Responses of Soil Microbial Survival Strategies and Functional Changes to Wet–Dry Cycle Events
Abstract
:1. Introduction
2. Materials and Methods
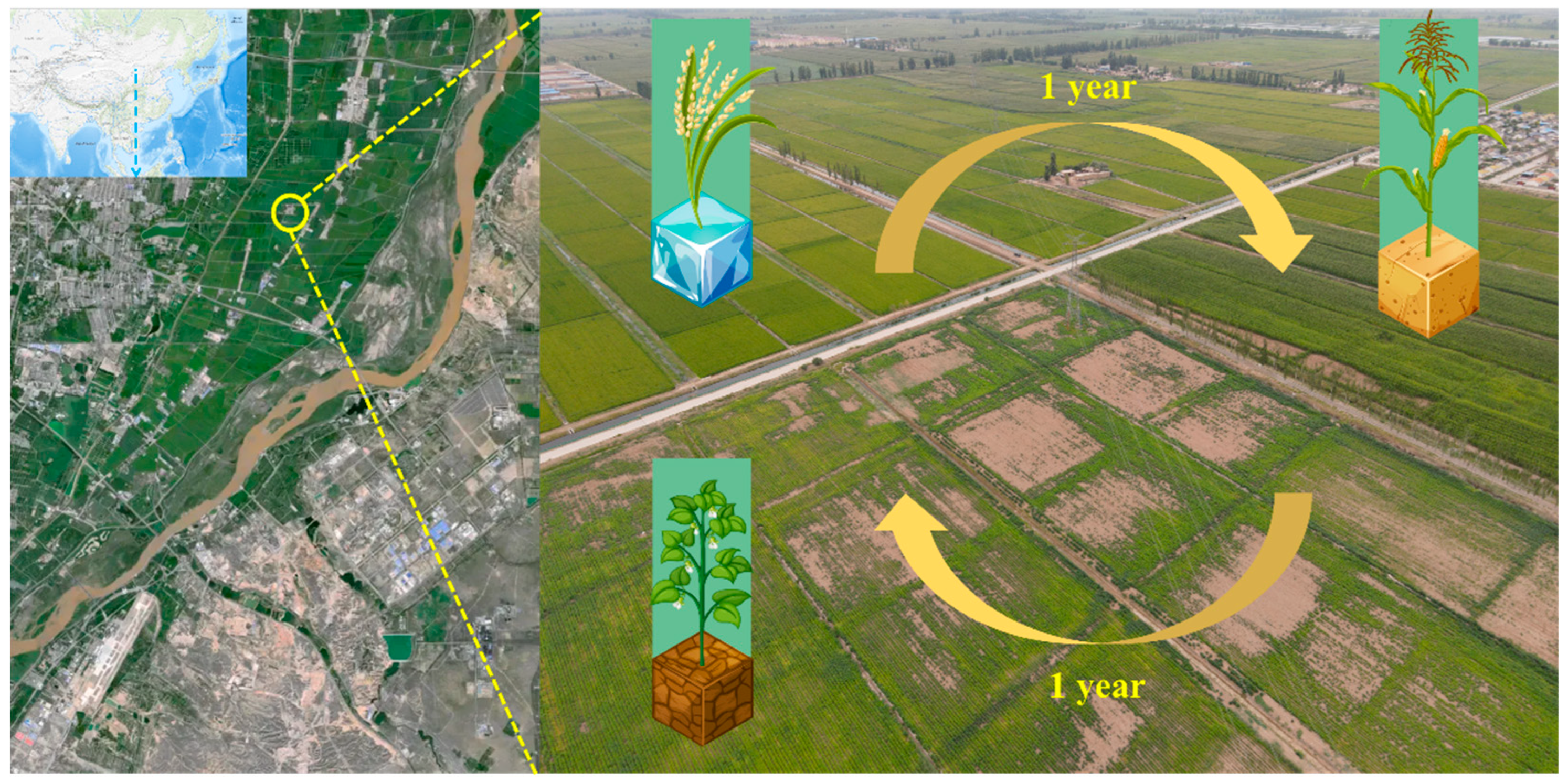
2.1. Study Site and Sample Collection
2.2. Analysis of Soil Physicochemical Properties
2.3. Determination of Soil Extracellular Enzyme Activities
2.4. High–Throughput Sequencing of Soil Bacteria and Fungi
2.5. Data Analysis
3. Results
3.1. Changes in Soil Physicochemical Properties and Enzyme Activities under Wet–Dry Events
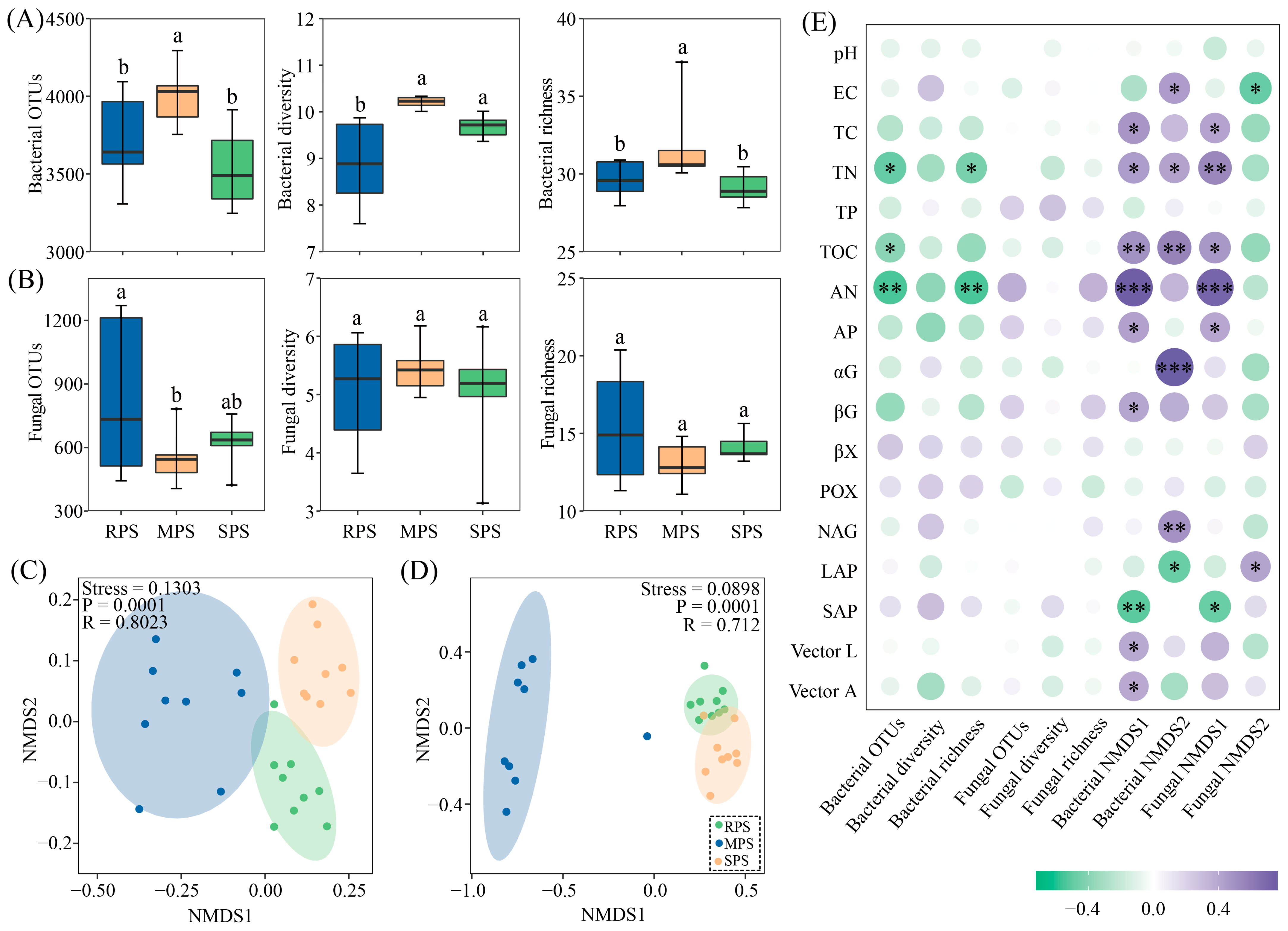
3.2. Soil Microbial Community Diversity and Composition under Wet–Dry Events
3.3. Changes in Soil r/K–Strategist Bacteria under Wet–Dry Events
3.4. Relationships between Soil r/K–Strategist Bacteria and Fungi under Wet–Dry Events
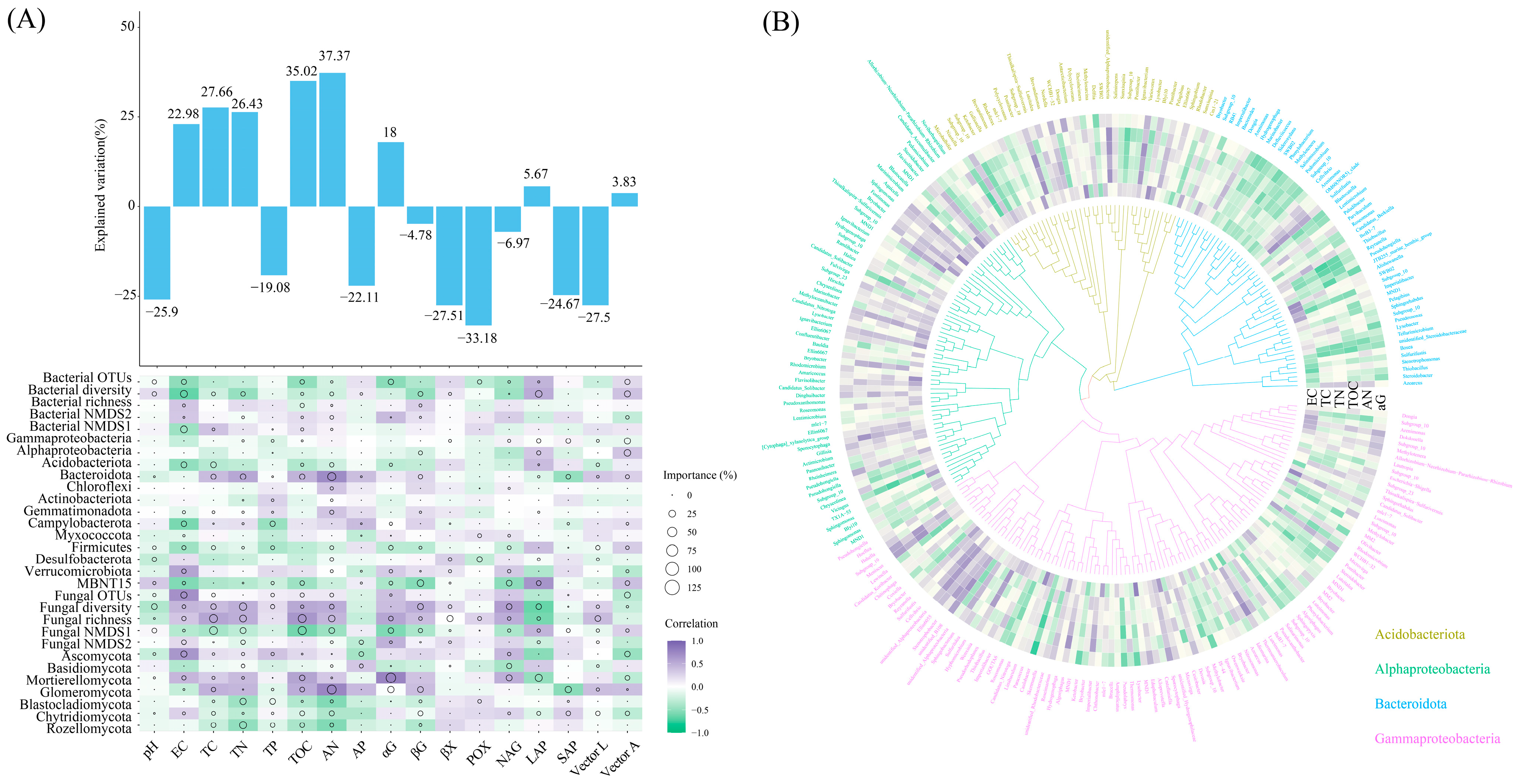
3.5. Main Environmental Factors Affecting Soil Microbial Community Structure under Wet–Dry Events
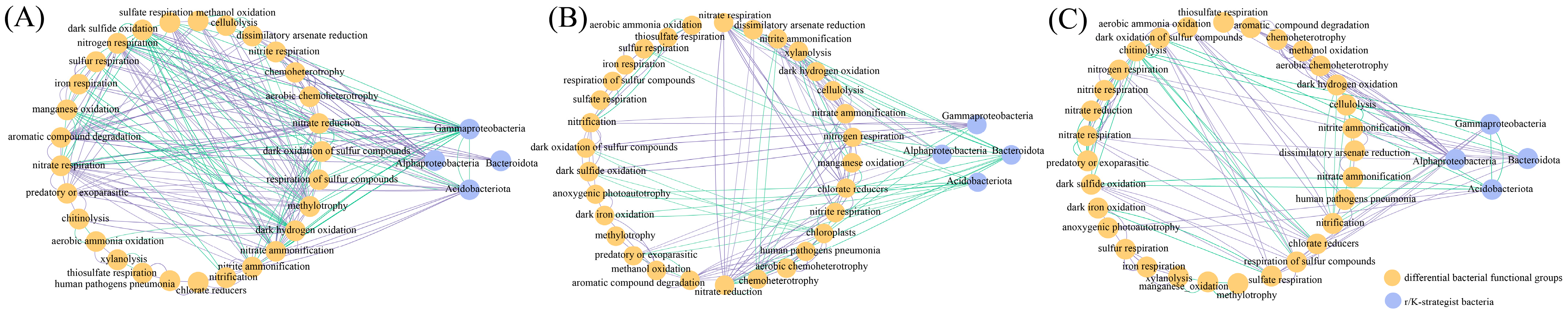
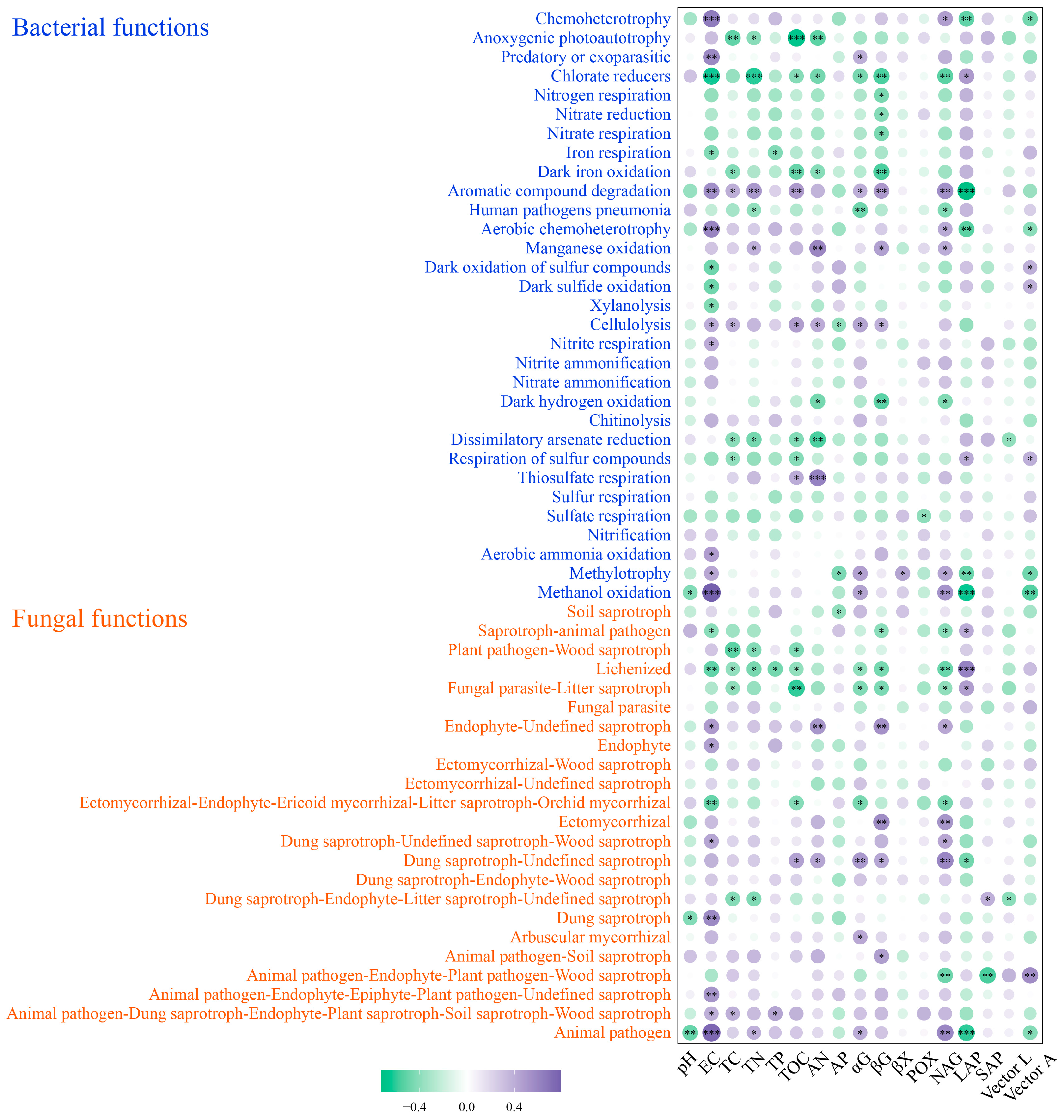
3.6. Changes in Soil Bacterial and Fungal Community Function under Wet–Dry Events
4. Discussion
4.1. Large Differences in Soil Physicochemical Properties and Enzyme Activities under Wet–Dry Events
4.2. Wet–Dry Events Drove the Transition of Soil r–Strategist Bacteria to K–Strategist Bacteria
4.3. Soil Environment Is an Important Affecting Microbial Function Changes under Wet–Dry Events
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wilschut, R.A.; van der Putten, W.H.; Garbeva, P.; Harkes, P.; Konings, W.; Kulkarni, P.; Martens, H.; Geisen, S. Root traits and belowground herbivores relate to plant–soil feedback variation among congeners. Nat. Commun. 2019, 10, 1564. [Google Scholar] [CrossRef] [PubMed]
- Crawford, K.M.; Hawkes, C.V. Soil precipitation legacies influence intraspecific plant–soil feedback. Ecology 2020, 101, e03142. [Google Scholar] [CrossRef] [PubMed]
- Cowan, J.A.; Grady, K.C.; Dijkstra, P.; Schwartz, E.; Gehring, C.A. Invasive and native grasses exert negative plant–soil feedbacks on the woody shrub Artemisia tridentata. Oecologia 2022, 199, 1007–1019. [Google Scholar] [CrossRef]
- Fry, E.L.; Johnson, G.N.; Hall, A.L.; Pritchard, W.J.; Bullock, J.M.; Bardgett, R.D. Drought neutralises plant–soil feedback of two mesic grassland forbs. Oecologia 2018, 186, 1113–1125. [Google Scholar] [CrossRef]
- Pfennigwerth, A.; van Nuland, M.; Bailey, J.; Schweitzer, J. Plant–soil feedbacks mediate shrub expansion in declining forests, but only in the right light. J. Ecol. 2017, 106, 179–194. [Google Scholar] [CrossRef]
- Lynn, J.S.; Duarte, D.A.; Rudgers, J.A. Soil microbes that may accompany climate warming increase alpine plant production. Oecologia 2019, 191, 493–504. [Google Scholar] [CrossRef]
- Snyder, A.E.; Harmon–Threatt, A.N. Reduced water–availability lowers the strength of negative plant–soil feedbacks of two Asclepias species. Oecologia 2019, 190, 425–432. [Google Scholar] [CrossRef]
