The Role of Chlorine in the Formation and Development of Tap Water Biofilms under Different Flow Regimes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental System and Conditions
2.2. Biofilm Measurements
2.3. Biomaterial Measurements
2.4. Statistical Analysis
3. Results
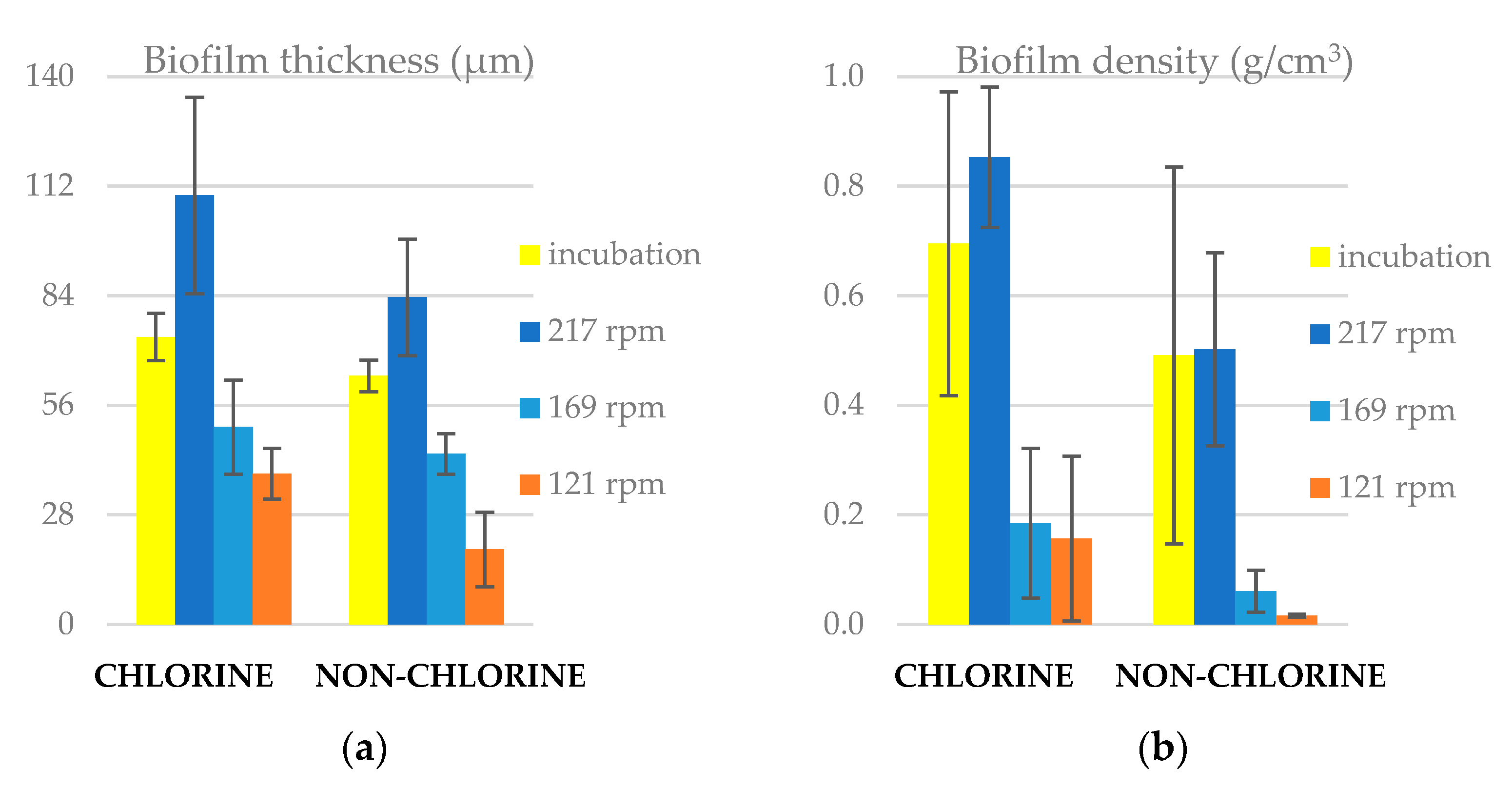
3.1. Biofilm Thickness and Density
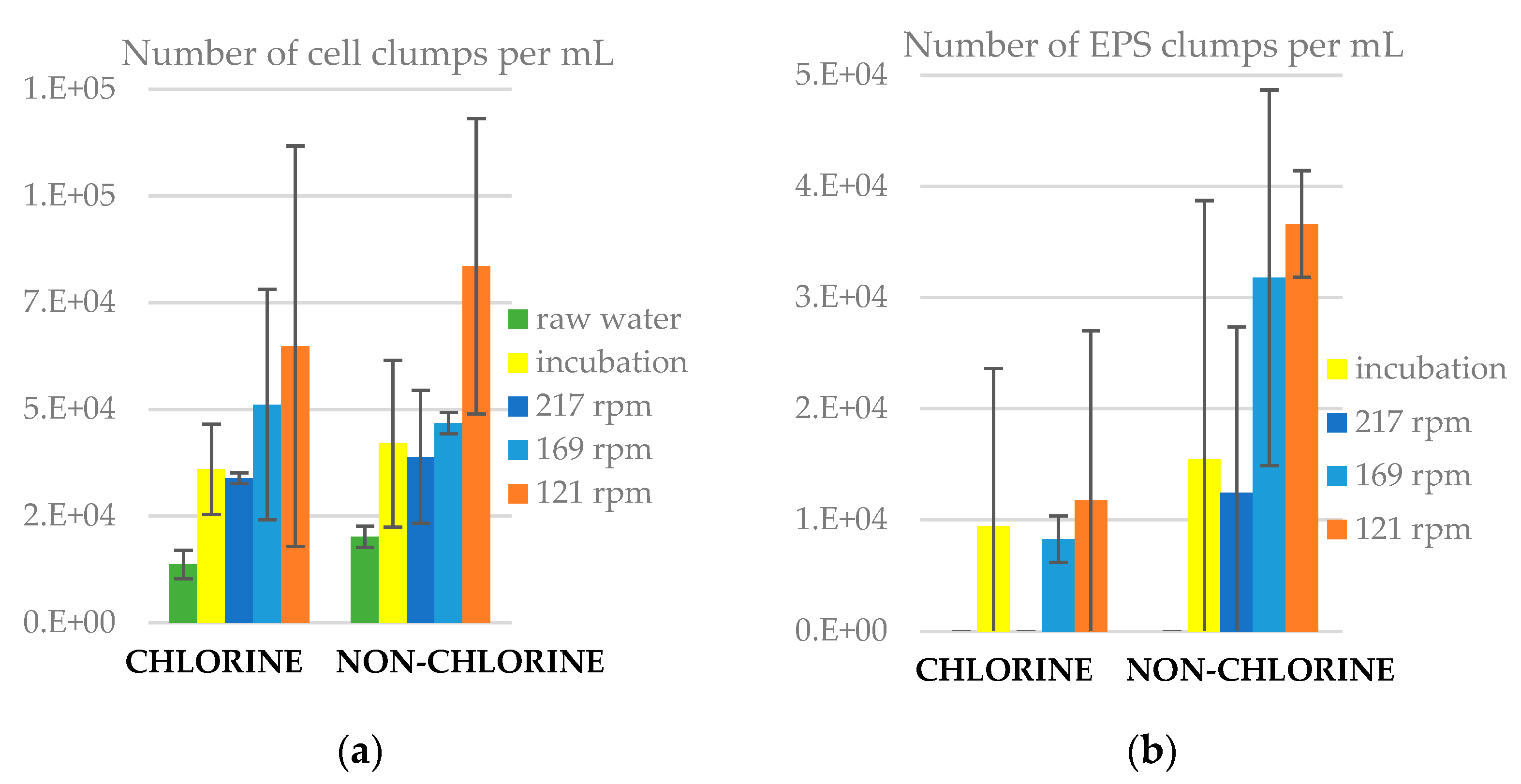
3.2. Number of Clumps on Slides
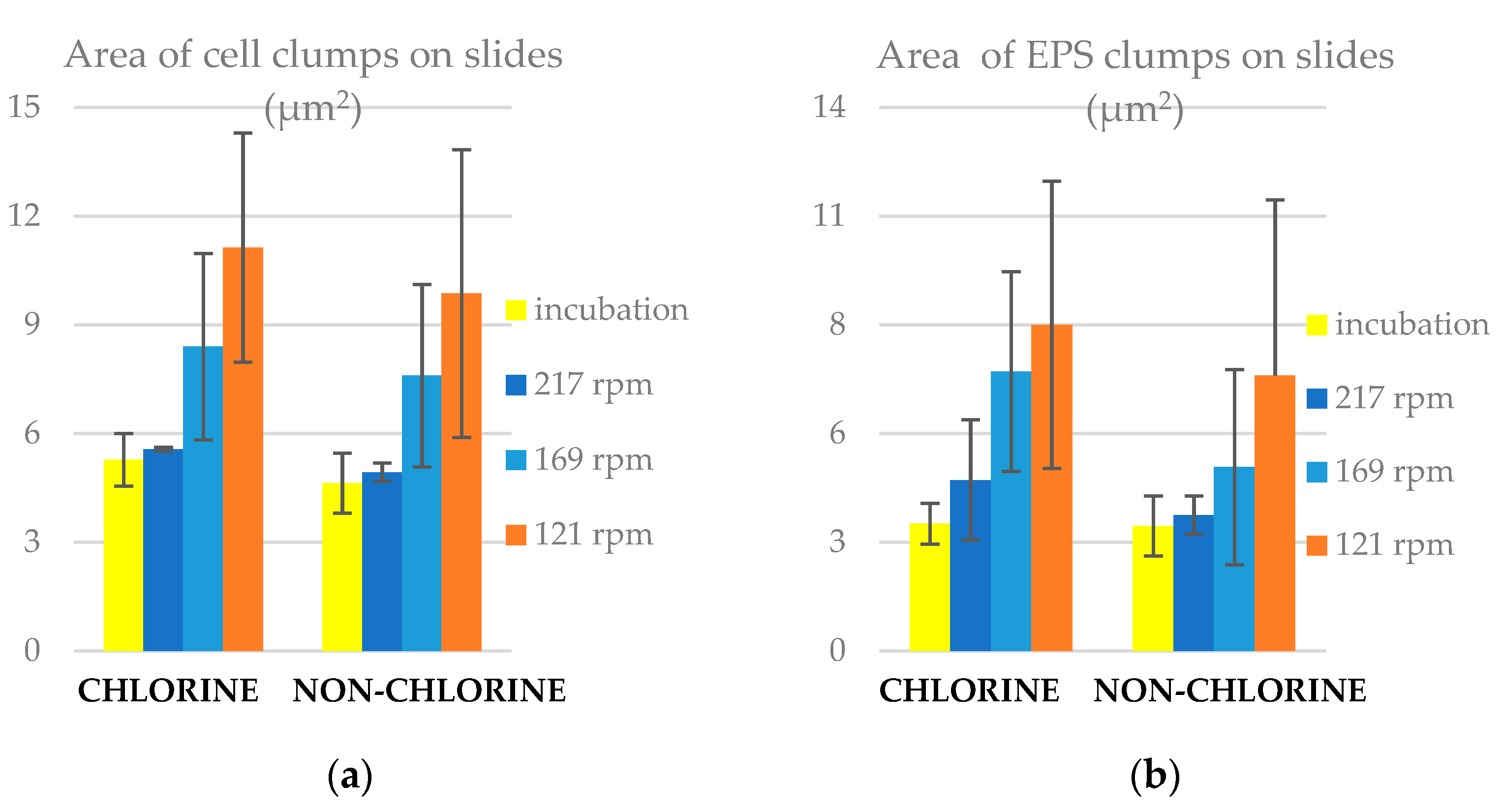
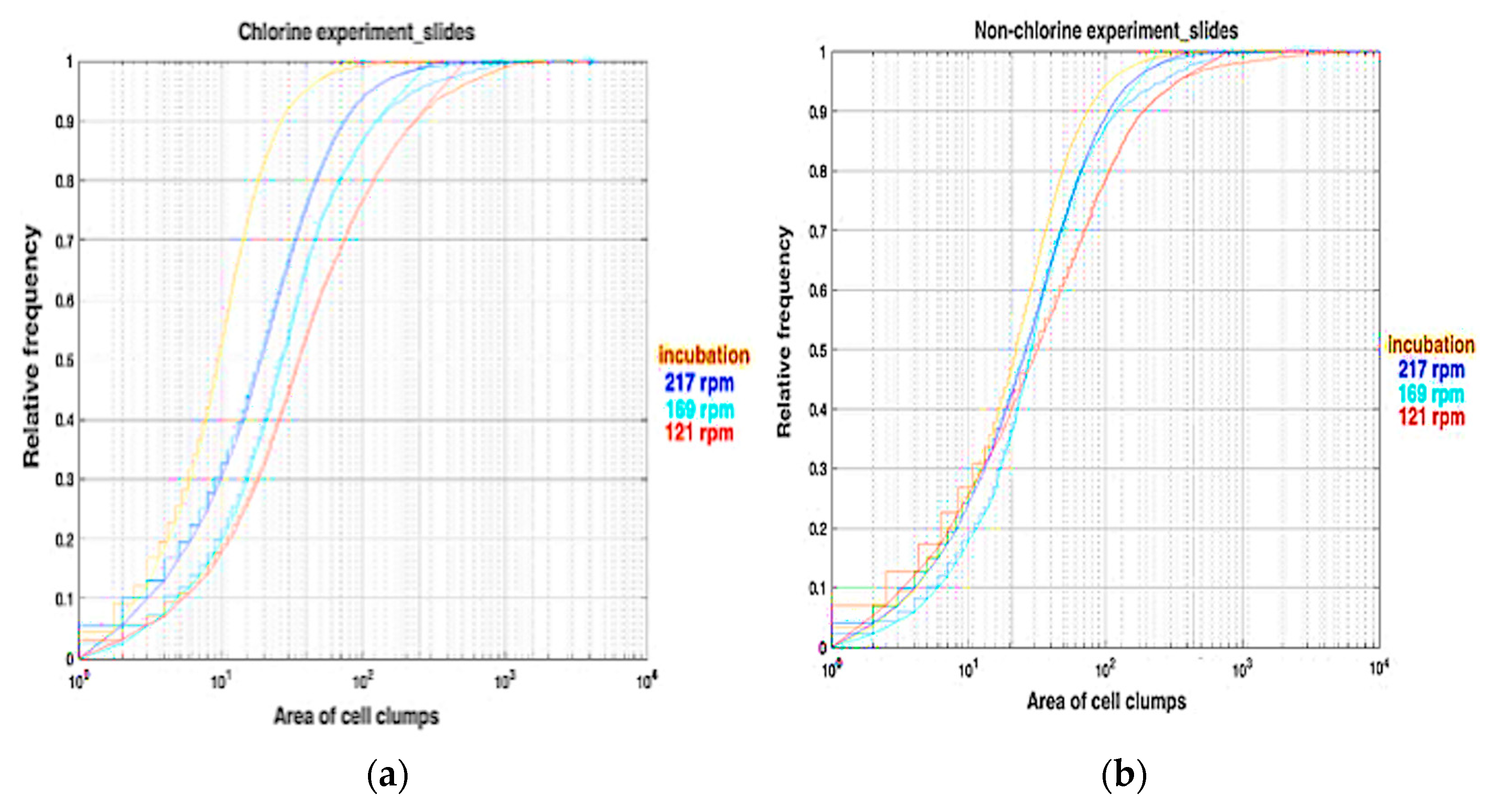
3.3. Area of Clumps on Slides
3.4. Coverage Area of Clumps on Slides
3.5. Cell Concentration in Water
3.6. Number of Clumps in Water
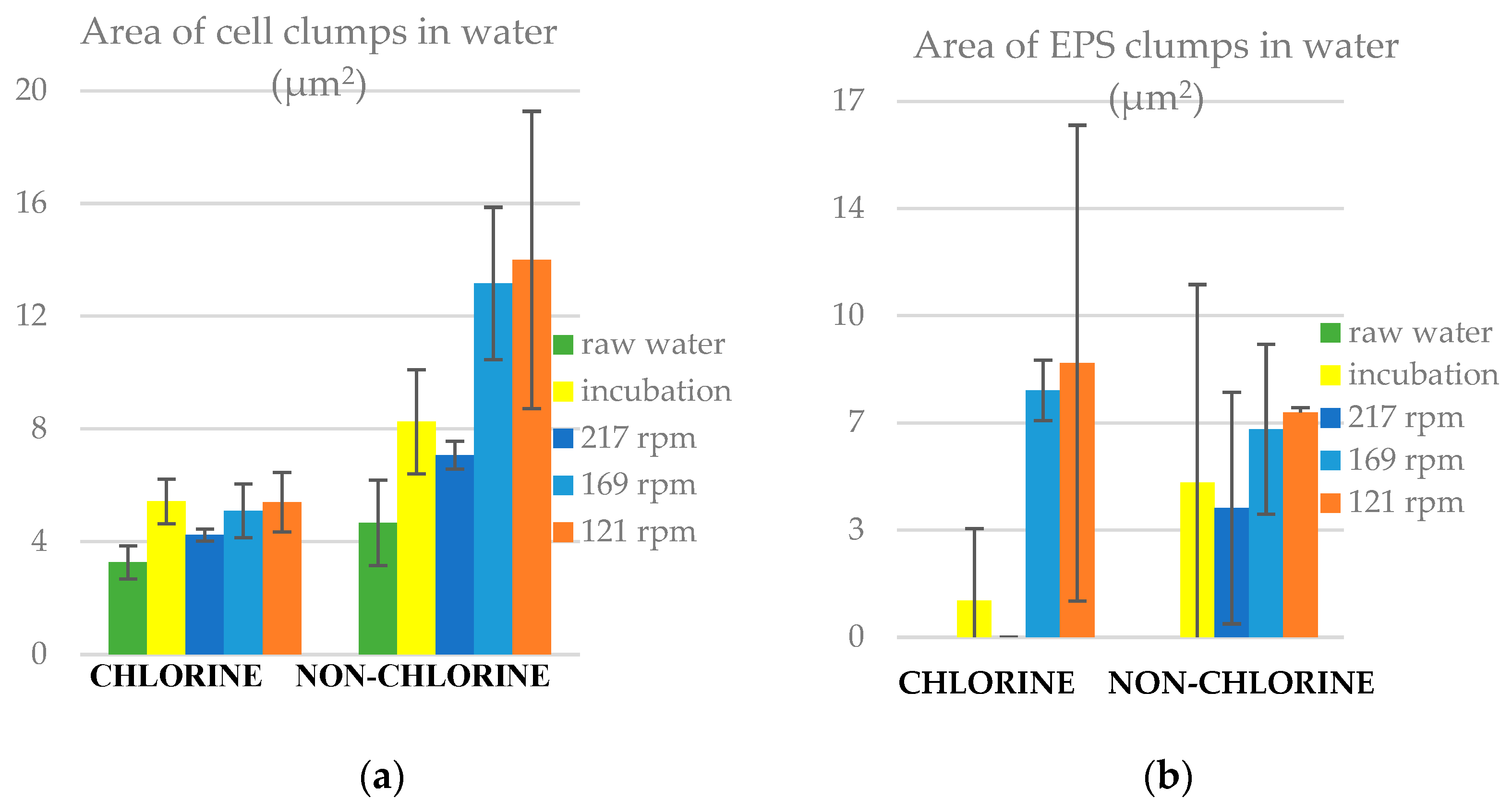
3.7. Area of Clumps in Water
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Flemming, H.C.; Percival, S.L.; Walker, J.T. Contamination potential of biofilms in water distribution systems. Innov. Conv. Adv. Water Treat. Process. 2002, 2, 271–280. [Google Scholar] [CrossRef]
- Hemdan, B.A.; El-Taweel, G.E.; Goswami, P.; Pant, D.; Sevda, S. The role of biofilm in the development and dissemination of ubiquitous pathogens in drinking water distribution systems: An overview of surveillance, outbreaks, and prevention. World J. Microbiol. Biotechnol. 2021, 37, 36. [Google Scholar] [CrossRef] [PubMed]
- Collier, S.A.; Deng, L.; Adam, E.A.; Benedict, K.M.; Beshearse, E.M.; Blackstock, A.J.; Bruce, B.B.; Derado, G.; Edens, C.; Fullerton, K.E.; et al. Estimate of Burden and Direct Healthcare Cost of Infectious Waterborne Disease in the United States. Emerg. Infect. Dis. 2021, 27, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Craun, G.F.; Brunkard, J.M.; Yoder, J.S.; Roberts, V.A.; Carpenter, J.; Wade, T.; Calderon, R.L.; Roberts, J.M.; Beach, M.J.; Roy, S.L. Causes of outbreaks associated with drinking water in the United States from 1971 to 2006. Clin. Microbiol. Rev. 2010, 23, 507–528. [Google Scholar] [CrossRef] [PubMed]
