Recombinant p40 Protein Promotes Expression of Occludin in HaCaT Keratinocytes: A Brief Communication
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Growth Conditions
2.2. DNA Extraction
2.3. Cloning, Expression, and Purification of p40
2.4. Cell Culture and Cell Treatment
2.5. MTT Assay
2.6. LDH Assay
2.7. TEER
2.8. Western Blot
2.9. Statistics
3. Results
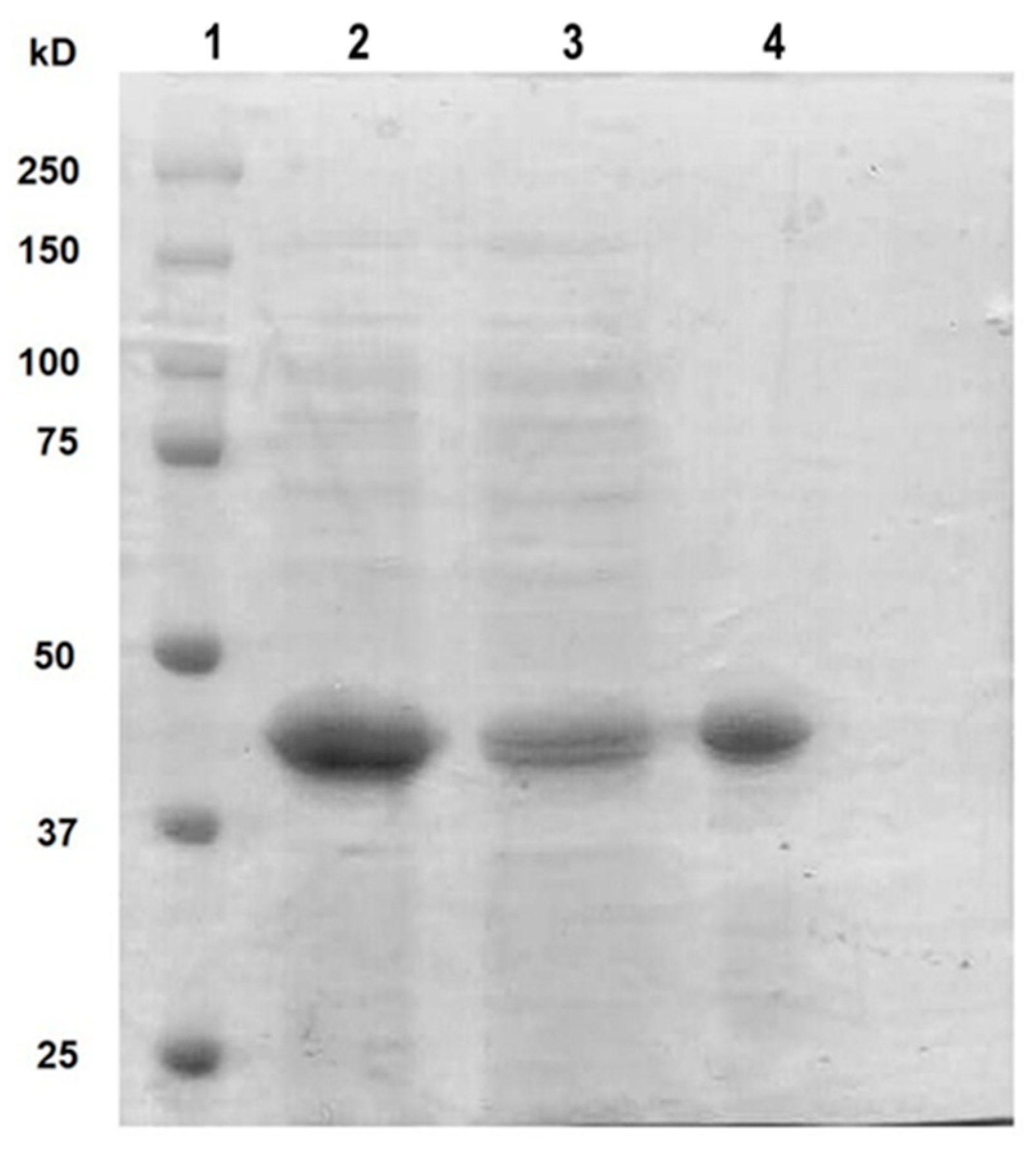
3.1. Cloning and Purification of rt-p40 from L. rhamnosus GR-1
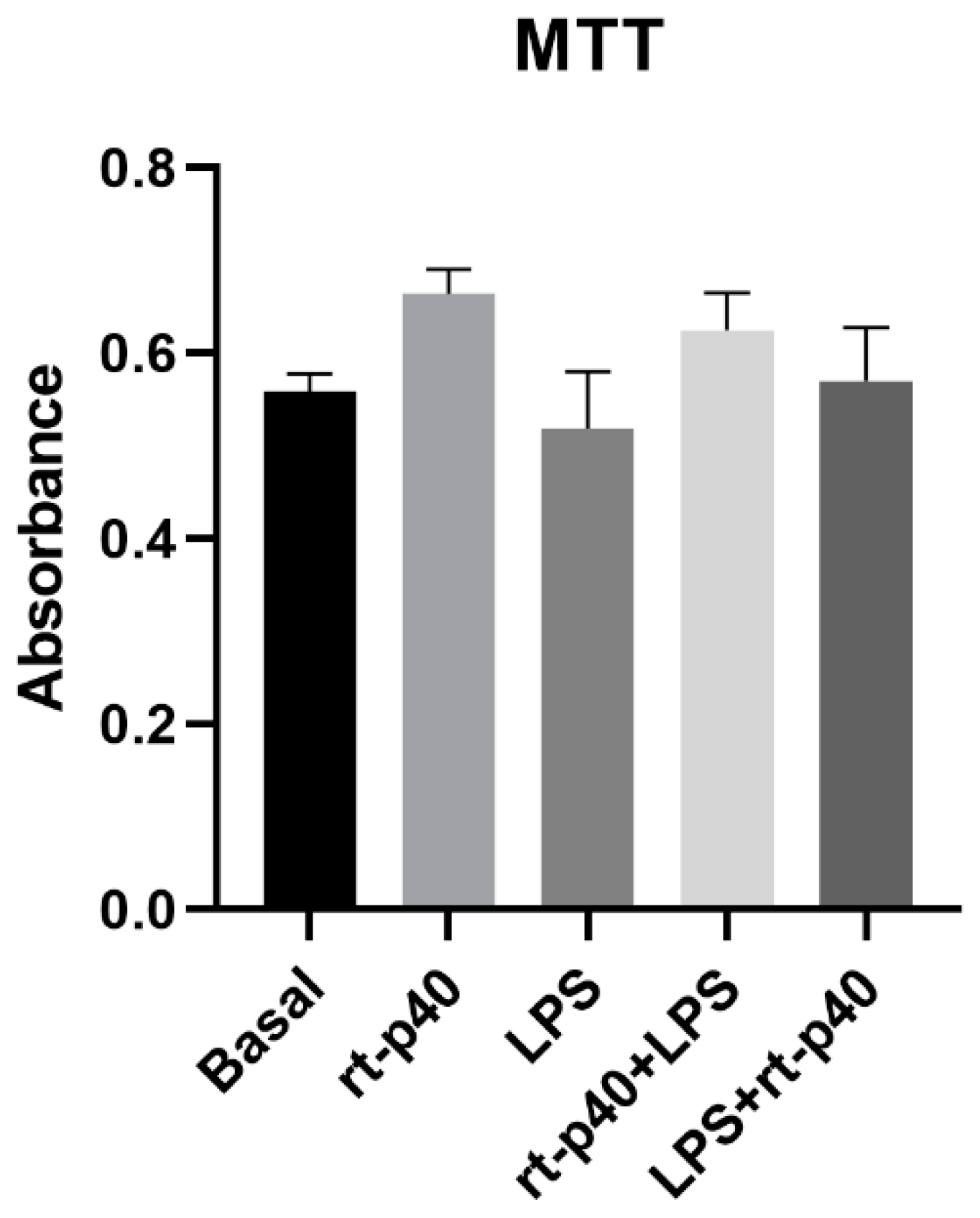
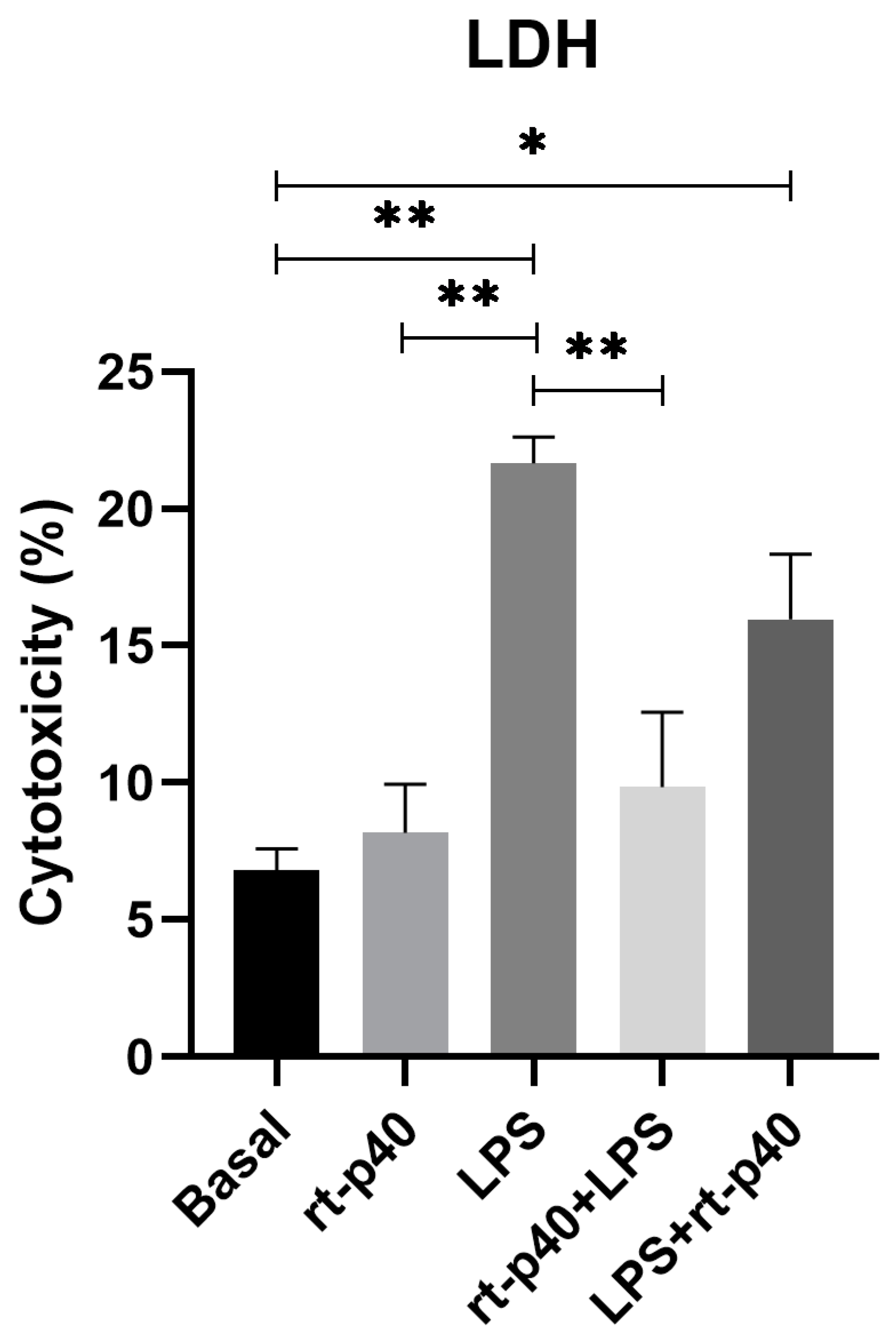
3.2. Cell Viability and Cell Cytotoxicity
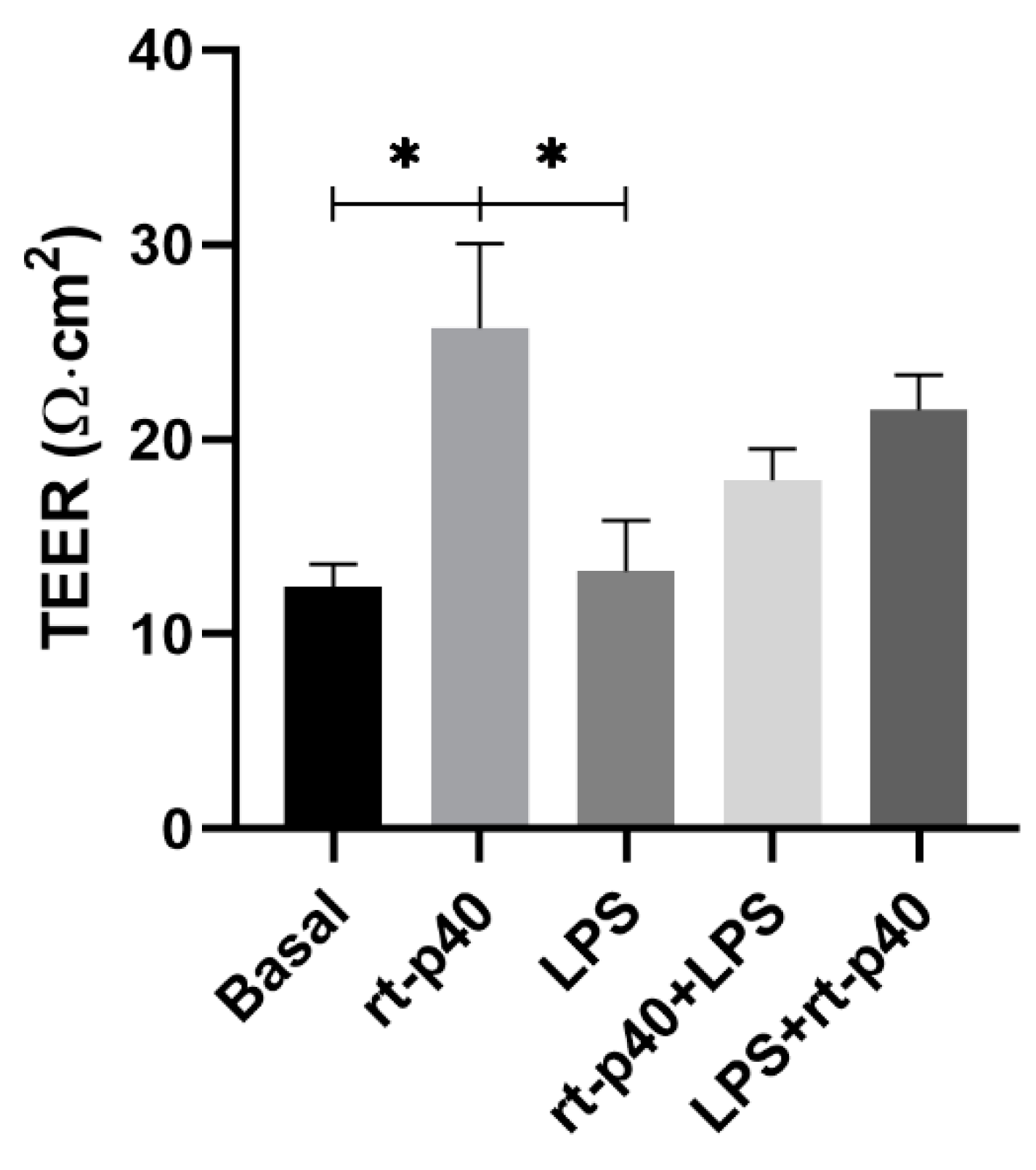
3.3. TEER
3.4. Expression of Tight Junction Proteins and EGFR Activation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lechuga, S.; Ivanov, A.I. Disruption of the Epithelial Barrier during Intestinal Inflammation: Quest for New Molecules and Mechanisms. Biochim. Biophys. Acta 2017, 1864, 1183–1194. [Google Scholar] [CrossRef] [PubMed]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, S.; Sabharwal, V.; Kaushik, P.; Joshi, A.; Aayushi, A.; Suri, M. Postbiotics: From Emerging Concept to Application. Front. Sustain. Food Syst. 2022, 6, 887642. [Google Scholar] [CrossRef]
- He, X.; Zeng, Q.; Puthiyakunnon, S.; Zeng, Z.; Yang, W.; Qiu, J.; Du, L.; Boddu, S.; Wu, T.; Cai, D.; et al. Lactobacillus Rhamnosus GG Supernatant Enhance Neonatal Resistance to Systemic Escherichia Coli K1 Infection by Accelerating Development of Intestinal Defense. Sci. Rep. 2017, 7, 43305. