1. Introduction
Epichloë endophytes commonly grow in the apoplast of the aerial tissues of a variety of cool-season grasses without causing disease [
1,
2]. Following an extended period of adaptive evolution, a mutually beneficial symbiotic relationship is established between endophytic fungi and cold season grasses. In this relationship, endophytes are capable of acquiring nutrients from their host plants and utilizing the host plant as their habitat sites [
3]. Endophytic fungi have been found to influence plant productivity, plant–plant interactions, the structure and biodiversity of plant communities, and the conservation and restoration of ecosystems [
4,
5]. Thus far, the importance of endophytic fungal communities in mitigating key abiotic stresses has been well studied. However, there are few reports on whether grasses and their symbiotic microorganisms can offset the harmful effects of root hemiparasitic plants in the broader context of biological stress.
Root hemiparasitic plants depend largely on haustoria to obtain nutrients and water from their host plants [
6,
7] and subsequently weaken their growth [
8,
9]. Hemiparasitic plants compete with host plants for light resources, which in turn alters the competitive relationship between host and non-host plants. This competition has a significant impact on the composition and structure of plant communities [
10,
11]. At present, the majority of root hemiparasitic plants have become obnoxious forbs that seriously harm agriculture and animal husbandry [
12,
13]. For example,
Striga hermonthica is parasitic on sorghum [
14], maize [
15], and other crops, resulting in an estimated yield reduction of 65% or even no harvest in some cases. In China, there are many kinds of root hemiparasitic plants, but most are scattered and rarely clustered [
16], which has resulted in insufficient attention being paid to the hazardous risks of this group. However, in recent years, some root hemiparasitic plant species have shown significant spreading trends, which pose a potential threat to the sustainable use of grasslands and the healthy development of animal husbandry [
17,
18]. A typical example is the expanded population of
P. kansuensis into Qinghai, Xinjiang, southwestern Gansu, western Sichuan, and western Tibet [
