The Effect of Lactiplantibacillus plantarum I-Enriched Diet on the Phenolic Content and Antioxidant Capacity of Queen Scallop (Aequipecten opercularis Linnaeus, 1758) Extracts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganisms
2.2. Reagents
2.3. Sampling and Keeping
2.4. Preparation of Wet Biomass for Supplementation and Feeding
2.5. Experimental Design
2.6. Determination of Total Phenols, Flavonoids, Non-Flavonoids, and Total Antioxidant Capacity
2.7. Statistical Analysis
3. Results
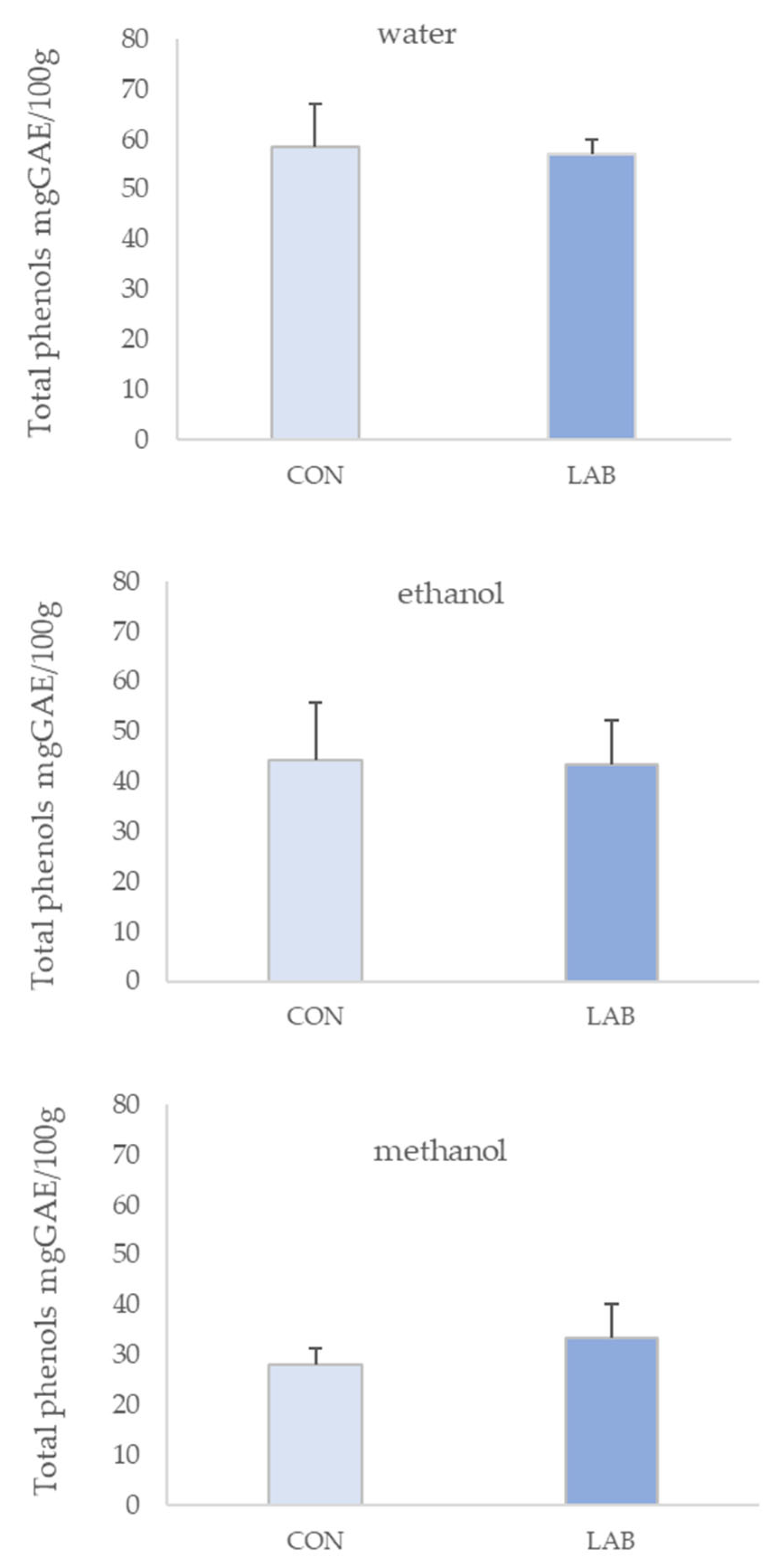
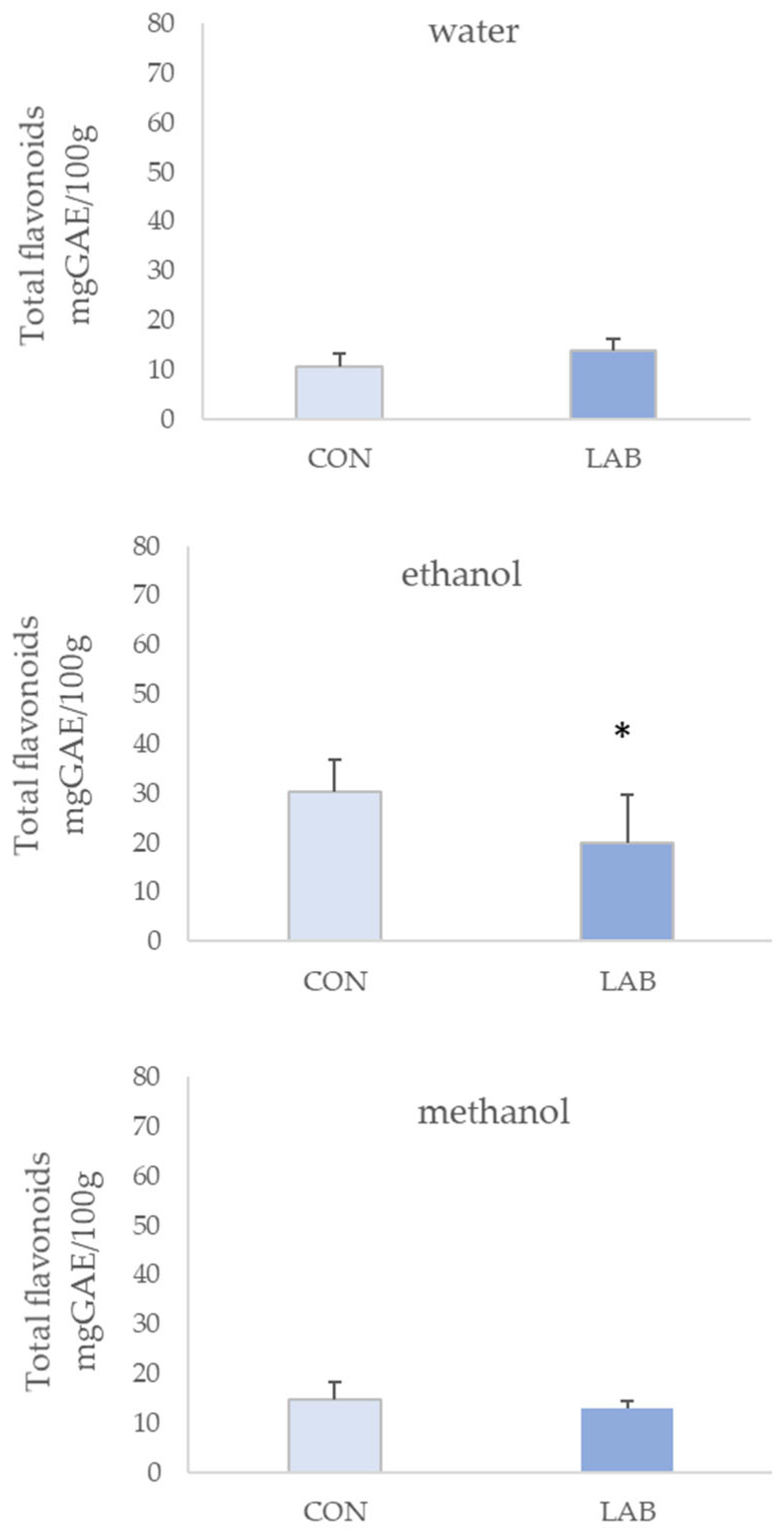
3.1. Phenolic Content in Queen Scallop Extracts
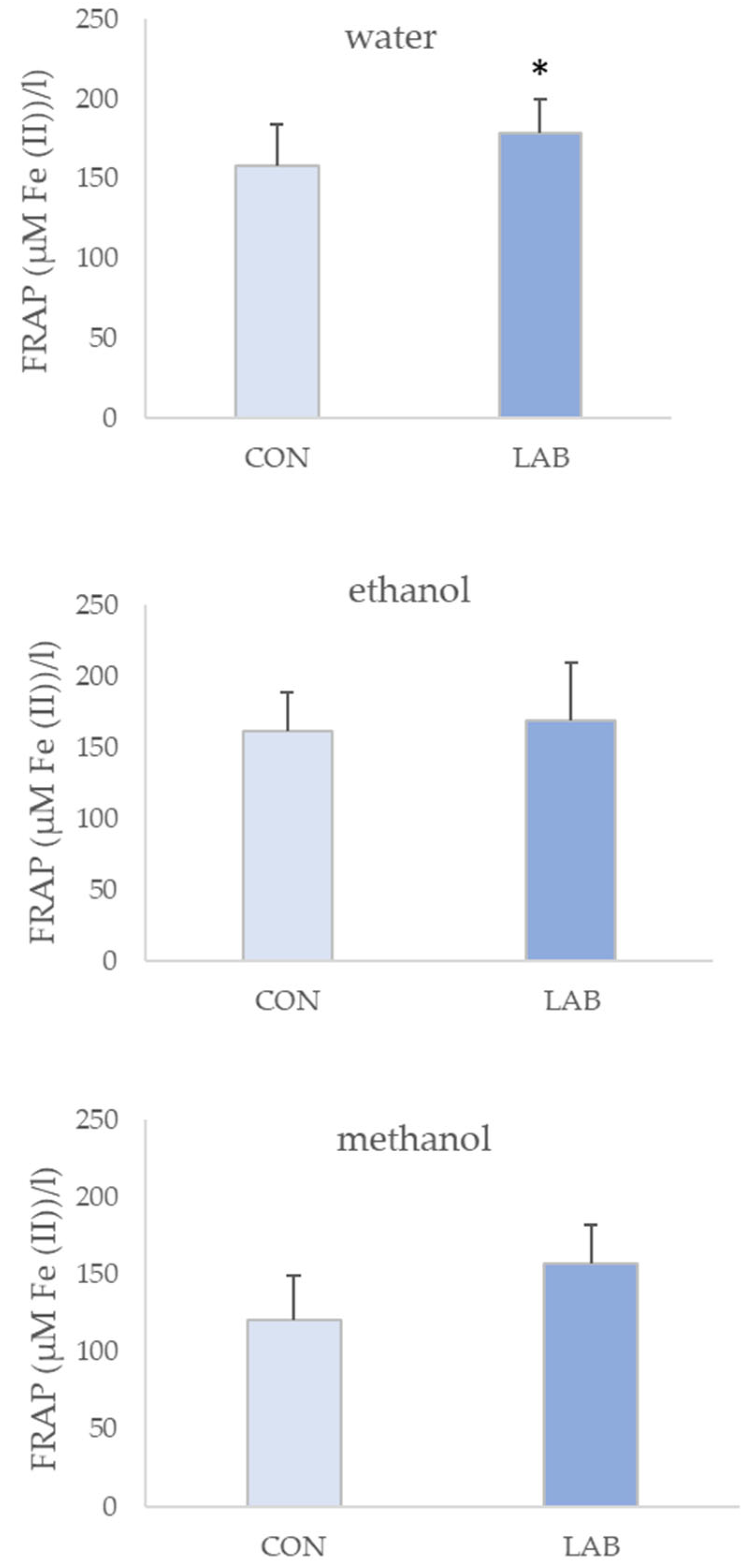
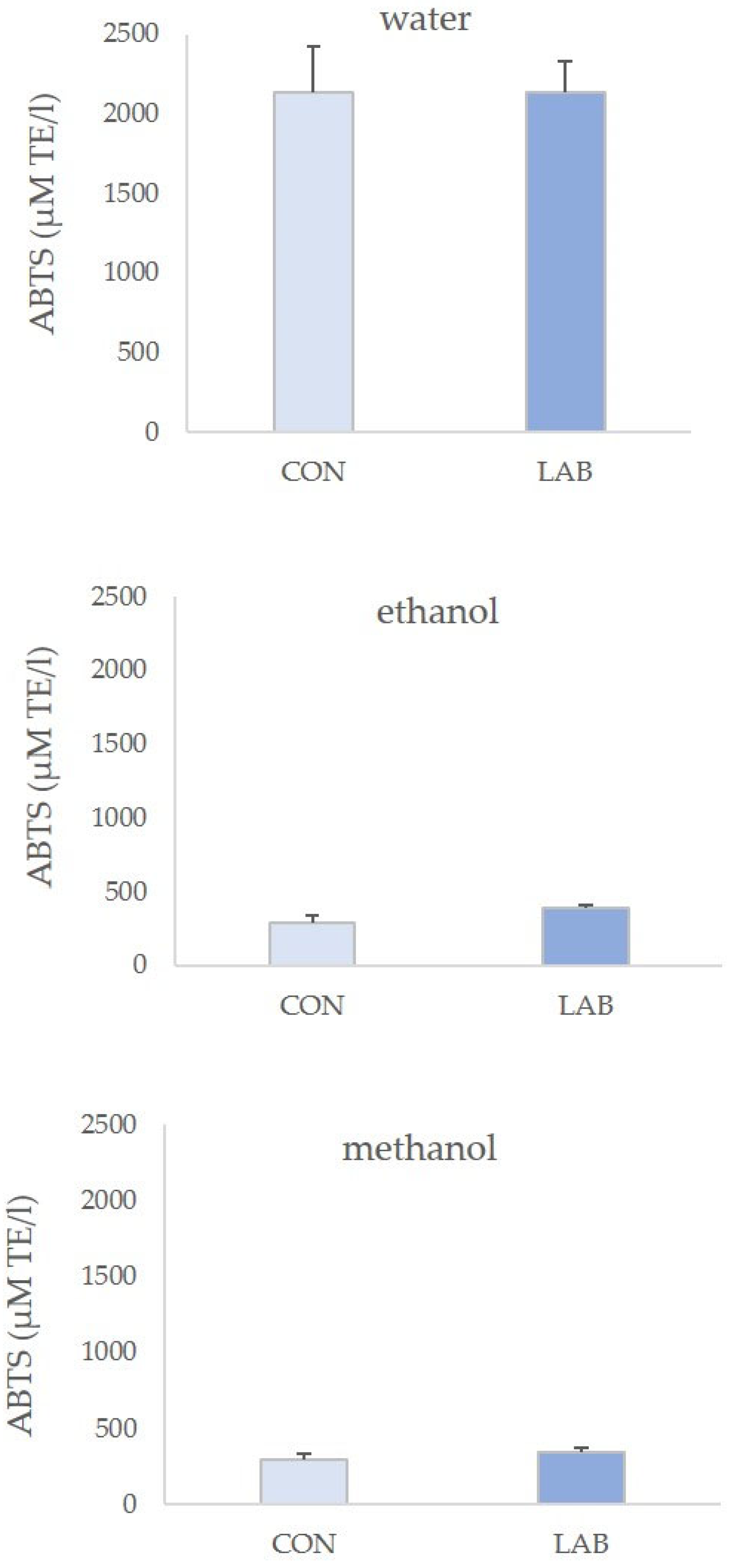
3.2. Antioxidant Capacity in Queen Scallop Extracts
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Saber, S.; Abd El-Fattah, E.E.; Yahya, G.; Gobba, N.A.; Maghmomeh, A.O.; Khodir, A.E.; Mourad, A.A.E.; Saad, A.S.; Mohammed, H.G.; Nouh, N.A.; et al. A Novel Combination Therapy Using Rosuvastatin and Lactobacillus Combats Dextran Sodium Sulfate-Induced Colitis in High-Fat Diet-Fed Rats by Targeting the TXNIP/NLRP3 Interaction and Influencing Gut Microbiome Composition. Pharmaceuticals 2021, 14, 341. [Google Scholar] [CrossRef]
- Yadav, M.; Mandeep; Shukla, P. Probiotics of diverse origin and their therapeutic applications: A review. J. Am. Coll. Nutr. 2020, 39, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Dahiya, D.; Nigam, P.S. Nutraceutical Combinational Therapy for Diarrhoea Control with Probiotic Beverages from Fermented Fruits, Vegetables and Cereals to Regain Lost Hydration, Nutrition and Gut Microbiota. Microorganisms 2023, 11, 2190. [Google Scholar] [CrossRef] [PubMed]
- Son, S.J.; Han, A.-R.; Sung, M.J.; Hong, S.M.; Lee, S.-H. Hermetia illucens Fermented with Lactobacillus plantarum KCCM12757P Alleviates Dextran Sodium Sulfate-Induced Colitis in Mice. Antioxidants 2023, 12, 1822. [Google Scholar] [CrossRef]
- Iorizzo, M.; Albanese, G.; Letizia, F.; Testa, B.; Tremonte, P.; Vergalito, F.; Lombardi, S.J.; Succi, M.; Coppola, R.; Sorrentino, E. Probiotic Potentiality from Versatile Lactiplantibacillus plantarum Strains as Resource to Enhance Freshwater Fish Health. Microorganisms 2022, 10, 463. [Google Scholar] [CrossRef] [PubMed]
