Extracellular Vesicles Secreted by Acanthamoeba culbertsoni Have COX and Proteolytic Activity and Induce Hemolysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Amoeba Culture
2.2. Extracellular Vesicle (EV) Isolation
2.3. EVs Characterization
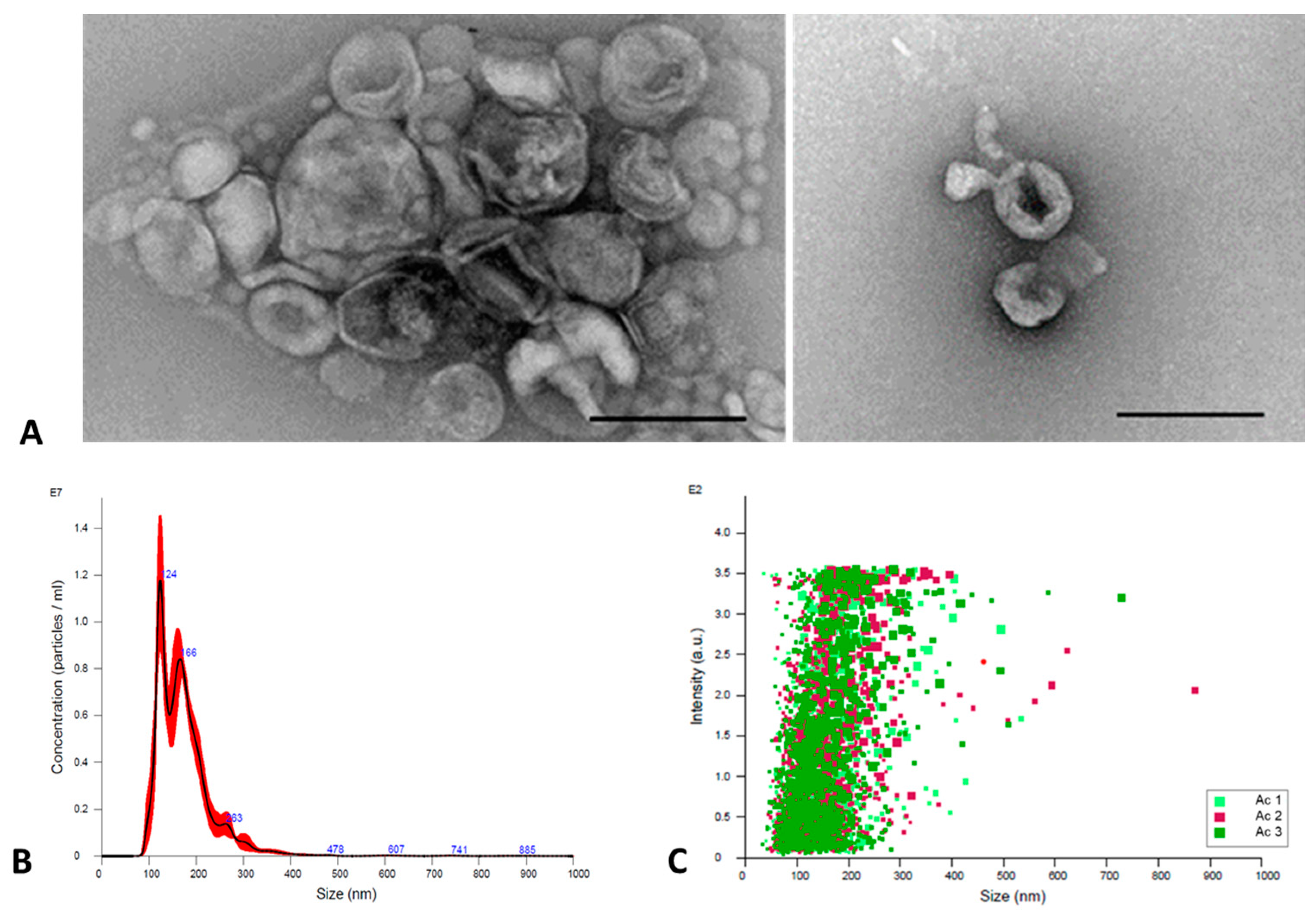
2.3.1. TEM
2.3.2. NTA
2.4. EVs Emission
2.4.1. Confocal Microscopy
2.4.2. TEM
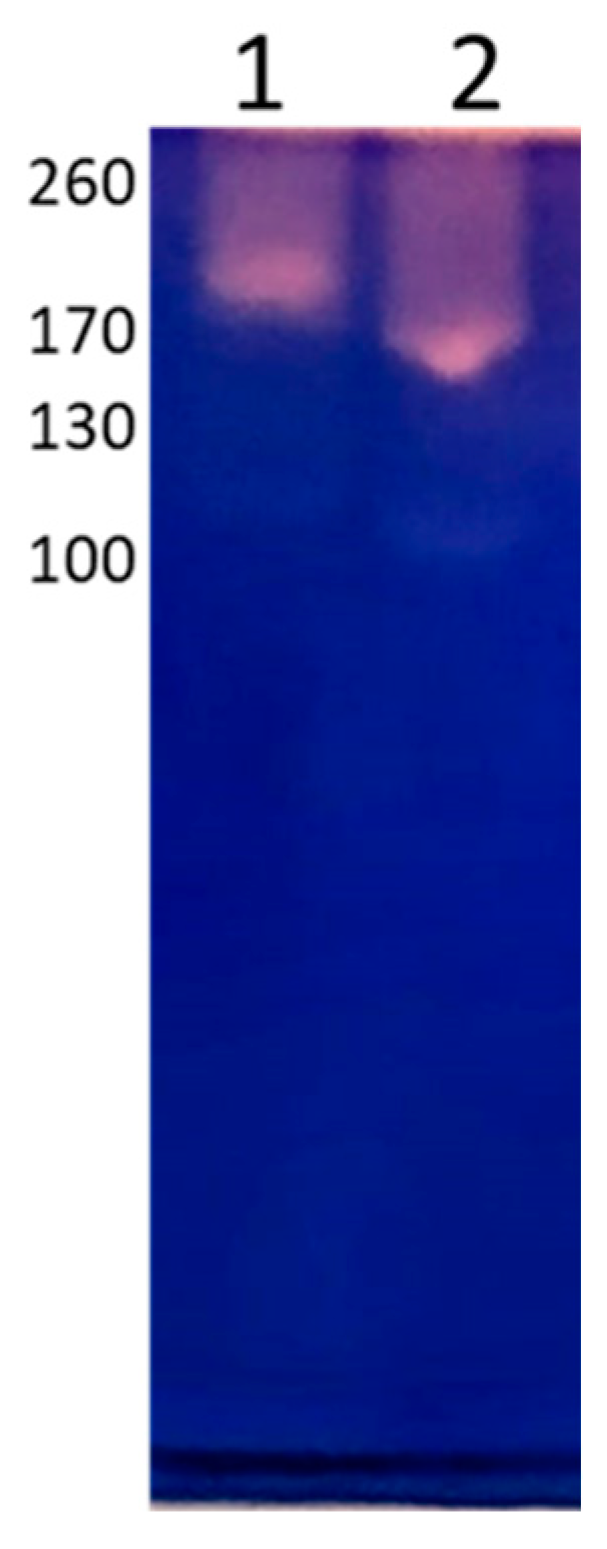
2.5. Determination of the Protein Pattern by Electrophoresis and Immunorecognition by Western Blot
2.6. Internalization of EVs in A. culbertsoni Trophozoites
2.7. Biological EVs Activity
2.7.1. Proteolytic Activity
2.7.2. Determination of Hemolysis Activity in EVs
2.7.3. Determination of Cyclooxygenase Activity in Trophozoites and EVs
2.7.4. Leishmanolysin-like Protein Detection in A. culbertsoni Trophozoites and EVs
2.8. Statistical Analysis
3. Results
3.1. Extracellular Vesicles (EVs) Characterization
3.2. EVs Emission
3.3. Protein Pattern by Electrophoresis and Immunorecognition by Western Blot
3.4. Internalization of EVs in A. culbertsoni Trophozoites
3.5. Biological EVs Activity
3.5.1. Proteolytic Activity
3.5.2. Hemolysis
3.5.3. Determination of Cyclooxygenase Activity in Trophozoites and EVs
3.5.4. Leishmanolysin-like Protein Detection in A. culbertsoni Trophozoites and EVs
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rosenberg, K.J.; Bertaux, K.; Krome, A.; Hartmann, A.; Scheu, S.; Bonkowski, M. Soil amoebae rapidly change bacterial community 660 composition in the rhizosphere of Arabidopsis thaliana. ISME J. 2009, 3, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Schuster, F.L.; Visvesvara, G.S. Opportunistic amoebae: Challenges in prophylaxis and treatment. Drug Resist. Updates 2004, 7, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Kot, K.; Lanocha-Arendarczyk, N.; Kosik-Bogacka, D. Immunopathogenicity of Acanthamoeba spp. in the Brain and Lungs. Int. J. Mol. Sci. 2021, 22, 1261. [Google Scholar] [CrossRef]
- Martinez, A.J.; Visvesvara, G.S. Free-living, amphizoic and opportunistic amebas. Brain Pathol. 1997, 7, 583–598. [Google Scholar] [CrossRef] [PubMed]
- Reddy, D.L.; van den Berg, E.; Grayson, W.; Mphahlele, M.; Frean, J. Clinical Improvement of Disseminated Acanthamoeba Infection in a Patient with Advanced HIV Using a Non-Miltefosine-Based Treatment Regimen in a Low-Resource Setting. Trop. Med. Infect. Dis. 2022, 7, 24. [Google Scholar] [CrossRef] [PubMed]
- Damhorst, G.L.; Watts, A.; Hernandez-Romieu, A.; Mel, N.; Palmore, M.; Ali, I.B.K.; Neill, S.G.; Kalapila, A.; Cope, J.R. Acanthamoeba castellanii encephalitis in a patient with AIDS: A case report and literature review. Lancet Infect. Dis. 2022, 22, e59–e65. [Google Scholar] [CrossRef] [PubMed]
