - | 0.05 | |
| Proteobacteria | Gammaproteobacteria | Enterobacteriales | Enterobacteriaceae | Trabulsiella | farmeri | 0.05 | |
| Proteobacteria | Gammaproteobacteria | Enterobacteriales | Enterobacteriaceae | Trabulsiella | - | 0.05 | |
| Proteobacteria | Gammaproteobacteria | - | - | - | - | 0.05 |
| Properties | Female | Male | ||
|---|---|---|---|---|
| cry1ab/ac(−) Cotton | cry1ab/ac(+) Cotton | cry1ab/ac(−) Cotton | cry1ab/ac(+) Cotton | |
| Clustering coefficient | 0.57 | 0.6 | 0.64 | 0.65 |
| Modularity | 0.19 | 0.15 | 0.17 | 0.14 |
| Positive edge percentage | 58.33 | 53.6 | 68.24 | 57.06 |
| Edge density | 0.27 | 0.42 | 0.23 | 0.41 |
| Natural connectivity | 0.03 | 0.08 | 0.06 | 0.07 |
| Vertex connectivity | 3 | 3 | 1 | 2 |
| Edge connectivity | 3 | 3 | 1 | 2 |
| Average dissimilarity | 0.95 | 0.91 | 0.95 | 0.92 |
| Average path length | 1.59 | 1.43 | 1.81 | 1.45 |
| Hubs | Clostridium and Pseudomonas | Clostridium and Faecalibacterium | Clostridium and Bacillus | Clostridium, Dysgonomonas, and Serratia |
| Number of clusters | 5 | 3 | 5 | 4 |
| Sex | Properties | Jacc | p (≤Jacc) | p (≥Jacc) |
|---|---|---|---|---|
| Female | Degree | 0.55 | 0.98 | 0.03 |
| Betweenness centrality | 1 | 1 | 0.33 | |
| Closeness centrality | 0.16 | 0.18 | 0.94 | |
| Eigenvector centrality | 0.55 | 0.98 | 0.03 | |
| ARI | 0.28 | - | 0.02 | |
| Male | Degree | 0.21 | 0.26 | 0.89 |
| Betweenness centrality | 0 | 1 | 1 | |
| Closeness centrality | 0.12 | 0.19 | 0.96 | |
| Eigenvector centrality | 0.21 | 0.26 | 0.89 | |
| ARI | 0 | - | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-López, J.; Alavez, V.; Cerritos, R.; Andraca-Gómez, G.; Fornoni, J.; Wegier, A. Residual Effects of Transgenic Cotton on the Intestinal Microbiota of Dysdercus concinnus. Microorganisms 2023, 11, 261. https://doi.org/10.3390/microorganisms11020261
Pérez-López J, Alavez V, Cerritos R, Andraca-Gómez G, Fornoni J, Wegier A. Residual Effects of Transgenic Cotton on the Intestinal Microbiota of Dysdercus concinnus. Microorganisms. 2023; 11(2):261. https://doi.org/10.3390/microorganisms11020261
Chicago/Turabian StylePérez-López, Javier, Valeria Alavez, René Cerritos, Guadalupe Andraca-Gómez, Juan Fornoni, and Ana Wegier. 2023. "Residual Effects of Transgenic Cotton on the Intestinal Microbiota of Dysdercus concinnus" Microorganisms 11, no. 2: 261. https://doi.org/10.3390/microorganisms11020261
APA StylePérez-López, J., Alavez, V., Cerritos, R., Andraca-Gómez, G., Fornoni, J., & Wegier, A. (2023). Residual Effects of Transgenic Cotton on the Intestinal Microbiota of Dysdercus concinnus. Microorganisms, 11(2), 261. https://doi.org/10.3390/microorganisms11020261






