Pore- and Core-Scale Recovery Performance of Consortium Bacteria from Low-Permeability Reservoir
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Microscopic Flooding Experiments
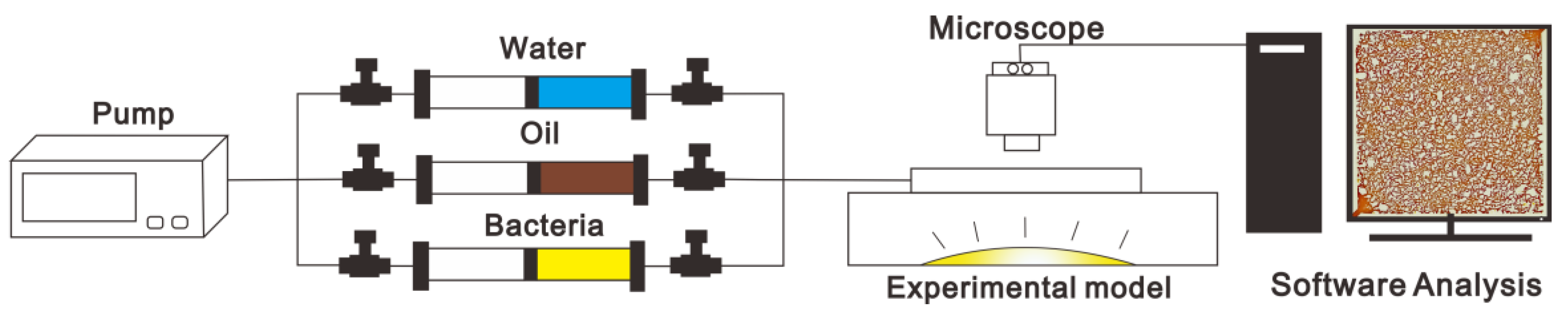
2.2.1. Apparatus
2.2.2. Procedure
2.3. Microscopic Pore-Throat Structure Analysis
2.3.1. Casting Thin Section
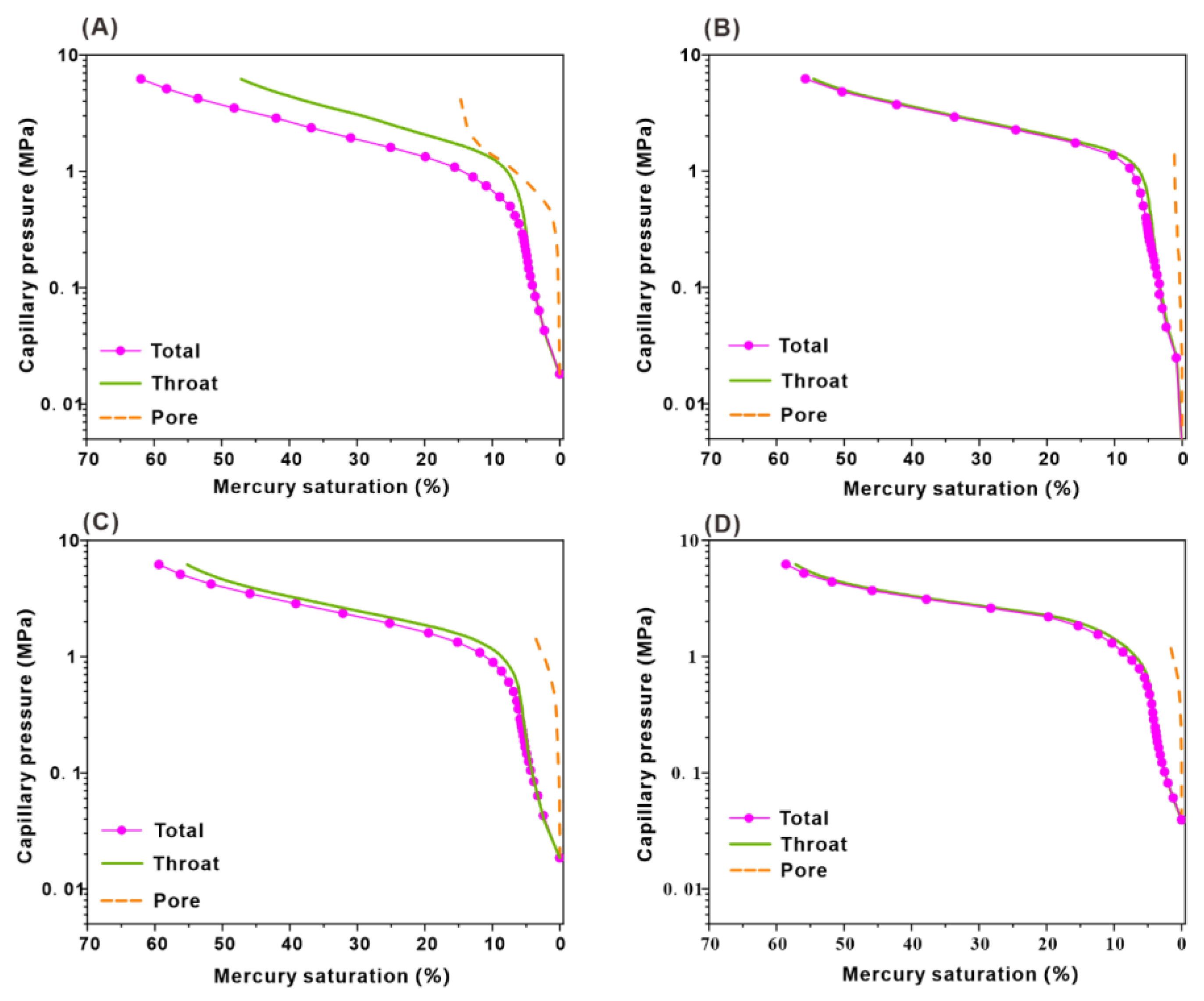
2.3.2. Rate-Controlled Porosimetry
2.4. Core Flooding Experiments
2.4.1. Apparatus
2.4.2. Procedure
3. Results
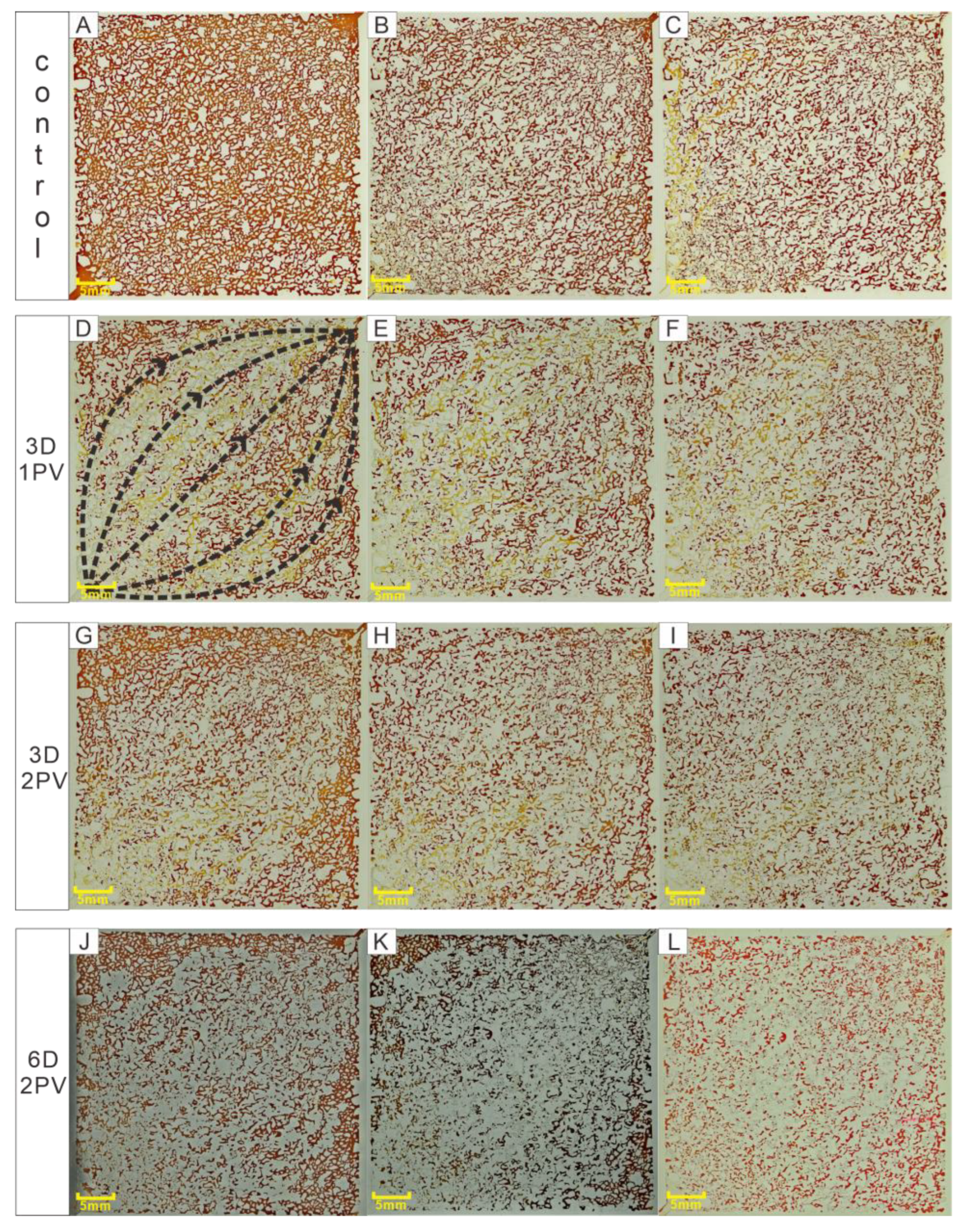
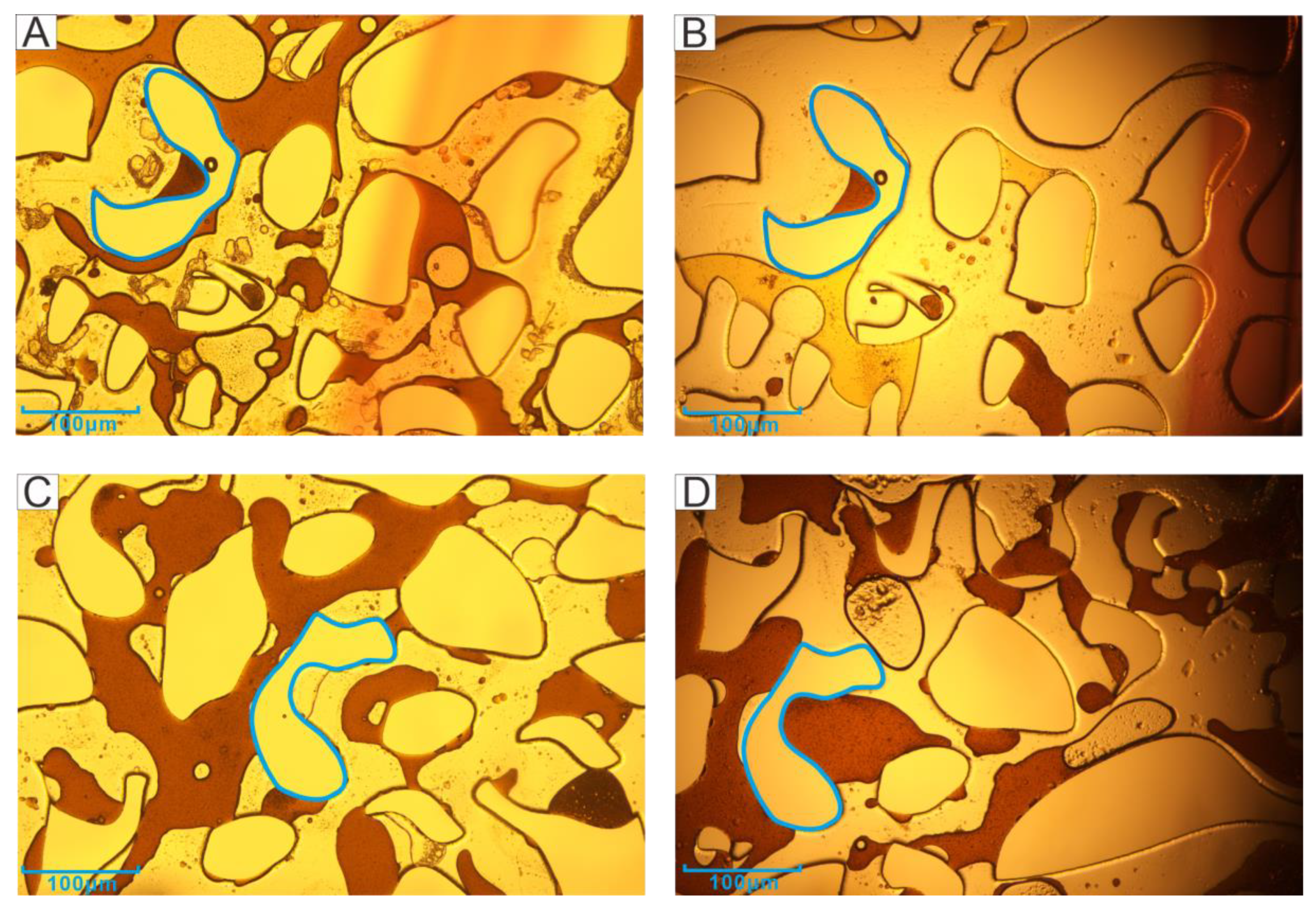
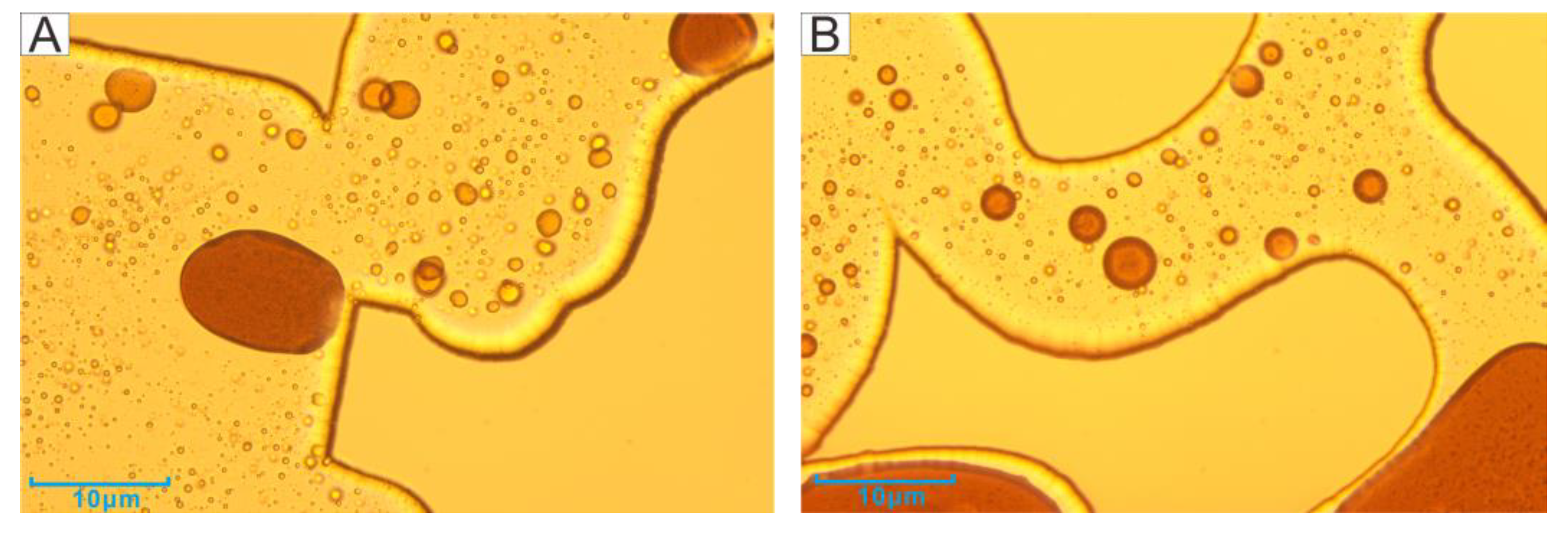
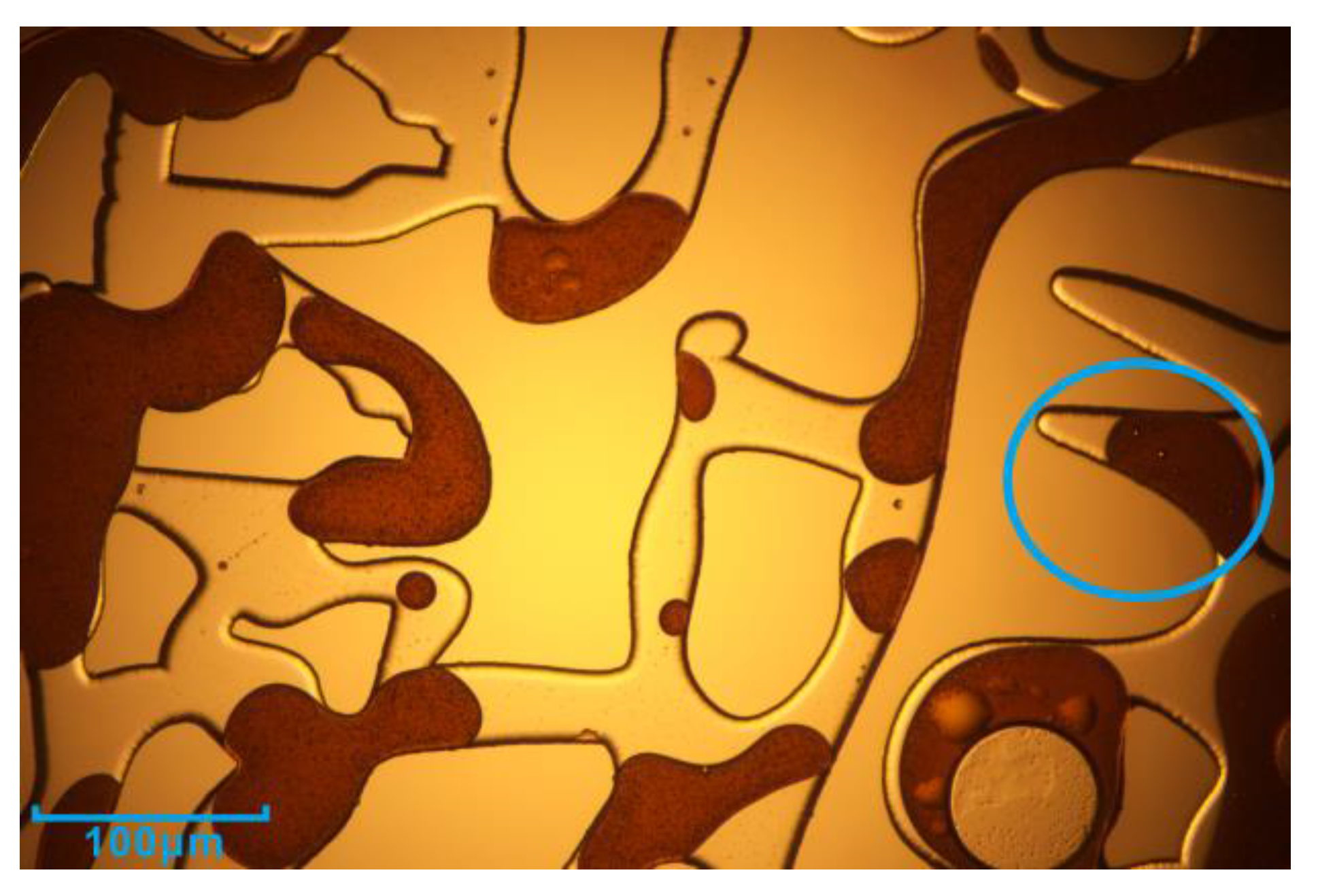
3.1. Microscopic Visualization Experiment
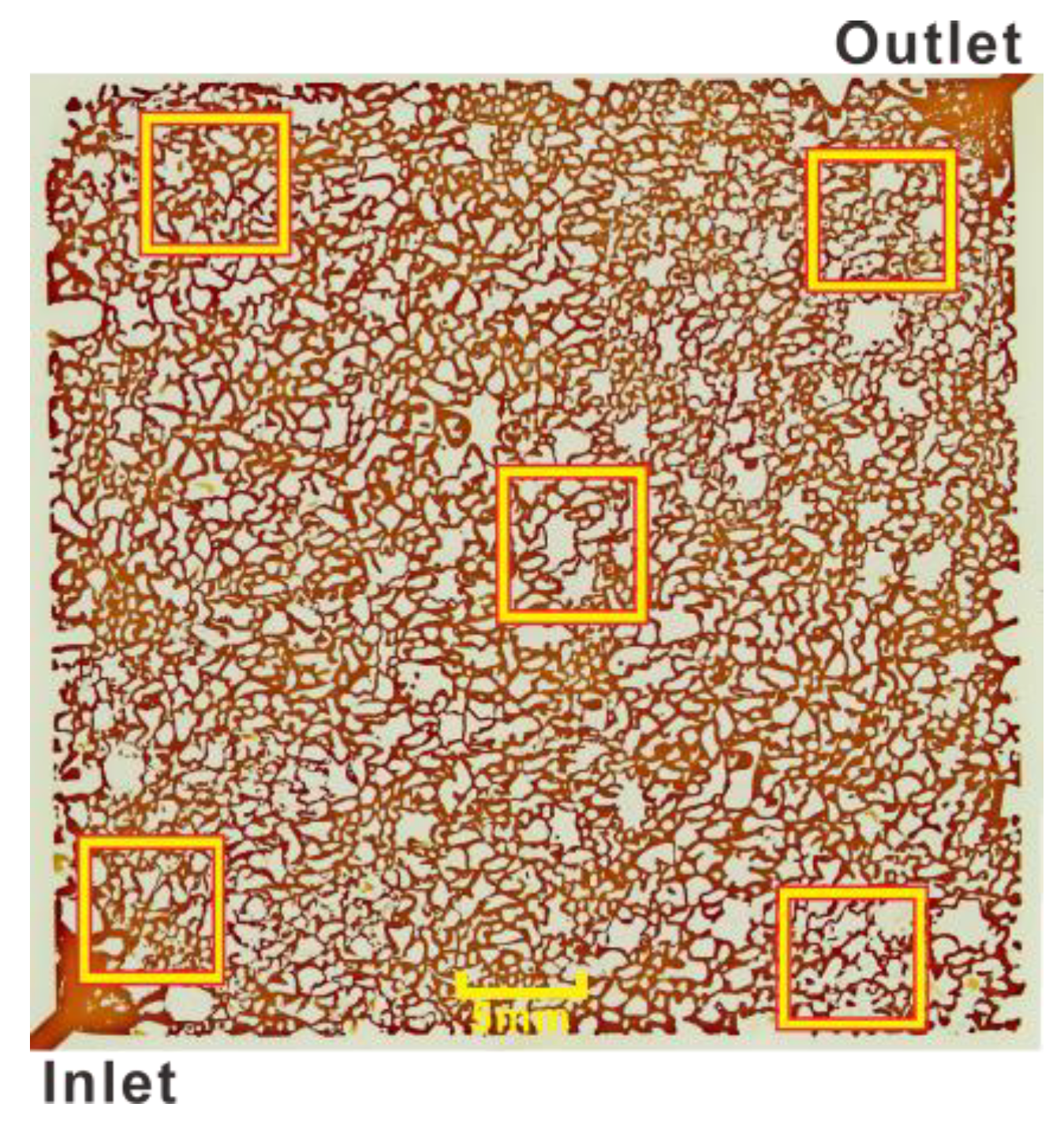
3.2. Residual Oil in Different Regions after Second Water Flooding
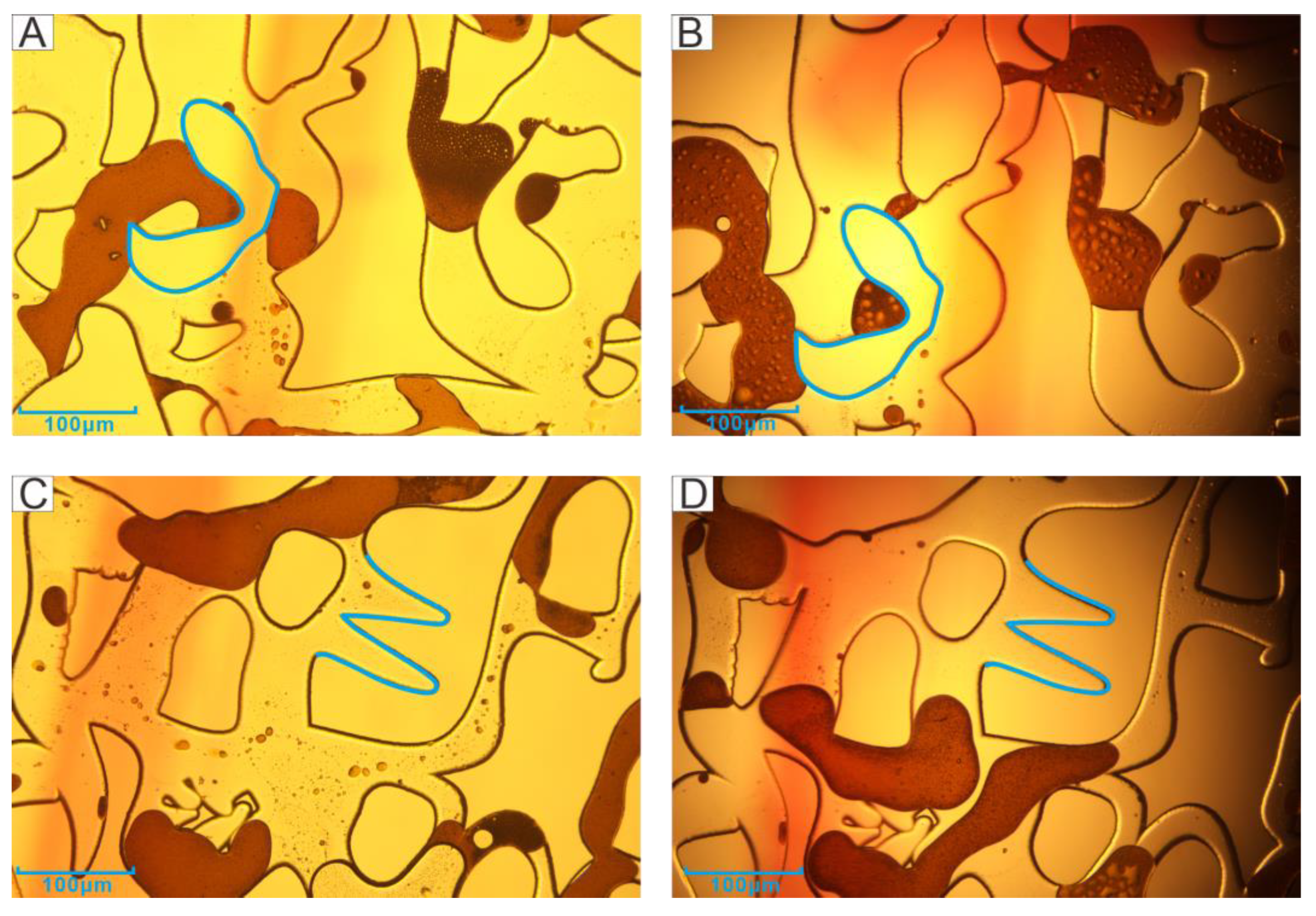
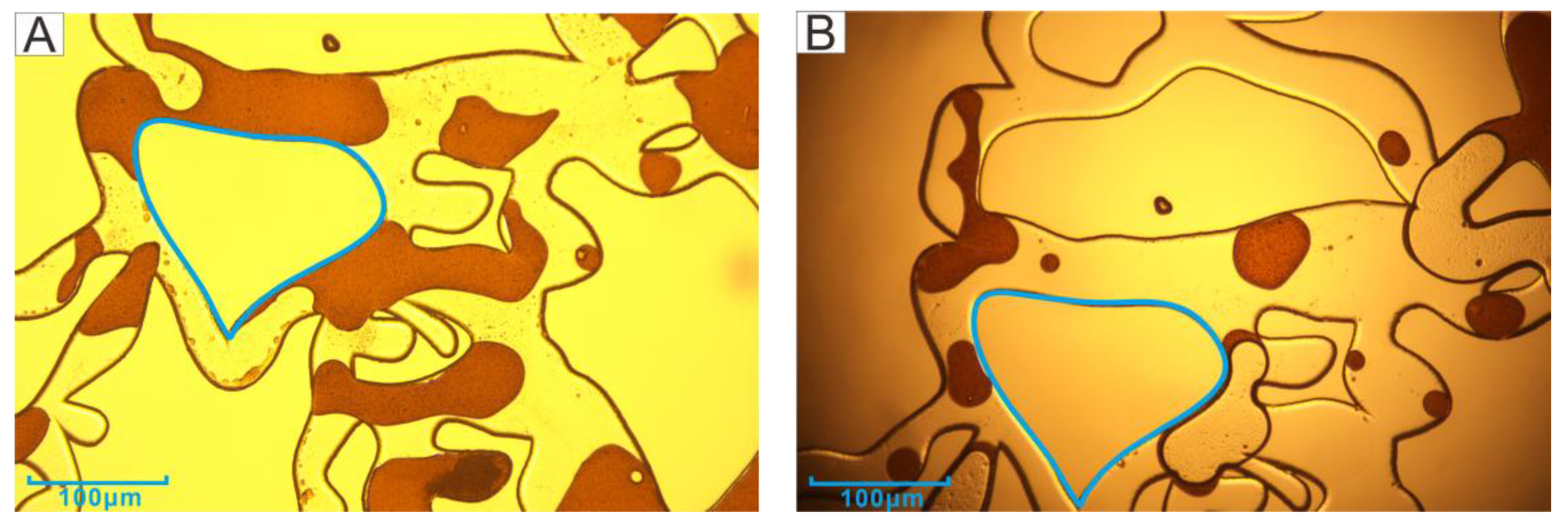
3.3. Petrology Characteristics
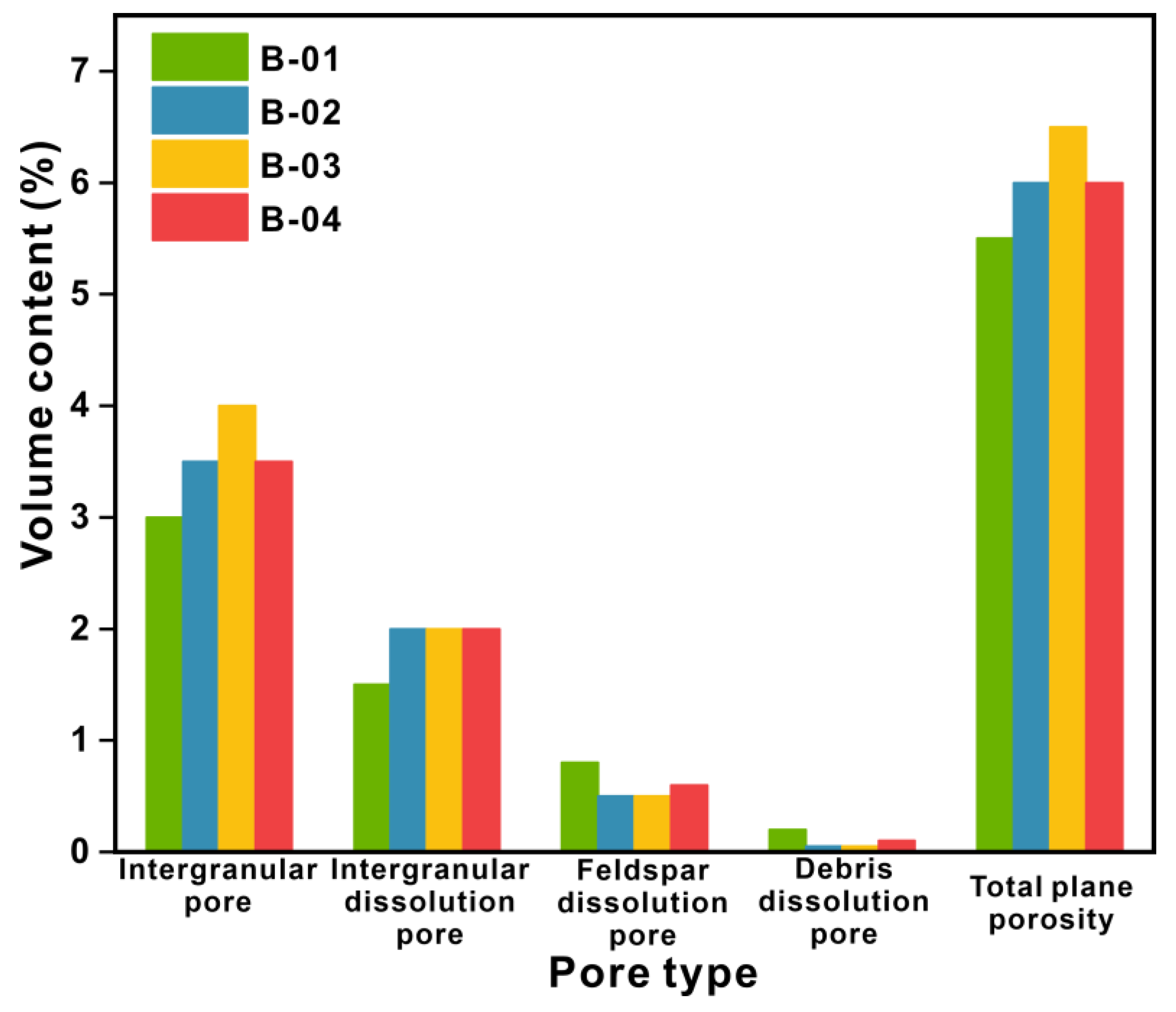
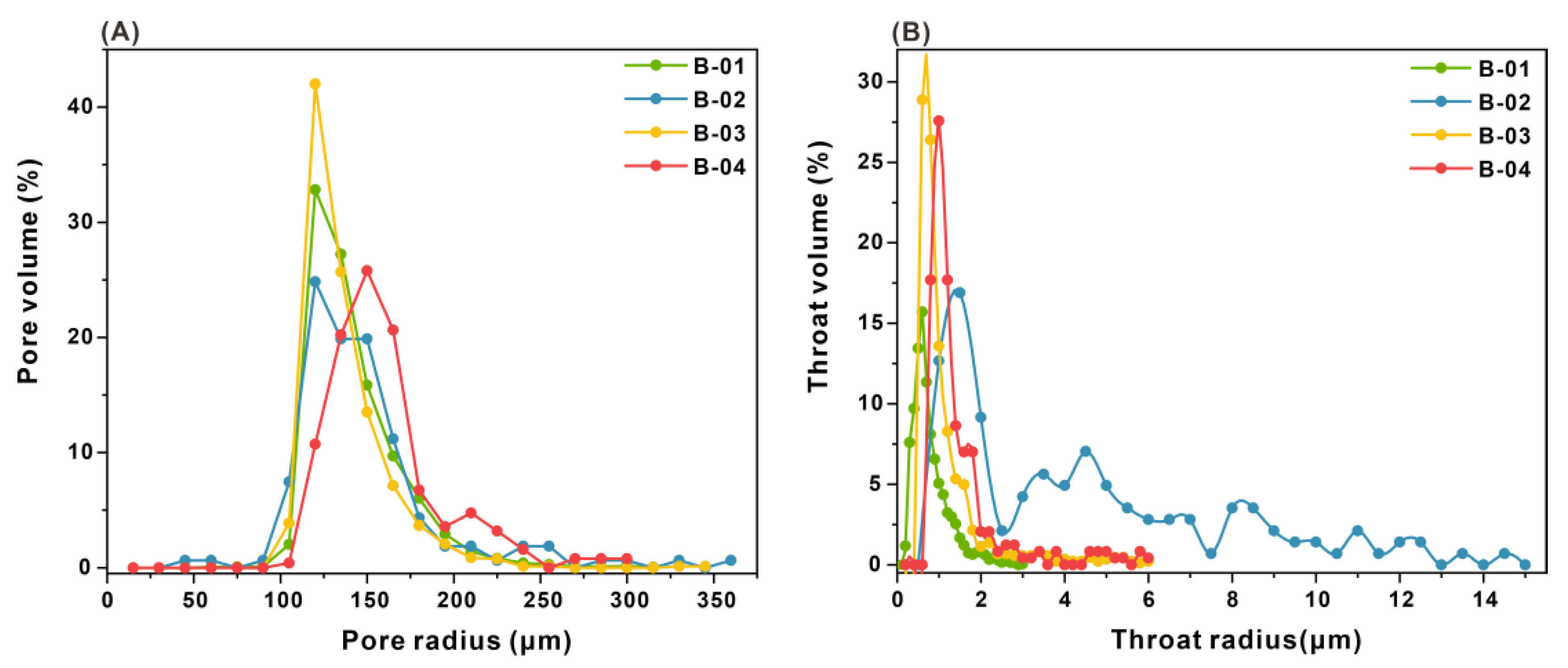
3.4. Characteristics of Pore and Throat
3.5. Core Flooding Experiments
4. Discussion
4.1. Effect of Emulsification on Recovery Efficiency
4.2. The Change of Wave Efficiency
4.3. The Potential Environmental Impact of Consortium Bacteria
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nikolova, C.; Gutierrez, T. Use of microorganisms in the recovery of oil from recalcitrant oil reservoirs: Current state of knowledge, technological advances and future perspectives. Front. Microbiol. 2020, 10, 2996. [Google Scholar] [CrossRef]
- Safdel, M.; Anbaz, M.A.; Daryasafar, A.; Jamialahmadi, M. Microbial enhanced oil recovery, a critical review on worldwide implemented field trials in different countries. Renew. Sustain. Energy Rev. 2017, 74, 159–172. [Google Scholar] [CrossRef]
- Huang, H.L.; Lin, J.Z.; Wang, W.D.; Li, S. Biopolymers Produced by Sphingomonas Strains and Their Potential Applications in Petroleum Production. Polymers 2022, 14, 1920. [Google Scholar] [CrossRef] [PubMed]
