In Vitro Leishmanicidal Activity of Copaiba Oil and Kojic Acid Combination on the Protozoan Leishmania (Leishmania) amazonensis and Host Cell
Abstract
:1. Introduction
2. Materials and Methods
2.1. Obtaining and Dilution of the Copaíba Oil and Kojic Acid
2.2. Tests with the Host Cell
2.2.1. Peritoneal Macrophage Culture
2.2.2. Macrophage Treatment and Cytotoxic Analysis
2.3. Macrophage Microbicidal Response after Treatment
2.3.1. Detection of Reactive Oxygen Species (ROS) Production
2.3.2. Indirect Nitric Oxide (NO) Production
2.4. Tests with Leishmania (Leishmania) amazonensis
2.4.1. Cultivation and Maintenance
2.4.2. Antipromastigote Assay
2.4.3. Microscopy Analysis
2.4.4. ROS Detection
2.4.5. Apoptosis/Necrosis Detection by Flow Cytometry
2.5. Interaction: Host Cell and Protozoan
2.5.1. Antiamastigote Assay
2.5.2. Indirect Nitric Oxide (NO) Production
2.5.3. Cytokine Profile Analysis
2.6. Statistical Analysis
3. Results
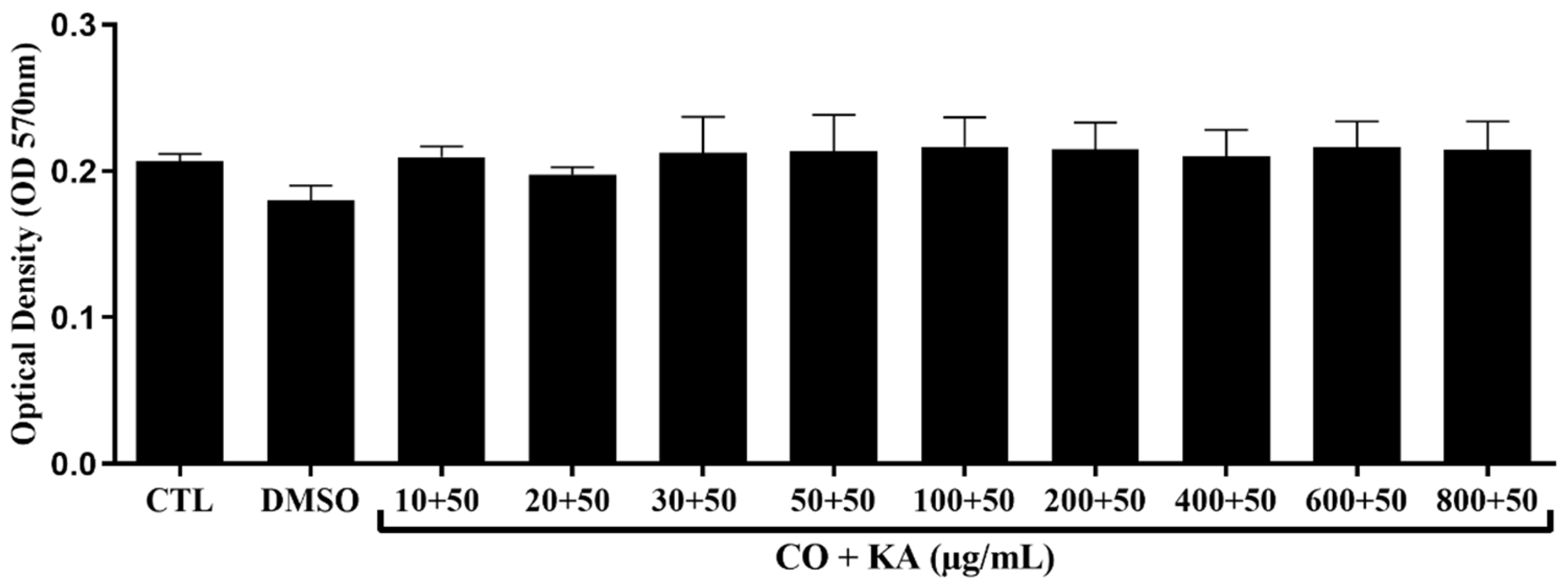
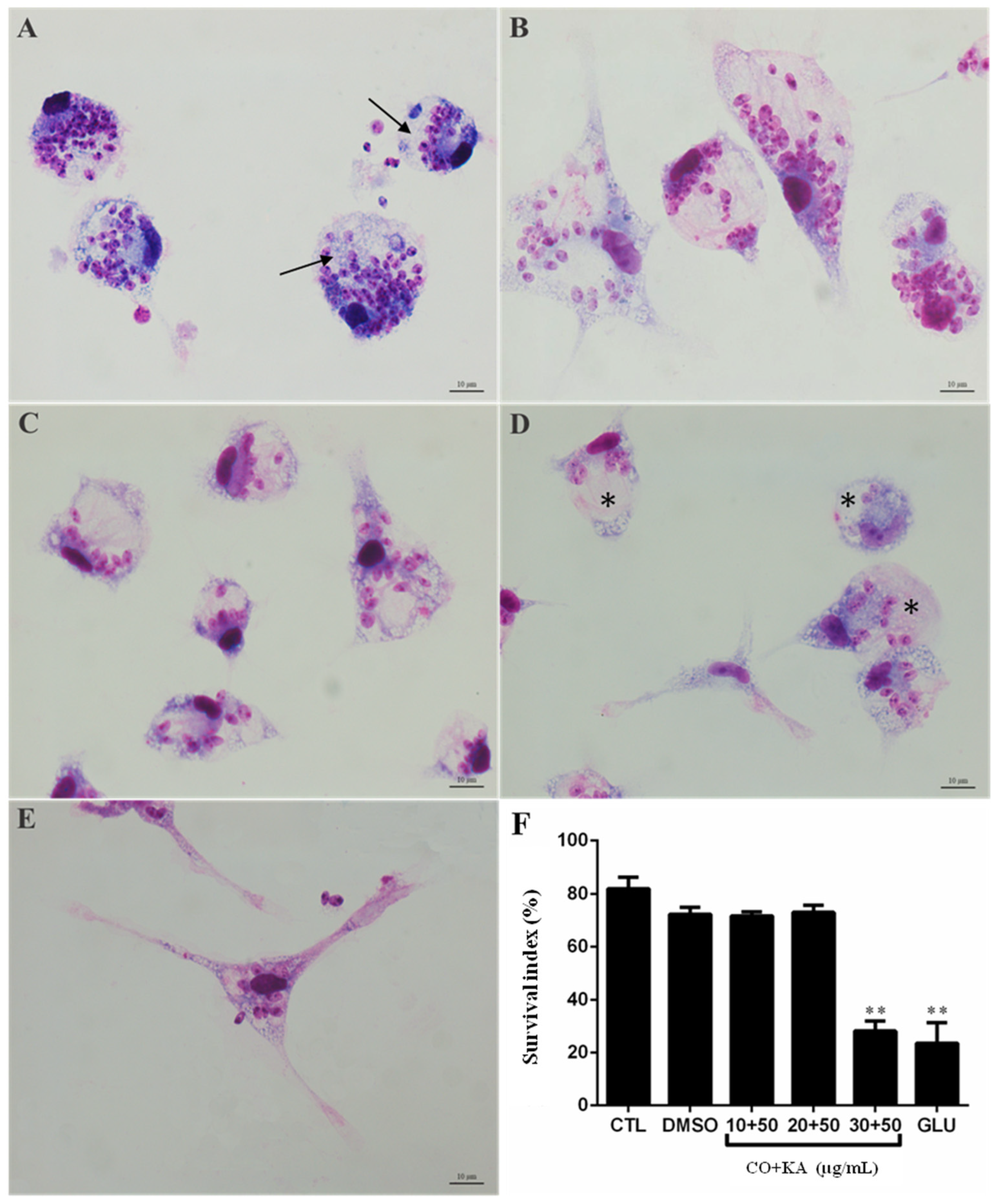
3.1. Macrophage Viability following Combination Treatment
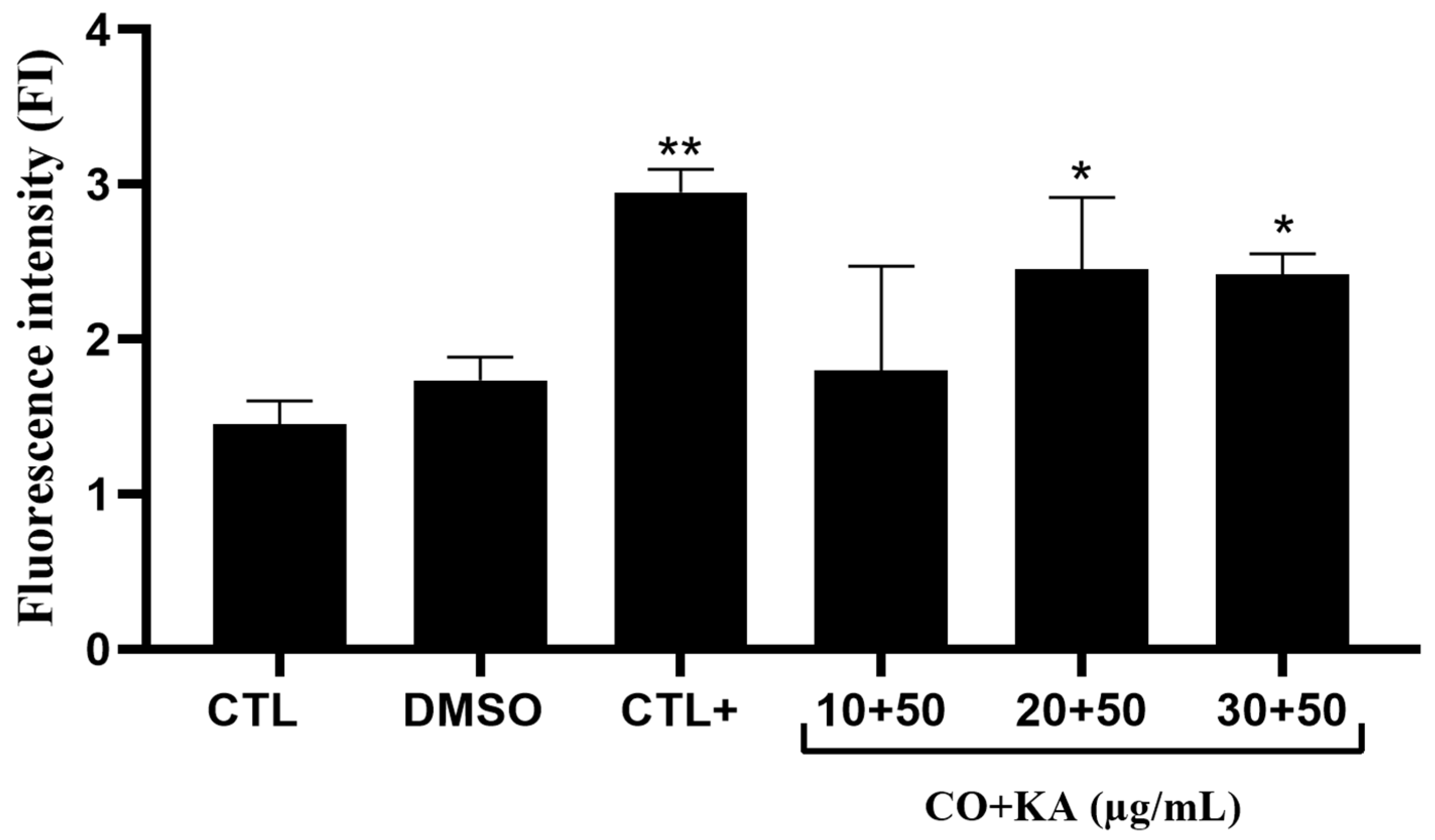
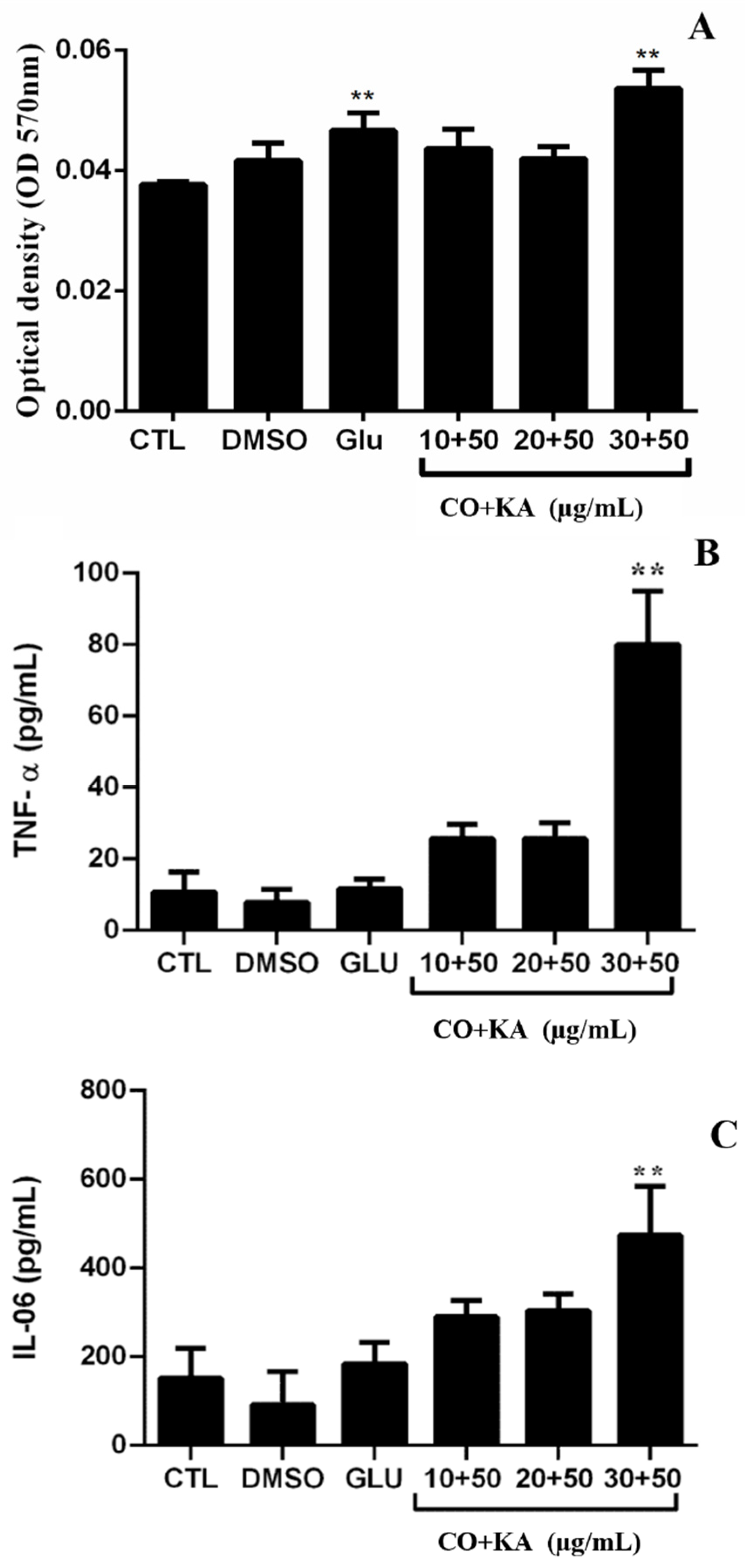
3.2. Microbicide Response of Macrophages
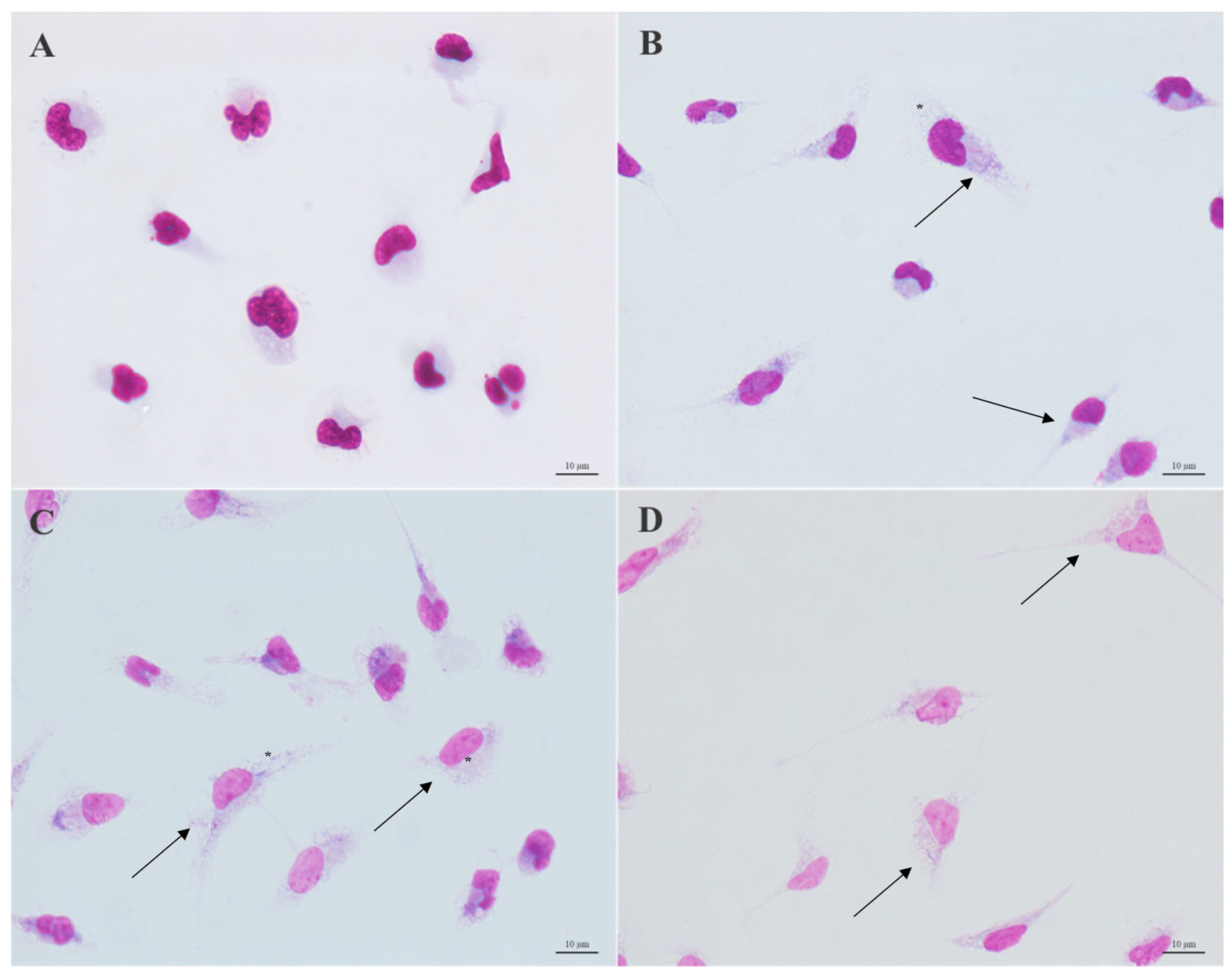
3.3. Morphological Analysis
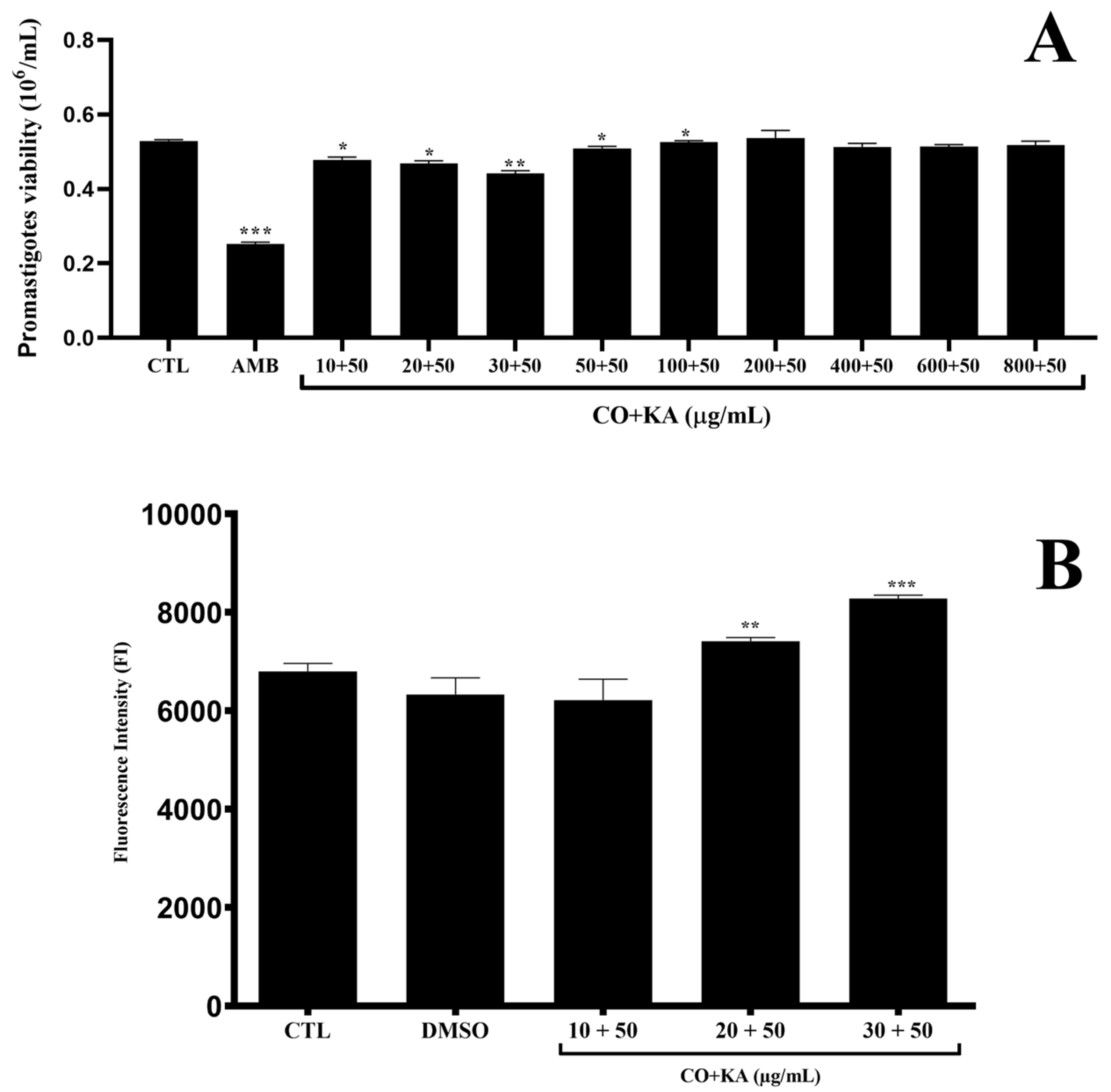
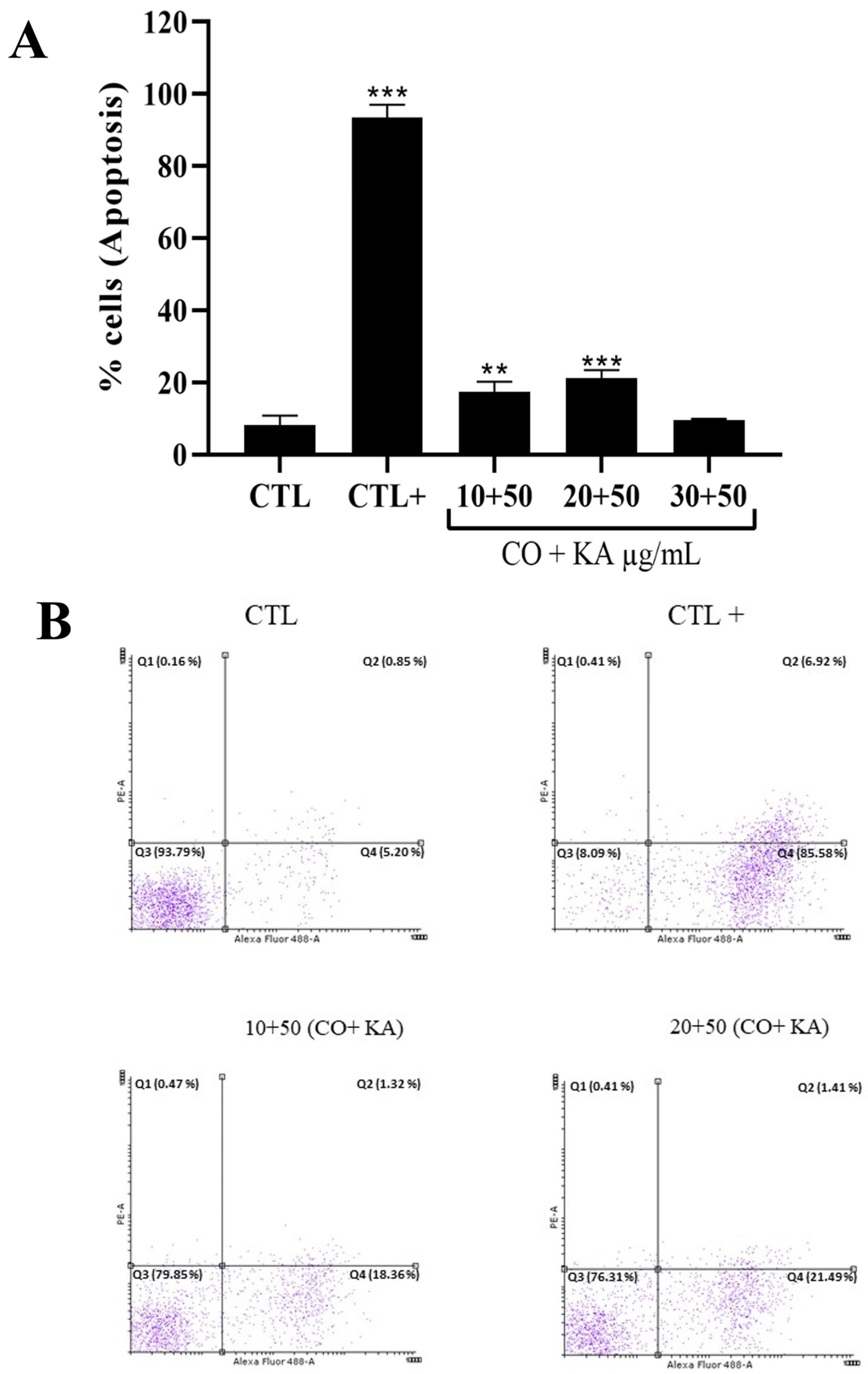