- Keane, N.A.; Lane, L.M.; Canniff, E.; Hare, D.; Doran, S.; Wallace, E.; Hutchinson, S.; Healy, M.L.; Hennessy, B.; Meaney, J.; et al. A Surviving Case of Acanthamoeba Granulomatous Amebic Encephalitis in a Hematopoietic Stem Cell Transplant Recipient. Am. J. Case Rep. 2020, 21, e923219. [Google Scholar] [CrossRef]
- Flores-Maldonado, C.; Gonzalez-Robles, A.; Salazar-Villatoro, L.; Omana-Molina, M.; Gallardo, J.M.; Gonzalez-Lazaro, M.; Hernandez-Ramirez, V.I.; Talamas-Rohana, P.; Lorenzo-Morales, J.; Martinez-Palomo, A. Acanthamoeba (T4) trophozoites cross the MDCK epithelium without cell damage but increase paracellular permeability and transepithelial resistance by modifying tight junction composition. Exp. Parasitol. 2017, 183, 69–75. [Google Scholar] [CrossRef]
- Omana-Molina, M.; Gonzalez-Robles, A.; Salazar-Villatoro, L.I.; Lorenzo-Morales, J.; Cristobal-Ramos, A.R.; Hernandez-Ramirez, V.I.; Talamas-Rohana, P.; Mendez Cruz, A.R.; Martinez-Palomo, A. Reevaluating the role of Acanthamoeba proteases in tissue invasion: Observation of cytopathogenic mechanisms on MDCK cell monolayers and hamster corneal cells. Biomed. Res. Int. 2013, 2013, 461329. [Google Scholar] [CrossRef]
- Omana-Molina, M.; Hernandez-Martinez, D.; Sanchez-Rocha, R.; Cardenas-Lemus, U.; Salinas-Lara, C.; Mendez-Cruz, A.R.; Colin-Barenque, L.; Aley-Medina, P.; Espinosa-Villanueva, J.; Moreno-Fierros, L.; et al. In vivo CNS infection model of Acanthamoeba genotype T4: The early stages of infection lack presence of host inflammatory response and are a slow and contact-dependent process. Parasitol. Res. 2017, 116, 725–733. [Google Scholar] [CrossRef]
- Omana-Molina, M.; Sanchez-Rocha, R.; Hernandez-Martinez, D.; Romero Grijalva, M.; Salinas-Lara, C.; Rodriguez-Sosa, M.; Juarez-Avelar, I.; Salazar-Villatoro, L.; Gonzalez-Robles, A.; Mendez-Cruz, A.R.; et al. Type 2 diabetes mellitus BALB/c mice are more susceptible to granulomatous amoebic encephalitis: Immunohistochemical study. Exp. Parasitol. 2017, 183, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Corsaro, D. Acanthamoeba Mannose and Laminin Binding Proteins Variation across Species and Genotypes. Microorganisms 2022, 10, 2162. [Google Scholar] [CrossRef] [PubMed]
- Garate, M.; Cubillos, I.; Marchant, J.; Panjwani, N. Biochemical characterization and functional studies of Acanthamoeba mannose-binding protein. Infect. Immun. 2005, 73, 5775–5781. [Google Scholar] [CrossRef] [PubMed]