- Amin, A.; Ali Reza, K.; Rohallah, H.; Jazini, M. Experimental study of microbial enhanced oil recovery in oil-wet fractured porous media. Oil Gas Sci. Technol. 2020, 75, 73. [Google Scholar]
- Kryachko, Y. Novel approaches to microbial enhancement of oil recovery. J. Biotechnol. 2018, 266, 118–123. [Google Scholar] [CrossRef]
- Nazina, T.N.; Feng, Q.X.; Kostryukova, N.K.; Shestakova, N.M.; Babich, T.L.; Ni, F.T.; Wang, J.Q.; Liu, M.; Ivanov, M.V. Microbiological and Production Characteristics of the Dagang High-Temperature Heavy Oil Reservoir (Block no. 1). Microbiology 2017, 86, 653–665. [Google Scholar] [CrossRef]
- Nnaemeka, O.; Franklin, N.; Stanley, O. A Review of Microbial Enhanced Oil Recovery Applications Projects. Oil Gas Res. 2018, 4, 152. [Google Scholar] [CrossRef]
- Omoniyi, O.A.; Abdulmalik, F. A review of microbial enhanced oil recovery: Current development and future prospects. Int. J. Sci. Eng. Res. 2015, 6, 1378–1389. [Google Scholar]
- Niu, J.J.; Liu, Q.; Lv, J.; Peng, B. Review on microbial enhanced oil recovery: Mechanisms, modeling and field trials. J. Pet. Sci. Eng. 2020, 192, 107350. [Google Scholar] [CrossRef]
- Ganesan, M.; Mani, R.; Sai, S.; Kasivelu, G.; Awasthi, M.K.; Rajagopal, R.; Wan Azelee, N.I.; Selvi, P.K.; Chang, S.W.; Ravindran, B. Bioremediation by oil degrading marine bacteria: An overview of supplements and pathways in key processes. Chemosphere 2022, 303, 134956. [Google Scholar] [CrossRef]
- Pandey, R.; Sharma, P.; Rathee, S.; Singh, H.P.; Batish, D.R.; Krishnamurthy, B.; Kohli, R.K. Isolation and characterization of a novel hydrocarbonoclastic and biosurfactant producing bacterial strain: Fictibacillus phosphorivorans RP3. 3 Biotech 2021, 11, 105. [Google Scholar] [CrossRef] [PubMed]
- Paul, I.; Mandal, T.; Mandal, D.D. Assessment of bacterial biosurfactant production and application in Enhanced Oil Recovery (EOR)—A green approach. Environ. Technol. Innov. 2022, 28, 102733. [Google Scholar] [CrossRef]