- Bertelli, C.; Courtois, S.; Rosikiewicz, M.; Piriou, P.; Aeby, S.; Robert, S.; Loret, J.F.; Greub, G. Reduced Chlorine in Drinking Water Distribution Systems Impacts Bacterial Biodiversity in Biofilms. Front. Microbiol. 2018, 9, 2520. [Google Scholar] [CrossRef]
- Douterelo, I.; Sharpe, R.; Boxall, J. Bacterial community dynamics during the early stages of biofilm formation in a chlorinated experimental drinking water distribution system: Implications for drinking water discolouration. J. Appl. Microbiol. 2014, 117, 286–301. [Google Scholar] [CrossRef]
- Liu, S.; Gunawan, C.; Barraud, N.; Rice, S.A.; Harry, E.J.; Amal, R. Understanding, Monitoring, and Controlling Biofilm Growth in Drinking Water Distribution Systems. Environ. Sci. Technol. 2016, 50, 8954–8976. [Google Scholar] [CrossRef]
- Nieuwenhuijsen, M.J.; Martinez, D.; Grellier, J.; Bennett, J.; Best, N.; Iszatt, N.; Vrijheid, M.; Toledano, M.B. Chlorination disinfection by-products in drinking water and congenital anomalies: Review and meta-analyses. Environ. Health Perspect. 2009, 117, 1486–1493. [Google Scholar] [CrossRef]
- Zhu, Z.; Shan, L.; Hu, F.; Li, Z.; Zhong, D.; Yuan, Y.; Zhang, J. Biofilm formation potential and chlorine resistance of typical bacteria isolated from drinking water distribution systems. RSC Adv. 2020, 10, 31295–31304. [Google Scholar] [CrossRef]
- Abberton, C.L.; Bereschenko, L.; van der Wielen, P.W.; Smith, C.J. Survival, Biofilm Formation, and Growth Potential of Environmental and Enteric Escherichia coli Strains in Drinking Water Microcosms. Appl. Environ. Microbiol. 2016, 82, 5320–5331. [Google Scholar] [CrossRef]
