Isolation, Antimicrobial Effect and Metabolite Analysis of Bacillus amyloliquefaciens ZJLMBA1908 against Citrus Canker Caused by Xanthomonas citri subsp. citri
Abstract
:1. Introduction
2. Material and Methods
2.1. Bacterial Strains and Plants
2.2. Isolation and Purification of Endophytic Bacteria
2.3. Screening for Antagonistic Bacteria against Plant Pathogens
2.4. Identification of Strain ZJLMBA1908
2.5. Preparation and Antimicrobial Activity Determination of ZJLMBA1908 Cell-Free Supernatant (CFS)
2.6. Preparation and MIC/MBC Determination against X. citri subsp. citri of Crude Extract (CE) from ZJLMBA1908 CFS
2.7. Biocontrol Assays of CE under Greenhouse Conditions
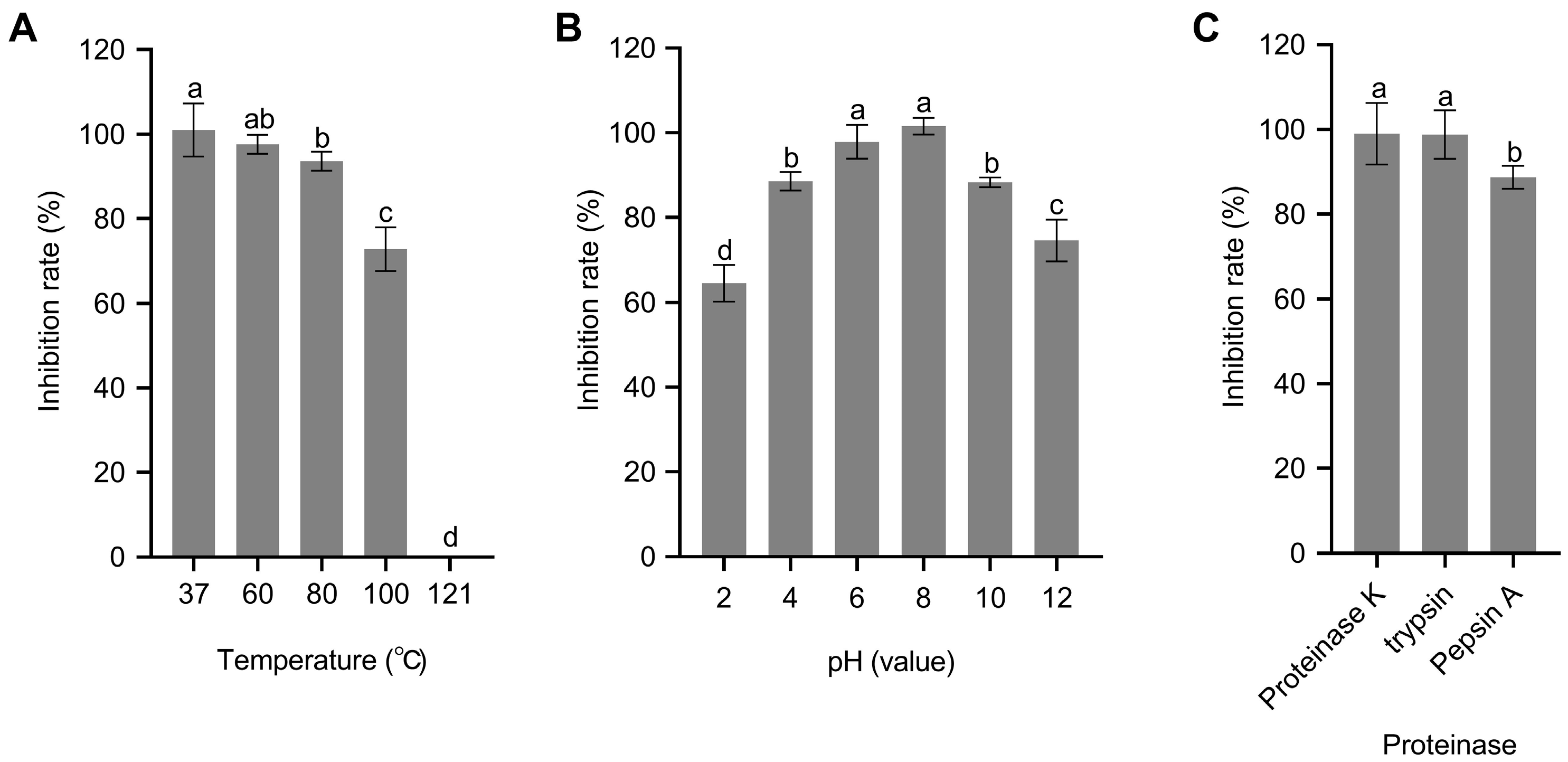
2.8. Determination of CE Stability to Thermal, pH and Proteinase Enzymes
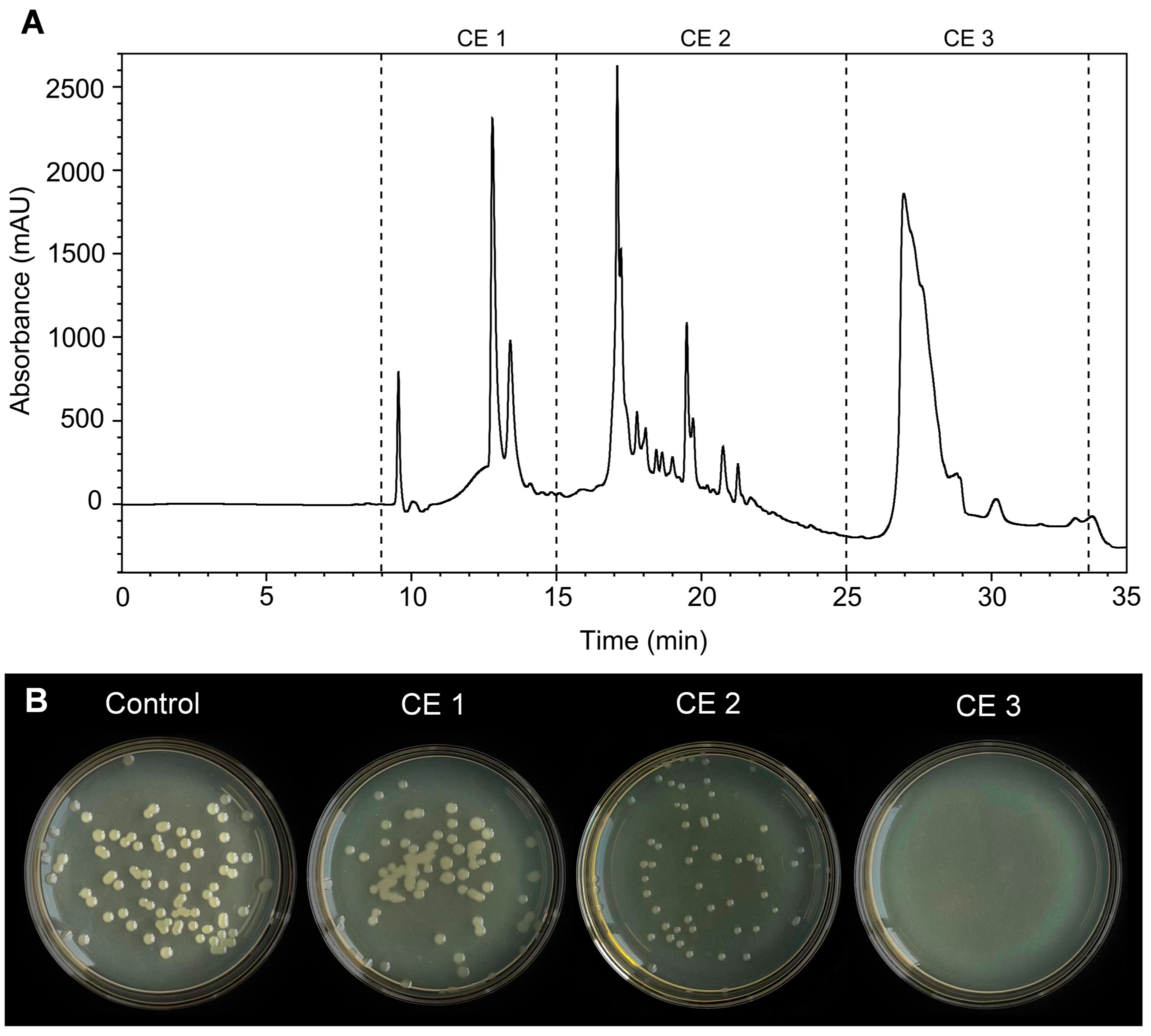
2.9. Purification of CE Using HPLC
2.10. Identification of CE Using LC–ESI–MS
2.11. Statistical Analysis
3. Results
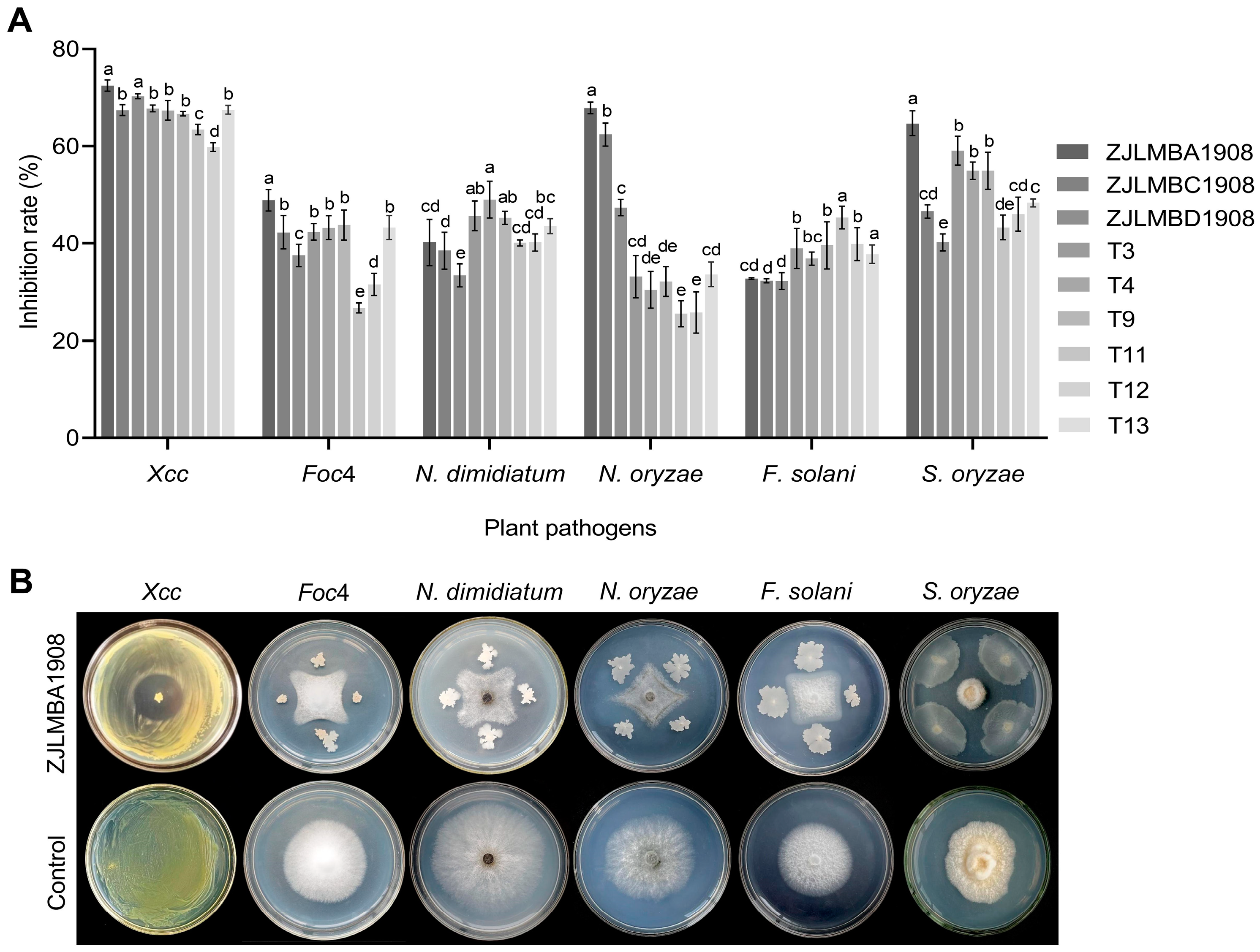
3.1. Antagonistic Effect of Different Endophytic Bacteria
3.2. Identification of Strain ZJLMBA1908
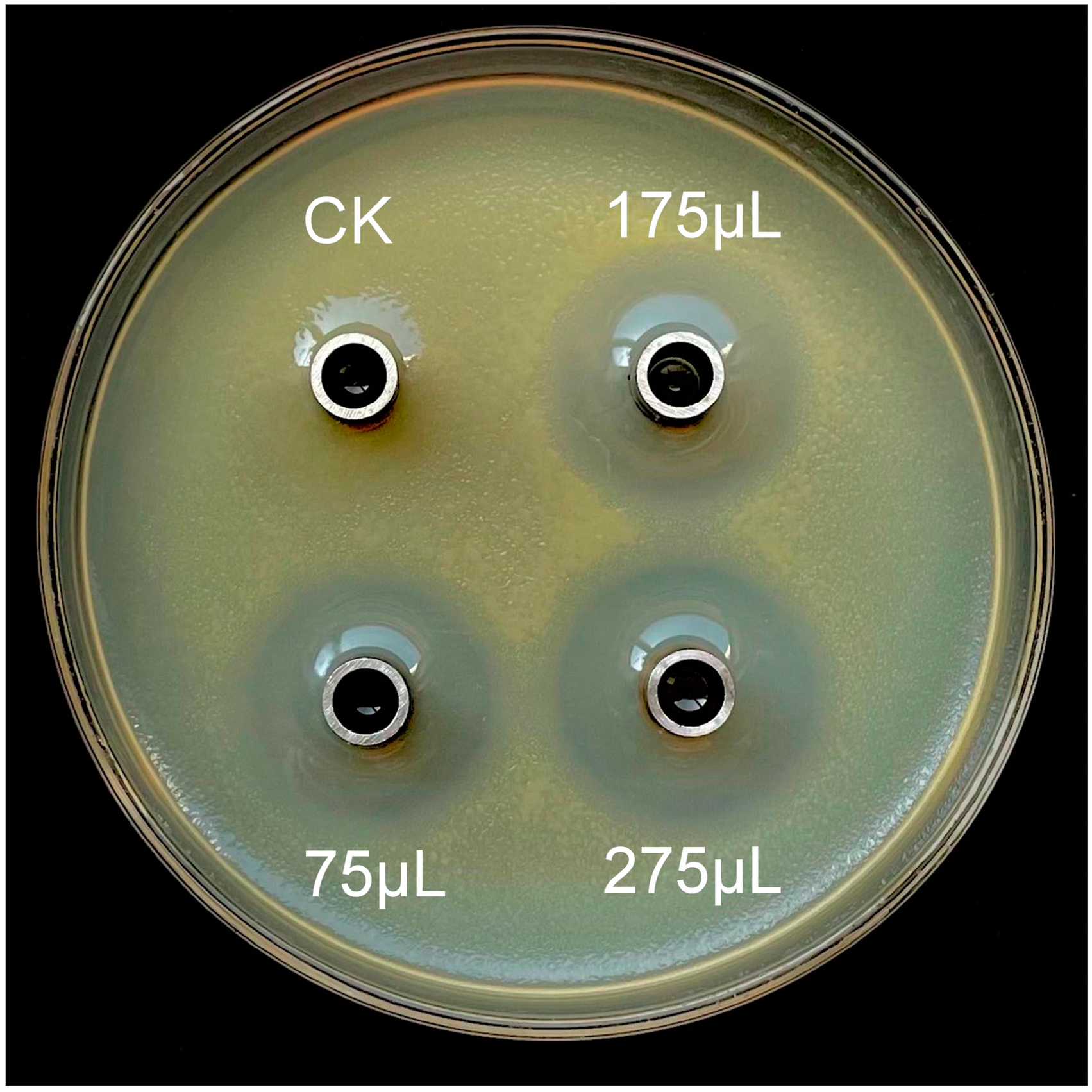
3.3. Antagonistic Effect Analysis of ZJLMBA1908 CFS
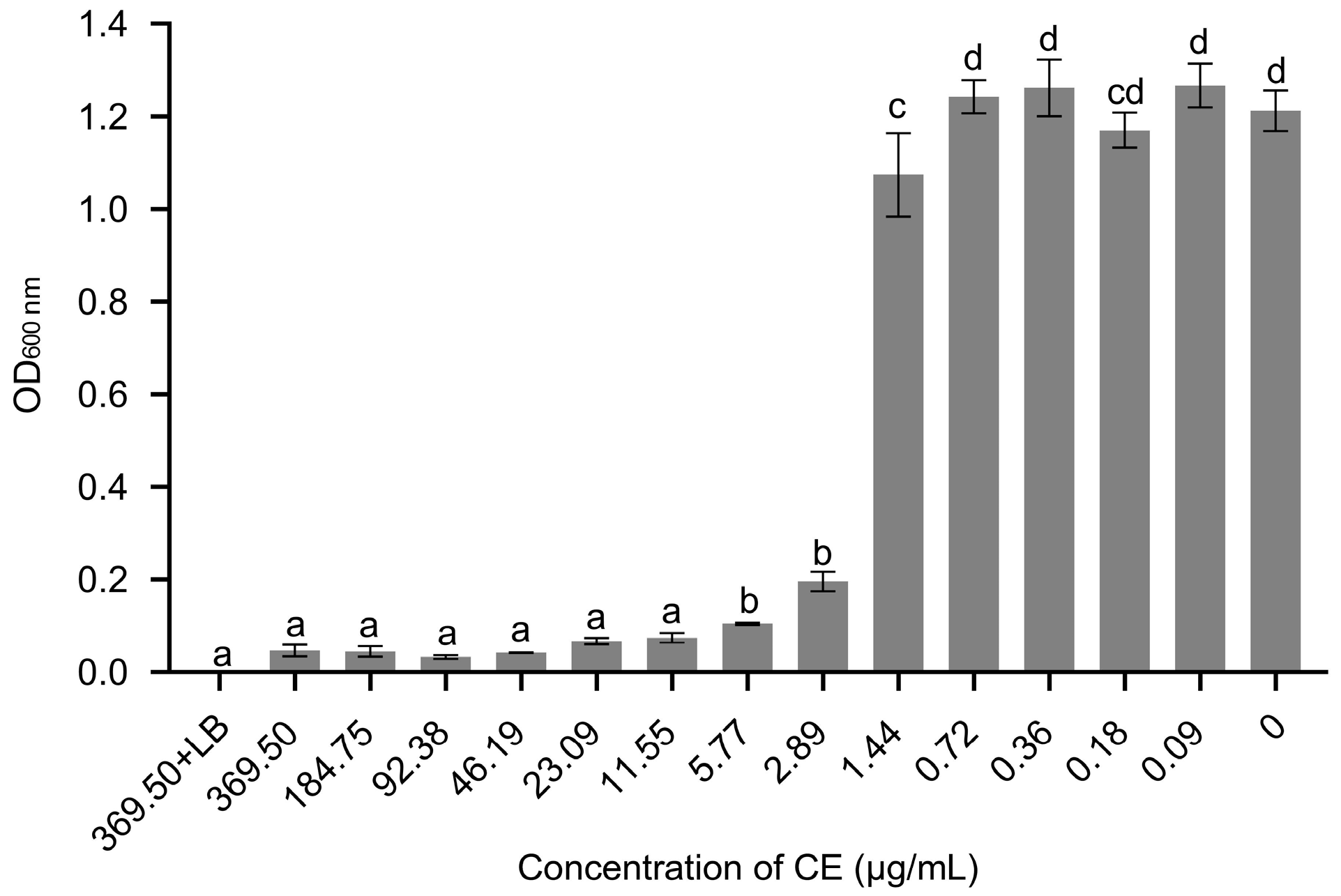
3.4. The MIC and MBC of Crude Extract (CE) against X. citri subsp. citri
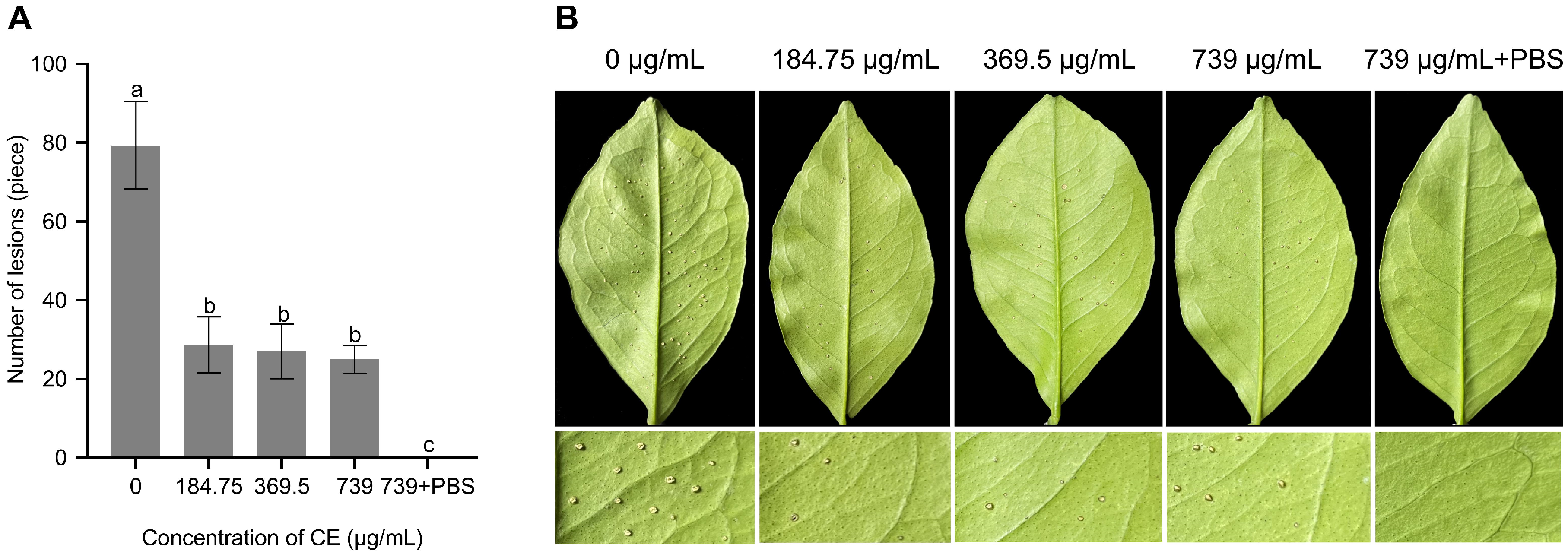
3.5. Evaluation of Biocontrol Efficiency of CE
3.6. CE Stability Analysis
3.7. Analysis of the Antibacterial Activity of the Purified CE
3.8. Identification of Antibacterial Components from CE3
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pitino, M.; Armstrong, C.M.; Duan, Y. Rapid screening for citrus canker resistance employing pathogen-associated molecular pattern-triggered immunity responses. Hortic. Res. 2015, 2, 15042. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, S.A.H.; Wang, J.; Malik, M.T.; Umar, U.-U.-D.; Ateeq-Ur-Rehman; Hasnain, A.; Sohail, M.A.; Shakeel, M.T.; Nauman, M.; Hafeez-ur-Rehman; et al. Citrus canker—distribution, taxonomy, epidemiology, disease cycle, pathogen biology, detection, and management: A critical review and future research agenda. Agronomy 2022, 12, 1075. [Google Scholar] [CrossRef]