3.4. Combination Promoted Antipromastigote Activity and Reactive Oxygen Species Production, Significant Morphological Alterations and Cell Death by Initial Apoptosis
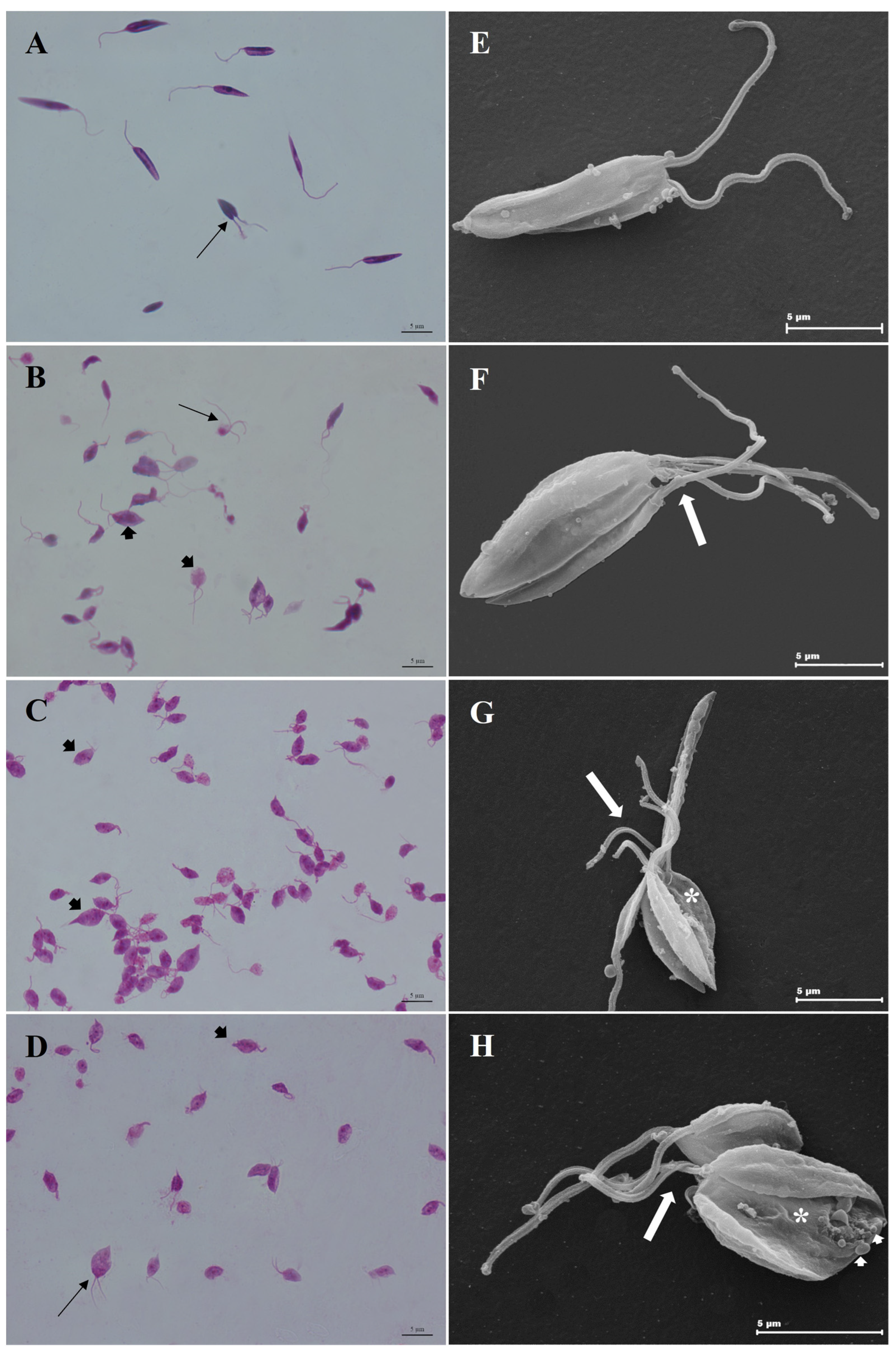
3.5. Combination of CO and KA Promoted Ultrastructural Alterations in Promastigotes
3.6. Combination Promotes Reduction in Amastigote Forms of Protozoan Leishmania (L.) amazonensis, after Interaction
3.7. Immunomodulatory Effect on Infected Macrophages
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- PAHO. Pan American Health Organization (2022). Leishmaniasis. Key Facts. Updated January 2022. Available online: https://www.paho.org/en/topics/leishmaniasis (accessed on 22 September 2023).
- WHO. World Health Organization (2023). Control of the Leishmaniases. Fact Sheets. Updated January 2023. Available online: http://www.who.int/mediacentre/factsheets/fs375/en/ (accessed on 22 September 2023).