- Dell’Anno, M.; Callegari, M.L.; Reggi, S.; Caprarulo, V.; Giromini, C.; Spalletta, A.; Coranelli, S.; Sgoifo Rossi, C.A.; Rossi, L. Lactobacillus plantarum and Lactobacillus reuteri as Functional Feed Additives to Prevent Diarrhoea in Weaned Piglets. Animals 2021, 11, 1766. [Google Scholar] [CrossRef]
- Kaktcham, P.M.; Temgoua, J.-B.; Zambou, F.N.; Diaz-Ruiz, G.; Wacher, C.; de Lourdes Pérez-Chabela, M. In Vitro Evaluation of the Probiotic and Safety Properties of Bacteriocinogenic and Non-Bacteriocinogenic Lactic Acid Bacteria from the Intestines of Nile Tilapia and Common Carp for Their Use as Probiotics in Aquaculture. Prob. Antimicrob. Prot. 2018, 10, 98–109. [Google Scholar] [CrossRef]
- Kazuń, B.; Kazuń, K.; Żylińska, J.; Siwicki, A.K. In Vitro Study of Lactobacillus plantarum Properties as a Potential Probiotic Strain and an Alternative Method to Antibiotic Treatment of Fish. Fish. Aquat. Life 2018, 26, 47–55. [Google Scholar] [CrossRef]
- Čanak, I.; Kovačić, I.; Žunec, A.; Jakopović, Ž.; Kostelac, D.; Markov, K.; Štifanić, M.; Burić, P.; Iveša, N.; Frece, J. Study of the Impact of Lactiplantibacillus plantarum I on the Health Status of Queen Scallop Aequipecten opercularis. Appl. Sci. 2023, 13, 7723. [Google Scholar] [CrossRef]
- Mirzabekyan, S.; Harutyunyan, N.; Manvelyan, A.; Malkhasyan, L.; Balayan, M.; Miralimova, S.; Chikindas, M.L.; Chistyakov, V.; Pepoyan, A. Fish Probiotics: Cell Surface Properties of Fish Intestinal Lactobacilli and Escherichia coli. Microorganisms 2023, 11, 595. [Google Scholar] [CrossRef]
- Ringø, E.; Doan, H.V.; Lee, S.; Song, S.K. Lactic acid bacteria in shellfish: Possibilities and challenges. Rev. Fish. Sci. Aquac. 2020, 28, 139–169. [Google Scholar] [CrossRef]
- Bentzon-Tilia, M.; Sonnenschein, E.C.; Gram, L. Monitoring and managing microbes in aquaculture—Towards a sustainable industry. Microb. Biotechnol. 2016, 9, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Roy, S.; Meena, D.K.; Sarkar, U.K. Application of probiotics in shrimp aquaculture: Importance, mechanisms of action, and methods of administration. Rev. Fish. Sci. Aquac. 2016, 24, 342–368. [Google Scholar] [CrossRef]
- Zorriehzahra, M.J.; Delshad, S.T.; Adel, M.; Tiwari, R.; Karthik, K.; Dhama, K.; Lazado, C.C. Probiotics as beneficial microbes in aquaculture: An update on their multiple modes of action: A review. Vet. Q. 2016, 36, 228–241. [Google Scholar] [CrossRef]