- Nelson, E.B.; Karp, M.A. Soil pathogen communities associated with native and non–native Phragmites australis populations in freshwater wetlands. Ecol. Evol. 2013, 3, 5254–5267. [Google Scholar] [CrossRef]
- Tan, L.S.; Ge, Z.M.; Zhou, X.H.; Li, S.H.; Li, X.Z.; Tang, J.W. Conversion of coastal wetlands, riparian wetlands, and peatlands increases greenhouse gas emissions: A global meta–analysis. Glob. Change Biol. 2020, 26, 1638–1653. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Lu, X.; Liu, B.Y.; Wu, D.T.; Fu, G.; Zhao, Y.T.; Sun, P.L. Spatial relationships between ecosystem services and socioecological drivers across a large–scale region: A case study in the Yellow River Basin. Sci. Total Environ. 2021, 766, 142480. [Google Scholar] [CrossRef]
- Lin, Q.W.; Wang, S.S.; Li, Y.C.; Riaz, L.; Yu, F.; Yang, Q.X.; Han, S.J.; Ma, J.M. Effects and mechanisms of land–types conversion on greenhouse gas emissions in the Yellow River floodplain wetland. Sci. Total Environ. 2022, 813, 152406. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, E.I.; Prieto–Davó, A.; López–Lozano, N.E.; Hernández–Eligio, A.; Vega–Alvarado, L.; Juárez, K.; García–González, A.S.; López, M.G.; Cervantes, F.J. Anaerobic methane oxidation driven by microbial reduction of natural organic matter in a tropical wetland. Appl. Environ. Microbiol. 2017, 83, e00645-17. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Miao, Y.J.; Gan, Y.D.; Wei, S.D.; Tan, S.J.; Rask, K.A.; Wang, L.H.; Dai, J.L.; Chen, W.F.; Ekelund, F. Soil bacterial community response to long–term land use conversion in Yellow River Delta. Appl. Soil Ecol. 2020, 156, 103709. [Google Scholar] [CrossRef]
- Jing, C.L.; Xu, Z.C.; Zou, P.; Tang, Q.; Li, Y.Q.; You, X.W.; Zhang, C.S. Coastal halophytes alter properties and microbial community structure of the saline soils in the Yellow River Delta, China. Appl. Soil Ecol. 2019, 134, 1–7. [Google Scholar] [CrossRef]
- Jiao, S.; Lu, Y.H. Soil pH and temperature regulate assembly processes of abundant and rare bacterial communities in agricultural ecosystems. Environ. Microbiol. 2020, 22, 1052–1065. [Google Scholar] [CrossRef]
- Qi, J.J.; Chen, B.B.; Gao, J.M.; Peng, Z.H.; Jiao, S.; Wei, G.H. Responses of soil bacterial community structure and function to dry–wet cycles more stable in paddy than in dryland agricultural ecosystems. Glob. Ecol. Biogeogr. 2021, 31, 362–377. [Google Scholar] [CrossRef]
- Liu, S.G.; García–Palacios, P.; Tedersoo, L.; Guirado, E.; van der Heijden, M.G.A.; Wagg, C.; Chen, D.M.; Wang, Q.K.; Wang, J.T.; Singh, B.K.; et al. Phylotype diversity within soil fungal functional groups drives ecosystem stability. Nat. Ecol. Evol. 2022, 6, 900–909. [Google Scholar] [CrossRef]