- Sharma, J.; Kapley, A.; Sundar, D.; Srivastava, P. Characterization of a potent biosurfactant produced from Franconibacter sp. IITDAS19 and its application in enhanced oil recovery. Colloids Surf. B Biointerfaces 2022, 214, 112453. [Google Scholar] [CrossRef] [PubMed]
- Sakthipriya, N.; Kumar, G.; Agrawal, A.; Doble, M.; Sangwai, J.S. Impact of Biosurfactants, Surfactin, and Rhamnolipid Produced from Bacillus subtilis and Pseudomonas aeruginosa, on the Enhanced Recovery of Crude Oil and Its Comparison with Commercial Surfactants. Energy Fuels 2021, 35, 9883–9893. [Google Scholar]
- Zhao, W.Q.; Zhao, L.; Jia, P.; Wang, P.; Hou, J. Influence of micro-heterogeneity of fractured-porous reservoirs on the water flooding mobilization law. Sustain. Energy Technol. Assess. 2022, 53, 102694. [Google Scholar] [CrossRef]
- Kadnikov, V.V.; Ravin, N.V.; Sokolova, D.S.; Semenova, E.M.; Bidzhieva, S.K.; Beletsky, A.V.; Ershov, A.P.; Babich, T.L.; Khisametdinov, M.R.; Mardanov, A.V.; et al. Metagenomic and Culture-Based Analyses of Microbial Communities from Petroleum Reservoirs with High-Salinity Formation Water, and Their Biotechnological Potential. Biology 2023, 12, 1300. [Google Scholar] [CrossRef] [PubMed]
- Zou, C.N.; Zhu, R.K.; Bia, B.; Yang, Z.; Hou, L.H.; Cha, M.; Fu, J.H.; Shao, Y.; Liu, L.Y.; Cao, H.; et al. Significance, Geologic Characteristics, Resource Potential and Future Challenges of Tight Oil and Shale Oil. Bull. Mineral. Petrol. Geochem. 2015, 34, 3–17. [Google Scholar]
- Khademolhosseini, R.; Jafari, A.; Mousavi, S.M.; Hajfarajollah, H.; Noghabi, K.A.; Manteghian, M. Physicochemical characterization and optimization of glycolipid biosurfactant production by a native strain of Pseudomonas aeruginosa HAK01 and its performance evaluation for the MEOR process. RSC Adv. 2019, 9, 7932–7947. [Google Scholar] [CrossRef]
- Jing, W.L.; Fu, S.S.; Zhang, L.; Li, A.F.; Ren, X.X.; Xu, C.; Gao, Z.H. Pore scale experimental and numerical study of surfactant flooding for enhanced oil recovery. J. Pet. Sci. Eng. 2021, 196, 107999. [Google Scholar] [CrossRef]
