Association Analysis of Gut Microbiota and Prognosis of Patients with Acute Ischemic Stroke in Basal Ganglia Region
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients Enrollment
2.2. Image Acquisition
2.3. Demographics and Clinical Data Collection
2.4. Fecal Sample Collection and Gut Microbiota Analysis
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Patients
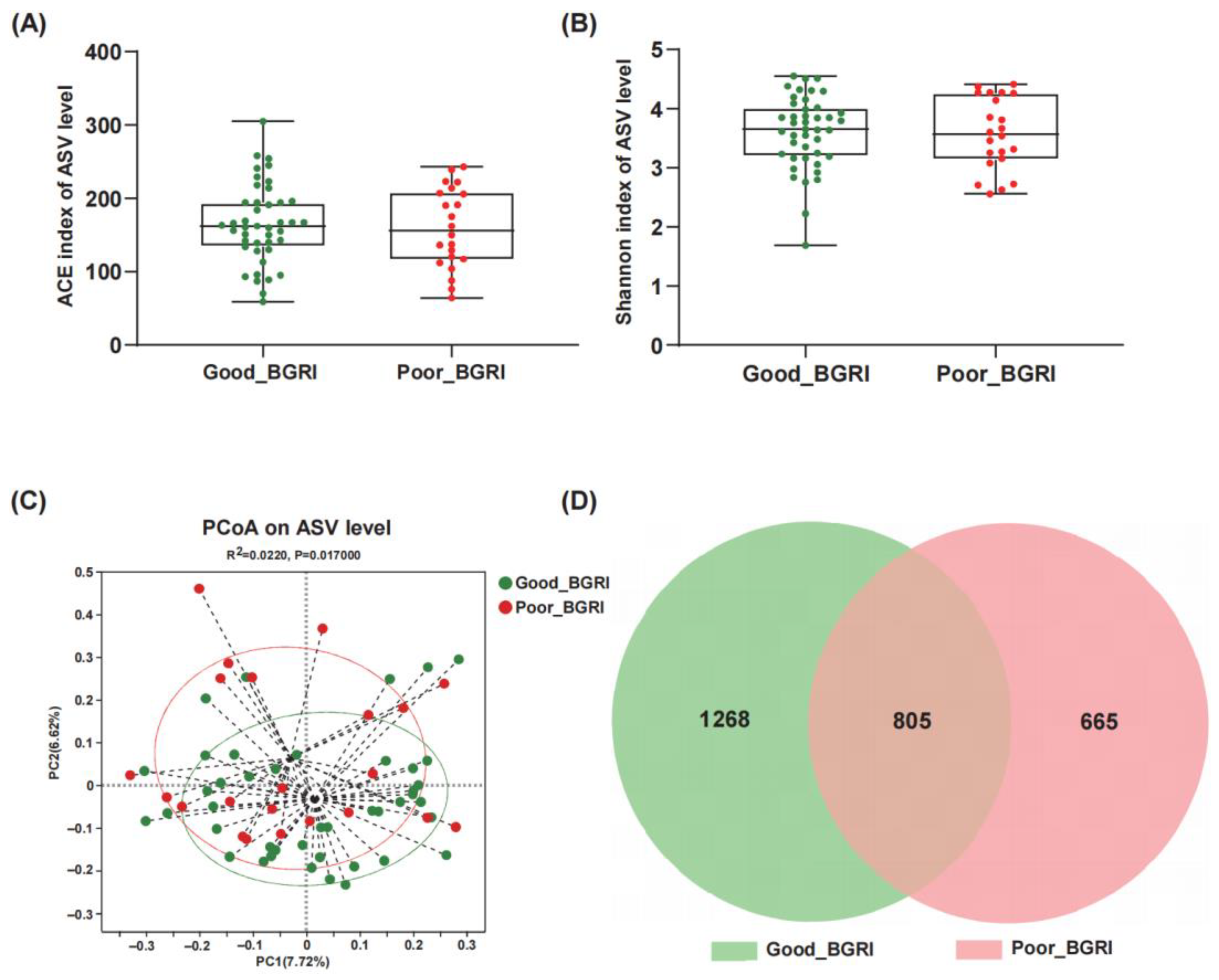
3.2. The Different Gut Microbiota Diversities between Good-BGRI Group and Poor-BGRI Group
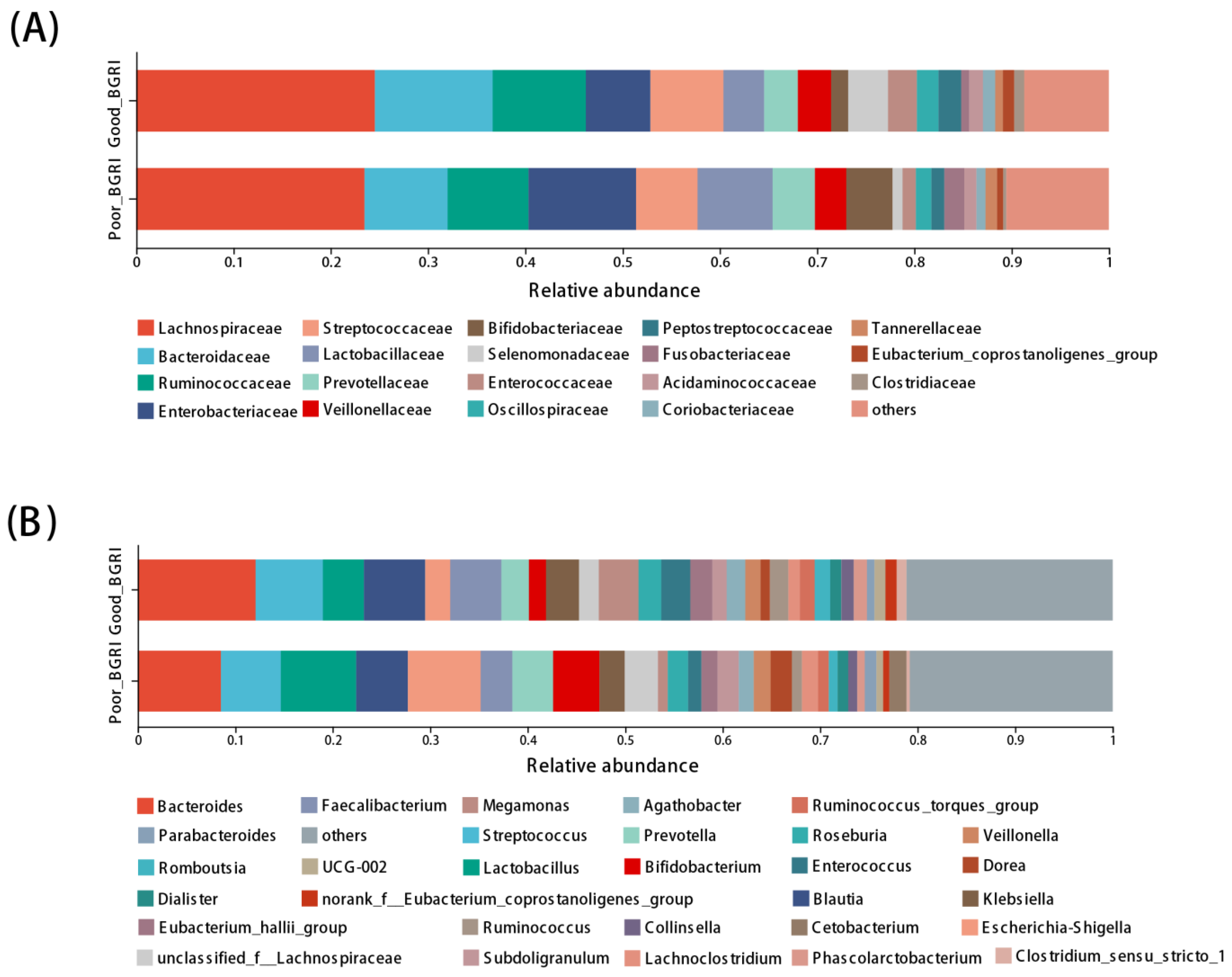
3.3. Different Compositions at Family and Genus Levels between Good-BGRI and Poor-BGRI Groups
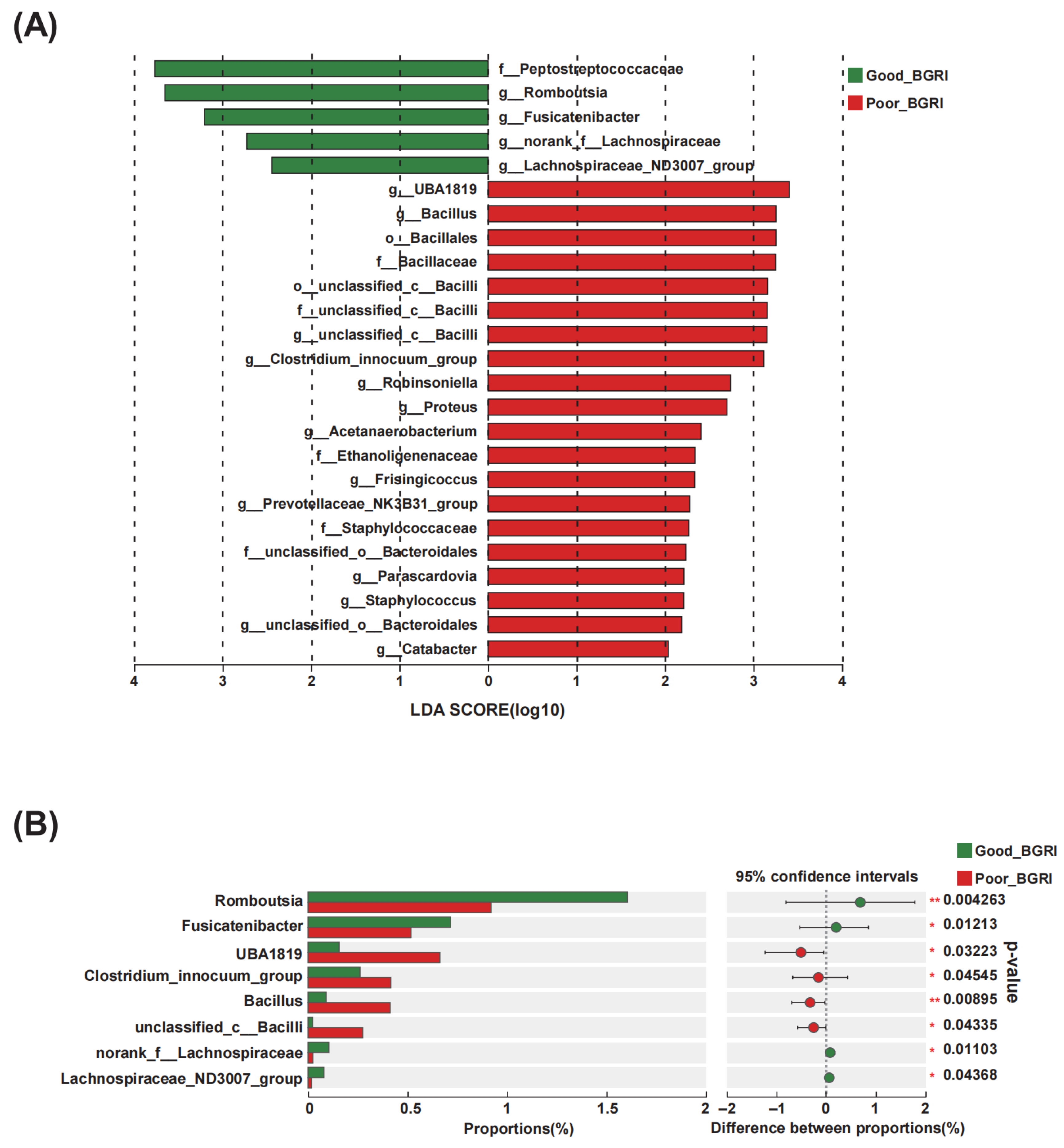
3.4. The Differential Gut Microbiota in Good-BGRI Group
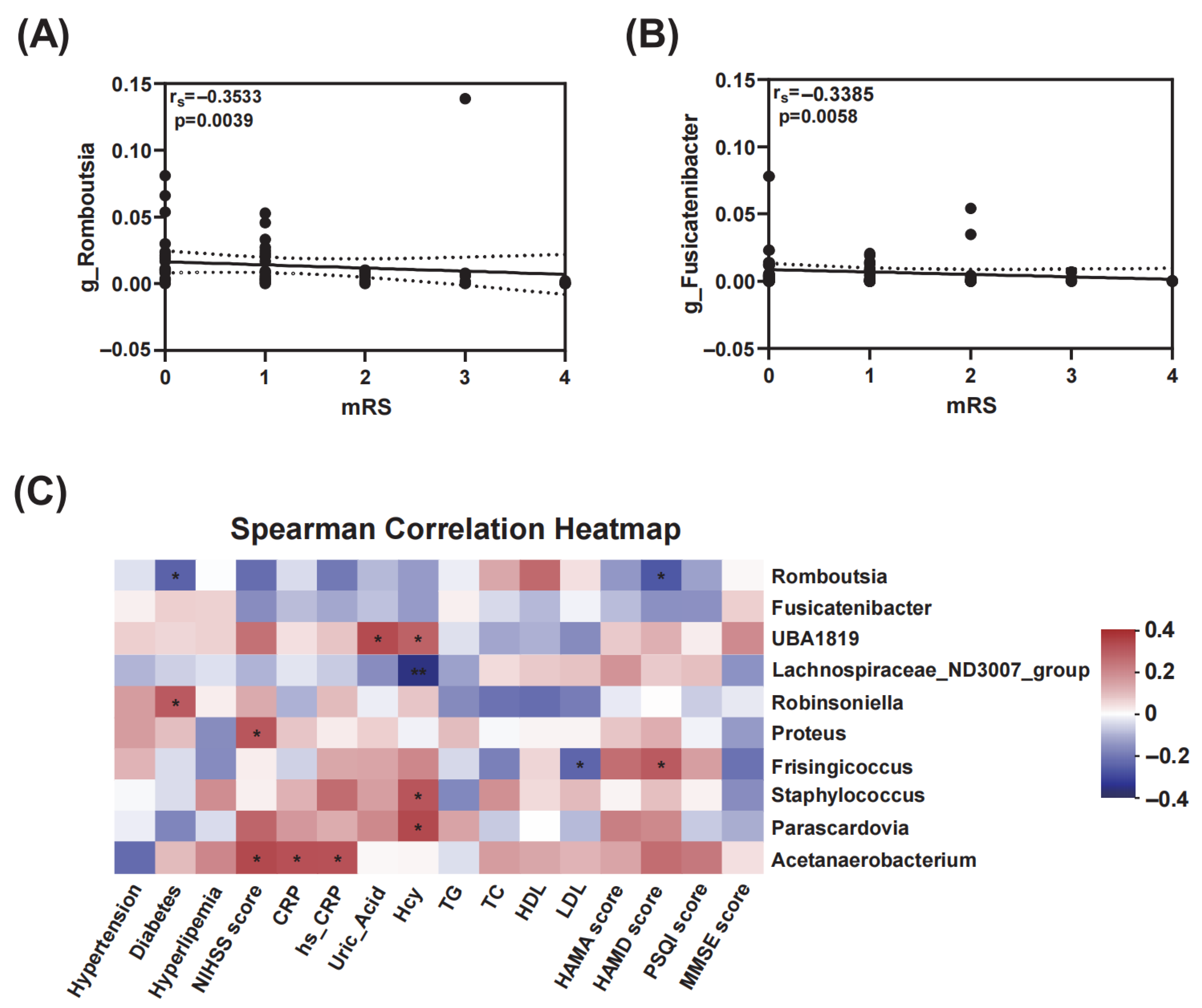
3.5. Correlations of Gut Microbiota with mRS Scores and Clinical Indicators
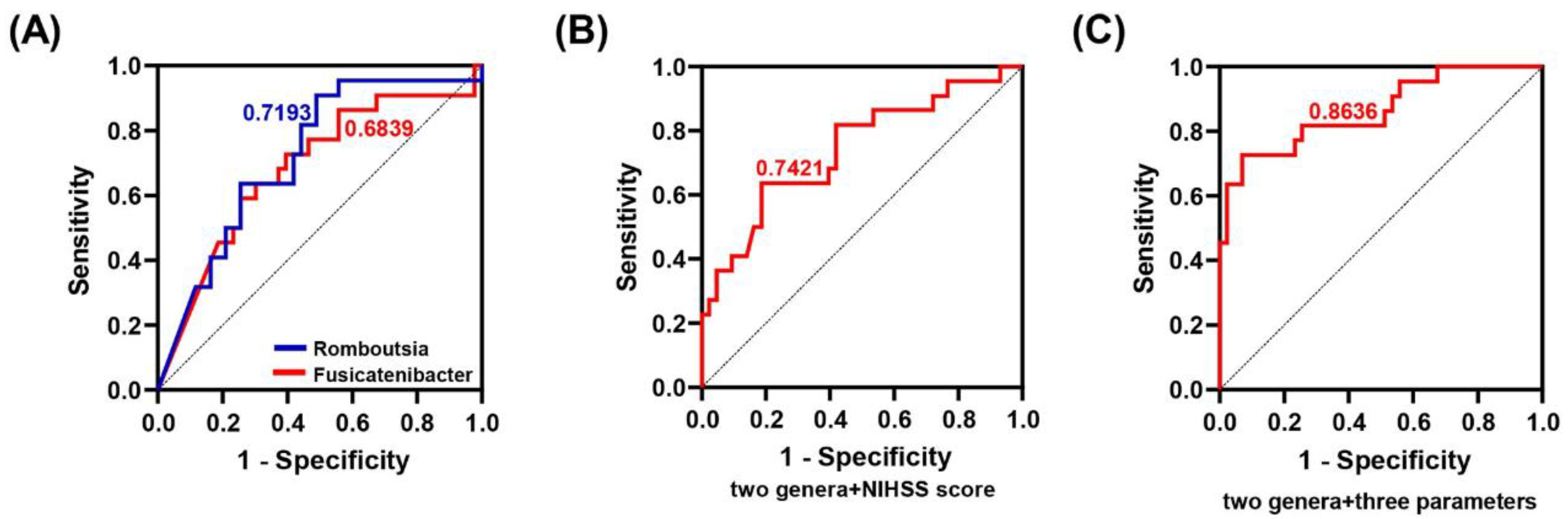
3.6. Predictive Performance of Differential Gut Microbiota from Good-BGRI Group and Clinical Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Ding, Q.; Liu, S.; Yao, Y.; Liu, H.; Cai, T.; Han, L. Global, regional, and national burden of ischemic stroke, 1990–2019. Neurology 2022, 98, e279–e290. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Jiang, B.; Sun, H.; Ru, X.; Sun, D.; Wang, L.; Wang, L.; Jiang, Y.; Li, Y.; Wang, Y.; et al. Prevalence, incidence, and mortality of stroke in China: Results from a nationwide population-based survey of 480 687 adults. Circulation 2017, 135, 759–771. [Google Scholar] [CrossRef] [PubMed]
- Alexander, G.E.; DeLong, M.R.; Strick, P.L. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Ann. Rev. Neurosci. 1986, 9, 357–381. [Google Scholar] [CrossRef] [PubMed]
- Delgado, P.; Riba-Llena, I.; Tovar, J.L.; Jarca, C.I.; Mundet, X.; Lopez-Rueda, A.; Orfila, F.; Llussa, J.; Manresa, J.M.; Alvarez-Sabin, J.; et al. Prevalence and associated factors of silent brain infarcts in a Mediterranean cohort of hypertensives. Hypertension 2014, 64, 658–663. [Google Scholar] [CrossRef] [PubMed]