- Jiao, S.; Yang, Y.F.; Xu, Y.Q.; Zhang, J.; Lu, Y.H. Balance between community assembly processes mediates species coexistence in agricultural soil microbiomes across eastern China. ISME J. 2020, 14, 202–216. [Google Scholar] [CrossRef]
- Pascault, N.; Ranjard, L.; Kaisermann, A.; Bachar, D.; Christen, R.; Terrat, S.; Mathieu, O.; Lévêque, J.; Mougel, C.; Hénault, C.; et al. Stimulation of different functional groups of bacteria by various plant residues as a driver of soil priming effect. Ecosystems 2013, 16, 810–822. [Google Scholar] [CrossRef]
- Pan, Y.Q.; Kang, P.; Tan, M.; Hu, J.P.; Zhang, Y.Q.; Zhang, J.L.; Song, N.P.; Li, X.R. Root exudates and rhizosphere soil bacterial relationships of Nitraria tangutorum are linked to k–strategists bacterial community under salt stress. Front. Plant Sci. 2022, 13, 997292. [Google Scholar] [CrossRef]
- Fierer, N.; Bradford, M.A.; Jackson, R.B. Toward an ecological classification of soil bacteria. Ecology 2007, 88, 1354–1364. [Google Scholar] [CrossRef] [PubMed]
- Zhalnina, K.; Louie, K.B.; Hao, Z.; Mansoori, N.; da Rocha, U.N.; Shi, S.J.; Cho, H.; Karaoz, U.; Loqué, D.; Bowen, B.P.; et al. Dynamic root exudate chemistry and microbial substrate preferences drive patterns in rhizosphere microbial community assembly. Nat. Microbiol. 2018, 3, 470–480. [Google Scholar] [CrossRef]
- Rath, K.M.; Fierer, N.; Murphy, D.V.; Rousk, J. Linking bacterial community composition to soil salinity along environmental gradients. ISME J. 2019, 13, 836–846. [Google Scholar] [CrossRef]
- van der Putten, W.H.; Bradford, M.A.; Pernilla Brinkman, E.; van de Voorde, T.F.J.; Veen, G.F. Where, when and how plant–soil feedback matters in a changing world. Funct. Ecol. 2016, 30, 1109–1121. [Google Scholar] [CrossRef]
- Bardgett, R. Plant trait–based approaches for interrogating belowground function. Biol. Environ. 2017, 117B, 1. [Google Scholar] [CrossRef]
- Kivlin, S.N.; Bedoya, R.; Hawkes, C.V. Heterogeneity in arbuscular mycorrhizal fungal communities may contribute to inconsistent plant–soil feedback in a Neotropical forest. Plant Soil 2018, 432, 29–44. [Google Scholar] [CrossRef]
- Mooshammer, M.; Wanek, W.; Zechmeister–Boltenstern, S.; Richter, A. Stoichiometric imbalances between terrestrial decomposer communities and their resources: Mechanisms and implications of microbial adaptations to their resources. Front. Microbiol. 2014, 5, 22. [Google Scholar] [CrossRef] [PubMed]
- Zechmeister–Boltenstern, S.; Keiblinger, K.; Mooshammer, M.; Penuelas, J.; Richter, A.; Sardans, J.; Wanek, W. The application of ecological stoichiometry to plant–microbial–soil organic matter transformations. Ecol. Monogr. 2015, 85, 133–155. [Google Scholar] [CrossRef]
- Bünemann, E.K.; Bongiorno, G.; Bai, Z.G.; Creamer, R.E.; De Deyn, G.; de Goede, R.; Fleskens, L.; Geissen, V.; Kuyper, T.W.; Mäder, P.; et al. Soil quality–A critical review. Soil Biol. Biochem. 2018, 120, 105–125. [Google Scholar] [CrossRef]