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Cao, H.; Cover, T.L.; Whitehead, R.; Washington, M.K.; Polk, D.B. Soluble Proteins Produced by Probiotic Bacteria Regulate Intestinal Epithelial Cell Survival and Growth. Gastroenterology 2007, 132, 562–575. [Google Scholar] [CrossRef]
- Seth, A.; Yan, F.; Polk, D.B.; Rao, R.K. Probiotics Ameliorate the Hydrogen Peroxide-Induced Epithelial Barrier Disruption by a PKC- and MAP Kinase-Dependent Mechanism. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 294, G1060–G1069. [Google Scholar] [CrossRef]
- Yan, F.; Cao, H.; Cover, T.L.; Washington, M.K.; Shi, Y.; Liu, L.; Chaturvedi, R.; Peek, R.M.; Wilson, K.T.; Polk, D.B. Colon-Specific Delivery of a Probiotic-Derived Soluble Protein Ameliorates Intestinal Inflammation in Mice through an EGFR-Dependent Mechanism. J. Clin. Investig. 2011, 121, 2242–2253. [Google Scholar] [CrossRef]
- Kelley, L.A.; Mezulis, S.; Yates, C.M.; Wass, M.N.; Sternberg, M.J.E. The Phyre2 Web Portal for Protein Modeling, Prediction and Analysis. Nat. Protoc. 2015, 10, 845–858. [Google Scholar] [CrossRef]
- Bäuerl, C.; Pérez-Martínez, G.; Yan, F.; Polk, D.B.; Monedero, V. Functional Analysis of the P40 and P75 Proteins from Lactobacillus Casei BL23. J. Mol. Microbiol. Biotechnol. 2010, 19, 231–241. [Google Scholar] [CrossRef]
- Bäuerl, C.; Abitayeva, G.; Sosa-Carrillo, S.; Mencher-Beltrán, A.; Navarro-Lleó, N.; Coll-Marqués, J.M.; Zúñiga-Cabrera, M.; Shaikhin, S.; Pérez-Martinez, G. P40 and P75 Are Singular Functional Muramidases Present in the Lactobacillus Casei /Paracasei/Rhamnosus Taxon. Front. Microbiol. 2019, 10, 1420. [Google Scholar] [CrossRef]