- Gattringer, T.; Eppinger, S.; Pinter, D.; Pirpamer, L.; Berghold, A.; Wünsch, G.; Ropele, S.; Wardlaw, J.M.; Enzinger, C.; Fazekas, F. Morphological MRI characteristics of recent small subcortical infarcts. Int. J. Stroke 2015, 10, 1037–1043. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.K.; Chen, Y.K.; Mok, V.; Chu, W.C.; Ungvari, G.S.; Ahuja, A.T.; Wong, K.S. Acute basal ganglia infarcts in poststroke fatigue: An MRI study. J. Neurol. 2010, 257, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Biesbroek, J.M.; Shi, L.; Liu, W.; Kuijf, H.J.; Chu, W.W.; Abrigo, J.M.; Lee, R.K.; Leung, T.W.; Lau, A.Y.; et al. Strategic infarct location for post-stroke cognitive impairment: A multivariate lesion-symptom mapping study. J. Cereb. Blood Flow. Metab. 2018, 38, 1299–1311. [Google Scholar] [CrossRef] [PubMed]
- Dmytriw, A.A.; Dibas, M.; Schirmer, C.M.; Settecase, F.; Heran, M.K.S.; Efendizade, A.; Kühn, A.L.; Puri, A.S.; Ospel, J.; Menon, B.; et al. Age and acute ischemic stroke outcome in north American patients with COVID-19. J. Am. Heart Assoc. 2021, 10, e021046. [Google Scholar] [CrossRef]
- Kapral, M.K.; Fang, J.; Hill, M.D.; Silver, F.; Richards, J.; Jaigobin, C.; Cheung, A.M. Sex differences in stroke care and outcomes: Results from the Registry of the Canadian Stroke Network. Stroke 2005, 36, 809–814. [Google Scholar] [CrossRef]
- Willey, J.Z.; Ryu, W.-S.; Hong, K.-S.; Jeong, S.-W.; Park, J.E.; Kim, B.J.; Kim, J.-T.; Lee, K.B.; Park, T.H.; Park, S.-S.; et al. Association of ischemic stroke onset time with presenting severity, acute progression, and long-term outcome: A cohort study. PLoS Med. 2022, 19, e1003910. [Google Scholar] [CrossRef]
- Ding, G.Y.; Xu, J.H.; He, J.H.; Nie, Z.Y. Clinical scoring model based on age, NIHSS, and stroke-history predicts outcome 3 months after acute ischemic stroke. Front. Neurol. 2022, 13, 935150. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, S.; Lindley, R.I.; Carcel, C.; Sato, S.; Delcourt, C.; Wang, X.; Chalmers, J.; Anderson, C.S. NIHSS cut point for predicting outcome in supra- vs infratentorial acute ischemic stroke. Neurology 2018, 91, e1695–e1701. [Google Scholar] [CrossRef]
- Li, N.; Wang, X.; Sun, C.; Wu, X.; Lu, M.; Si, Y.; Ye, X.; Wang, T.; Yu, X.; Zhao, X.; et al. Change of intestinal microbiota in cerebral ischemic stroke patients. BMC Microbiol. 2019, 19, 191. [Google Scholar] [CrossRef] [PubMed]
- Gu, M.; Chen, N.; Sun, H.; Li, Z.; Chen, X.; Zhou, J.; Zhang, Y. Roseburia abundance associates with severity, evolution and outcome of acute ischemic stroke. Front. Cell Infect. Microbiol. 2021, 11, 669322. [Google Scholar] [CrossRef] [PubMed]