- Pan, Y.Q.; Kang, P.; Hu, J.P.; Song, N.P. Bacterial community demonstrates stronger network connectivity than fungal community in desert–grassland salt marsh. Sci. Total Environ. 2021, 798, 149118. [Google Scholar] [CrossRef]
- Kang, P.; Pan, Y.Q.; Ran, Y.C.; Li, W.N.; Shao, M.X.; Zhang, Y.Q.; Ji, Q.B.; Ding, X.D. Soil saprophytic fungi could be used as an important ecological indicator for land management in desert steppe. Ecol. Indic. 2023, 150, 110224. [Google Scholar] [CrossRef]
- Bao, S.D. Soil and Agricultural Chemistry Analysis; China Agriculture Publication: Beijing, China, 2000. [Google Scholar]
- Saiya–Cork, K.R.; Sinsabaugh, R.L.; Zak, D.R. The effects of long term nitrogen deposition on extracellular enzyme activity in an Acer saccharum forest soil. Soil Biol. Biochem. 2002, 34, 1309–1315. [Google Scholar] [CrossRef]
- Yu, S.; He, Z.L.; Stoffella, P.J.; Calvert, D.V.; Yang, X.E.; Banks, D.J.; Baligar, V.C. Surface runoff phosphorus (P) loss in relation to phosphatase activity and soil P fractions in Florida sandy soils under citrus production. Soil Biol. Biochem. 2006, 38, 619–628. [Google Scholar] [CrossRef]
- Moorhead, D.L.; Sinsabaugh, R.L.; Hill, B.H.; Weintraub, M.N. Vector analysis of ecoenzyme activities reveal constraints on coupled C, N and P dynamics. Soil Biol. Biochem. 2016, 93, 1–7. [Google Scholar] [CrossRef]
- Claesson, M.J.; O’Sullivan, O.; Wang, Q.; Nikkilä, J.; Marchesi, J.R.; Smidt, H.; de Vos, W.M.; Ross, R.P.; O’Toole, P.W. Comparative analysis of pyrosequencing and a phylogenetic microarray for exploring microbial community structures in the human distal intestine. PLoS ONE 2009, 4, e6669. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.Y.; Yuan, M.S.; Tang, L.; Shen, Y.F.; Yu, Q.; Li, S.Q. Integrated microbiology and metabolomics analysis reveal responses of soil microorganisms and metabolic functions to phosphorus fertilizer on semiarid farm. Sci. Total Environ. 2022, 817, 152878. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg–Lyons, D.; Huntley, J.; Fierer, N.; Owens, S.M.; Betley, J.; Fraser, L.; Bauer, M.; et al. Ultra–high–throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 2012, 6, 1621–1624. [Google Scholar] [CrossRef]
- Bell, T.H.; El–Din Hassan, S.; Lauron–Moreau, A.; Al–Otaibi, F.; Hijri, M.; Yergeau, E.; St–Arnaud, M. Linkage between bacterial and fungal rhizosphere communities in hydrocarbon–contaminated soils is related to plant phylogeny. ISME J. 2014, 8, 331–343. [Google Scholar] [CrossRef]
- Gu, Y.F.; Bai, Y.; Xiang, Q.X.; Yu, X.M.; Zhao, K.; Zhang, X.P.; Li, C.N.; Liu, S.Q.; Chen, Q. Degradation shaped bacterial and archaeal communities with predictable taxa and their association patterns in Zoige wetland at Tibet plateau. Sci. Rep. 2018, 8, 3884. [Google Scholar] [CrossRef]