- BRASIL. Ministério da Saúde. Secretaria de Vigilância em Saúde. Departamento de Vigilância Epidemiológica. Casos de Leishmaniose Tegumentar. Brasil, Grandes Regiões e Unidades Federadas. 1990 a 2020 Brasília/DF, 202. Available online: https://www.gov.br/saude/pt-br/media/pdf/2021/novembro/17/lt-casos.pdf (accessed on 20 September 2023).
- De Vries, H.J.; Schallig, H.D. Cutaneous leishmaniasis: A 2022 updated narrative review into diagnosis and management developments. Am. J. Clin. Dermatol. 2022, 23, 823–840. [Google Scholar] [CrossRef]
- Loría-Cervera, E.N.; Andrade-Narvaez, F. The role of monocytes/macrophages in Leishmania infection: A glance at the human response. Acta Trop. 2020, 207, 105456. [Google Scholar] [CrossRef]
- Cruz, A.K.; Freitas-Castro, F. Genome and transcriptome analyses of Leishmania spp.: Opening Pandora’s box. Curr. Opin. Microbiol. 2019, 52, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Costa-da-Silva, A.C.; Nascimento, D.O.; Ferreira, J.R.M.; Pinto, K.G.; Freire-de-Lima, L.; Morrot, A.; Decote-Ricardo, D.; Filardy, A.A.; Freire-de-Lima, G. Immune Response in Leishmaniasis: An Overview. Trop. Med. Infect. Dis. 2022, 7, 54. [Google Scholar] [CrossRef] [PubMed]
- Gurjar, D.; Patra, S.K.; Bodhale, N.; Lenka, N.; Saha, B. Leishmania intercepts IFN-γR signaling at multiple levels in macrophages. Cytokine 2022, 157, 155956. [Google Scholar] [CrossRef] [PubMed]
- Tomiotto-Pellissier, F.; Bortoleti, B.T.D.S.; Assolini, J.P.; Gonçalves, M.D.; Carloto, A.C.M.; Miranda-Sapla, M.M.; Conchon-Costa, I.; Bordignon, J.; Pavanelli, W.R. Macrophage Polarization in Leishmaniasis: Broadening Horizons. Front. Immunol. 2018, 9, 225–229. [Google Scholar] [CrossRef] [PubMed]
- BRASIL. Manual de Vigilância da Leishmaniose Tegumentar Americana. Série A. Normas e Manuais Técnicos. Brasília: Editora MS, 2ª Ed. Atualizada, 2018. Available online: http://bvsms.saude.gov.br/bvs/publicacoes/manual_vigilancia_leishmaniosetegumentar_americana.pdf (accessed on 20 September 2023).
- Passero, L.F.; Cruz, L.A.; Santos-Gomes, G.; Rodrigues, E.; Laurenti, M.D.; Lago, J.H.G. Conventional versus natural alternative treatments for leishmaniasis: A review. Curr. Top. Med. Chem. 2018, 18, 1275–1286. [Google Scholar] [CrossRef]
- Torres-Guerrero, E.; Quintanilla-Cedillo, M.R.; Ruiz-Esmenjaud, J.; Arenas, R. Leishmaniasis: A review. F1000Research 2017, 6, 750. [Google Scholar] [CrossRef]
- Ameen, M. Cutaneous leishmaniasis: Advances in disease pathogenesis, diagnostics and therapeutics. Cli. Exp. Derm. 2010, 35, 699–705. [Google Scholar] [CrossRef]
- Zulfiqar, B.; Shelper, T.B.; Avery, V.M. Leishmaniasis drug discovery: Recent progress and challenges in assay development. Drug Discov. Today 2017, 10, 1516–1531. [Google Scholar] [CrossRef]
- Da Silva, B.J.M.; Hage, A.A.P.; Silva, E.O.; Rodrigues, A.P.D. Medicinal plants from the Brazilian Amazonian region and their antileishmanial activity: A review. J. Integ. Med. 2018, 16, 211–222. [Google Scholar] [CrossRef]
- Moraes, L.S.; Donza, M.R.H.; Rodrigues, A.P.D.; Silva, B.J.M.; Brasil, D.S.B.; Zoghbi, M.G.B.; Andrade, E.H.A.; Guilhon, G.M.S.P.; Silva, E.O. Leishmanicidal Activity of (+) Phyllanthidine and the Phytochemical Profile of Margaritaria nobilis (Phyllanthaceae). Molecules 2015, 20, 22157–22169. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, R.R.P.; Da Silva, B.J.M.; Rodrigues, A.P.D.; Farias, L.H.S.; Da Silva, M.N.; Alves, D.T.V.; Bastos, G.N.T.; Do Nascimento, J.L.M.; Silva, E.O. In vitro biological action of aqueous extract from roots of Physalis angulata against Leishmania (Leishmania) amazonensis. BMC Comp. Alt. Med. 2015, 15, 249. [Google Scholar] [CrossRef] [PubMed]
- Parvez, S.; Kang, M.; Chung, H.S.; Cho, C.; Hong, M.C.; Shin, M.K.; Bae, H. Survey and mechanism of skin depigmenting and lightening agents. Phytother. Res. 2006, 20, 921–934. [Google Scholar] [CrossRef] [PubMed]
- Jacquel, A.; Obba, S.; Boyer, L.; Dufies, M.; Robert, G.; Gounon, P.; Auberger, P. Autophagy is required for CSF-1–induced macrophagic differentiation and acquisition of phagocytic functions. Blood J. Amer. Soc. Hematol. 2012, 119, 4527–4531. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.P.D.; Carvalho, A.S.; Santos, A.S.; Alves, C.N.; Nascimento, J.L.; Silva, E.O. Kojic acid, a secondary metabolite from sp., acts as an inducer of macrophage activation. Cell Biol. Int. 2011, 1, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.P.D.; Farias, L.H.S.; Carvalho, A.S.C.; Santos, A.S.; Nascimento, J.L.M.; Silva, E.O. A Novel Function for Kojic Acid, a Secondary Metabolite from Aspergillus Fungi, as Antileishmanial Agent. PLoS ONE 2014, 9, e91259. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.O.; Ueda-Nakamura, T.; Dias Filho, B.P.; Veiga Junior, V.F.; Pinto, A.C.; Nakamura, C.V. Effect of Brazilian copaiba oils on Leishmania Amazonensis. J. Ethnopharmacol. 2008, 120, 204–208. [Google Scholar] [CrossRef]