- Spychala, M.S.; Venna, V.R.; Jandzinski, M.; Doran, S.J.; Durgan, D.J.; Ganesh, B.P.; Ajami, N.J.; Putluri, N.; Graf, J.; Bryan, R.M.; et al. Age-related changes in the gut microbiota influence systemic inflammation and stroke outcome. Ann. Neurol. 2018, 84, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhong, Y.; Zhu, H.; Mahgoub, O.K.; Jian, Z.; Gu, L.; Xiong, X. Different gender-derived gut microbiota influence stroke outcomes by mitigating inflammation. J. Neuroinflamm. 2022, 19, 245. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Han, Z.Y.; Tian, D.F.; Liu, G.L.; Wang, Z.Y.; Lin, J.F.; Chang, Z.; Zhang, D.D.; Xie, Y.Z.; Sun, Y.K.; et al. Xinglou Chengqi Decoction improves neurological function in experimental stroke mice as evidenced by gut microbiota analysis and network pharmacology. Chin. J. Nat. Med. 2021, 19, 881–899. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Liu, X.; Chen, C.; Lin, J.; Li, A.; Guo, K.; An, D.; Zhou, D.; Hong, Z. Alteration of gut microbiota in patients with epilepsy and the potential index as a biomarker. Front. Microbiol. 2020, 11, 517797. [Google Scholar] [CrossRef]
- Lubomski, M.; Xu, X.; Holmes, A.J.; Muller, S.; Yang, J.Y.H.; Davis, R.L.; Sue, C.M. Nutritional intake and gut microbiome composition predict Parkinson’s disease. Front. Aging Neurosci. 2022, 14, 881872. [Google Scholar] [CrossRef]
- Chen, J.; Chi, B.; Ma, J.; Zhang, J.; Gu, Q.; Xie, H.; Kong, Y.; Yao, S.; Liu, J.; Sun, J.; et al. Gut microbiota signature as predictors of adverse outcomes after acute ischemic stroke in patients with hyperlipidemia. Front. Cell. Infect. Microbiol. 2022, 12, 1073113. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, X.; Li, Q.; Chen, J.; Liu, S.; Zhao, W.; Cai, H.; Zhu, J.; Yu, Y. Brain network topology and structural-functional connectivity coupling mediate the association between gut microbiota and cognition. Front. Neurosci. 2022, 16, 814477. [Google Scholar] [CrossRef] [PubMed]
- Reichelt, A.C.; Loughman, A.; Bernard, A.; Raipuria, M.; Abbott, K.N.; Dachtler, J.; Van, T.T.H.; Moore, R.J. An intermittent hypercaloric diet alters gut microbiota, prefrontal cortical gene expression and social behaviours in rats. Nutr. Neurosci. 2020, 23, 613–627. [Google Scholar] [CrossRef] [PubMed]