- Behlau, F.; Hong, J.C.; Jones, J.B.; Graham, J.H. Evidence for acquisition of copper resistance genes from different sources in citrus-associated Xanthomonads. Phytopathology 2013, 103, 409–418. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, P.A. Biological control of plant diseases. Australas. Plant Path. 2017, 46, 293–304. [Google Scholar] [CrossRef]
- Caicedo, J.C.; Villamizar, S.; Ferro, M.I.T.; Kupper, K.C.; Ferro, J.A. Bacteria from the citrus phylloplane can disrupt cell–cell signalling in Xanthomonas citri and reduce citrus canker disease severity. Plant Pathol. 2016, 65, 782–791. [Google Scholar] [CrossRef]
- Rabbee, M.F.; Islam, N.; Baek, K.-H. Biocontrol of citrus bacterial canker caused by Xanthomonas citri subsp. citri by Bacillus velezensis. Saudi J. Biol. Sci. 2022, 29, 2363–2371. [Google Scholar] [CrossRef] [PubMed]
- Wattana-Amorn, P.; Charoenwongsa, W.; Williams, C.; Crump, M.P.; Apichaisataienchote, B. Antibacterial activity of cyclo(L-Pro-L-Tyr) and cyclo(D-Pro-L-Tyr) from Streptomyces sp. strain 22-4 against phytopathogenic bacteria. Nat. Prod. Res. 2016, 30, 1980–1983. [Google Scholar] [CrossRef]
- Gholami, D.; Aminzadeh, S.; Alavi, S.M.; Kazemipour, N.; Ghoroghi, A.; Emruzi, Z. Comparison of antibiotics and bacteriocins antibacterial activity on Xanthomonas citri subsp. citri. Iran. J. Fish. Sci. 2018, 17, 162–178. [Google Scholar]
- Eid, A.M.; Fouda, A.; Abdel-Rahman, M.A.; Salem, S.S.; Elsaied, A.; Oelmüller, R.; Hijri, M.; Bhowmik, A.; Elkelish, A.; Hassan, S.E.-D. Harnessing bacterial endophytes for promotion of plant growth and biotechnological applications: An overview. Plants 2021, 10, 935. [Google Scholar] [CrossRef]
- Daungfu, O.; Youpensuk, S.; Lumyong, S. Endophytic bacteria isolated from citrus plants for biological control of citrus canker in lime plants. Trop. Life Sci. Res. 2019, 30, 73–88. [Google Scholar] [CrossRef]
- Chen, K.; Tian, Z.; Luo, Y.; Cheng, Y.; Long, C.A. Antagonistic activity and the mechanism of Bacillus amyloliquefaciens DH-4 against citrus green mold. Phytopathology 2018, 108, 1253–1262. [Google Scholar] [CrossRef]
- Bashan, Y.; de-Bashan, L.E.; Prabhu, S.; Hernández, J.-P. Advances in plant growth-promoting bacterial inoculant technology: Formulations and practical perspectives (1998–2013). Plant Soil 2014, 378, 1–33. [Google Scholar] [CrossRef]
- Pellegrini, M.; Pagnani, G.; Bernardi, M.; Mattedi, A.; Spera, D.M.; Gallo, M.D. Cell-Free supernatants of plant growth-promoting bacteria: A review of their use as biostimulant and microbial biocontrol agents in sustainable agriculture. Sustainability 2020, 12, 9917. [Google Scholar] [CrossRef]