- Fish, K.E.; Collins, R.; Green, N.H.; Sharpe, R.L.; Douterelo, I.; Osborn, A.M.; Boxall, J.B. Characterisation of the physical composition and microbial community structure of biofilms within a model full-scale drinking water distribution system. PLoS ONE 2015, 10, e0115824. [Google Scholar] [CrossRef]
- Wang, Y.H.; Wu, Y.H.; Yu, T.; Zhao, X.H.; Tong, X.; Bai, Y.; Huo, Z.Y.; Hu, H.Y. Effects of chlorine disinfection on the membrane fouling potential of bacterial strains isolated from fouled reverse osmosis membranes. Sci. Total Environ. 2019, 693, 133579. [Google Scholar] [CrossRef] [PubMed]
- Mir, J.; Morato, J.; Ribas, F. Resistance to chlorine of freshwater bacterial strains. J. Appl. Microbiol. 1997, 82, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Packman, A. Characterizing the Interactions between Pathogens and Biofilms in Distribution Systems; Northwestern University, The Water Research Foundation: Evanston, IL, USA, 2015. [Google Scholar]
- Sedlak, D.L.; von Gunten, U. Chemistry. The chlorine dilemma. Science 2011, 331, 42–43. [Google Scholar] [CrossRef]
- Fish, K.E.; Reeves-McLaren, N.; Husband, S.; Boxall, J. Unchartered waters: The unintended impacts of residual chlorine on water quality and biofilms. NPJ Biofilms Microbiomes 2020, 6, 34. [Google Scholar] [CrossRef] [PubMed]
- Fish, K.E.; Boxall, J.B. Biofilm Microbiome (Re)Growth Dynamics in Drinking Water Distribution Systems Are Impacted by Chlorine Concentration. Front. Microbiol. 2018, 9, 2519. [Google Scholar] [CrossRef] [PubMed]
- White, C.; Tancos, M.; Lytle, D.A. Microbial community profile of a lead service line removed from a drinking water distribution system. Appl. Environ. Microbiol. 2011, 77, 5557–5561. [Google Scholar] [CrossRef]
- Su, H.C.; Liu, Y.S.; Pan, C.G.; Chen, J.; He, L.Y.; Ying, G.G. Persistence of antibiotic resistance genes and bacterial community changes in drinking water treatment system: From drinking water source to tap water. Sci. Total Environ. 2018, 616–617, 453–461. [Google Scholar] [CrossRef]