- Sato, R.; Bryan, R.N.; Fried, L.P. Neuroanatomic and functional correlates of depressed mood: The Cardiovascular Health Study. Am. J. Epidemiol. 1999, 150, 919–929. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Zuo, L.; Zhu, W.; Jing, J.; Zhang, Z.; Ding, L.; Wang, F.; Jian, C.; Wang, Y.; Li, Z.; et al. The distinct disrupted plasticity in structural and functional network in mild stroke with basal ganglia region infarcts. Brain Imaging Behav. 2021, 16, 2199–2219. [Google Scholar] [CrossRef] [PubMed]
- Sacco, R.L.; Kasner, S.E.; Broderick, J.P.; Caplan, L.R.; Connors, J.J.; Culebras, A.; Elkind, M.S.V.; George, M.G.; Hamdan, A.D.; Higashida, R.T.; et al. An updated definition of stroke for the 21st century. Stroke 2013, 44, 2064–2089. [Google Scholar] [CrossRef] [PubMed]
- Kwah, L.K.; Diong, J. National Institutes of Health Stroke Scale (NIHSS). J. Physiother. 2014, 60, 61. [Google Scholar] [CrossRef] [PubMed]
- Duvekot, M.H.C.; Venema, E.; Rozeman, A.D.; Moudrous, W.; Vermeij, F.H.; Biekart, M.; Lingsma, H.F.; Maasland, L.; Wijnhoud, A.D.; Mulder, L.; et al. Comparison of eight prehospital stroke scales to detect intracranial large-vessel occlusion in suspected stroke (PRESTO): A prospective observational study. Lancet Neurol. 2021, 20, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Hendrix, P.; Melamed, I.; Collins, M.; Lieberman, N.; Sharma, V.; Goren, O.; Zand, R.; Schirmer, C.M.; Griessenauer, C.J. NIHSS 24 h after mechanical thrombectomy predicts 90-day functional outcome. Clin. Neuroradiol. 2022, 32, 401–406. [Google Scholar] [CrossRef]
- Clarke, R.; Collins, R.; Lewington, S.; Donald, A.; Alfthan, G.; Tuomilehto, J.; Arnesen, E.; Bonaa, K.; Blacher, J.; Boers, H.; et al. Homocysteine and risk of ischemic heart disease and stroke: A meta-analysis. JAMA 2002, 288, 2015–2022. [Google Scholar] [CrossRef]
- Wu, X.Q.; Ding, J.; Ge, A.Y.; Liu, F.F.; Wang, X.; Fan, W. Acute phase homocysteine related to severity and outcome of atherothrombotic stroke. Eur. J. Intern. Med. 2013, 24, 362–367. [Google Scholar] [CrossRef]
- Nam, K.W.; Kim, C.K.; Yu, S.; Oh, K.; Chung, J.W.; Bang, O.Y.; Kim, G.M.; Jung, J.M.; Song, T.J.; Kim, Y.J.; et al. Plasma total homocysteine level is related to unfavorable outcomes in ischemic stroke with atrial fibrillation. J. Am. Heart Assoc. 2022, 11, e022138. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Dong, Z.; Cheng, M.; Zhao, Y.; Wang, M.; Sai, N.; Wang, X.; Liu, H.; Huang, G.; Zhang, X. Homocysteine exaggerates microglia activation and neuroinflammation through microglia localized STAT3 overactivation following ischemic stroke. J. Neuroinflamm. 