- Fira, D.; Dimkić, I.; Berić, T.; Lozo, J.; Stanković, S. Biological control of plant pathogens by Bacillus species. J. Biotechnol. 2018, 285, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, D.B.; Tounsi, S.; Gharsallah, H.; Hammami, A.; Frikha-Gargouri, O. Bacillus amyloliquefaciens strain 32a as a source of lipopeptides for biocontrol of Agrobacterium tumefaciens strains. J. Appl. Microbiol. 2015, 119, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Liu, T.; Zheng, Y.; Song, R.; Nan, T.; Yang, X.; Huang, L.; Yuan, Y. A mycorrhizal bacteria strain isolated from Polyporus umbellatus exhibits broad-spectrum antifungal activity. Front. Plant Sci. 2022, 13, 954160. [Google Scholar] [CrossRef]
- Li, Y.; Cai, Y.J.; Yu, Q.H.; Kong, S.L.; Zhou, X.Q.; Zhang, Y.H. Citrus bacterial canker disease: Pathogen identification and bactericides screening. Chin. Agri. Sci. Bull. 2021, 37, 146–153. [Google Scholar]
- Zhang, J.; Gu, S.B.; Zhang, T.R.; Wu, Y.; Ma, J.L.; Zhao, L.; Li, X.; Zhang, J. Characterization and antibacterial modes of action of bacteriocins from Bacillus coagulans CGMCC 9951 against Listeria monocytogenes. LWT-Food Sci. Technol. 2022, 160, 113272. [Google Scholar] [CrossRef]
- Cappuccino, J.C.; Sherman, N. Microbiology: A Laboratory Manual, 3rd ed.; Benjamin-Cummings Pub. Co.: New York, NY, USA, 1991. [Google Scholar]
- Holt, J.G.; Krieg, N.R.; Sneath, P.H.A.; Staley, J.T.; Williams, S.T. Bergey’s Manual of Determinative Bacteriology, 9th ed.; Williams and Wilkins Pub.: Philadelphia, PA, USA, 1994. [Google Scholar]
- Ludwig, W. Nucleic acid techniques in bacterial systematics and identification. Int. J. Food Microbiol. 2007, 120, 225–236. [Google Scholar] [CrossRef]
- Chun, J.; Bae, K.S. Phylogenetic analysis of Bacillus subtilis and related taxa based on partial gyrA gene sequences. Anton. Leeuw. Int. J. G. 2000, 78, 123–127. [Google Scholar] [CrossRef]
- Feng, D.; Zhang, A.; Yang, Y.; Yang, P. Coumarin-containing hybrids and their antibacterial activities. Arch. Pharm. 2020, 353, e1900380. [Google Scholar] [CrossRef] [PubMed]