19].
P. kansuensis is an endemic annual or biennial root hemiparasitic herb found in China [
20]. Since 2000, the native species of
P. kansuensis has been expanding rapidly, which has threatened the local livestock industry [
17,
21]. The hemiparasitic nature of its roots is an exclusive survival strategy employed by
P. kansuensis. Specifically, it relies on its specialized structure called haustoria to obtain a portion of water and nutrients from gramineous and leguminous plants [
22], resulting in the biomass of gramineous and leguminous plants rapidly decreasing [
20,
23,
24,
25]. Recent studies have demonstrated that plant symbiotic microorganisms have the ability to greatly enhance their host’s tolerance to root parasitic stress [
26,
27]. In a study conducted by Sui et al. [
28], it was discovered that the growth performance of both hosts and parasites in
Trifolium repens and
Pedicularis rex/tricolor legume pairs was greatly enhanced through inoculation with
Glomus mosseae. This inoculation also helped alleviate the harm caused by
P. rex/tricolor. At present, most studies focus on
Epichloë endophytes in improving host grass resistance ability [
29]. However, there are currently few reports on the regulation of carbon uptake and allocation by endophytic fungi in grasses and root hemiparasitic systems.
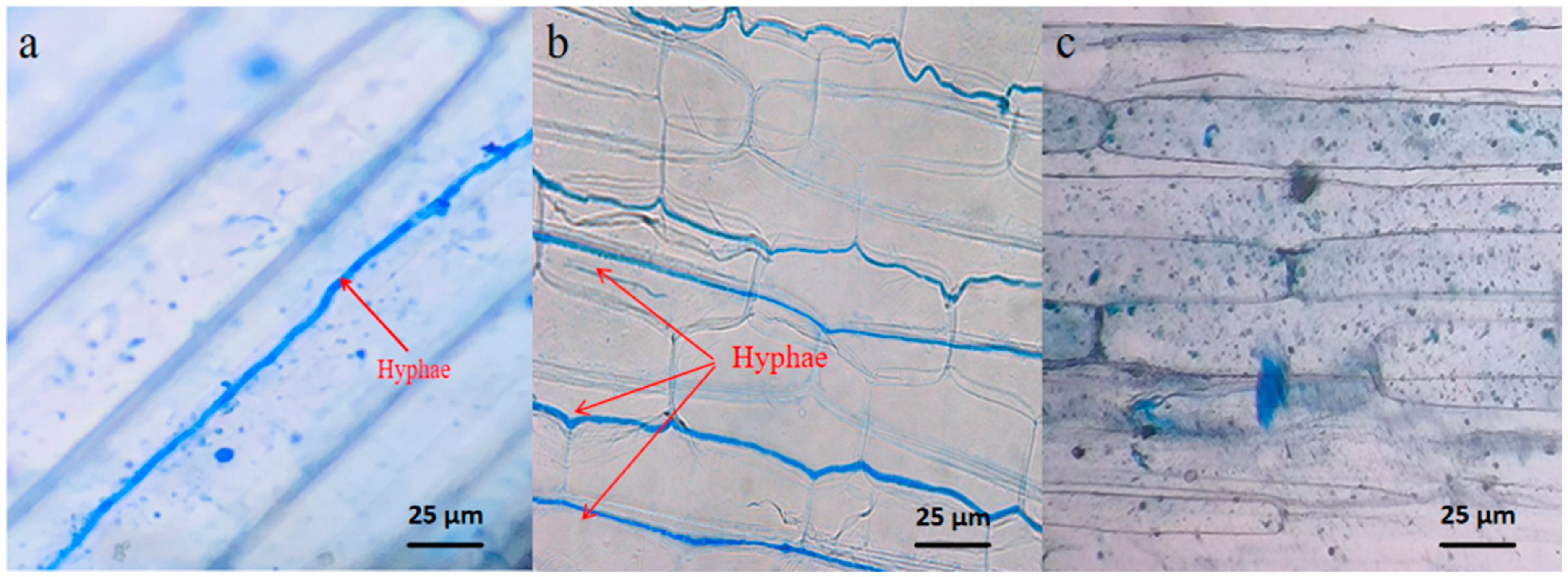
Our previous study found that
P. kansuensis could establish root parasitic relationships with 18 sympatric plants and that
S. purpurea was revealed to be the main host of
P. kansuensis [
30]. Meanwhile, the infection rate of endophytic fungi in the degraded grassland of
S. purpurea was found to be more than 90%. The type of endophytic fungi responsible for the infection was identified as
Epichloë inebrians [
31]. Therefore, in this study, we utilized endophyte-infected (E+) and endophyte-free (E−)
S. purpurea as our materials to study the plant growth of
S. purpurea under different intensities of
P. kansuensis parasitization and normal growth conditions. Accordingly, this study aims to address the following questions: (1) What are the growth characteristics of E+ and E−
S. purpurea and