- Kennett, M.J.; Hook, R.R., Jr.; Franklin, C.L.; Riley, L.K. Acanthamoeba castellanii: Characterization of an adhesin molecule. Exp. Parasitol. 1999, 92, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Rocha-Azevedo, B.D.; Jamerson, M.; Cabral, G.A.; Silva-Filho, F.C.; Marciano-Cabral, F. Acanthamoeba interaction with extracellular matrix glycoproteins: Biological and biochemical characterization and role in cytotoxicity and invasiveness. J. Eukaryot. Microbiol. 2009, 56, 270–278. [Google Scholar] [CrossRef]
- Brittingham, A.; Chen, G.; McGwire, B.S.; Chang, K.P.; Mosser, D.M. Interaction of Leishmania gp63 with cellular receptors for fibronectin. Infect. Immun. 1999, 67, 4477–4484. [Google Scholar] [CrossRef]
- Hernandez-Ramirez, V.I.; Estrada-Figueroa, L.A.; Medina, Y.; Lizarazo-Taborda, M.R.; Toledo-Leyva, A.; Osorio-Trujillo, C.; Morales-Mora, D.; Talamas-Rohana, P. A monoclonal antibody against a Leishmania mexicana COX-like enzymatic activity also recognizes similar proteins in different protozoa of clinical importance. Parasitol. Res. 2023, 122, 479–492. [Google Scholar] [CrossRef]
- Coronado-Velazquez, D.; Silva-Olivares, A.; Castro-Munozledo, F.; Lares-Jimenez, L.F.; Rodriguez-Anaya, L.Z.; Shibayama, M.; Serrano-Luna, J. Acanthamoeba mauritaniensis genotype T4D: An environmental isolate displays pathogenic behavior. Parasitol. Int. 2020, 74, 102002. [Google Scholar] [CrossRef]
- Gonzalez-Robles, A.; Omana-Molina, M.; Salazar-Villatoro, L.; Flores-Maldonado, C.; Lorenzo-Morales, J.; Reyes-Batlle, M.; Arnalich-Montiel, F.; Martinez-Palomo, A. Acanthamoeba culbertsoni isolated from a clinical case with intraocular dissemination: Structure and in vitro analysis of the interaction with hamster cornea and MDCK epithelial cell monolayers. Exp. Parasitol. 2017, 183, 245–253. [Google Scholar] [CrossRef]
- Omana-Molina, M.; Navarro-Garcia, F.; Gonzalez-Robles, A.; Serrano-Luna, J.J.; Campos-Rodriguez, R.; Martinez-Palomo, A.; Tsutsumi, V.; Shibayama, M. Induction of morphological and electrophysiological changes in hamster cornea after in vitro interaction with trophozoites of Acanthamoeba spp. Infect. Immun. 2004, 72, 3245–3251. [Google Scholar] [CrossRef]
- Castelan-Ramirez, I.; Salazar-Villatoro, L.; Chavez-Munguia, B.; Salinas-Lara, C.; Sanchez-Garibay, C.; Flores-Maldonado, C.; Hernandez-Martinez, D.; Anaya-Martinez, V.; Avila-Costa, M.R.; Mendez-Cruz, A.R.; et al. Schwann Cell Autophagy and Necrosis as Mechanisms of Cell Death by Acanthamoeba. Pathogens 2020, 9, 458. [Google Scholar] [CrossRef] [PubMed]