- Sunagawa, S.; Coelho, L.P.; Chaffron, S.; Kultima, J.R.; Labadie, K.; Salazar, G.; Djahanschiri, B.; Zeller, G.; Mende, D.R.; Alberti, A.; et al. Ocean plankton. Structure and function of the global ocean microbiome. Science 2015, 348, 1261359. [Google Scholar] [CrossRef]
- Jiao, S.; Chu, H.Y.; Zhang, B.G.; Wei, X.R.; Chen, W.M.; Wei, G.H. Linking soil fungi to bacterial community assembly in arid ecosystems. iMeta 2022, 1, e2. [Google Scholar] [CrossRef]
- Garland, G.; Banerjee, S.; Edlinger, A.; Oliveira, E.; Herzog, C.; Wittwer, R.; Philippot, L.; Maestre, F.T.; Van der Heijden, M. A closer look at the functions behind ecosystem multifunctionality: A review. J. Ecol. 2020, 109, 600–613. [Google Scholar] [CrossRef]
- Baker, B.; Mohler, C. Weed management by upstate New York organic farmers: Strategies, techniques and research priorities. Renew. Agr. Food Syst. 2014, 30, 1–10. [Google Scholar] [CrossRef]
- Byrne, B.M. Structural Equation Modeling with AMOS: Basic Concepts, Applications, and Programming, 3rd ed.; Routledge: New York, NY, USA, 2016. [Google Scholar]
- Martins, C.S.C.; Nazaries, L.; Delgado–Baquerizo, M.; Macdonald, C.A.; Anderson, I.C.; Singh, B.K. Rainfall frequency and soil water availability regulate soil methane and nitrous oxide fluxes from a native forest exposed to elevated carbon dioxide. Funct. Ecol. 2021, 35, 1833–1847. [Google Scholar] [CrossRef]
- Yang, Y.; Li, T.; Wang, Y.Q.; Cheng, H.; Chang, S.X.; Liang, C.; An, S.S. Negative effects of multiple global change factors on soil microbial diversity. Soil Biol. Biochem. 2021, 156, 108229. [Google Scholar] [CrossRef]
- Yemadje, P.L.; Guibert, H.; Chevallier, T.; Deleporte, P.; Bernoux, M. Effect of biomass management regimes and wetting–drying cycles on soil carbon mineralization in a Sudano–Sahelian region. J. Arid Environ. 2016, 127, 1–6. [Google Scholar] [CrossRef]
- Zang, H.D.; Xiao, M.L.; Wang, Y.D.; Ling, N.; Wu, J.S.; Ge, T.D.; Kuzyakov, Y. Allocation of assimilated carbon in paddies depending on rice age, chase period and N fertilization: Experiment with 13CO2 labelling and literature synthesis. Plant Soil 2019, 445, 113–123. [Google Scholar] [CrossRef]
- Li, J.L.; Li, Y.E.; Wan, Y.F.; Wang, B.; Waqas, M.A.; Cai, W.W.; Guo, C.; Zhou, S.H.; Su, R.S.; Qin, X.B.; et al. Combination of modified nitrogen fertilizers and water saving irrigation can reduce greenhouse gas emissions and increase rice yield. Geoderma 2018, 315, 1–10. [Google Scholar] [CrossRef]
- Zhou, S.; Sun, H.F.; Bi, J.G.; Zhang, J.N.; Riya, S.H.; Hosomi, M. Effect of water–saving irrigation on the N2O dynamics and the contribution of exogenous and endogenous nitrogen to N2O production in paddy soil using 15N tracing. Soil Tillage Res. 2020, 200, 104610. [Google Scholar] [CrossRef]
- Dassen, S.; Cortois, R.; Martens, H.; de Hollander, M.; Kowalchuk, G.A.; van der Putten, W.H.; De Deyn, G.B. Differential responses of soil bacteria, fungi, archaea and protists to plant species richness and plant functional group identity. Mol. Ecol. 2017, 26, 4085–4098. [Google Scholar] [CrossRef]