- Wu, D.; Ju, B.S.; Chen, X.L.; Fu, Y.K.; Brantson, E.T. Experimental evaluation of different strategies for improving oil recovery at high-water-cut by quantitative etched-glass micro-models. Energy Explor. Exploit. 2016, 34, 586–605. [Google Scholar] [CrossRef]
- Zhou, B.; Kang, W.L.; Wang, Q.X.; Li, X.X.; He, Y.Q.; Jia, R.X.; Wang, K.H.; Zhao, Z.Z.; Yang, H.B.; Li, Z. Enhanced oil recovery performance and mechanism of a wormlike micelles flooding system with zwitterionic-anionic surfactants. J. Mol. Liq. 2022, 362, 119726. [Google Scholar] [CrossRef]
- Wang, X.; Gan, M.; Yang, X.; Zhang, P.; Peng, X.; Ju, Y.; Kou, Y.; Yu, X.; Zheng, L.; Wang, C. Mechanism of enhanced oil recovery by fuzzy-ball fluid as a novel oil-displacement agent. Energy Rep. 2023, 9, 1447–1463. [Google Scholar] [CrossRef]
- Mofrad, S.K.; Saeedi Dehaghani, A.H. An experimental investigation into enhancing oil recovery using smart water combined with anionic and cationic surfactants in carbonate reservoir. Energy Rep. 2020, 6, 543–549. [Google Scholar] [CrossRef]
- Bian, Z.W.; Zhi, Z.N.; Zhang, X.C.; Qu, Y.Q.; Wei, L.S.; Wu, H.N.; Wu, Y.F. Performance Evaluation of Bacterial Consortia from Low-Permeability Reservoir in Ordos Basin. Int. J. Energy Res. 2023, 2023, 4728717. [Google Scholar] [CrossRef]
- Yu, X.L.; Niu, H.; Li, H.; Du, C.N.; Song, Z.Y.; Kong, D.B.; Zhu, W.Y. Pore-Scale Flow Fields of the Viscosity-Lost Partially Hydrolyzed Polyacrylamide Solution Caused by Sulfide Ion. Energies 2022, 15, 2048. [Google Scholar] [CrossRef]
- Li, H.; Zhu, W.Y.; Song, Z.Y. 2-D pore-scale oil recovery mechanisms of the anionic and nonionic surfactants. Colloids Surf. A Physicochem. Eng. Asp. 2022, 655, 130245. [Google Scholar] [CrossRef]
- Song, Z.Y.; Zhu, W.Y.; Wang, X.; Guo, S.X. 2-D Pore-Scale Experimental Investigations of Asphaltene Deposition and Heavy Oil Recovery by CO2 Flooding. Energy Fuels 2018, 32, 3194–3201. [Google Scholar] [CrossRef]