- Yan, F.; Polk, D.B. Characterization of a Probiotic-Derived Soluble Protein Which Reveals a Mechanism of Preventive and Treatment Effects of Probiotics on Intestinal Inflammatory Diseases. Gut Microbes 2012, 3, 25–28. [Google Scholar] [CrossRef]
- Cicenia, A.; Santangelo, F.; Gambardella, L.; Pallotta, L.; Iebba, V.; Scirocco, A.; Marignani, M.; Tellan, G.; Carabotti, M.; Corazziari, E.S.; et al. Protective Role of Postbiotic Mediators Secreted by Lactobacillus Rhamnosus GG Versus Lipopolysaccharide-Induced Damage in Human Colonic Smooth Muscle Cells. J. Clin. Gastroenterol. 2016, 50, S140–S144. [Google Scholar] [CrossRef] [PubMed]
- Kareem, K.Y.; Ling, F.H.; Chwen, L.T.; Foong, O.M.; Anjas Asmara, S. Inhibitory Activity of Postbiotic Produced by Strains of Lactobacillus Plantarum Using Reconstituted Media Supplemented with Inulin. Gut Pathog. 2014, 6, 23. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, F.; Ishida, Y.; Sawada, D.; Ashida, N.; Sugawara, T.; Sakai, M.; Goto, T.; Kawada, T.; Fujiwara, S. Fragmented Lactic Acid Bacterial Cells Activate Peroxisome Proliferator-Activated Receptors and Ameliorate Dyslipidemia in Obese Mice. J. Agric. Food Chem. 2016, 64, 2549–2559. [Google Scholar] [CrossRef] [PubMed]