- Li, S.B.; Fang, M.; Zhou, R.C.; Huang, J. Characterization and evaluation of the endophyte Bacillus B014 as a potential biocontrol agent for the control of Xanthomonas axonopodis pv. dieffenbachiae—Induced blight of Anthurium. Biol. Control 2012, 63, 9–16. [Google Scholar] [CrossRef]
- Wang, N.N.; Yan, X.; Gao, X.N.; Niu, H.J.; Kang, Z.S.; Huang, L.L. Purification and characterization of a potential antifungal protein from Bacillus subtilis E1R-J against Valsa mali. World J. Microb. Biotechnol. 2016, 32, 63. [Google Scholar] [CrossRef]
- Im, S.M.; Yu, N.H.; Joen, H.W.; Kim, S.O.; Park, H.W.; Park, A.R.; Kim, J.-C. Biological control of tomato bacterial wilt by oxydifficidin and difficidin-producing Bacillus methylotrophicus DR-08. Pestic. Biochem. Physiol. 2020, 163, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Ying, T.T.; Wu, P.J.; Gao, L.L.; Wang, C.C.; Zhang, T.H.; Liu, S.S.; Huang, R.Q. Isolation and characterization of a new strain of Bacillus amyloliquefaciens and its effect on strawberry preservation. LWT-Food Sci. Technol. 2022, 165, 113712. [Google Scholar] [CrossRef]
- Yu, X.Y.; Armstrong, C.M.; Zhou, M.G.; Duan, Y.P. Bismerthiazol inhibits Xanthomonas citri subsp. citri growth and induces differential expression of citrus defense-related genes. Phytopathology 2016, 106, 693–701. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, A.G.; Spago, F.R.; Simionato, A.S.; Navarro, M.O.P.; da Silva, C.S.; Barazetti, A.R.; Cely, M.V.T.; Tischer, C.A.; San Martin, J.A.B.; de Jesus Andrade, C.G.T.; et al. Bioactive organocopper compound from Pseudomonas aeruginosa inhibits the growth of Xanthomonas citri subsp. citri. Front. Microbiol. 2016, 7, 113. [Google Scholar] [CrossRef]
- Ye, P.X.; Wang, J.W.; Liu, M.M.; Li, P.; Gu, Q. Purification and characterization of a novel bacteriocin from Lactobacillus paracasei ZFM54. LWT-Food Sci. Technol. 2021, 143, 111125. [Google Scholar] [CrossRef]
- Feng, C.L.; Lu, L.Z.; Liu, D.D.; Ning, Y.W.; Wang, Z.X. Purification, structure and characterization of the novel antimicrobial lipopeptides produced by Paenibacillus ehimensis HD. LWT-Food Sci. Technol. 2023, 177, 114603. [Google Scholar] [CrossRef]
- Tsugawa, H.; Satoh, A.; Uchino, H.; Cajka, T.; Arita, M.; Arita, M. Mass spectrometry data repository enhances novel metabolite discoveries with advances in computational metabolomics. Metabolites 2019, 9, 119. [Google Scholar] [CrossRef]