- Zhu, W.Y.; Zhao, J.X.; Han, H.Y.; Sun, G.Z.; Song, Z.Y. High-Pressure Microscopic Investigation on the Oil Recovery Mechanism by In Situ Biogases in Petroleum Reservoirs. Energy Fuels 2015, 29, 7866–7874. [Google Scholar] [CrossRef]
- Xie, J.Y.; Zhang, J.J.; Xiang, W.; Fang, Y.P.; Xue, Y.J.; Cao, J.X.; Tian, R.F. Effect of microscopic pore structures on ultrasonic velocity in tight sandstone with different fluid saturation. Pet. Sci. 2022, 19, 2683–2694. [Google Scholar] [CrossRef]
- SY/T 5913-2021; Standards of Rock Thin Section Preparation of Oil and Gas Industry. China National Energy Administration: Beijing, China, 2021.
- SY/T 5368-2016; Standards of Identification for Thin Section of Rocks of Oil and Gas Industry. China National Energy Administration: Beijing, China, 2016.
- Wang, Y.J.; Zhao, X.D.; Zhou, B.Y.; Li, Q.; Liu, Z.L.; Li, L.; Li, X.S.; Ji, X.B. Evaluation of pore structure in low permeability sandstone reservoir based on high pressure-constant velocity mercury injection. Fault-Block Oil Gas Field 2022, 29, 824–830. [Google Scholar]
- Cerepi, A.; Humbert, L.; Burlot, R. Dynamics of capillary flow and transport properties in porous media by time-controlled porosimetry. Colloids Surf. A Physicochem. Eng. Asp. 2002, 206, 425–444. [Google Scholar] [CrossRef]
- Cao, S.Q. Research on Reservoir Characteristics of Yan 9 Oil-Bearing Layer-Fuxian Formation in Hujianshan, Ordos Basin. Master’s Thesis, Xi’an Shiyou University, Xi’an, China, 2017. [Google Scholar]
- Zhang, W.K.; Shi, Z.J.; Tian, Y.M.; Xie, D.; Li, W.J. The combination of high-pressure mercury injection and rate-controlled mercury injection to characterize the pore-throat structure in tight sandstone reservoirs. Fault-Block Oil Gas Field 2021, 28, 14–20. [Google Scholar]
- Bian, Z.W.; Wu, H.N.; Wei, L.S.; Zhi, Z.N.; Wu, Y.F. Isolation and Characterization of Viscosity-Reducing and Biosurfactant-Producing Bacteria in Low-Permeability Reservoir. Int. J. Energy Res. 2023, 2023, 3223516. [Google Scholar]