- Chandy, J.P.; Angles, M.L. Determination of nutrients limiting biofilm formation and the subsequent impact on disinfectant decay. Water Res. 2001, 35, 2677–2682. [Google Scholar] [CrossRef]
- Tsagkari, E.; Sloan, W.T. Turbulence accelerates the growth of drinking water biofilms. Bioprocess Biosyst. Eng. 2018, 41, 757–770. [Google Scholar] [CrossRef]
- García-Ávila, F.; Avilés-Añazco, A.; Ordoñez-Jara, J.; Guanuchi-Quezada, C.; Flores del Pino, L.; Ramos-Fernández, L. Modeling of residual chlorine in a drinking water network in times of pandemic of the SARS-CoV-2 (COVID-19). Sustain. Environ. Res. 2021, 31, 12. [Google Scholar] [CrossRef]
- Tsagkari, E.; Sloan, W.T. Impact of Methylobacterium in the drinking water microbiome on removal of trihalomethanes. Int. Biodeterior. Biodegrad. 2019, 141, 10–16. [Google Scholar] [CrossRef]
- Chamberlain, E.; Adams, C. Oxidation of sulfonamides, macrolides, and carbadox with free chlorine and monochloramine. Water Res. 2006, 40, 2517–2526. [Google Scholar] [CrossRef] [PubMed]
- Szabo, J.G.; Rice, E.W.; Bishop, P.L. Persistence and decontamination of Bacillus atrophaeus subsp. globigii spores on corroded iron in a model drinking water system. Appl. Environ. Microbiol. 2007, 73, 2451–2457. [Google Scholar]
- Garny, K.; Horn, H.; Neu, T.R. Interaction between biofilm development, structure and detachment in rotating annular reactors. Bioprocess. Biosyst. Eng. 2008, 31, 619–629. [Google Scholar] [CrossRef]
- Tsagkari, E.; Keating, C.; Couto, J.M.; Sloan, W.T. A Keystone Methylobacterium Strain in Biofilm Formation in Drinking Water. Water 2017, 9, 778. [Google Scholar] [CrossRef]