- Marin, V.R.; Ferrarezi, J.H.; Vieira, G.; Sass, D.C. Recent advances in the biocontrol of Xanthomonas spp. World J. Microb. Biotechnol. 2019, 35, 72. [Google Scholar] [CrossRef]
- Tariq, M.; Khan, A.; Asif, M.; Khan, F.; Ansari, T.; Shariq, M.; Siddiqui, M.A. Biological control: A sustainable and practical approach for plant disease management. Acta Agric. Scand. Sect. B Soil Plant Sci. 2020, 70, 507–524. [Google Scholar] [CrossRef]
- WoldemariamYohannes, K.; Wan, Z.; Yu, Q.L.; Li, H.Y.; Wei, X.T.; Liu, Y.L.; Wang, J.; Sun, B.G. Prebiotic, probiotic, antimicrobial, and functional food applications of Bacillus amyloliquefaciens. J. Agric. Food Chem. 2020, 68, 14709–14727. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Li, Q.; Xu, Z.H.; Zhang, N.; Shen, Q.R.; Zhang, R.F. Responses of beneficial Bacillus amyloliquefaciens SQR9 to different soilborne fungal pathogens through the alteration of antifungal compounds production. Front. Microbiol. 2014, 5, 636. [Google Scholar] [CrossRef]
- Torres, M.J.; Brandan, C.P.; Petroselli, G.; Erra-Balsells, R.; Audisio, M.C. Antagonistic effects of Bacillus subtilis subsp. subtilis and B. amyloliquefaciens against Macrophomina phaseolina: SEM study of fungal changes and UV-MALDI-TOF MS analysis of their bioactive compounds. Microbiol. Res. 2016, 182, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Rabbee, M.F.; Ali, M.S.; Baek, K.-H. Endophyte Bacillus velezensis isolated from Citrus spp. controls streptomycin-resistant Xanthomonas citri subsp. citri that causes citrus bacterial canker. Agronomy 2019, 9, 470. [Google Scholar] [CrossRef]
- Nugroho, Y.A.; Suharjono, S.; Widyaningsih, S. Biological control of citrus canker pathogen Xanthomonas citri subsp. citri using Rangpur lime endophytic bacteria. Egypt. J. Biol. Pest Control 2022, 32, 63. [Google Scholar] [CrossRef]
- Wang, X.; Liang, L.Q.; Shao, H.; Ye, X.X.; Yang, X.B.; Chen, X.Y.; Shi, Y.; Zhang, L.H.; Xu, L.H.; Wang, J.X. Isolation of the novel strain Bacillus amyloliquefaciens F9 and identification of lipopeptide extract components responsible for activity against Xanthomonas citri subsp. citri. Plants 2022, 11, 457. [Google Scholar] [CrossRef]
- Lin, H.T.; Liang, Y.; Kaliaperumal, K.; Xiong, Q.; Duan, S.; Jiang, Y.M.; Zhang, J. Linoleic acid from the endophytic fungus Diaporthe sp. HT-79 inhibits the growth of Xanthomonas citri subsp. citri by destructing the cell membrane and producing reactive oxygen species (ROS). Pestic. Biochem. Physiol. 2023, 192, 105423. [Google Scholar] [CrossRef]