- Sawada, H.; Furushiro, M.; Hirai, K.; Motoike, M.; Watanabe, T.; Yokokura, T. Purification and Characterization of an Antihypertensive Compound from Lactobacillus Casei. Agric. Biol. Chem. 1990, 54, 3211–3219. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shin, H.S.; Park, S.Y.; Lee, D.K.; Kim, S.A.; An, H.M.; Kim, J.R.; Kim, M.J.; Cha, M.G.; Lee, S.W.; Kim, K.J.; et al. Hypocholesterolemic Effect of Sonication-Killed Bifidobacterium Longum Isolated from Healthy Adult Koreans in High Cholesterol Fed Rats. Arch. Pharm. Res. 2010, 33, 1425–1431. [Google Scholar] [CrossRef] [PubMed]
- Tiptiri-Kourpeti, A.; Spyridopoulou, K.; Santarmaki, V.; Aindelis, G.; Tompoulidou, E.; Lamprianidou, E.E.; Saxami, G.; Ypsilantis, P.; Lampri, E.S.; Simopoulos, C.; et al. Lactobacillus Casei Exerts Anti-Proliferative Effects Accompanied by Apoptotic Cell Death and up-Regulation of TRAIL in Colon Carcinoma Cells. PLoS ONE 2016, 11, e0147960. [Google Scholar] [CrossRef] [PubMed]
- Vidal, K.; Donnet-Hughes, A.; Granato, D. Lipoteichoic Acids from Lactobacillus Johnsonii Strain La1 and Lactobacillus Acidophilus Strain La10 Antagonize the Responsiveness of Human Intestinal Epithelial HT29 Cells to Lipopolysaccharide and Gram-Negative Bacteria. Infect. Immun. 2002, 70, 2057–2064. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S. Antioxidative Activity of Lactic Acid Bacteria in Yogurt. Afr. J. Microbiol. Res. 2011, 5, 5194–5201. [Google Scholar] [CrossRef]
- Yan, F.; Polk, D.B. Probiotic Bacterium Prevents Cytokine-Induced Apoptosis in Intestinal Epithelial Cells. J. Biol. Chem. 2002, 277, 50959–50965. [Google Scholar] [CrossRef]