- Delgado–Baquerizo, M.; Grinyer, J.; Reich, P.B.; Singh, B.K. Relative importance of soil properties and microbial community for soil functionality: Insights from a microbial swap experiment. Funct. Ecol. 2016, 30, 1862–1873. [Google Scholar] [CrossRef]
- Caldwell, B.; Mohler, C.L.; Ketterings, Q.M.; DiTommaso, A. Yields and profitability during and after transition in organic grain cropping systems. Agron. J. 2014, 106, 871–880. [Google Scholar] [CrossRef]
- Jernigan, A.B.; Wickings, K.; Mohler, C.L.; Caldwell, B.A.; Pelzer, C.J.; Wayman, S.; Ryan, M.R. Legacy effects of contrasting organic grain cropping systems on soil health indicators, soil invertebrates, weeds, and crop yield. Agric. Syst. 2020, 177, 102719. [Google Scholar] [CrossRef]
- Dong, Z.J.; Li, H.B.; Xiao, J.N.; Sun, J.L.; Liu, R.L.; Zhang, A.P. Soil multifunctionality of paddy field is explained by soil pH rather than microbial diversity after 8–years of repeated applications of biochar and nitrogen fertilizer. Sci. Total Environ. 2022, 853, 158620. [Google Scholar] [CrossRef]
- Feng, J.; Xu, X.; Wu, J.J.; Zhang, Q.; Zhang, D.D.; Li, Q.X.; Long, C.Y.; Chen, Q.; Chen, J.W.; Cheng, X.L. Inhibited enzyme activities in soil macroaggregates contribute to enhanced soil carbon sequestration under afforestation in central China. Sci. Total Environ. 2018, 640–641, 653–661. [Google Scholar] [CrossRef]
- Vazquez, E.; Benito, M.; Masaguer, A.; Espejo, R.; Díaz–Pinés, E.; Teutscherova, N. Long–term effects of no tillage and Ca–amendment on the activity of soil proteases and β–glucosidase in a Mediterranean agricultural field. Eur. J. Soil Biol. 2019, 95, 103135. [Google Scholar] [CrossRef]
- Wang, H.X.; Wu, C.Y.; Chen, D.S.; Liu, H.Y.; Sun, X.M.; Zhang, S.G. Changes in soil carbon and nutrients and related extracellular enzymes in successive rotations of Japanese larch plantations. Catena 2021, 204, 105386. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Li, Y.; Sun, X.X.; Adams, J.M.; Wang, L.F.; Zhang, H.J.; Chu, H.Y. More robust Co–occurrence patterns and stronger dispersal limitations of bacterial communities in wet than dry seasons of riparian wetlands. mSystems 2023, 8, e0118722. [Google Scholar] [CrossRef]
- Zhou, Z.H.; Wang, C.K.; Luo, Y. Effects of forest degradation on microbial communities and soil carbon cycling: A global meta–analysis. Glob. Ecol. Biogeogr. 2018, 27, 110–124. [Google Scholar] [CrossRef]
- Luo, X.Z.; Hou, E.Q.; Zhang, L.L.; Kuang, Y.W.; Wen, D.Z. Altered soil microbial properties and functions after afforestation increase soil carbon and nitrogen but not phosphorus accumulation. Biol. Fert. Soils 2023, 59, 645–658. [Google Scholar] [CrossRef]