- Fish, K.; Osborn, A.M.; Boxall, J.B. Biofilm structures (EPS and bacterial communities) in drinking water distribution systems are conditioned by hydraulics and influence discolouration. Sci. Total Environ. 2017, 593–594, 571–580. [Google Scholar] [CrossRef]
- Agudelo-Vera, C.; Avvedimento, S.; Boxall, J.; Creaco, E.; De Kater, H.; Di Nardo, A.; Djukic, A.; Douterelo, I.; Fish, K.E.; Rey, P.L.; et al. Drinking Water Temperature around the Globe: Understanding, Policies, Challenges and Opportunities. Water 2020, 12, 1049. [Google Scholar] [CrossRef]
- Ripolles-Avila, C.; Hascoët, A.S.; Guerrero-Navarro, A.E.; Rodríguez-Jerez, J.J. Establishment of incubation conditions to optimize the in vitro formation of mature Listeria monocytogenes biofilms on food-contact surfaces. Food Control 2018, 92, 240–248. [Google Scholar] [CrossRef]
- Zlatanovic, L.; van der Hoek, J.P.; Vreeburg, J.H.G. An experimental study on the influence of water stagnation and temperature change on water quality in a full-scale domestic drinking water system. Water Res. 2017, 123, 761–772. [Google Scholar] [CrossRef]
- Childs, P.R.N. Rotating Cylinders, Annuli, and Spheres. In Rotating Flow; Elsevier Science & Technology Books: Shanghai, China, 2011; Chapter 6. [Google Scholar]
- Kaye, J.; Elgar, E.C. Modes of Adiabatic and Diabatic Fluid Flow in an Annulus with an Inner Rotating Cylinder; M.I.T. Research Laboratory of Heat Transfer in Electronics, Massachusetts Institute of Technology, Research Laboratory of Heat Transfer in Electronics: Cambridge, MA, USA, 1957; pp. 753–765. [Google Scholar]
- Bird, R.B.; Steward, E.W.; Lightfoot, N.E. Transport Phenomena; Wiley: New York, NY, USA, 1960; 780p. [Google Scholar]
- Munson, B.R.; Young, D.F.; Okiisshi, T.H. Fundamentals of Fluid Mechanics, 1st ed.; John Wiley and Sons: Hoboken, NJ, USA, 1998. [Google Scholar]