2017, 14, 187. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Wang, X.; He, M.; Qin, X.; Tang, G.; Huo, Y.; Li, J.; Fu, J.; Huang, X.; Cheng, X.; et al. Homocysteine and stroke risk: Modifying effect of methylenetetrahydrofolate reductase C677T polymorphism and folic acid intervention. Stroke 2017, 48, 1183–1190. [Google Scholar] [CrossRef]
- Sharrief, A.Z.; Sánchez, B.N.; Lisabeth, L.D.; Skolarus, L.E.; Zahuranec, D.B.; Baek, J.; Garcia, N.; Case, E.; Morgenstern, L.B. The impact of pre-stroke depressive symptoms, fatalism, and social support on disability after stroke. J. Stroke Cerebrovasc. Dis. 2017, 26, 2686–2691. [Google Scholar] [CrossRef] [PubMed]
- Hamano, T.; Li, X.; Lönn, S.L.; Nabika, T.; Shiwaku, K.; Sundquist, J.; Sundquist, K. Depression, stroke and gender: Evidence of a stronger association in men. J. Neurol. Neurosurg. Psychiatry 2015, 86, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Taylor-Rowan, M.; Momoh, O.; Ayerbe, L.; Evans, J.J.; Stott, D.J.; Quinn, T.J. Prevalence of pre-stroke depression and its association with post-stroke depression: A systematic review and meta-analysis. Psychol. Med. 2019, 49, 685–696. [Google Scholar] [CrossRef] [PubMed]
- Arzani, M.; Jahromi, S.R.; Ghorbani, Z.; Vahabizad, F.; Martelletti, P.; Ghaemi, A.; Sacco, S.; Togha, M. Gut-brain axis and migraine headache: A comprehensive review. J. Headache Pain 2020, 21, 15. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; He, L.; Wang, C.; Zhang, T.; Guo, H.; Zhang, H.; Song, Y.; Chen, B. Altered gut microbiota and its metabolites correlate with plasma cytokines in schizophrenia inpatients with aggression. BMC Psychiatry 2022, 22, 629. [Google Scholar] [CrossRef]
- Ding, M.; Lang, Y.; Shu, H.; Shao, J.; Cui, L. Microbiota-gut-brain axis and epilepsy: A review on mechanisms and potential therapeutics. Front. Immunol. 2021, 12, 742449. [Google Scholar] [CrossRef]
- Sun, H.; Gu, M.; Li, Z.; Chen, X.; Zhou, J. Gut microbiota dysbiosis in acute ischemic stroke associated with 3-month unfavorable outcome. Front. Neurol. 2021, 12, 799222. [Google Scholar] [CrossRef]
- Gerritsen, J.; Umanets, A.; Staneva, I.; Hornung, B.; Ritari, J.; Paulin, L.; Rijkers, G.T.; de Vos, W.M.; Smidt, H. Romboutsia hominis sp. nov., the first human gut-derived representative of the genus Romboutsia, isolated from ileostoma effluent. Int. J. Syst. Evol. Microbiol. 2018, 68, 3479–3486. [Google Scholar] [CrossRef]