- Herrero-Ja’uregui, C.; Casado, M.A.; Zoghbi, M.G.B.; Martins-da-Silva, R.C. Chemical Variability of Copaifera reticulata DUCKE Oleoresin. Chem. Biodivers. 2011, 8, 674–685. [Google Scholar] [CrossRef]
- Fotakis, G.; Timbrell, J.A. In vitro cytotoxicity assays: Comparison of LDH, neutral red, MTT and protein assay in hepatoma cell lines following exposure to cadmium chloride. Toxicol. Lett. 2006, 160, 171–177. [Google Scholar] [CrossRef]
- Ahn, H.Y.; Fairfull-Smith, K.E.; Morrow, B.J.; Lussini, V.; Kim, B.; Bondar, M.V.; Belfield, K.D. Two-photon fluorescence microscopy imaging of cellular oxidative stress using profluorescent nitroxides. J. Amer. Chem. Soc. 2012, 134, 4721–4730. [Google Scholar] [CrossRef] [PubMed]
- Antinarelli, L.M.R.; Dias, R.M.P.; Souza, I.O.; Lima, W.P.; Gameiro, J.; Silva, A.D.; Coimbra, E.S. 4-Aminoquinoline Derivatives as Potential Antileishmanial Agents. Chem. Biol. Drug Des. 2015, 86, 704–714. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, J.M.; Hirai, Y.; Hirai, H.; Hoffmann, K.F. Schistosome egbg production is dependent upon the activities of two developmentally regulated tyrosinases. FASEB J. 2007, 21, 823–835. [Google Scholar] [CrossRef]
- Srivastava, S.; Mishra, J.; Gupta, A.K.; Singh, A.; Shankar, P.; Singh, S. Laboratory confirmed miltefosine resistant cases of visceral leishmaniasis from India. Parasites Vectors 2017, 10, 49. [Google Scholar] [CrossRef]
- Yoo, D.S.; Lee, J.; Choi, S.S.; Rho, H.S.; Cho, D.H.; Shin, W.C.; Cho, J.Y. A modulatory effect of novel kojic acid derivatives on cancer cell proliferation and macrophage activation. Die Pharm. Int. J. Pharm. Sci. 2010, 65, 261–266. [Google Scholar]
- Saghaie, L.; Pourfarzam, M.; Fassihi, A.; Sartippour, B. Synthesis and tyrosinase inhibitory properties of some novel derivatives of kojic acid. Res. Pharm. Sci. 2013, 8, 233. [Google Scholar]
- Montazeri, M.; Emamid, S.; Asgarian-Omrane, H.; Azizif, S.; Sharifa, M.; Sarvia, S.; Rezaeia, F.; Sadeghia, M.; Gohardehia, S.; Daryani, A. In vitro and in vivo evaluation of kojic acid against Toxoplasma gondii in experimental models of acute toxoplasmosis. Exp. Parasitol. 2019, 200, 7–12. [Google Scholar] [CrossRef]
- Veiga Júnior, V.F.; Rosas, E.C.; Carvalho, M.V.; Henriques, M.G.M.O.; Pinto, A.C. Chemical composition and anti-inflammatory activity of copaíba oils from Copaifera cearensis Huber ex Ducke, Copaifera reticulata Ducke and Copaifera multijuga Hayne—A comparative study. J. Etnopharmacol. 2007, 112, 248–254. [Google Scholar] [CrossRef]
- Gomes-Silva, A.; Bittar, R.C.; Nogueira, R.S.; Amato, V.S.; Mattos, M.S.; Oliveira-Neto, M.P.; Coutinho, S.G.; Cruz, A.M. Can interferon-ɣ and interleukin-10 balance be associated with severity of human Leishmania (Viannia) braziliensis infection? Br. Soc. Immunol. Cli. Exp. Immun. 2007, 149, 440–444. [Google Scholar] [CrossRef]
- Soares, D.C.; Portella, N.A.; Ramos, M.F.; Siani, A.C.; Saraiva, E.M. Trans-β-Caryophyllene: An Effective Antileishmanial Compound Found in Commercial Copaiba Oil (Copaifera spp.). Evid. Based Complement. Altern. Med. 2013, 2013, 761323. [Google Scholar] [CrossRef]
- Robinson, J.M.; Seguchi, H.; Badwey, J.A. Active oxygen and nitrogen species in biology: From cytocidal agents or signaling intermediates. Histochem. Cell Biol. 2004, 122, 273–275. [Google Scholar] [CrossRef]
- Chacón-vargas, K.F.; Sánchez-Torres, L.E.; Chávez-González, M.L.; Adame-Gallegos, J.R.; Nevárez-Moorillón, G.V. Mexican Oregano (Lippia berlandieri Schauer and Poliomintha longiflora Gray) Essential Oils Induce Cell Death by Apoptosis in Leishmania (Leishmania) mexicana Promastigotes. Molecules 2022, 27, 5183. [Google Scholar] [CrossRef]
- Charras, G.T. A short history of blebbing. J. Microsc. 2008, 3, 466–478. [Google Scholar] [CrossRef] [PubMed]
- Do Campo, R.; Moreno, S.N. The acidocalcisome. Mol. Biochem. Parasitol. 2001, 114, 151–159. [Google Scholar] [CrossRef]
- Ruiz, F.A.; Rodrigues, C.O.; Do Campo, R. Rapid changes in polyphosphate content within acidocalcisomes in response to cell growth, differentiation, and environmental stress in Trypanosoma cruzi. J. Biol. Chem. 2001, 276, 26114–26121. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.O.; Ueda-Nakamura, T.; Dias Filho, B.P.; Veiga Junior, V.F.; Pinto, A.C.; Nakamura, C.V. Copaiba Oil: An Alternative to Development of New Drugs against Leishmaniasis. Evid. Based Complem. Alt. Med. 2011, 2012, 898419. [Google Scholar] [CrossRef] [PubMed]
- Naderer, T.; McConville, M.J. The Leishmania-macrophage interaction: A metabolic perspective. Cell. Microbiol. 2008, 10, 301–308. [Google Scholar] [CrossRef]
- Calixto, S.L.; Glanzmann, N.; Xavier Silveira, M.M.; Trindade Granato, J.; Gorza Scopel, K.K.; Torres De Aguiar, T.; Da Matta, R.A.; Macedo, G.C.; Da Silva, A.D.; Coimbra, E.S. Novel organic salts based on quinoline derivatives: The in vitro activity trigger apoptosis inhibiting autophagy in Leishmania spp. Chem. Biol. Interact. 2018, 293, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.A.M.; Mottram, J.C.; Coombs, G.H. Distinct roles in autophagy and importance in infectivity of the two ATG4 cysteine peptidases of Leishmania major. J. Biol. Chem. 2013, 288, 3678–3690. [Google Scholar] [CrossRef] [PubMed]