- Characklis, W.G.; Turakhia, M.H.; Zelver, N. Transport and interfacial transfer phenomena. In Biofilms; Characklis, W.G., Marshall, K.C., Eds.; John Wiley & Sons: New York, NY, USA, 1990; p. 288. [Google Scholar]
- Staudt, C.; Horn, H.; Hempel, D.C.; Neu, T.R. Volumetric measurements of bacterial cells and extracellular polymeric substance glycoconjugates in biofilms. Biotechnol. Bioeng. 2004, 88, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Chao, Y.Q.; Zhang, T. Optimization of fixation methods for observation of bacterial cell morphology and surface ultrastructures by atomic force microscopy. Appl. Microbiol. Biotechnol. 2011, 92, 381–392. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Kepner, R.L.; Pratt, J.R. Use of Fluorochromes for Direct Enumeration of Total Bacteria in Environmental-Samples—Past and Present. Microbiol. Rev. 1994, 58, 603–615. [Google Scholar] [CrossRef]
- Srinivasan, S.; Harrington, G.W.; Xagoraraki, I.; Goel, R. Factors affecting bulk to total bacteria ratio in drinking water distribution systems. Water Res. 2008, 42, 3393–3404. [Google Scholar] [CrossRef]
- Momba, M.N.B.; Kfir, R.; Venter, S.N.; Cloete, T.E. An overview of biofilm formation in distribution systems and its impact on the deterioration of water quality. Water SA 2000, 26, 59–66. [Google Scholar]
- Tsagkari, E.; Connelly, S.; Liu, Z.; McBride, A.; Sloan, W.T. The role of shear dynamics in biofilm formation. NPJ Biofilms Microbiomes 2022, 8, 33. [Google Scholar] [CrossRef]
- Tsai, Y.P. Interaction of chlorine concentration and shear stress on chlorine consumption, biofilm growth rate and particle number. Bioresour. Technol. 2006, 97, 1912–1919. [Google Scholar] [CrossRef]
- Behnke, S.; Parker, A.E.; Woodall, D.; Camper, A.K. Comparing the chlorine disinfection of detached biofilm clusters with those of sessile biofilms and planktonic cells in single- and dual-species cultures. Appl. Environ. Microbiol. 2011, 77, 7176–7184. [Google Scholar] [CrossRef]