- Cui, W.; Xu, L.; Huang, L.; Tian, Y.; Yang, Y.; Li, Y.; Yu, Q. Changes of gut microbiota in patients at different phases of stroke. CNS Neurosci. Ther. 2023, 29, 3416–3429. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Huang, P.; Du, J.; He, Y.; Liu, J.; He, G.; Cui, S.; Zhang, W.; Li, G.; Chen, S. Specific gut microbiota alterations in essential tremor and its difference from Parkinson’s disease. NPJ Parkinson’s Dis. 2022, 8, 98. [Google Scholar] [CrossRef]
- Sorbara, M.T.; Littmann, E.R.; Fontana, E.; Moody, T.U.; Kohout, C.E.; Gjonbalaj, M.; Eaton, V.; Seok, R.; Leiner, I.M.; Pamer, E.G. Functional and genomic variation between human-derived isolates of Lachnospiraceae reveals inter- and intra-species diversity. Cell Host Microbe 2020, 28, 134–146.e134. [Google Scholar] [CrossRef] [PubMed]
- Weis, S.; Schwiertz, A.; Unger, M.M.; Becker, A.; Fassbender, K.; Ratering, S.; Kohl, M.; Schnell, S.; Schafer, K.H.; Egert, M. Effect of Parkinson’s disease and related medications on the composition of the fecal bacterial microbiota. NPJ Parkinson’s Dis. 2019, 5, 28. [Google Scholar] [CrossRef] [PubMed]
- Nishiwaki, H.; Ito, M.; Hamaguchi, T.; Maeda, T.; Kashihara, K.; Tsuboi, Y.; Ueyama, J.; Yoshida, T.; Hanada, H.; Takeuchi, I.; et al. Short chain fatty acids-producing and mucin-degrading intestinal bacteria predict the progression of early Parkinson’s disease. NPJ Parkinson’s Dis. 2022, 8, 65. [Google Scholar] [CrossRef] [PubMed]
- Wanapaisan, P.; Chuansangeam, M.; Nopnipa, S.; Mathuranyanon, R.; Nonthabenjawan, N.; Ngamsombat, C.; Thientunyakit, T.; Muangpaisan, W. Association between gut microbiota with mild cognitive impairment and Alzheimer’s disease in a Thai population. Neurodegener. Dis. 2022, 22, 43–54. [Google Scholar] [CrossRef]
- Chen, X.; Wu, Q.; Gao, X.; Wang, H.; Zhu, J.; Xia, G.; He, Y.; Song, W.; Xu, K. Gut microbial dysbiosis associated with type 2 diabetes aggravates acute ischemic stroke. mSystems 2021, 6, e01304-21. [Google Scholar] [CrossRef]
- Bai, J.; Wan, Z.; Zhang, Y.; Wang, T.; Xue, Y.; Peng, Q. Composition and diversity of gut microbiota in diabetic retinopathy. Front. Microbiol. 2022, 13, 926926. [Google Scholar] [CrossRef]
- Ye, X.; Liu, Y.; Hu, J.; Gao, Y.; Ma, Y.; Wen, D. Chlorogenic acid-induced gut microbiota improves metabolic endotoxemia. Front. Endocrinol. 2021, 12, 762691. [Google Scholar] [CrossRef]