- Sreedharan, S.M.; Rishi, N.; Singh, R. Microbial lipopeptides: Properties, mechanics and engineering for novel lipopeptides. Microbiol. Res. 2023, 271, 127363. [Google Scholar] [CrossRef]
- Zhang, D.J.; Liu, R.F.; Li, Y.G.; Tao, L.M.; Tian, L. Two new antifungal cyclic lipopeptides from Bacillus marinus B-9987. Chem. Pharm. Bull. 2010, 58, 1630–1634. [Google Scholar] [CrossRef] [PubMed]
- Thevissen, K.; Hillaert, U.; Meert, E.M.; Chow, K.K.; Cammue, B.P.; Van Calenbergh, S.; François, I.E. Fungicidal activity of truncated analogues of dihydrosphingosine. Bioorg. Med. Chem. Lett. 2008, 18, 3728–3730. [Google Scholar] [CrossRef] [PubMed]
- Cushnie, T.P.; Cushnie, B.; Lamb, A.J. Alkaloids: An overview of their antibacterial, antibiotic-enhancing and antivirulence activities. Int. J. Antimicrob. Agents 2014, 44, 377–386. [Google Scholar] [CrossRef] [PubMed]
- do Nascimento, P.G.; Lemos, T.L.; Almeida, M.C.; de Souza, J.M.; Bizerra, A.M.; Santiago, G.M.; da Costa, J.G.; Coutinho, H.D. Lithocholic acid and derivatives: Antibacterial activity. Steroids 2015, 104, 8–15. [Google Scholar] [CrossRef]
- Santhakumari, S.; Jayakumar, R.; Logalakshmi, R.; Prabhu, N.M.; Abdul Nazar, A.K.; Karutha Pandian, S.; Veera Ravi, A. In vitro and in vivo effect of 2,6-Di-tert-butyl-4-methylphenol as an antibiofilm agent against quorum sensing mediated biofilm formation of Vibrio spp. Int. J. Food Microbiol. 2018, 281, 60–71. [Google Scholar] [CrossRef]
- Mrudulakumari Vasudevan, U.; Lee, E.Y. Flavonoids, terpenoids, and polyketide antibiotics: Role of glycosylation and biocatalytic tactics in engineering glycosylation. Biotechnol. Adv. 2020, 41, 107550. [Google Scholar] [CrossRef]
- Sahoo, C.R.; Sahoo, J.; Mahapatra, M.; Lenka, D.; Sahu, P.K.; Dehury, B.; Padhy, R.N.; Paidesetty, S.K. Coumarin derivatives as promising antibacterial agent(s). Arab. J. Chem. 2021, 14, 102922. [Google Scholar] [CrossRef]
- Xie, L.G.; Zhu, G.D.; Shang, J.J.; Chen, X.M.; Zhang, C.T.; Ji, X.L.; Zhang, Q.; Wei, Y. An overview on the biological activity and anti-cancer mechanism of lovastatin. Cell. Signal. 2021, 87, 110122. [Google Scholar] [CrossRef]


| Characteristics | Results | Characteristics | Results |