P. kansuensis? (2) What are the total photosynthetic carbon and distribution characteristics of
S. purpurea when transferred to
P. kansuensis? (3) How does photosynthetic carbon sequestration and allocation differ between E+ and E−
S. purpurea?
4. Discussion
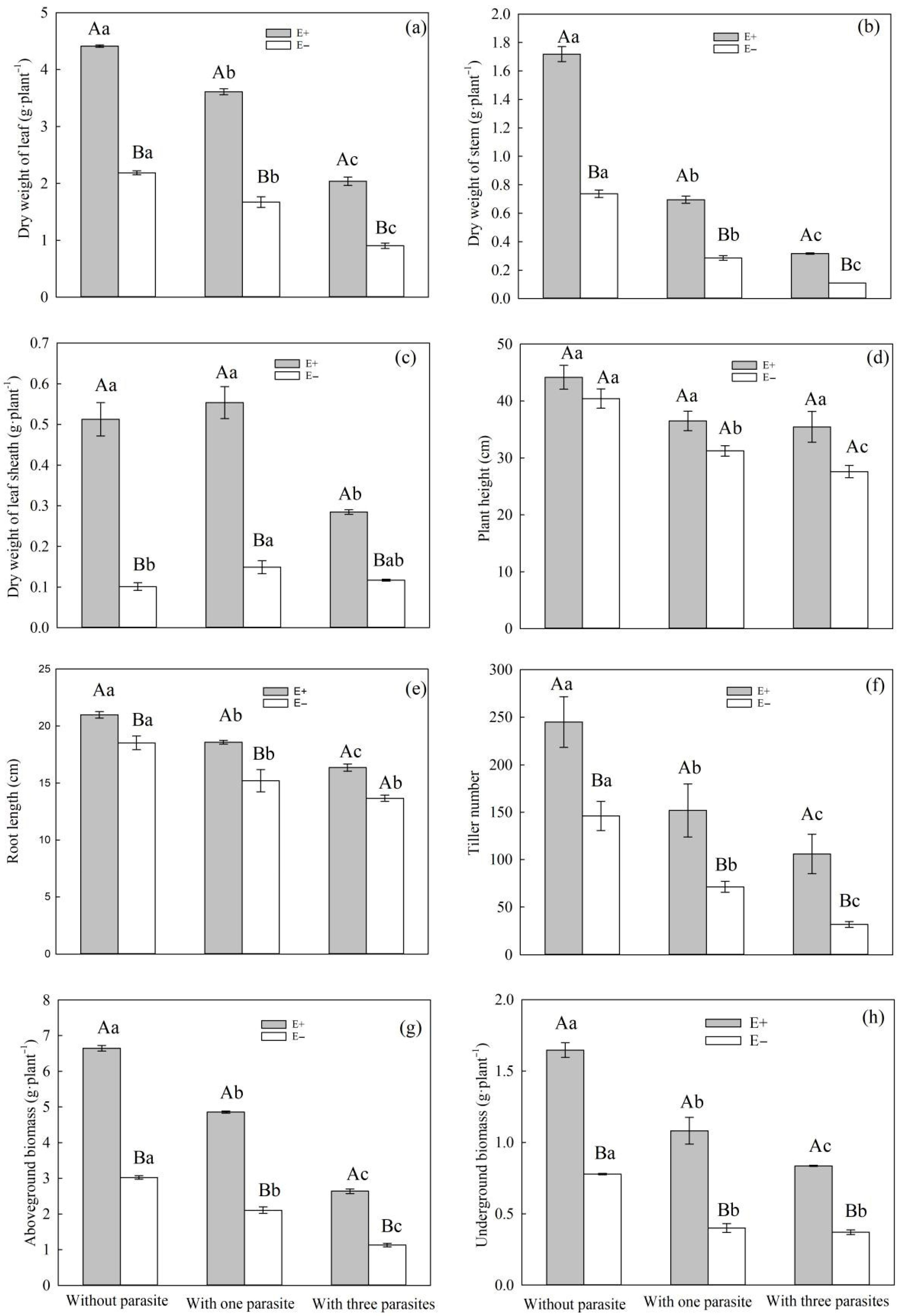
Root parasite plants usually rob their hosts of water, carbohydrates, and nutrients to meet their own growth needs [
7,
38]. Numerous studies have demonstrated that root parasitic plants can negatively impact the growth characteristics of their host plants [
39,
40]. In our experiment, we observed that when
P. kansuensis parasitized
S. purpurea, there was a decrease in the leaf weight, stem weight, sheath weight, aboveground biomass, underground biomass, plant height, root length, and tillering number of
S. purpurea (
Figure 3a–h). Meanwhile, as the parasitic density of
P. kansuensis increased, the inhibitory effect also increased gradually.
Some studies have suggested that endophytic fungi can enhance a plant’s ability to withstand both biotic and abiotic stresses [
41,
42,
43]. In the context of root parasitic stress, our study revealed that E+
S. purpurea exhibited a greater leaf weight, stem weight, sheath weight, aboveground biomass, underground biomass, root length, and tillering number compared to E− plants (
Figure 3a–h). In conclusion, we found that endophytic fungal infection can enhance the tolerance of
S. purpurea to the root hemiparasitic stress caused by
P. kansuensis. Furthermore, when
P. kansuensis parasitized
S. purpurea with an endophytic fungal infection, it had varying effects on the plant’s height, root length, aboveground biomass, and underground biomass.
It is worth noting that after
P. kansuensis parasitized E+
S. purpurea, we observed that its stems and leaves easily withered, but new stems and leaves could be regrown. This could be due to the infection of endophytic fungi inhibiting
P. kansuensis from getting nutrients from
S. purpurea, thereby causing the living environment of
P. kansuensis to deteriorate. However, in the case of
P. kansuensis parasitized with E+
S. purpurea, its aboveground and underground biomasses were higher than those of
P. kansuensis parasitized with E−
S. purpurea (