- Alsam, S.; Sissons, J.; Dudley, R.; Khan, N.A. Mechanisms associated with Acanthamoeba castellanii (T4) phagocytosis. Parasitol. Res. 2005, 96, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, G.A.; Magliano, A.C.M.; Pral, E.M.F.; Alfieri, S.C. Elastase secretion in Acanthamoeba polyphaga. Acta Trop. 2009, 112, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Matin, A.; Jung, S.J. Phospholipase activities in clinical and environmental isolates of Acanthamoeba. Korean J. Parasitol. 2011, 49, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.A.; Siddiqui, R. Acanthamoeba affects the integrity of human brain microvascular endothelial cells and degrades the tight junction proteins. Int. J. Parasitol. 2009, 39, 1611–1616. [Google Scholar] [CrossRef]
- Sissons, J.; Alsam, S.; Goldsworthy, G.; Lightfoot, M.; Jarroll, E.K.; Khan, N.A. Identification and properties of proteases from an Acanthamoeba isolate capable of producing granulomatous encephalitis. BMC Microbiol. 2006, 6, 42. [Google Scholar] [CrossRef]
- Kot, K.; Kolodziej, D.; Kupnicka, P.; Kosik-Bogacka, D.I.; Lanocha-Arendarczyk, N. Concentrations of PGE2 and TXB2 in the Eyes of Mice with Disseminated Acanthamoebiasis. Pathogens 2022, 11, 438. [Google Scholar] [CrossRef]
- Chávez-Munguía, B.; Salazar-Villatoro, L.; Omaña-Molina, M.; Espinosa-Cantellano, M.; Ramírez-Flores, E.; Lorenzo-Morales, J.; Martínez-Palomo, A. Acanthamoeba culbertsoni: Electron-Dense Granules in a Highly Virulent Clinical Isolate. J. Eukaryot. Microbiol. 2016, 63, 744–750. [Google Scholar] [CrossRef]
- Carrera-Bravo, C.; Koh, E.Y.; Tan, K.S.W. The roles of parasite-derived extracellular vesicles in disease and host-parasite communication. Parasitol. Int. 2021, 83, 102373. [Google Scholar] [CrossRef]
- Montaner, S.; Galiano, A.; Trelis, M.; Martin-Jaular, L.; Del Portillo, H.A.; Bernal, D.; Marcilla, A. The Role of Extracellular Vesicles in Modulating the Host Immune Response during Parasitic Infections. Front. Immunol. 2014, 5, 433. [Google Scholar] [CrossRef]
- Costa, A.O.; Chagas, I.A.R.; de Menezes-Neto, A.; Rêgo, F.D.; Nogueira, P.M.; Torrecilhas, A.C.; Furst, C.; Fux, B.; Soares, R.P. Distinct immunomodulatory properties of extracellular vesicles released by different strains of Acanthamoeba. Cell Biol. Int. 2021, 45, 1060–1071. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.C.; Tsai, C.Y.; Huang, J.M.; Wu, S.R.; Chu, L.J.; Huang, K.Y. Quantitative proteomic analysis and functional characterization of Acanthamoeba castellanii exosome-like vesicles. Parasit. Vectors. 2019, 12, 467. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, D.S.; Ferreira, M.D.S.; Liedke, S.C.; Gomes, K.X.; de Oliveira, G.A.; Leão, P.E.L.; Cesar, G.V.; Seabra, S.H.; Cortines, J.R.; Casadevall, A.; et al. Extracellular vesicles and vesicle-free secretome of the protozoa Acanthamoeba castellanii under homeostasis and nutritional stress and their damaging potential to host cells. Virulence 2018, 9, 818–836. [Google Scholar] [CrossRef]