- Kurita, N.; Yamashiro, K.; Kuroki, T.; Tanaka, R.; Urabe, T.; Ueno, Y.; Miyamoto, N.; Takanashi, M.; Shimura, H.; Inaba, T.; et al. Metabolic endotoxemia promotes neuroinflammation after focal cerebral ischemia. J. Cereb. Blood Flow. Metab. 2020, 40, 2505–2520. [Google Scholar] [CrossRef] [PubMed]
- Xie, A.; Ensink, E.; Li, P.; Gordevicius, J.; Marshall, L.L.; George, S.; Pospisilik, J.A.; Aho, V.T.E.; Houser, M.C.; Pereira, P.A.B.; et al. Bacterial butyrate in Parkinson’s disease is linked to epigenetic changes and depressive symptoms. Mov. Disord. 2022, 37, 1644–1653. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhu, Y.H.; Zhou, D.; Wu, Q.; Song, D.; Dicksved, J.; Wang, J.F. Oral administration of a select mixture of Bacillus probiotics affects the gut microbiota and goblet cell function following Escherichia coli challenge in newly weaned pigs of genotype MUC4 that are supposed to be enterotoxigenic E. coli F4ab/ac receptor negative. Appl. Environ. Microbiol. 2017, 83, e02747-16. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C.; Zhong, X.; Xu, T.; Xu, T.; Zhang, Y. Sex-specific relationship between serum uric acid and risk of stroke: A dose-response meta-analysis of prospective studies. J. Am. Heart Assoc. 2017, 6, e005042. [Google Scholar] [CrossRef]
- Chen, Y.; Pei, C.; Chen, Y.; Xiao, X.; Zhang, X.; Cai, K.; Deng, S.; Liang, R.; Xie, Z.; Li, P.; et al. Kidney tea ameliorates hyperuricemia in mice via altering gut microbiota and restoring metabolic profile. Chem. Biol. Interact. 2023, 376, 110449. [Google Scholar] [CrossRef]
- Tan, J.K.; Macia, L.; Mackay, C.R. Dietary fiber and SCFAs in the regulation of mucosal immunity. J. Allergy Clin. Immunol. 2023, 151, 361–370. [Google Scholar] [CrossRef]
- Tan, C.; Wu, Q.; Wang, H.; Gao, X.; Xu, R.; Cui, Z.; Zhu, J.; Zeng, X.; Zhou, H.; He, Y.; et al. Dysbiosis of gut microbiota and short-chain fatty acids in acute ischemic stroke and the subsequent risk for poor functional outcomes. J. Parenter. Enteral Nutr. 2021, 45, 518–529. [Google Scholar] [CrossRef]
- Lee, J.; d’Aigle, J.; Atadja, L.; Quaicoe, V.; Honarpisheh, P.; Ganesh, B.P.; Hassan, A.; Graf, J.; Petrosino, J.; Putluri, N.; et al. Gut microbiota-derived short-chain fatty acids promote poststroke recovery in aged mice. Circ. Res. 2020, 127, 453–465. [Google Scholar] [CrossRef]