- Yoda, K.; Miyazawa, K.; Hosoda, M.; Hiramatsu, M.; Yan, F.; He, F. Lactobacillus GG-Fermented Milk Prevents DSS-Induced Colitis and Regulates Intestinal Epithelial Homeostasis through Activation of Epidermal Growth Factor Receptor. Eur. J. Nutr. 2014, 53, 105–115. [Google Scholar] [CrossRef]
- Wang, L.; Cao, H.; Liu, L.; Wang, B.; Walker, W.A.; Acra, S.A.; Yan, F. Activation of Epidermal Growth Factor Receptor Mediates Mucin Production Stimulated by P40, a Lactobacillus Rhamnosus GG-Derived Protein. J. Biol. Chem. 2014, 289, 20234–20244. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, L.; Moore, D.J.; Shen, X.; Peek, R.M.; Acra, S.A.; Li, H.; Ren, X.; Polk, D.B.; Yan, F. An LGG-Derived Protein Promotes IgA Production through Upregulation of APRIL Expression in Intestinal Epithelial Cells. Mucosal Immunol. 2017, 10, 373–384. [Google Scholar] [CrossRef]
- Bernhofer, M.; Dallago, C.; Karl, T.; Satagopam, V.; Heinzinger, M.; Littmann, M.; Olenyi, T.; Qiu, J.; Schütze, K.; Yachdav, G.; et al. PredictProtein-Predicting Protein Structure and Function for 29 Years. Nucleic Acids Res. 2021, 49, W535–W540. [Google Scholar] [CrossRef]
- Claes, I.J.J.; Schoofs, G.; Regulski, K.; Courtin, P.; Chapot-Chartier, M.-P.; Rolain, T.; Hols, P.; von Ossowski, I.; Reunanen, J.; de Vos, W.M.; et al. Genetic and Biochemical Characterization of the Cell Wall Hydrolase Activity of the Major Secreted Protein of Lactobacillus Rhamnosus GG. PLoS ONE 2012, 7, e31588. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Al-Sadi, R.; Said, H.M.; Ma, T.Y. Lipopolysaccharide Causes an Increase in Intestinal Tight Junction Permeability in Vitro and in Vivo by Inducing Enterocyte Membrane Expression and Localization of TLR-4 and CD14. Am. J. Pathol. 2013, 182, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Wang, Y.; Wang, X.; Wang, C.; Bao, K.; Ji, L.; Jiang, G.; Hong, M. Calycosin Suppresses Epithelial Derived Initiative Key Factors and Maintains Epithelial Barrier in Allergic Inflammation via TLR4 Mediated NF-ΚB Pathway. Cell. Physiol. Biochem. 2017, 44, 1106–1119. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Zhang, G.; Liu, R.; Wang, Y.; Tsapieva, A.N.; Zhang, L.; Han, J. Heat-Killed Lacticaseibacillus Paracasei Repairs Lipopolysaccharide-Induced Intestinal Epithelial Barrier Damage via MLCK/MLC Pathway Activation. Nutrients 2023, 15, 1758. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Ahmad, A.A.; Yang, Y.; Liang, Z.; Shen, W.; Feng, M.; Shen, J.; Lan, X.; Ding, X. Lactobacillus Rhamnosus CY12 Enhances Intestinal Barrier Function by Regulating Tight Junction Protein Expression, Oxidative Stress, and Inflammation Response in Lipopolysaccharide-Induced Caco-2 Cells. Int. J. Mol. Sci. 2022, 23, 11162. [Google Scholar] [CrossRef]
- Yang, X.; Dang, X.; Zhang, X.; Zhao, S. Liquiritin Reduces Lipopolysaccharide-Aroused HaCaT Cell Inflammation Damage via Regulation of MicroRNA-31/MyD88. Int. Immunopharmacol. 2021, 101, 108283. [Google Scholar] [CrossRef] [PubMed]