- Abdel-Latif, H.M.; Yilmaz, E.; Dawood, M.A.; Ringø, E.; Ahmadifar, E.; Yilmaz, S. Shrimp vibriosis and possible control measures using probiotics, postbiotics, prebiotics, and synbiotics: A review. Aquaculture 2022, 551, 737951. [Google Scholar] [CrossRef]
- Hu, G.; Wang, Y.; Xue, R.; Liu, T.; Zhou, Z.; Yang, Z. Effects of the Exopolysaccharide from Lactiplantibacillus plantarum HMX2 on the Growth Performance, Immune Response, and Intestinal Microbiota of Juvenile Turbot, Scophthalmus maximus. Foods 2023, 12, 2051. [Google Scholar] [CrossRef]
- Yilmaz, S.; Yilmaz, E.; Dawood, M.A.; Ringø, E.; Ahmadifar, E.; Abdel-Latif, H.M. Probiotics, prebiotics, and synbiotics used to control vibriosis in fish: A review. Aquaculture 2022, 547, 737514. [Google Scholar] [CrossRef]
- Marimuthu, V.; Shanmugam, S.; Sarawagi, A.D.; Kumar, A.; Kim, I.H.; Balasubramanian, B. A glimpse on influences of feed additives in aquaculture. eFood 2022, 3, e6. [Google Scholar] [CrossRef]
- Balcázar, J.L.; Vendrell, D.; de Blas, I.; Ruiz-Zarzuela, I.; Muzquiz, J.L.; Girones, O. Characterization of probiotic properties of lactic acid bacteria isolated from intestinal microbiota of fish. Aquaculture 2008, 278, 188–191. [Google Scholar] [CrossRef]
- Do, Q.D.; Angkawijaya, A.E.; Tran-Nguyen, P.L.; Huynh, L.H.; Soetaredjo, F.E.; Ismadji, S.; Ju, Y.H. Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J. Food Drug Anal. 2013, 22, 296–302. [Google Scholar] [CrossRef]
- Kusmayadi, A.; Leong, Y.K.; Yen, H.W.; Huang, C.Y.; Chang, J.S. Microalgae as sustainable food and feed sources for animals and humans—Biotechnological and environmental aspects. Chemosphere 2021, 271, 129800. [Google Scholar] [CrossRef] [PubMed]
- Ercolano, G.; De Cicco, P.; Ianaro, A. New drugs from the sea: Pro-apoptotic activity of sponges and algae derived compounds. Mar. Drugs 2019, 17, 31. [Google Scholar] [CrossRef] [PubMed]
- Rajauria, G.; Jaiswal, A.K.; Abu-Ghannam, N.; Gupta, S. Antimicrobial, antioxidant and free radical scavenging capacity of brown seaweed Himanthaliae longata from Western Coast of Ireland. J. Food Bioch. 2013, 37, 322–335. [Google Scholar] [CrossRef]
- Ramamoorthy, M.; Sankar, G.; Jeyapriya, S.N. Evaluation of nutritional profile and anti-oxidant activity of Meretrix meretrix Asiatic hard clam along the Parangipettai coast of Tamilnadu. Sci. Acad. 2023, 4, 15–35. [Google Scholar]