- Liu, J.F.; Mbadinga, S.M.; Yang, S.Z.; Gu, J.D.; Mu, B.Z. Chemical Structure, Property and Potential Applications of Biosurfactants Produced by Bacillus subtilis in Petroleum Recovery and Spill Mitigation. Int. J. Mol. Sci. 2015, 16, 4814–4837. [Google Scholar] [CrossRef] [PubMed]
- Veshareh, M.J.; Ayatollahi, S. Microorganisms effect on the wettability of carbonate oil-wet surfaces: Implications for MEOR, smart water injection and reservoir souring mitigation strategies. J. Pet. Explor. Prod. Technol. 2020, 10, 1539–1550. [Google Scholar] [CrossRef]
- Kögler, F.; Dopffel, N.; Mahler, E.; Hartmann, F.S.F.; Schulze-Makuch, D.; Visser, F.; Frommherz, B.; Herold, A.; Alkan, H. Influence of surface mineralogy on the activity of Halanaerobium sp. during microbial enhanced oil recovery (MEOR). Fuel 2021, 290, 119973. [Google Scholar] [CrossRef]
- Mejia, L.; Tagavifar, M.; Xu, K.; Mejia, M.; Du, Y.; Balhoff, M. Surfactant flooding in oil-wet micromodels with high permeability fractures. Fuel 2019, 241, 1117–1128. [Google Scholar] [CrossRef]
- Ma, T.; Feng, H.; Wu, H.; Li, Z.; Jiang, J.; Xu, D.; Meng, Z.; Kang, W. Property evaluation of synthesized anionic-nonionic gemini surfactants for chemical enhanced oil recovery. Colloids Surf. A Physicochem. Eng. Asp. 2019, 581, 123800. [Google Scholar] [CrossRef]
- Xu, J.X.; Zhang, H.; Ren, G.L.; Lang, Y.J. Construction of composite oil displacing strains and determination of oil displacement performance. Chin. J. Microecol. 2016, 28, 1123–1125+1129. [Google Scholar]
- Xu, F.; Chen, Q.; Ma, M.Q.; Wang, Y.C.; Yu, F.W.; Li, J.J. Displacement mechanism of polymeric surfactant in chemical cold flooding for heavy oil based on microscopic visualization experiments. Adv. Geo-Energy Res. 2020, 4, 77–85. [Google Scholar] [CrossRef]
- Bryan, J.; Lkantzas, A. Enhanced Heavy-Oil Recovery by Alkali-Surfactant Flooding. In Proceedings of the SPE Annual Technical Conference and Exhibition, Anaheim, CA, USA, 11–14 November 2007. [Google Scholar]