- Retana Moreira, L.; Vargas Ramírez, D.; Linares, F.; Prescilla Ledezma, A.; Vaglio Garro, A.; Osuna, A.; Lorenzo Morales, J.; Abrahams Sandí, E. Isolation of Acanthamoeba T5 from Water: Characterization of Its Pathogenic Potential, Including the Production of Extracellular Vesicles. Pathogens 2020, 9, 144. [Google Scholar] [CrossRef]
- Culbertson, C.G.; Smith, J.W.; Cohen, H.K.; Minner, J.R. Experimental infection of mice and monkeys by Acanthamoeba. Am. J. Pathol. 1959, 35, 185–197. [Google Scholar]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Pierson, T.; Matrakas, D.; Taylor, Y.U.; Manyam, G.; Morozov, V.N.; Zhou, W.; van Hoek, M.L. Proteomic characterization and functional analysis of outer membrane vesicles of Francisella novicida suggests possible role in virulence and use as a vaccine. J. Proteome Res. 2011, 10, 954–967. [Google Scholar] [CrossRef]
- Estrada-Figueroa, L.A.; Díaz-Gandarilla, J.A.; Hernández-Ramírez, V.I.; Arrieta-González, M.M.; Osorio-Trujillo, C.; Rosales-Encina, J.L.; Toledo-Leyva, A.; Talamás-Rohana, P. Leishmania mexicana gp63 is the enzyme responsible for cyclooxygenase (COX) activity in this parasitic protozoa. Biochimie 2018, 151, 73–84. [Google Scholar] [CrossRef]
- Van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Nievas, Y.R.; Lizarraga, A.; Salas, N.; Cóceres, V.M.; de Miguel, N. Extracellular vesicles released by anaerobic protozoan parasites: Current situation. Cell Microbiol. 2020, 22, e13257. [Google Scholar] [CrossRef]
- Nievas, Y.R.; Coceres, V.M.; Midlej, V.; de Souza, W.; Benchimol, M.; Pereira-Neves, A.; Vashisht, A.A.; Wohlschlegel, J.A.; Johnson, P.J.; de Miguel, N. Membrane-shed vesicles from the parasite Trichomonas vaginalis: Characterization and their association with cell interaction. Cell Mol. Life Sci. 2018, 75, 2211–2226. [Google Scholar] [CrossRef] [PubMed]
- Gavinho, B.; Sabatke, B.; Feijoli, V.; Rossi, I.V.; da Silva, J.M.; Evans-Osses, I.; Palmisano, G.; Lange, S.; Ramirez, M.I. Peptidylarginine Deiminase Inhibition Abolishes the Production of Large Extracellular Vesicles From Giardia intestinalis, Affecting Host-Pathogen Interactions by Hindering Adhesion to Host Cells. Front. Cell. Infect. Microbiol. 2020, 10, 417. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Godínez, C.; Ríos-Valencia, D.G.; García-Aguirre, S.; Martínez-Calvillo, S.; Carrero, J.C. Immunomodulatory effect of extracellular vesicles from Entamoeba histolytica trophozoites: Regulation of NETs and respiratory burst during confrontation with human neutrophils. Front. Cell. Infect. Microbiol. 2022, 12, 1018314. [Google Scholar] [CrossRef] [PubMed]