|---|---|---|---|
| Catalase activity | + | D-mannose | + |
| Starch hydrolysis | + | D-xylose | + |
| Voges–Proskauer | + | D-Glucose | + |
| Methyl red | + | Sucrose | + |
| Gelatin liquefaction | + | D-galactose | + |
| Phenylalanine deaminase | − | Salt tolerance test with 0.1% NaCl | + |
| Anaerobic culture | − | Salt tolerance test with 5% NaCl | + |
| 3 °C growth | − | Salt tolerance test with 10% NaCl | + |
| Sorbitol | + | Salt tolerance test with 20% NaCl | − |
| CFS (µL) | X. citri subsp. citri | |
|---|---|---|
| Inhibition Diameter (mm) 1,2 | Inhibition Rate (%) 1,2 | |
| 75 | 22.67 ± 0.38 b | 25.19 ± 0.01 b |
| 175 | 24.38 ± 0.69 ab | 27.08 ± 0.01 ab |
| 275 | 24.94 ± 0.31 a | 27.71 ± 0.00 a |
| CFS (µL) | Inhibition Rate (%) 1,2 | ||||
|---|---|---|---|---|---|
| Foc4 | N. dimidiatum | N. oryzae | F. solani | S. oryzae | |
| 150 | 17.41 ± 0.48 d | 2.90 ± 1.09 d | 4.25 ± 0.15 e | 1.97 ± 0.55 e | 6.35 ± 0.19 e |
| 300 | 21.94 ± 0.36 c | 8.52 ± 0.42 c | 7.89 ± 0.09 d | 6.33 ± 0.51 d | 9.35 ± 0.03 d |
| 600 | 25.10 ± 0.64 b | 10.93 ± 1.76 c | 17.62 ± 0.58 c | 11.12 ± 0.43 c | 17.15 ± 1.05 c |
| 1200 | 25.82 ± 0.72 b | 24.29 ± 0.32 b | 40.66 ± 0.20 b | 42.13 ± 0.65 b | 30.22 ± 0.45 b |
| 2400 | 33.72 ± 0.67 a | 40.32 ± 0.13 a | 63.75 ± 0.76 a | 53.86 ± 1.62 a | 39.79 ± 1.08 a |
| Group | Metabolite | Ion Type | Measured (m/z) | Molecular Formula | Peak Area |
|---|---|---|---|---|---|
| Fatty acids | Palmitic acid | [M−H]− | 255.2428 | C16H32O2 | 7,943,267.400 |
| Pentadecanoic acid | [M−H]− | 241.2269 | C15H30O2 | 527,287.553 | |
| Linoleic acid | [M−H]− | 279.2437 | C18H32O2 | 324,303.341 | |
| Petroselinic acid | [M−H]− | 281.2605 | C18H34O2 | 284,596.411 | |
| Myristic acid | [M−H]− | 277.2086 | C14H28O2 | 259,333.257 | |
| Docosahexanoic acid | [M−H]− | 327.2402 | C22H32O2 | 72,556.094 | |
| α-Linolenic acid | [M−H]− | 277.2271 | C18H30O2 | 32,681.762 | |
| Lipopeptides | Surfactin C | [M+H]+ | 1036.687 | C53H93N7O13 | 505,074.195 |
| Surfactin B | [M+Na]+ | 1044.651 | C52H91N7O13 | 70,432.186 | |
| Surfactin C | [M+Na]+ | 1058.671 | C53H93N7O13 | 69,917.200 | |
| Surfactin A | [M+H]+ | 1008.656 | C51H89N7O13 | 63,120.98 | |
| Maribasin B | [M+H]+ | 1057.564 | C49H76N12O14 | 37,023.161 | |
| Sphingosine | Dihydrosphingosine | [M+H]+ | 302.3047 | C18H39NO2 | 411,920.009 |