- Liu, Z.Y.; Li, Y.Q.; Luan, H.X.; Gao, W.B.; Guo, Y.; Chen, Y.H. Pore scale and macroscopic visual displacement of oil-in-water emulsions for enhanced oil recovery. Chem. Eng. Sci. 2019, 197, 404–414. [Google Scholar] [CrossRef]
- Kong, D.; Li, Y.; Yu, M.; Ma, R.; Guo, H.; Peng, Y.; Xu, S.; Yan, H. Experimental investigation on block and transport characteristics of foam in porous media for enhanced oil recovery processes. Colloids Surf. A Physicochem. Eng. Asp. 2019, 570, 22–31. [Google Scholar] [CrossRef]
- Walker, D.L.; Britton, C.; Kim, D.H.; Dufour, S.; Weerasooriya, U.; Pope, G.A. The impact of microemulsion viscosity on oil recovery. In Proceedings of the SPE Improved Oil Recovery Symposium, Tulsa, OK, USA, 14–18 April 2012. [Google Scholar]
- Guillen, V.R.; Romero, M.I.; Carvalho, M.D.S.; Alvarado, V. Capillary-driven mobility control in macro emulsion flow in porous media. Int. J. Multiph. Flow 2012, 43, 62–65. [Google Scholar] [CrossRef]
- Gaur, S.; Gupta, S.; Jain, A. Characterization and oil recovery application of biosurfactant produced during bioremediation of waste engine oil by strain Pseudomonas aeruginosa gi|KP 16392| isolated from Sambhar salt lake. bioremediat. J. 2021, 25, 308–325. [Google Scholar] [CrossRef]
- Zhao, D.R.; Jin, X.X.; Yu, T.B. Analysis of influencing factors and treatment measures of oilfield produced water quality. Petrochem. Ind. Appl. 2023, 42, 74–77. [Google Scholar]
- Zhang, W.; Hu, X.L.; Qiu, X.; Liu, D.; Feng, L.; Ma, L.Y.; Hu, J.; Wang, H.M. Microbiomes in Zhansan area, Shengli Oilfield, Shandong Province, China. Chin. Sci. Bull. 2019, 64, 1930–1942. [Google Scholar] [CrossRef]


| Sample | Initial Crude Oil % | EORE * (W)% | EORE * (M)% | EORE * (S)% |
|---|---|---|---|---|
| Control | 30.27 | 42.98 | / | 8.69 |
| 1PV 3D | 26.49 | 53.82 | 5.65 | 9.18 |
| 2PV 3D | 34.91 | 52.07 | 27.04 | 21.18 |