- Meyle, J.; Gültig, K.; Rascher, G.; Wolburg, H. Transepithelial Electrical Resistance and Tight Junctions of Human Gingival Keratinocytes. J. Periodontal Res. 1999, 34, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Moran, M.C.; Pandya, R.P.; Leffler, K.A.; Yoshida, T.; Beck, L.A.; Brewer, M.G. Characterization of Human Keratinocyte Cell Lines for Barrier Studies. JID Innov. 2021, 1, 100018. [Google Scholar] [CrossRef] [PubMed]
- Al-Sadi, R.; Khatib, K.; Guo, S.; Ye, D.; Youssef, M.; Ma, T. Occludin Regulates Macromolecule Flux across the Intestinal Epithelial Tight Junction Barrier. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 300, G1054–G1064. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Liu, L.; Dempsey, P.J.; Tsai, Y.-H.; Raines, E.W.; Wilson, C.L.; Cao, H.; Cao, Z.; Liu, L.; Polk, D.B. A Lactobacillus Rhamnosus GG-Derived Soluble Protein, P40, Stimulates Ligand Release from Intestinal Epithelial Cells to Transactivate Epidermal Growth Factor Receptor. J. Biol. Chem. 2013, 288, 30742–30751. [Google Scholar] [CrossRef]
- Spano, J.P.; Fagard, R.; Soria, J.C.; Rixe, O.; Khayat, D.; Milano, G. Epidermal Growth Factor Receptor Signaling in Colorectal Cancer: Preclinical Data and Therapeutic Perspectives. Ann. Oncol. 2005, 16, 189–194. [Google Scholar] [CrossRef]
- Janani, B.; Vijayakumar, M.; Priya, K.; Kim, J.H.; Prabakaran, D.S.; Shahid, M.; Al-Ghamdi, S.; Alsaidan, M.; Othman Bahakim, N.; Hassan Abdelzaher, M.; et al. EGFR-Based Targeted Therapy for Colorectal Cancer—Promises and Challenges. Vaccines 2022, 10, 499. [Google Scholar] [CrossRef]


| Name | Sequence | Length (bp) | %GC | TM Q5 °C | Purpose | Reference |