- Preece, C.; Verbruggen, E.; Liu, L.; Weedon, J.T.; Peñuelas, J. Effects of past and current drought on the composition and diversity of soil microbial communities. Soil Biol. Biochem. 2019, 131, 28–39. [Google Scholar] [CrossRef]
- Sun, W.M.; Xiao, E.Z.; Krumins, V.; Häggblom, M.M.; Dong, Y.R.; Pu, Z.L.; Li, B.Q.; Wang, Q.; Xiao, T.F.; Li, F.B. Rhizosphere microbial response to multiple metal(ioid)s in different contaminated arable Soils indicates crop–specific metal–microbe interactions. Appl. Environ. Microbiol. 2018, 84, e00701-18. [Google Scholar] [CrossRef]
- Karimi, B.; Maron, P.A.; Chemidlin–Prevost Boure, N.; Bernard, N.; Gilbert, D.; Ranjard, L. Microbial diversity and ecological networks as indicators of environmental quality. Environ. Chem. Lett. 2017, 15, 265–281. [Google Scholar] [CrossRef]
- Freilich, M.A.; Wieters, E.; Broitman, B.R.; Marquet, P.A.; Navarrete, S.A. Species co–occurrence networks: Can they reveal trophic and non–trophic interactions in ecological communities? Ecology 2018, 99, 690–699. [Google Scholar] [CrossRef] [PubMed]
- de Vries, F.T.; Wallenstein, M.D. Below–ground connections underlying above–ground food production: A framework for optimising ecological connections in the rhizosphere. J. Ecol. 2017, 105, 913–920. [Google Scholar] [CrossRef]
- Zeng, Y.; Fang, Z.T.; Liu, J.W.; Dong, Y.F.; Li, F.S. Nitrous oxide emission in relation to paddy soil microbial communities in south China under different irrigation and nitrogen strategies. Commun. Soil Sci. Plan. 2019, 50, 1278–1291. [Google Scholar] [CrossRef]
- Orwin, K.H.; Dickie, I.A.; Wood, J.R.; Bonner, K.I.; Holdaway, R.J. Soil microbial community structure explains the resistance of respiration to a dry–rewet cycle, but not soil functioning under static conditions. Funct. Ecol. 2016, 30, 1430–1439. [Google Scholar] [CrossRef]
- Garibotti, I.A.; Gonzalez Polo, M.; Tabeni, S. Linking biological soil crust attributes to the multifunctionality of vegetated patches and interspaces in a semiarid shrubland. Funct. Ecol. 2018, 32, 1065–1078. [Google Scholar] [CrossRef]
- Guan, P.T.; Mahamood, M.; Atwill, Y.Y.; Wu, D.H. Land conversion regulates the effects of long–term climate warming on soil micro–food web communities. Agr. Ecosyst. Environ. 2021, 314, 107426. [Google Scholar] [CrossRef]
- Peralta, A.L.; Ludmer, S.; Kent, A.D. Hydrologic history influences microbial community composition and nitrogen cycling under experimental drying/wetting treatments. Soil Biol. Biochem. 2013, 66, 29–37. [Google Scholar] [CrossRef]
- Wang, L.; Hao, D.C.; Fan, S.S.; Xie, H.T.; Bao, X.L.; Jia, Z.J.; Wang, L.F. N2O emission and nitrification/denitrification bacterial communities in upland black soil under combined effects of early and immediate moisture. Agriculture 2022, 12, 330. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Dong, L.Z.; Zhang, H.J.; Deng, Y.Y.; Hu, B.; Wang, W. Distinct roles of bacteria and fungi in mediating soil extracellular enzymes under long–term nitrogen deposition in temperate plantations. For. Ecol. Manag. 2023, 529, 120658. [Google Scholar] [CrossRef]