- Tavares, G.S.; Mendonça, D.V.; Lage, D.P.; Antinarelli, L.M.; Soyer, T.G.; Senna, A.J.; Matos, G.F.; Dias, D.S.; Ribeiro, P.A.F.; Batista, J.P.T.; et al. In vitro and in vivo antileishmanial activity of a fluoroquinoline derivate against Leishmania infantum and Leishmania amazonensis species. Acta Trop. 2019, 191, 29–37. [Google Scholar] [CrossRef]
- Da Silva, B.J.M.; Pereira, S.W.G.; Rodrigues, A.P.D.; Do Nascimento, J.L.M.; Silva, E.O. In vitro antileishmanial effects of Physalis angulata root extract on Leishmania infantum. J. Integ. Med. 2018, 16, 404–410. [Google Scholar] [CrossRef]
- Shadab, M.; Jha, B.; Asad, M.; Deepthi, M.; Kamran, M.; Ali, N. Apoptosis-like cell death in Leishmania donovani treated with KalsomeTM10, a new liposomal amphotericin B. PLoS ONE 2017, 12, e0171306. [Google Scholar] [CrossRef] [PubMed]
- Kian, D.; Lancheros, C.A.C.; Assolini, J.P.; Arakawa, N.S.; Veiga-Júnior, V.F.; Nakamura, C.V.; Pinge-Filho, P.; Conchon-Costa, I.; Pavanelli, W.R.; Yamada-Ogatta, S.F.; et al. Trypanocidal activity of copaiba oil and kaurenoic acid does not depend on macrophage killing machinery. Biomed. Pharmacother. 2018, 103, 1294–1301. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, J.P.; Rodrigues, A.P.D.; Farias, L.H.S.; Frade, P.C.R.; Da Silva, B.J.M.; Do Nascimento, J.L.M.; Silva, E.O. Biological effects of kojic acid on human monocytes in vitro. Biomed. Pharmacother. 2018, 101, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Mukbel, R.M.; Patten, C.J.R.; Gibson, K.; Ghosh, M.; Petersen, C.; Jones, D.E. Macrophage killing of Leishmania amazonensis amastigotes requires both nitric oxide and superoxide. Ame. J. Trop. Med. Hyg. 2007, 76, 669–675. [Google Scholar] [CrossRef]
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2009, 8, 958–969. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Uzonna, J.E. The early interaction of Leishmania with macrophages and dendritic cells and its influence on the host immune response. Front. Cell Inf. Microb. 2012, 2, 83. [Google Scholar] [CrossRef]
- Bian, Z.; Gong, Y.; Huang, T.; Lee, C.Z.W.; Bian, L.; Bai, Z.; Shi, H.; Zeng, Y.; Liu, C.; He, J.; et al. Deciphering human macrophage development at single-cell resolution. Nature 2020, 582, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Almeida, F.S.; Vanderley, S.E.R.; Comberlang, F.C.; Andrade, A.G.; Cavalcante-Silva, L.H.A.; Silva, E.D.S.; Palmeira, P.H.S.; Amaral, I.P.G.D.; Keesen, T.S.L. Leishmaniasis: Immune Cells Crosstalk in Macrophage Polarization. Trop. Med. Infect. Dis. 2023, 8, 276. [Google Scholar] [CrossRef] [PubMed]
- Santiago, K.B.; Conti, B.J.; Andrade, B.F.M.T.; Da Silva, J.J.M.; Rogez, H.L.G.; Crevelin, E.J.; Moraes, L.A.B.; Veneziani, R.; Ambrósio, S.R.; Bastos, J.K.; et al. Immunomodulatory action of Copaifera spp. oleoresins on cytokine production by human monocytes. Biomed. Pharmacother. 2015, 70, 12–18. [Google Scholar] [CrossRef]
- Guimarães, L.R.; Rodrigues, A.P.D.; Marinho, P.S.; Muller, A.H.; Guilhon, G.M.S.P.; Santos, L.S.; Nascimento, J.L.M.; Silva, E.O. Activity of the julocrotine, a glutarimide alkaloid from Croton pullei var. glabrior, on Leishmania (L.) amazonensis. Parasitol. Res. 2010, 107, 1075–1081. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moraes, L.S.d.; Galué-Parra, A.J.; Hage, A.A.P.; Moura, H.A.; Garcia, M.S.A.; Macêdo, C.G.; Rodrigues, A.P.D.; Guilhon, G.M.S.P.; Silva, E.O.d. In Vitro Leishmanicidal Activity of Copaiba Oil and Kojic Acid Combination on the Protozoan Leishmania (Leishmania) amazonensis and Host Cell. Microorganisms 2023, 11, 2925. https://doi.org/10.3390/microorganisms11122925
Moraes LSd, Galué-Parra AJ, Hage AAP, Moura HA, Garcia MSA, Macêdo CG, Rodrigues APD, Guilhon GMSP, Silva EOd. In Vitro Leishmanicidal Activity of Copaiba Oil and Kojic Acid Combination on the Protozoan Leishmania (Leishmania) amazonensis and Host Cell. Microorganisms. 2023; 11(12):2925. https://doi.org/10.3390/microorganisms11122925
Chicago/Turabian StyleMoraes, Lienne Silveira de, Adan Jesús Galué-Parra, Amanda Anastácia Pinto Hage, Hévila Aragão Moura, Marcus Savio Araujo Garcia, Caroline Gomes Macêdo, Ana Paula Drummond Rodrigues, Giselle Maria Skelding Pinheiro Guilhon, and Edilene Oliveira da Silva. 2023. "In Vitro Leishmanicidal Activity of Copaiba Oil and Kojic Acid Combination on the Protozoan Leishmania (Leishmania) amazonensis and Host Cell" Microorganisms 11, no. 12: 2925. https://doi.org/10.3390/microorganisms11122925
APA StyleMoraes, L. S. d., Galué-Parra, A. J., Hage, A. A. P., Moura, H. A., Garcia, M. S. A., Macêdo, C. G., Rodrigues, A. P. D., Guilhon, G. M. S. P., & Silva, E. O. d. (2023). In Vitro Leishmanicidal Activity of Copaiba Oil and Kojic Acid Combination on the Protozoan Leishmania (Leishmania) amazonensis and Host Cell. Microorganisms, 11(12), 2925. https://doi.org/10.3390/microorganisms11122925