Figure 4c,d); the reason for this difference may be that the endophytic fungus infection may enhance the photosynthetic capacity of
S. purpurea under stressful conditions. This can lead to the accumulation of more carbohydrates, which would enable
S. purpurea and root parasitic plants to meet their growth requirements. Consequently, endophytic fungi help alleviate the root hemiparasitic damage caused by
P. kansuensis on
S. purpurea.
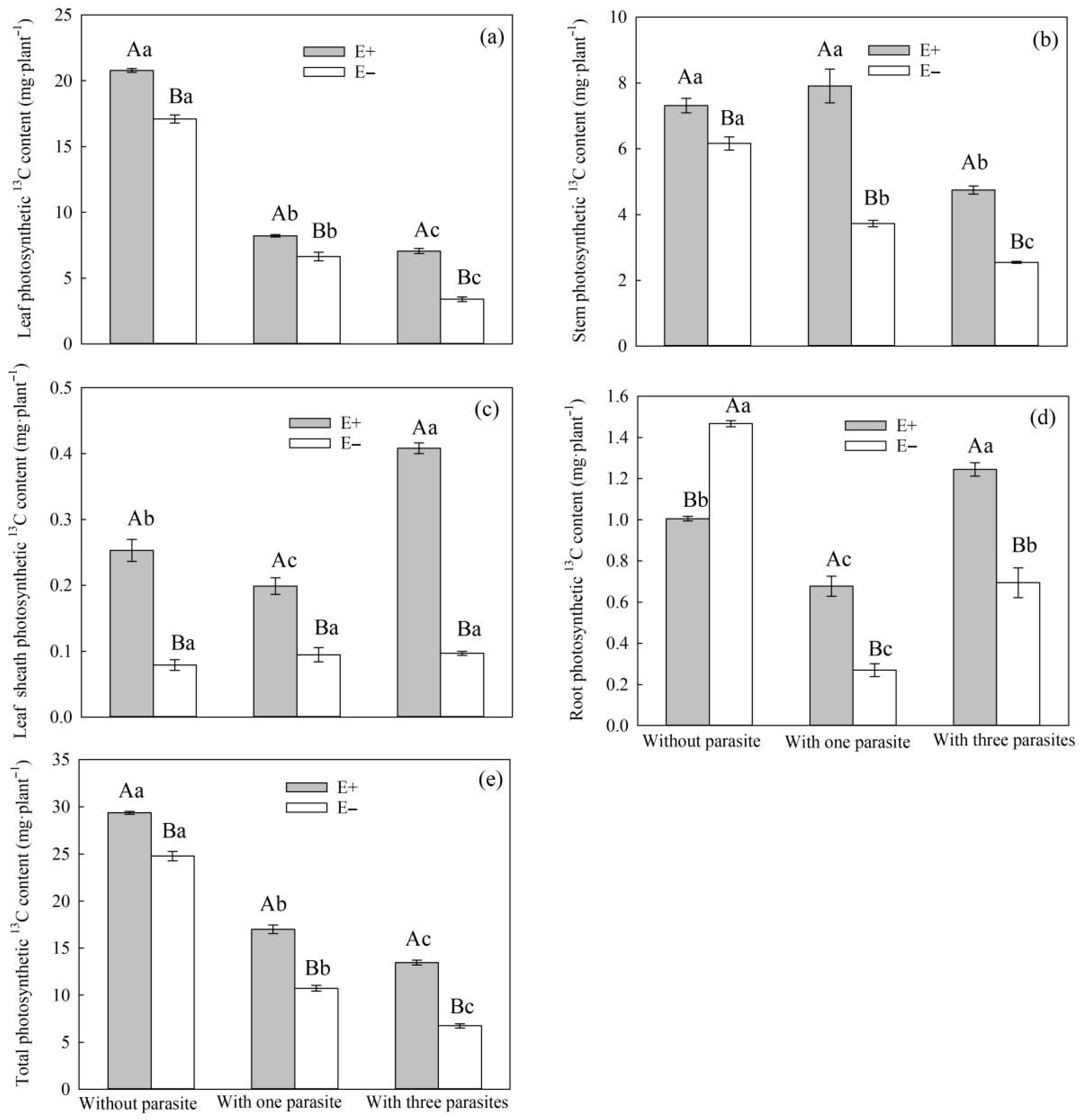
In this study, we found that when the parasitic density of P. kansuensis was varied, the aboveground, underground, and total biomasses of the E+ S. purpurea and P. kansuensis parasitism systems were higher compared to the E− S. purpurea and P. kansuensis parasitism systems. The results of this study suggest that endophytic fungi enhance the light capture capacity of the aboveground part of S. purpurea by influencing its growth characteristics. Additionally, endophytic fungi promote root growth and improve a plant’s ability to compete for nutrient absorption in the subsurface. By reducing the number of haustoria of P. kansuensis per unit area, the nutrient plunder from S. purpurea is minimized, resulting in an increased accumulation of dry matter in the host–parasitic plant system.
The process of plant growth is influenced by both material accumulation and physiological metabolism, which are, in turn, affected by plant photosynthesis. Approximately 90% of the materials required for plant growth are derived from organic compounds produced through photosynthesis, which typically have a carbon content ranging from 40 to 50% [
44]. Photosynthetic carbon is the starting point of carbon metabolism in plants, which is the most important metabolic activity in the process of plant growth and development. This function mainly includes the assimilation of inorganic carbon (CO
2) into organic carbon and the metabolic processes of carbohydrate transformation, transport, accumulation, and decomposition among different plant tissues [
45,
46]. Thus, the quantitative allocation and fixation of photosynthetic carbon is of great significance for studying the allocation of fixed carbon in plants, while also serving as an important means by which to analyze the influence of external factors on plant growth [
46,
47].