- Lê, H.G.; Kang, J.M.; Võ, T.C.; Yoo, W.G.; Na, B.K. Naegleria fowleri Extracellular Vesicles Induce Proinflammatory Immune Responses in BV-2 Microglial Cells. Int. J. Mol. Sci. 2023, 24, 13623. [Google Scholar] [CrossRef]
- Lertjuthaporn, S.; Somkird, J.; Lekmanee, K.; Atipimonpat, A.; Sukapirom, K.; Sawasdipokin, H.; Tiewcharoen, S.; Pattanapanyasat, K.; Khowawisetsut, L. Extracellular Vesicles from Naegleria fowleri Induce IL-8 Response in THP-1 Macrophage. Pathogens 2022, 11, 632. [Google Scholar] [CrossRef]
- Retana Moreira, L.; Steller Espinoza, M.F.; Chacón Camacho, N.; Cornet-Gomez, A.; Sáenz-Arce, G.; Osuna, A.; Lomonte, B.; Abrahams Sandí, E. Characterization of Extracellular Vesicles Secreted by a Clinical Isolate of Naegleria fowleri and Identification of Immunogenic Components within Their Protein Cargo. Biology 2022, 11, 983. [Google Scholar] [CrossRef]
- De Pontes, L.G.; Altei, W.F.; Galan, A.; Bilić, P.; Guillemin, N.; Kuleš, J.; Horvatić, A.; Ribeiro, L.N.M.; de Paula, E.; Pereira, V.B.R.; et al. Extracellular vesicles in infectious diseases caused by protozoan parasites in buffaloes. J. Venom. Anim. Toxins Incl. Trop. Dis. 2020, 26, e20190067. [Google Scholar] [CrossRef]
- Kalra, H.; Drummen, G.; Mathivanan, S. Focus on Extracellular Vesicles: Introducing the Next Small Big Thing. Int. J. Mol. Sci. 2016, 17, 170. [Google Scholar] [CrossRef]
- Visvesvara, G.S.; Balamuth, W. Comparative studies on related free-living and pathogenic amebae with special reference to Acanthamoeba. J. Protozool. 1975, 22, 245–256. [Google Scholar] [CrossRef]
- Mulcahy, L.; Pink, R.; Carter, D. Routes and mechanisms of extracellular vesicle uptake. J. Extracell. Vesicles. 2014, 3, 24641. [Google Scholar] [CrossRef]
- Fitzner, D.; Schnaars, M.; van Rossum, D.; Krishnamoorthy, G.; Dibaj, P.; Bakhti, M.; Regen, T.; Hanisch, U.K.; Simons, M. Selective transfer of exosomes from oligodendrocytes to microglia by macropinocytosis. J. Cell Sci. 2011, 124, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Douanne, N.; Dong, G.; Amin, A.; Bernardo, L.; Blanchette, M.; Langlais, D.; Olivier, M.; Fernandez-Prada, C. Leishmania parasites exchange drug-resistance genes through extracellular vesicles. Cell Rep. 2022, 40, 111121. [Google Scholar] [CrossRef] [PubMed]