- Zayed, K.M.; Ghareeb, M.A.; Habib, M.R.; El-Einin, H.M.A.; Ali, R.E.M.; El-Karim, R.M.G.; Sabour, R.; Hamed, A.A. Biological activity, chemical profiling and molecular docking of tissue extracts of the sea snail Trochus erithreus. J. Appl. Pharm. Sci. 2023, 13, 199–210. [Google Scholar] [CrossRef]
- Mamelona, J.; Pelletier, E.; Girard-Lalancette, K.; Legault, J.; Karboune, S.; Kermasha, S. Quantification of phenolic contents and antioxidant capacity of Atlantic sea cucumber, Cucumaria frondosa. Food Chem. 2007, 104, 1040–1047. [Google Scholar] [CrossRef]
- Rozirwan; Nanda; Nugroho, R.Y.; Diansyah, G.; Muhtadi, I.; Fauziyah; Putri, W.A.E.; Agussalim, A. Phytochemical composition, total phenolic content and antioxidant activity of Anadara granosa (Linnaeus, 1758) collected from the east coast of South Sumatra, Indonesia. Baghdad Sci. J. 2023, 20, 1258. [Google Scholar] [CrossRef]
- Krishnamoorthy, V.; Chuen, L.Y.; Sivayogi, V.; Kathiresan, S.; Bahari, M.B.; Raju, G.; Parasuraman, S. Exploration of antioxidant capacity of extracts of Perna viridis, a marine bivalve. Pharmacogn. Mag. 2019, 15, 402–409. [Google Scholar] [CrossRef]
- Halliwell, B. Biochemistry of oxidative stress. Biochem. Soc. Trans. 2007, 35, 1147–1150. [Google Scholar] [CrossRef]
- Tan, K.S.; Zhang, B.; Zhang, H.; Zheng, H. Total antioxidant capacity of commercially important bivalves: A case study in Southern China. Aquac. Res. 2021, 53, 657–664. [Google Scholar] [CrossRef]
- Sansone, C.; Brunet, C. Promises and Challenges of Microalgal Antioxidant Production. Antioxidants 2019, 8, 199. [Google Scholar] [CrossRef] [PubMed]
- Manduzio, H.; Rocher, B.; Durand, F.; Galap, C.; Leboulenger, F. The point about oxidative stress in molluscs. Invertebr. Surviv. J. 2005, 2, 91–104. [Google Scholar]
- Mateos, R.; Pérez-Correa, J.R.; Domínguez, H. Bioactive Properties of Marine Phenolics. Mar. Drugs 2020, 18, 501. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Rhodes, C.J.; Moncol, J.; Izakovic, M.; Mazur, M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interac. 2006, 160, 1–40. [Google Scholar] [CrossRef]
- Holtin, K.; Kuehnle, M.; Rehbein, J.; Schuler, P.; Nicholson, G.; Albert, K. Determination of astaxanthin and astaxanthin esters in the microalgae Haematococcus pluvialis by LC-(APCI)MS and characterization of predominant carotenoid isomers by NMR spectroscopy. Anal. Bioanal. Chem. 2009, 395, 1613–1622. [Google Scholar] [CrossRef] [PubMed]