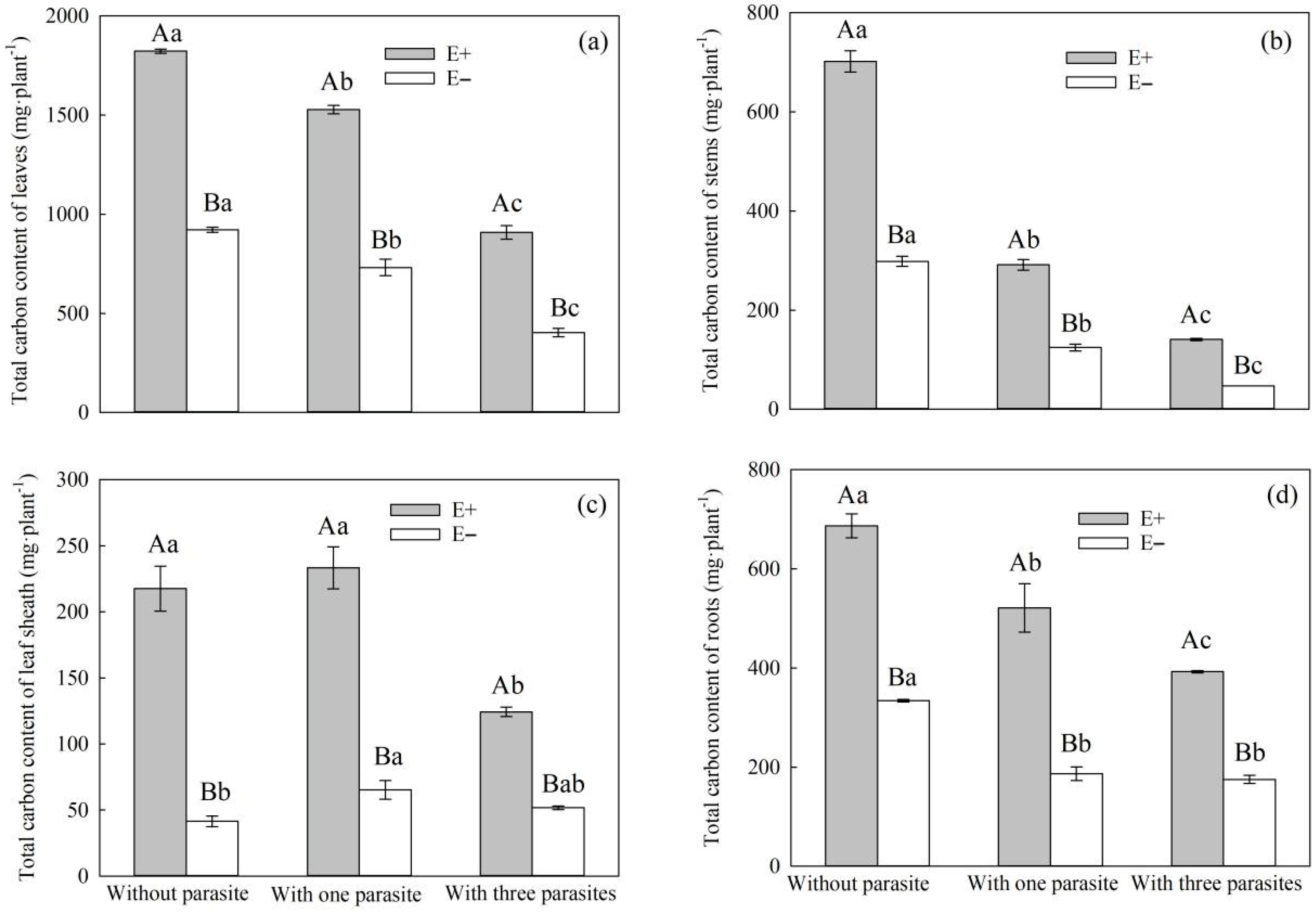
Parasitic plants use the root haustorium to establish a parasitic relationship with the host, and part of the carbohydrate produced by the host through photosynthesis is ingested by parasitic plants through phloem channels [
7,
48]. Studies have shown that root hemiparasitic plants can directly inhibit the photosynthetic metabolism of hosts and reduce their aboveground biomass [
49,
50,
51,
52]. In this study, with the increase in the parasitic density of
P. kansuensis, the total carbon contents of the leaves, stems, and roots of
S. purpurea decreased, and the total carbon contents of E+
S. purpurea leaves, stems, leaf sheaths, and roots were higher than those of E− plants (
Figure 6a–d). This is consistent with Suryanarayanan et al.’s conclusion that endophytic fungi have a positive effect on the photosynthetic characteristics of plants [
53]. In addition, with the increase in the density of
P. kansuensis, the total carbon content of the stem leaves and roots of
P. kansuensis increased, regardless of whether there was a host, and the total carbon contents of the stem leaves and roots of
P. kansuensis parasitizing E+
S. purpurea were higher than those of
P. kansuensis parasitica E− plants. However, the total carbon contents of the stem leaves and roots of
P. kansuensis parasitica E−
S. purpurea were lower than those of plants without a parasite (
Figure 8a,b). On the one hand, the inhibition of photosynthesis in E−
S. purpurea under root hemiparasitic stress could have led to a decrease in the carbon allocation absorbed by
P. kansuensis. On the other hand, in the pot experiment, both
S. purpurea and
P. kansuensis may have competed for nutrients, resulting in insufficient nutrient availability for both species. In contrast, the host-free
P. kansuensis could fully absorb the required nutrients.
Up to now, the positive effect of endophytic fungi on photosynthetic carbon fixation has been widely accepted [
53], but its mechanism needs to be further explored.