- Zhang, H.; Tian, Y.; Kang, M.; Chen, C.; Song, Y.; Li, H. Effects of chlorination/chlorine dioxide disinfection on biofilm bacterial community and corrosion process in a reclaimed water distribution system. Chemosphere 2019, 215, 62–73. [Google Scholar] [CrossRef]
- Kelly, J.J.; Minalt, N.; Culotti, A.; Pryor, M.; Packman, A. Temporal variations in the abundance and composition of biofilm communities colonizing drinking water distribution pipes. PLoS ONE 2014, 9, e98542. [Google Scholar] [CrossRef] [PubMed]
- Rosario-Ortiz, F.; Rose, J.; Speight, V.; Gunten, U.V.; Schnoor, J. How do you like your tap water? Science 2016, 351, 912–914. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Y.; Shan, R.; Chen, G.; Liu, L. Linking flow velocity-regulated EPS production with early-stage biofilm formation in drinking water distribution systems. Water Supply 2020, 20, 1253–1263. [Google Scholar] [CrossRef]
- Morgenroth, E.; Wilderer, P.A. Influence of detachment mechanisms on competition in biofilms. Water Res. 2000, 34, 417–426. [Google Scholar] [CrossRef]
- Cogan, N.G.; Harro, J.M.; Stoodley, P.; Shirtliff, M.E. Predictive Computer Models for Biofilm Detachment Properties in Pseudomonas aeruginosa. Mbio 2016, 7, e00815-16. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Sevillano-Rivera, M.C.; Calus, S.T.; Bautista-de Los Santos, Q.M.; Eren, A.M.; Van Der Wielen, P.W.; Ijaz, U.Z.; Pinto, A.J. Disinfection exhibits systematic impacts on the drinking water microbiome. Microbiome 2020, 8, 42. [Google Scholar] [CrossRef]
- Falkinham, J.O.; Pruden, A.; Edwards, M. Opportunistic Premise Plumbing Pathogens: Increasingly Important Pathogens in Drinking Water. Pathogens 2015, 4, 373–386. [Google Scholar] [CrossRef]