- Sellami, M.; Rebah, F.B.; Gargouri, Y.; Miled, N. Lipid composition and antioxidant activity of liver oils from ray species living in Tunisian coasts. Arab. J. Chem. 2018, 11, 233–239. [Google Scholar] [CrossRef]
- Goh, S.-H.; Yusoff, F.M.; Loh, S.-P. A Comparison of the Antioxidant Properties and Total Phenolic Content in a Diatom, Chaetoceros sp. and a Green Microalga, Nannochloropsis sp. J. Agric. Sci. 2010, 2, 123. [Google Scholar] [CrossRef]
- Sultana, B.; Anwar, F.; Ashraf, M. Effect of extraction solvent/technique on the antioxidant activity of selected medicinal plant extracts. Molecules 2009, 14, 2167–2180. [Google Scholar] [CrossRef]
- Generalić Mekinić, I.; Gotovac, M.; Skroza, D.; Ljubenkov, I.; Burčul, F.; Katalinić, V. Effect of the extraction solvent on the oleuropein content and antioxidant properties of olive leaf (cv. Oblica, Lastovka and Levantinka) extracts. Croat. J. Food Sci. Technol. 2014, 6, 7–14. [Google Scholar]
- Goldsmith, C.; Vuon, G.Q.; Stathopoulos, C.; Roach, P.; Scarlett, C. Optimization of the aqueous extraction of phenolic compounds from olive leaves. Antioxidants 2014, 3, 700–712. [Google Scholar] [CrossRef]
- Fatimah Zaharah, M.Y.; Rabeta, M.S. Antioxidant and antimicrobial activities of squid ink powder. Food Res. 2018, 2, 82–88. [Google Scholar] [CrossRef]
- Nguyen, M.T.; Tran, T.N.A.; Nguyen, H.V. Antibacterial and antioxidant activities of the extracts from marine snail Hemifusus colosseus (Lamarck, 1816). Aquac. Aquar. Conserv. Legis. 2022, 15, 251–260. [Google Scholar]
- Tan, K.S.; Leng, X.; Zhao, Y.; Liu, H.X.; Cheng, D.W.; Ma, H.Y.; Li, S.K.; Zheng, H.P. Amino acid variations in polymorphic noble scallops, Chlamys nobilis. J. Food Process. Pres. 2019, 43, e14262. [Google Scholar] [CrossRef]
- da Silva Martins, A.C.; Flores, J.A.; Porto, C.; Junior, W.W.; Monserrat, J.M. Antioxidant and oxidative damage responses in different organs of Pacific white shrimp Litopenaeus vannamei (Boone, 1931) reared in a biofloc technology system. Mar. Freshw. Behav. Physiol. 2015, 48, 279–288. [Google Scholar] [CrossRef]