| Phytosphingosine | [M+H]+ | 318.2997 | C18H39NO3 | 247,879.301 | |
| Phenols | 2,6-di-tert-butyl-4-methylphenol | [M−H]− | 219.1839 | C15H24O | 563,053.549 |
| 5-caffeoylquinic acid | [M+Na]+ | 377.0894 | C16H18O9 | 20,313.456 | |
| Flavonoids | Procyanidin A1 | [M+H]+ | 577.1314 | C30H24O12 | 219,703.147 |
| Glabrol | [M+H]+ | 393.2101 | C25H28O4 | 92,746.922 | |
| Genistein | [M+H]+ | 271.0595 | C15H10O5 | 25,979.231 | |
| Alkaloids | Agelasine | [M+NH4]+ | 475.3233 | C26H40ClN5 | 214,877.055 |
| Vedelianin | [M+H]+ | 481.2607 | C29H36O6 | 72,421.615 | |
| Okaramine J | [M+H]+ | 525.2859 | C32H36N4O3 | 49,245.988 | |
| Steroids | Lithocholic acid | [M−H]− | 375.2863 | C24H40O3 | 304,811.408 |
| Cinnamic acid | Cinnamaldehyde | [M+H]+ | 133.0651 | C9H8O | 35,660.410 |
| 4-methoxycinnamic acid | [M−H]− | 177.0638 | C10H10O3 | 117,098.160 | |
| Terpenoids | Cucurbitacin B | [M+Na]+ | 581.3050 | C32H46O8 | 28,239.383 |
| Ginsenoside Rk2 | [M+H]+ | 605.4328 | C36H60O7 | 26,324.767 | |
| Esters | Lovastatin | [M+Na]+ | 427.2459 | C24H36O5 | 19,164.271 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ke, X.; Wu, Z.; Liu, Y.; Liang, Y.; Du, M.; Li, Y. Isolation, Antimicrobial Effect and Metabolite Analysis of Bacillus amyloliquefaciens ZJLMBA1908 against Citrus Canker Caused by Xanthomonas citri subsp. citri. Microorganisms 2023, 11, 2928. https://doi.org/10.3390/microorganisms11122928
Ke X, Wu Z, Liu Y, Liang Y, Du M, Li Y. Isolation, Antimicrobial Effect and Metabolite Analysis of Bacillus amyloliquefaciens ZJLMBA1908 against Citrus Canker Caused by Xanthomonas citri subsp. citri. Microorganisms. 2023; 11(12):2928. https://doi.org/10.3390/microorganisms11122928
Chicago/Turabian StyleKe, Xinru, Zilin Wu, Yucheng Liu, Yonglin Liang, Manling Du, and Ya Li. 2023. "Isolation, Antimicrobial Effect and Metabolite Analysis of Bacillus amyloliquefaciens ZJLMBA1908 against Citrus Canker Caused by Xanthomonas citri subsp. citri" Microorganisms 11, no. 12: 2928. https://doi.org/10.3390/microorganisms11122928
APA StyleKe, X., Wu, Z., Liu, Y., Liang, Y., Du, M., & Li, Y. (2023). Isolation, Antimicrobial Effect and Metabolite Analysis of Bacillus amyloliquefaciens ZJLMBA1908 against Citrus Canker Caused by Xanthomonas citri subsp. citri. Microorganisms, 11(12), 2928. https://doi.org/10.3390/microorganisms11122928