- Waak, M.B.; Hozalski, R.M.; Hallé, C.; LaPara, T.M. Comparison of the microbiomes of two drinking water distribution systems-with and without residual chloramine disinfection. Microbiome 2019, 7, 87. [Google Scholar] [CrossRef]
- Zhang, H.C.; Chang, F.; Shi, P.; Ye, L.; Zhou, Q.; Pan, Y.; Li, A. Antibiotic Resistome Alteration by Different Disinfection Strategies in a Full-Scale Drinking Water Treatment Plant Deciphered by Metagenomic Assembly. Environ. Sci. Technol. 2019, 53, 2141–2150. [Google Scholar] [CrossRef]
- Saravanan, P.; Nancharaiah, Y.V.; Venugopalan, V.P.; Rao, T.S.; Jayachandran, S. Biofilm formation by Pseudoalteromonas ruthenica and its removal by chlorine. Biofouling 2006, 22, 371–381. [Google Scholar] [CrossRef]
- Loret, J.F.; Robert, S.; Thomas, V.; Levi, Y.; Cooper, A.J.; McCoy, W.F. Comparison of disinfectants for biofilm, protozoa and Legionella control. J. Water Health 2005, 3, 423–433. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsagkari, E.; Sloan, W. The Role of Chlorine in the Formation and Development of Tap Water Biofilms under Different Flow Regimes. Microorganisms 2023, 11, 2680. https://doi.org/10.3390/microorganisms11112680
Tsagkari E, Sloan W. The Role of Chlorine in the Formation and Development of Tap Water Biofilms under Different Flow Regimes. Microorganisms. 2023; 11(11):2680. https://doi.org/10.3390/microorganisms11112680
Chicago/Turabian StyleTsagkari, Erifyli, and William Sloan. 2023. "The Role of Chlorine in the Formation and Development of Tap Water Biofilms under Different Flow Regimes" Microorganisms 11, no. 11: 2680. https://doi.org/10.3390/microorganisms11112680
APA StyleTsagkari, E., & Sloan, W. (2023). The Role of Chlorine in the Formation and Development of Tap Water Biofilms under Different Flow Regimes. Microorganisms, 11(11), 2680. https://doi.org/10.3390/microorganisms11112680