- Twu, O.; de Miguel, N.; Lustig, G.; Stevens, G.C.; Vashisht, A.A.; Wohlschlegel, J.A.; Johnson, P.J. Trichomonas vaginalis exosomes deliver cargo to host cells and mediate host∶parasite interactions. PLoS Pathog. 2013, 9, e1003482. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Morgado, P.; Zhang, H.; Ehrenkaufer, G.; Manna, D.; Singh, U. Characterization of Extracellular Vesicles from Entamoeba histolytica Identifies Roles in Intercellular Communication That Regulates Parasite Growth and Development. Infect. Immun. 2020, 88, e00349-20. [Google Scholar] [CrossRef]
- Harrison, J.L.; Ferreira, G.A.; Raborn, E.S.; Lafrenaye, A.D.; Marciano-Cabral, F.; Cabral, G.A. Acanthamoeba culbertsoni elicits soluble factors that exert anti-microglial cell activity. Infect. Immun. 2010, 78, 4001–4011. [Google Scholar] [CrossRef]
- Li, H.; Edin, M.L.; Gruzdev, A.; Cheng, J.; Bradbury, J.A.; Graves, J.P.; DeGraff, L.M.; Zeldin, D.C. Regulation of T helper cell subsets by cyclooxygenases and their metabolites. Prostaglandins Other Lipid Mediat. 2013, 104–105, 74–83. [Google Scholar] [CrossRef]
- Hadas, E.; Mazur, T. Biosynthesis of prostaglandins in pathogenic and nonpathogenic strains of Acanthamoeba spp. Parasitol. Res. 1997, 83, 296–299. [Google Scholar] [CrossRef]
- Siddiqui, R.; Lakhundi, S.; Iqbal, J.; Khan, N.A. Effect of non-steroidal anti-inflammatory drugs on biological properties of Acanthamoeba castellanii belonging to the T4 genotype. Exp. Parasitol. 2016, 168, 45–50. [Google Scholar] [CrossRef]
- Malvezi, A.D.; Panis, C.; da Silva, R.V.; de Freitas, R.C.; Lovo-Martins, M.I.; Tatakihara, V.L.; Zanluqui, N.G.; Neto, E.C.; Goldenberg, S.; Bordignon, J.; et al. Inhibition of cyclooxygenase-1 and cyclooxygenase-2 impairs Trypanosoma cruzi entry into cardiac cells and promotes differential modulation of the inflammatory response. Antimicrob. Agents Chemother. 2014, 58, 6157–6164. [Google Scholar] [CrossRef]
- Teixeira, J.E.; Sateriale, A.; Bessoff, K.E.; Huston, C.D. Control of Entamoeba histolytica adherence involves metallosurface protease 1, an M8 family surface metalloprotease with homology to leishmanolysin. Infect. Immun. 2012, 80, 2165–2176. [Google Scholar] [CrossRef]
- Oliveira, S.S.C.; Correia, C.A.; Santos, V.S.; da Cunha, E.F.F.; de Castro, A.A.; Ramalho, T.C.; Devereux, M.; McCann, M.; Branquinha, M.H.; Santos, A.L.S. Silver(I) and Copper(II) 1,10-Phenanthroline-5,6-dione Complexes as Promising Antivirulence Strategy against Leishmania: Focus on Gp63 (Leishmanolysin). Trop. Med. Infect. Dis. 2023, 8, 348. [Google Scholar] [CrossRef] [PubMed]
- Dey, I.; Keller, K.; Belley, A.; Chadee, K. Identification and characterization of a cyclooxygenase-like enzyme from Entamoeba histolytica. Proc. Natl. Acad. Sci. USA 2003, 100, 13561–13566. [Google Scholar] [CrossRef] [PubMed]