- Martins, D.; Barros, L.; Carvalho, A.M.; Ferreira, I.C.F.R. Nutritional and in vitro antioxidant properties of edible wild greens in Iberian peninsula traditional diet. Food Chem. 2011, 125, 488–494. [Google Scholar] [CrossRef]
- Xu, Z.; Regenstein, J.M.; Xie, D.; Lu, W.J.; Ren, X.C.; Yuan, J.J.; Mao, L.C. The oxidative stress and antioxidant responses of Litopenaeus vannamei to low temperature and air exposure. Fish Shellfish Immunol. 2018, 72, 564–571. [Google Scholar] [CrossRef]
- Taylor, M.A.; Maher, W.A.; Ubrihien, R.P. Mortality, condition index and cellular responses of Anadara trapezia to combined salinity and temperature stress. J. Exp. Mar. Biol. Ecol. 2017, 497, 172–179. [Google Scholar] [CrossRef]
- Iglesias, P.; Louro, Á.; Román, G. The effect of depth on the reproductive and reserve storage cycles of the pectinids Aequipecten opercularis (L., 1758) and Chlamys varia (L., 1758) in Galicia, Northwest Spain. J. Shellfish Res. 2012, 31, 677–684. [Google Scholar] [CrossRef]
- Jideani, A.I.O.; Silungwe, H.; Takalani, T.; Omolola, A.O.; Udeh, H.O.; Anyasi, T.A. Antioxidant-rich natural fruit and vegetable products and human health. Int. J. Food Prop. 2021, 24, 41–67. [Google Scholar] [CrossRef]
- Baroni, L.; Sarni, A.R.; Zuliani, C. Plant Foods Rich in Antioxidants and Human Cognition: A Systematic Review. Antioxidants 2021, 10, 714. [Google Scholar] [CrossRef]
- Lourenço, S.C.; Moldão-Martins, M.; Alves, V.D. Antioxidants of Natural Plant Origins: From Sources to Food Industry Applications. Molecules 2019, 24, 4132. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ren, Z.; Zhang, J.; Chuang, C.-C.; Kandaswamy, E.; Zhou, T.; Zuo, L. Role of ROS and Nutritional Antioxidants in Human Diseases. Front Physiol. 2018, 9, 477. [Google Scholar] [CrossRef] [PubMed]
- Kovačić, I.; Bilić, J.; Dudaš, S.; Poljuha, D. Phenolic content and antioxidant capacity of istrian olive leaf infusions. Poljopr./Agric. 2017, 23, 38–45. [Google Scholar] [CrossRef]
- Tan, K.S.; Liu, H.L.; Zhang, H.K.; Li, S.K.; Ma, H.Y.; Zheng, H.P. Carotenoid content and lipid nutritional quality variation in tissues of male and female polymorphic (golden and brown) noble scallop Chlamys nobilis. Aquaculture 2021, 536, 736483. [Google Scholar] [CrossRef]
- Tan, K.S.; Ma, H.Y.; Li, S.K.; Zheng, H.P. Bivalves as future source of sustainable natural omega-3 polyunsaturated fatty acids. Food Chem. 2020, 311, 125907. [Google Scholar] [CrossRef] [PubMed]