|---|---|---|---|---|---|---|
| P40NCOI | GGGTGAGGTCAATCATGAAATTCAATAAA-GCAATGATGAC | 33 | 48 | 76 | Cloning | This work |
| P40XHOI | TAAACTCGAGCCGGTG-GATGTAAACGTAGCTG | 32 | 50 | 74 | Cloning | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domínguez-Díaz, C.; Avila-Arrezola, K.E.; Rodríguez, J.A.; del-Toro-Arreola, S.; Delgado-Rizo, V.; Fafutis-Morris, M. Recombinant p40 Protein Promotes Expression of Occludin in HaCaT Keratinocytes: A Brief Communication. Microorganisms 2023, 11, 2913. https://doi.org/10.3390/microorganisms11122913
Domínguez-Díaz C, Avila-Arrezola KE, Rodríguez JA, del-Toro-Arreola S, Delgado-Rizo V, Fafutis-Morris M. Recombinant p40 Protein Promotes Expression of Occludin in HaCaT Keratinocytes: A Brief Communication. Microorganisms. 2023; 11(12):2913. https://doi.org/10.3390/microorganisms11122913
Chicago/Turabian StyleDomínguez-Díaz, Carolina, Karina Elizabeth Avila-Arrezola, Jorge A. Rodríguez, Susana del-Toro-Arreola, Vidal Delgado-Rizo, and Mary Fafutis-Morris. 2023. "Recombinant p40 Protein Promotes Expression of Occludin in HaCaT Keratinocytes: A Brief Communication" Microorganisms 11, no. 12: 2913. https://doi.org/10.3390/microorganisms11122913
APA StyleDomínguez-Díaz, C., Avila-Arrezola, K. E., Rodríguez, J. A., del-Toro-Arreola, S., Delgado-Rizo, V., & Fafutis-Morris, M. (2023). Recombinant p40 Protein Promotes Expression of Occludin in HaCaT Keratinocytes: A Brief Communication. Microorganisms, 11(12), 2913. https://doi.org/10.3390/microorganisms11122913