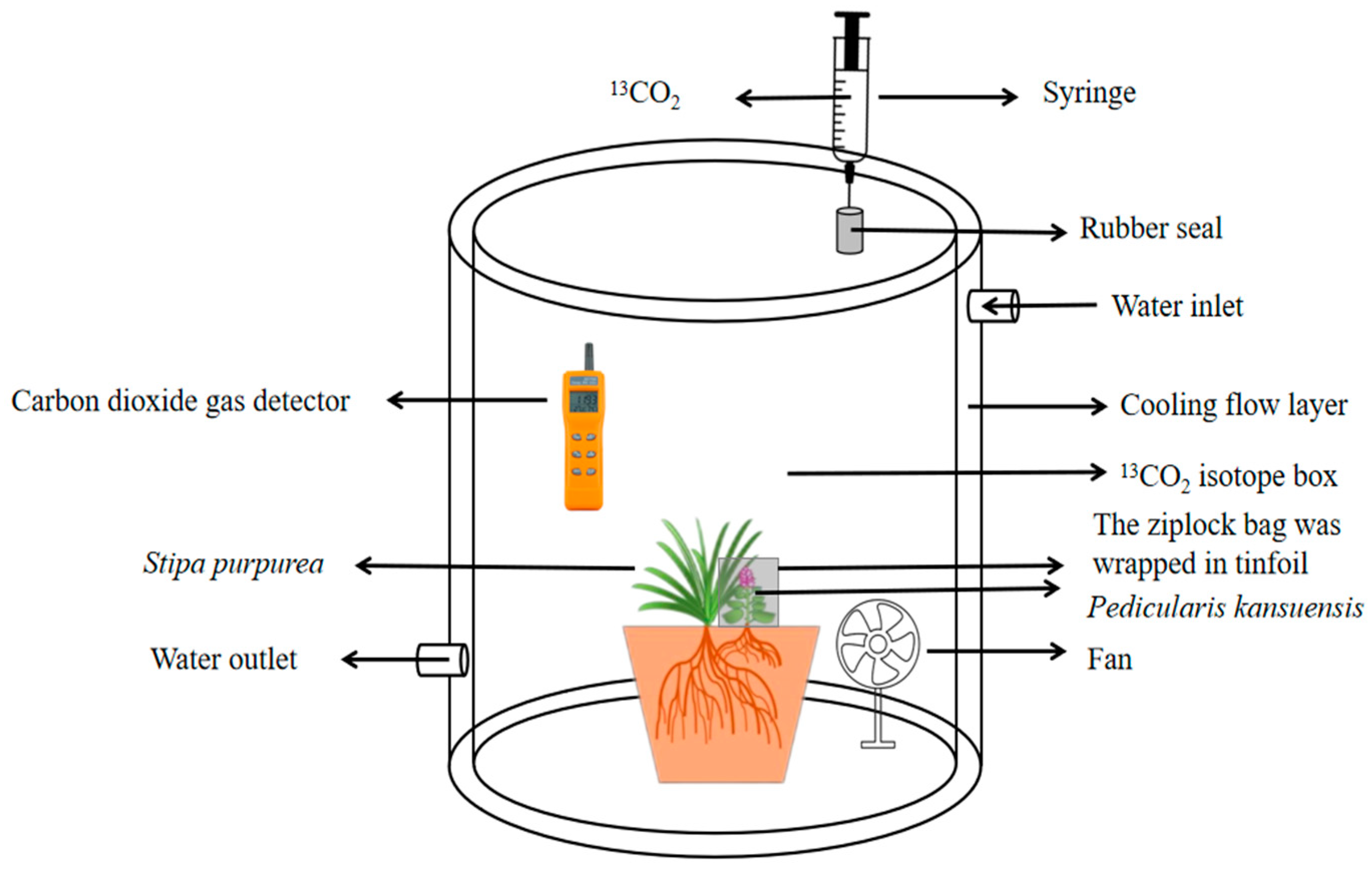
13C pulse labeling can be used to study the distribution of photosynthetic carbon in plants at a certain period in time [
47,
54,
55]. Therefore,
13C pulse labeling can be used to study the photosynthetic carbon allocation in the parasitic system established by host–parasite plants [
37]. In this study,
13CO
2 isotope labeling was used to quantitatively analyze the photosynthetic
13C amount and
13C allocation rate of
P. kansuensis that was transferred from
S. purpurea and the total carbon content of
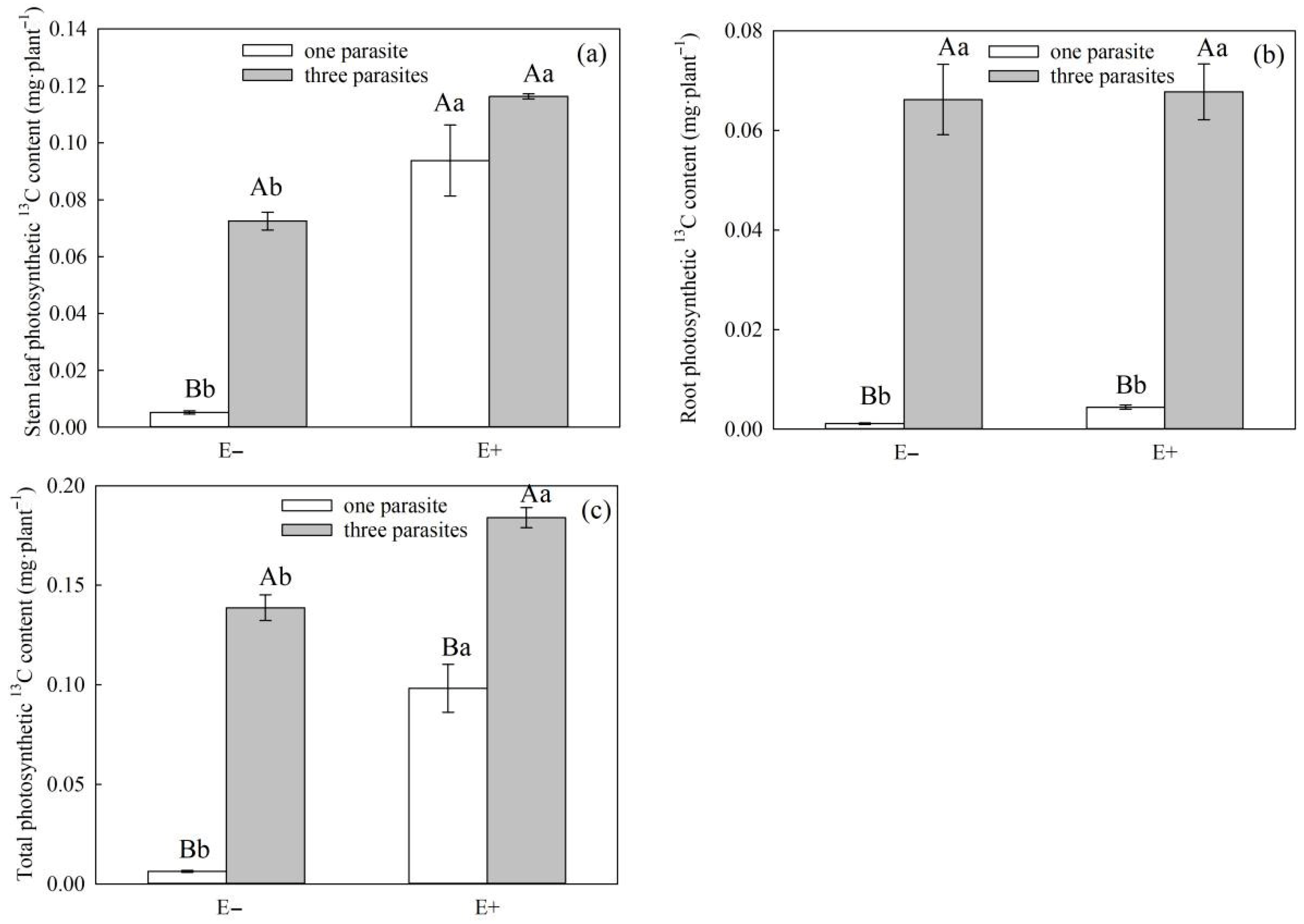
P. kansuensis. In our experiment, the stem photosynthetic 13C of high-density parasitic E+
S. purpurea was significantly higher compared to that of non-parasitic E+ plants (
Figure 8b,
p < 0.05), while that of low-density parasitic E+
S. purpurea was higher than that of non-parasitic E+
S. purpurea, although the differences were not significant (
Figure 8b,
p > 0.05). This may be the result of the transition from a mutualistic relationship to a competitive one between endophytic fungi and
S. purpurea. Some studies have shown that endophytic fungi are most frequently distributed in the stem internode and leaf sheath [
56], and the difference in the photosynthetic
13C in the stem and leaf sheath of E+
S. purpurea under different parasitic densities may be the result of different distribution densities of endophytic fungi. At the same time, compared with the high-density parasitism in
P. kansuensis, carbon fixed by photosynthesis and carbohydrates formed by carbon cycling can meet the growth requirements of
S. purpurea, endophytic fungi, and root parasitism plants. However, in our study, the carbon and carbohydrate synthesized from the stems and leaves of
S. purpurea could not meet the above three requirements at the same time under the high-density parasitic stress of
P. kansuensis. To maintain its normal growth,
S. purpurea preferentially delivers more carbon and carbohydrates to the roots, which is consistent with the result that the root biomass of E+
S. purpurea was higher than that of E−
S. purpurea under heavy root parasitic stress. This conclusion is also consistent with the survival strategy that plants preferentially distribute nutrients to the roots, through which they also obtain nutrient resources in the soil, under the condition of nutrient scarcity [
57].