- Ruano, F.; Ramos, P.; Quaresma, M.; Bandarra, N.M.; da Fonseca, I.P. Evolution of fatty acid profile and condition index in mollusc bivalves submitted to different depuration periods. Rev. Port. Cienc. Vet. 2012, 111, 75–84. [Google Scholar]
- Food and Agriculture Organisation (FAO). The State of World Fisheries and Aquaculture; FAO: Rome, Italy, 2018. [Google Scholar]
- Brand, A.R. Scallop ecology: Distributions and behaviour. In Scallops: Biology, Ecology, Aquaculture and Fisheries; Shumway, S.E., Parsons, G.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; Volume 40, pp. 469–533. [Google Scholar]
- Outeiro, L.; Otero, J.; Alonso-Fernández, A.; Bañón, R.; Palacios-Abrantes, J. Quantifying abundance trends and environmental effects on a population of queen scallop Aequipecten opercularis targeted by artisanal fishers in a coastal upwelling area (Ría de Arousa, NW Spain) using a Bayesian spatial model. Fish Res. 2021, 240, 105963. [Google Scholar] [CrossRef]
- Duncan, P.F.; Brand, A.R.; Strand, Ø.; Foucher, E. The European scallop fisheries for Pecten maximus, Aequipecten opercularis, Chlamys islandica, and Mimachlamys varia. In Scallops: Biology, Ecology, Aquaculture and Fisheries; Shumway, S.E., Parsons, G.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; Volume 40, pp. 781–858. [Google Scholar]
- Kovačić, I.; Žunec, A.; Matešković, M.; Burić, P.; Iveša, N.; Štifanić, M.; Frece, J. Commercial Quality, Biological Indices and Biochemical Composition of Queen Scallop Aequipecten opercularis in Culture. Fishes 2023, 8, 48. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Ough, C.S.; Amerine, M.A. Methods for Analysis is of Musts and Wine; John Wiley & Sons: New York, NY, USA, 1988. [Google Scholar]
- Poljuha, D.; Šola, I.; Bilić, J.; Dudaš, S.; Bilušić, T.; Markić, J.; Rusak, G. Phenolic composition, antioxidant capacity, energy content and gastrointestinal stability of Croatian wild edible plants. Eur. Food Res. Technol. 2015, 241, 573–585. [Google Scholar] [CrossRef]
- Darmastuti, A.; Hasan, P.N.; Wikandari, R.; Utami, T.; Rahayu, E.S.; Suroto, D.A. Adhesion Properties of Lactobacillus plantarum Dad-13 and Lactobacillus plantarum Mut-7 on Sprague Dawley Rat Intestine. Microorganisms 2021, 9, 2336. [Google Scholar] [CrossRef] [PubMed]


| Scheme 2 | Mean Squares (s2) | |||
|---|---|---|---|---|