| 2PV 6D | 34.91 | 45.57 | 3.97 | 44.77 |
| No. | Porosity (%) | Permeability (mD) | Average Pore-Throat Radius Ratio | Displacement Pressure (MPa) |
|---|---|---|---|---|
| B-01 | 21.06 | 1.82 | 259.85 | 0.29 |
| B-02 | 19.36 | 0.26 | 66.98 | 0.50 |
| B-03 | 20.47 | 0.75 | 169.08 | 0.42 |
| B-04 | 19.41 | 0.35 | 143.86 | 0.78 |
| No. | WS (g) | Oil Weight of Water Flooding (g) | Efficiency of Water Flooding % | Oil Weight of Second Water Flooding (g) | Efficiency of Second Water Flooding % |
|---|---|---|---|---|---|
| B-01 | 3.698 | 0.804 | 21.74 | Trace | / |
| B-02 | 3.866 | 0.86 | 22.25 | 0.083 | 2.15 |
| B-03 | 3.926 | 0.856 | 21.8 | 0.258 | 6.98 |
| No. | Saturated oil quality (g) | Oil weight of MEOR | Efficiency of MEOR | Oil weight of second water flooding (g) | Efficiency of second water flooding % |
| B-04 | 3.974 | 1.304 | 32.81 | 0.107 | 2.69 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bian, Z.; Song, Z.; Zhi, Z.; Zhang, X.; Qu, Y.; Chai, R.; Wu, H.; Wu, Y. Pore- and Core-Scale Recovery Performance of Consortium Bacteria from Low-Permeability Reservoir. Microorganisms 2023, 11, 2738. https://doi.org/10.3390/microorganisms11112738
Bian Z, Song Z, Zhi Z, Zhang X, Qu Y, Chai R, Wu H, Wu Y. Pore- and Core-Scale Recovery Performance of Consortium Bacteria from Low-Permeability Reservoir. Microorganisms. 2023; 11(11):2738. https://doi.org/10.3390/microorganisms11112738
Chicago/Turabian StyleBian, Ziwei, Zhiyong Song, Zena Zhi, Xiangchun Zhang, Yiqian Qu, Ruiyang Chai, Hanning Wu, and Yifei Wu. 2023. "Pore- and Core-Scale Recovery Performance of Consortium Bacteria from Low-Permeability Reservoir" Microorganisms 11, no. 11: 2738. https://doi.org/10.3390/microorganisms11112738
APA StyleBian, Z., Song, Z., Zhi, Z., Zhang, X., Qu, Y., Chai, R., Wu, H., & Wu, Y. (2023). Pore- and Core-Scale Recovery Performance of Consortium Bacteria from Low-Permeability Reservoir. Microorganisms, 11(11), 2738. https://doi.org/10.3390/microorganisms11112738