- Voth, B.R.; Kelly, B.L.; Joshi, P.B.; Ivens, A.C.; McMaster, W.R. Differentially expressed Leishmania major gp63 genes encode cell surface leishmanolysin with distinct signals for glycosylphosphatidylinositol attachment. Mol. Biochem. Parasitol. 1998, 93, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Briaud, P.; Carroll, R.K. Extracellular Vesicle Biogenesis and Functions in Gram-Positive Bacteria. Infect. Immun. 2020, 88, e00433-20. [Google Scholar] [CrossRef] [PubMed]
- Drurey, C.; Maizels, R.M. Helminth extracellular vesicles: Interactions with the host immune system. Mol. Immunol. 2021, 137, 124–133. [Google Scholar] [CrossRef]
- Liebana-Jordan, M.; Brotons, B.; Falcon-Perez, J.M.; Gonzalez, E. Extracellular Vesicles in the Fungi Kingdom. Int. J. Mol. Sci. 2021, 22, 7221. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sierra-López, F.; Castelan-Ramírez, I.; Hernández-Martínez, D.; Salazar-Villatoro, L.; Segura-Cobos, D.; Flores-Maldonado, C.; Hernández-Ramírez, V.I.; Villamar-Duque, T.E.; Méndez-Cruz, A.R.; Talamás-Rohana, P.; et al. Extracellular Vesicles Secreted by Acanthamoeba culbertsoni Have COX and Proteolytic Activity and Induce Hemolysis. Microorganisms 2023, 11, 2762. https://doi.org/10.3390/microorganisms11112762
Sierra-López F, Castelan-Ramírez I, Hernández-Martínez D, Salazar-Villatoro L, Segura-Cobos D, Flores-Maldonado C, Hernández-Ramírez VI, Villamar-Duque TE, Méndez-Cruz AR, Talamás-Rohana P, et al. Extracellular Vesicles Secreted by Acanthamoeba culbertsoni Have COX and Proteolytic Activity and Induce Hemolysis. Microorganisms. 2023; 11(11):2762. https://doi.org/10.3390/microorganisms11112762
Chicago/Turabian StyleSierra-López, Francisco, Ismael Castelan-Ramírez, Dolores Hernández-Martínez, Lizbeth Salazar-Villatoro, David Segura-Cobos, Catalina Flores-Maldonado, Verónica Ivonne Hernández-Ramírez, Tomás Ernesto Villamar-Duque, Adolfo René Méndez-Cruz, Patricia Talamás-Rohana, and et al. 2023. "Extracellular Vesicles Secreted by Acanthamoeba culbertsoni Have COX and Proteolytic Activity and Induce Hemolysis" Microorganisms 11, no. 11: 2762. https://doi.org/10.3390/microorganisms11112762
APA StyleSierra-López, F., Castelan-Ramírez, I., Hernández-Martínez, D., Salazar-Villatoro, L., Segura-Cobos, D., Flores-Maldonado, C., Hernández-Ramírez, V. I., Villamar-Duque, T. E., Méndez-Cruz, A. R., Talamás-Rohana, P., & Omaña-Molina, M. (2023). Extracellular Vesicles Secreted by Acanthamoeba culbertsoni Have COX and Proteolytic Activity and Induce Hemolysis. Microorganisms, 11(11), 2762. https://doi.org/10.3390/microorganisms11112762