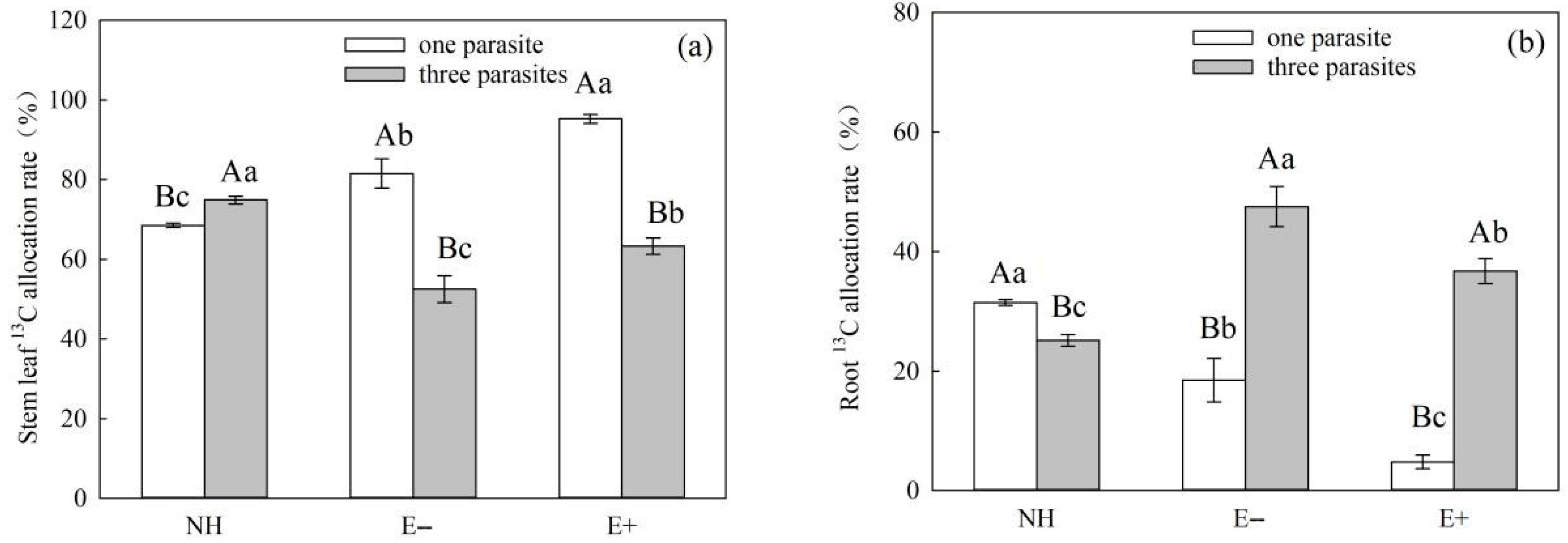
It Is worth noting that under conditions of heavy parasitism, the
13C allocation rates of the stem and root of E+
S. purpurea were lower than those of E−
S.
purpurea (
Figure 10a–d,
p > 0.05), although the differences were not significant. However, the photosynthetic
13C amounts in the stem and root of E+
S.
purpurea were significantly higher compared to those of E− plants (
Figure 8a–d,
p < 0.05). These results indicate that the carbon metabolic efficiency of E+
S. purpurea is higher than that of E− plants under the condition of heavy parasitism of
P. kansuensis, which further supports the idea that endophytic fungi can improve the photosynthetic capacity of host grasses and thus enhance the carbon utilization efficiency. Meanwhile, the photosynthetic
13C amounts in the stems, leaves, and whole plants of
P. kansuensis that were transferred from E+
S. purpurea were higher than those transferred from E−
S. purpurea, but there was no difference in the photosynthetic
13C amounts of
P. kansuensis transferred from the roots (
Figure 9a–c). Furthermore, the photosynthetic
13C allocation rate of
P. kansuensis transferred from
S. purpurea further supports the above view. Compared with
P. kansuensis, which was parasitic upon E−
S. purpurea, the photosynthetic allocation rate of
13C in the stem leaves of
P. kansuensis parasitized by E+
S. purpurea was higher, but the photosynthetic allocation rate of
13C was significantly decreased when the roots were transferred from
S. purpurea (
Figure 11a,b). These results further indicate that endophytic fungi regulate the distribution ratio of photosynthetic
13C in the roots and stem leaves of
P. kansuensis when transferred from
S. purpurea in an environment of heavy parasitism. In other words,
P. kansuensis’s stem leaves receive a preferential distribution of photosynthetic
13C, which makes the root’s primary source of carbon photosynthetic carbon, which is then used to offset the plant’s excessive uptake of
S. purpurea’s carbohydrates. At the same time, the regulatory mechanism of photosynthetic carbon allocation by endophytic fungi on the transfer of
P. kansuensis from
S. purpurea could further explain why
P. kansuensis accumulated more biomass when parasitized by E+
S. purpurea (
Figure 4).