| Phenolic Content | df | TP | TNF | TF |
| Diet | 1 | 4.13 | 6.19 | 543.98 * |
| Solvent | 2 | 2996.36 *** | 901.97 ** | 8381.62 *** |
| LAB*solvent | 2 | 54.80 | 3.39 | 1934.03 ** |
| Source of Variation | Mean Squares (s2) | |||
|---|---|---|---|---|
| Phenolic Content | df | FRAP | ABTS | DPPH |
| Diet | 1 | 0.006 * | 28.81 ** | 3.48 ** |
| Solvent | 2 | 0.004 * | 0.003 | 0.76 ** |
| LAB*solvent | 2 | 0.001 | 0.14 | 1.78 ** |
| Antioxidant Capacity | ||||
|---|---|---|---|---|
| Phenolic Content | df | FRAP | ABTS | DPPH |
| TP | Control | 0.591 * | 0.754 * | 0.701 * |
| LAB | 0.282 | 0.819 * | 0.732 * | |
| TNF | Control | 0.628 * | 0.546 * | 0.489 * |
| LAB | 0.077 | 0.727 * | 0.604 * | |
| TF | Control | 0.168 | −0.522 * | −0.536 * |
| LAB | −0.161 | −0.034 | 0.225 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kovačić, I.; Burić, P.; Žunec, A.; Bilić, J.; Prgić, A.; Čanak, I.; Iveša, N.; Štifanić, M.; Frece, J. The Effect of Lactiplantibacillus plantarum I-Enriched Diet on the Phenolic Content and Antioxidant Capacity of Queen Scallop (Aequipecten opercularis Linnaeus, 1758) Extracts. Microorganisms 2023, 11, 2723. https://doi.org/10.3390/microorganisms11112723
Kovačić I, Burić P, Žunec A, Bilić J, Prgić A, Čanak I, Iveša N, Štifanić M, Frece J. The Effect of Lactiplantibacillus plantarum I-Enriched Diet on the Phenolic Content and Antioxidant Capacity of Queen Scallop (Aequipecten opercularis Linnaeus, 1758) Extracts. Microorganisms. 2023; 11(11):2723. https://doi.org/10.3390/microorganisms11112723
Chicago/Turabian StyleKovačić, Ines, Petra Burić, Ante Žunec, Josipa Bilić, Anamarija Prgić, Iva Čanak, Neven Iveša, Mauro Štifanić, and Jadranka Frece. 2023. "The Effect of Lactiplantibacillus plantarum I-Enriched Diet on the Phenolic Content and Antioxidant Capacity of Queen Scallop (Aequipecten opercularis Linnaeus, 1758) Extracts" Microorganisms 11, no. 11: 2723. https://doi.org/10.3390/microorganisms11112723
APA StyleKovačić, I., Burić, P., Žunec, A., Bilić, J., Prgić, A., Čanak, I., Iveša, N., Štifanić, M., & Frece, J. (2023). The Effect of Lactiplantibacillus plantarum I-Enriched Diet on the Phenolic Content and Antioxidant Capacity of Queen Scallop (Aequipecten opercularis Linnaeus, 1758) Extracts. Microorganisms, 11(11), 2723. https://doi.org/10.3390/microorganisms11112723