| Soil Physicochemical Properties and Extracellular Enzyme Activities | RPS | MPS | SPS |
|---|---|---|---|
| pH | 8.11 ± 0.04a | 8.06 ± 0.04a | 7.04 ± 0.02a |
| EC (us/cm) | 815.67 ± 92.03b | 1554.00 ± 159.51a | 1378.67 ± 125.23a |
| TC (g/kg) | 25.36 ± 0.55b | 24.79 ± 0.86b | 28.92 ± 0.67a |
| TN (g/kg) | 0.88 ± 0.05b | 0.84 ± 0.05b | 1.17 ± 0.06a |
| TP (g/kg) | 0.71 ± 0.02a | 0.74 ± 0.02a | 0.76 ± 0.04a |
| TOC (g/kg) | 17.74 ± 0.82b | 18.01 ± 0.99b | 23.56 ± 0.65a |
| AN (mg/kg) | 55.16 ± 2.24b | 48.64 ± 1.93b | 68.03 ± 2.57a |
| AP (mg/kg) | 24.82 ± 0.70a | 22.38 ± 0.54a | 23.96 ± 1.24a |
| αG (ng/L) | 42.92 ± 1.09b | 45.43 ± 0.71a | 47.64 ± 0.71a |
| βG (IU/L) | 47.21 ± 1.23b | 47.49 ± 0.83b | 51.73 ± 0.63a |
| βX (IU/L) | 225.21 ± 4.37a | 224.73 ± 4.27a | 221.70 ± 5.86a |
| POX (IU/L) | 242.67 ± 5.59a | 249.11 ± 5.77a | 247.10 ± 7.11a |
| NAG (U/L) | 40.53 ± 0.78b | 42.93 ± 0.60a | 44.50 ± 0.79a |
| LAP (U/L) | 253.65 ± 4.36a | 244.67 ± 5.70a | 227.26 ± 5.05b |
| SAP (U/L) | 787.96 ± 21.09a | 853.59 ± 16.18a | 781.08 ± 19.46a |
| Vector L | 1.83 ± 0.009ab | 1.82 ± 0.006b | 1.84 ± 0.007a |
| Vector A | 52.48 ± 0.12a | 51.88 ± 0.16b | 52.14 ± 0.19ab |
| Network Parameters | RPS | MPS | SPS |
|---|---|---|---|
| Nodes | 800 | 1007 | 898 |
| Edges | 821 | 1233 | 865 |
| Positive edges | 572 | 671 | 489 |
| Negative edges | 249 | 562 | 376 |
| Number of modules | 264 | 254 | 309 |
| Modularity | 0.87 | 0.86 | 0.90 |
| Average path length | 7.40 | 9.64 | 10.53 |
| Graph diameter | 23.08 | 29.62 | 30.30 |
| Clustering coefficient | 0.32 | 0.32 | 0.39 |
| Degree centralization | 0.01 | 0.01 | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Mo, C.; Pan, Y.; Yang, P.; Ding, X.; Lei, Q.; Kang, P. Responses of Soil Microbial Survival Strategies and Functional Changes to Wet–Dry Cycle Events. Microorganisms 2023, 11, 2783. https://doi.org/10.3390/microorganisms11112783
Zhang Y, Mo C, Pan Y, Yang P, Ding X, Lei Q, Kang P. Responses of Soil Microbial Survival Strategies and Functional Changes to Wet–Dry Cycle Events. Microorganisms. 2023; 11(11):2783. https://doi.org/10.3390/microorganisms11112783
Chicago/Turabian StyleZhang, Yaqi, Chunyi Mo, Yaqing Pan, Pengbin Yang, Xiaodong Ding, Qian Lei, and Peng Kang. 2023. "Responses of Soil Microbial Survival Strategies and Functional Changes to Wet–Dry Cycle Events" Microorganisms 11, no. 11: 2783. https://doi.org/10.3390/microorganisms11112783
APA StyleZhang, Y., Mo, C., Pan, Y., Yang, P., Ding, X., Lei, Q., & Kang, P. (2023). Responses of Soil Microbial Survival Strategies and Functional Changes to Wet–Dry Cycle Events. Microorganisms, 11(11), 2783. https://doi.org/10.3390/microorganisms11112783