- Chen, R.; Xu, Y.; Wu, P.; Zhou, H.; Lasanajak, Y.; Fang, Y.; Tang, L.; Ye, L.; Li, X.; Cai, Z.; et al. Transplantation of fecal microbiota rich in short chain fatty acids and butyric acid treat cerebral ischemic stroke by regulating gut microbiota. Pharmacol. Res. 2019, 148, 104403. [Google Scholar] [CrossRef]
- Sadler, R.; Cramer, J.V.; Heindl, S.; Kostidis, S.; Betz, D.; Zuurbier, K.R.; Northoff, B.H.; Heijink, M.; Goldberg, M.P.; Plautz, E.J.; et al. Short-chain fatty acids improve poststroke recovery via immunological mechanisms. J. Neurosci. 2020, 40, 1162–1173. [Google Scholar] [CrossRef]
- Jiang, W.; Chen, J.; Gong, L.; Liu, F.; Zhao, H.; Yan, Z.; Li, Y.; Zhang, J.; Xiao, M.; Mu, J. Microbiota-derived short-chain fatty acids may participate in post-stroke depression by regulating host’s lipid metabolism. J. Psychiatr. Res. 2023, 161, 426–434. [Google Scholar] [CrossRef]
- Liu, Y.; Kong, C.; Gong, L.; Zhang, X.; Zhu, Y.; Wang, H.; Qu, X.; Gao, R.; Yin, F.; Liu, X.; et al. The association of post-stroke cognitive impairment and gut microbiota and its corresponding metabolites. J. Alzheimer’s Dis. 2020, 73, 1455–1466. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, M.; Li, J.; Liang, J.; Yang, M.; Xia, G.; Ren, Y.; Zhou, H.; Wu, Q.; He, Y.; et al. Gut microbiota is causally associated with poststroke cognitive impairment through lipopolysaccharide and butyrate. J. Neuroinflamm. 2022, 19, 76. [Google Scholar] [CrossRef]
| Characteristics | Good-BGRI (n = 43) | Poor-BGRI (n = 22) | p-Value |
|---|---|---|---|
| Demographic parameters | |||
| Age (years) | 63.4 (10.7) | 67.1 (13.4) | 0.231 |
| Male, n (%) | 23 (53.5%) | 10 (45.5%) | 0.540 |
| Married, n (%) | 38 (88.4%) | 16 (72.7%) | 0.214 |
| Educational level, n (%) | 0.742 | ||
| Illiteracy | 9 (20.9%) | 3 (13.6%) | |
| Primary school | 18 (41.9%) | 11 (50.0%) | |
| Junior high school or above | 16 (37.2%) | 8 (36.4%) | |
| Previous history | |||
| Hyperlipidemia, n (%) | 24 (55.8%) | 9 (40.9%) | 0.255 |
| Diabetes, n (%) | 12 (27.9%) | 8 (36.4%) | 0.485 |
| Hypertension, n (%) | 26 (58.1%) | 17 (77.3%) | 0.127 |
| Hospital examination | |||
| SBP (mmHg) | 151.0 (25.0) | 167.5 (37.5) | 0.364 |
| DBP (mmHg) | 88.0 (13.0) | 86.5 (22.0) | 0.584 |
| MAP (mmHg) | 109.8 (12.0) | 110.3 (17.3) | 0.881 |
| CRP (mg/L) | 3.13 (0.32) | 3.30 (4.12) | 0.158 |
| Hs-CRP (mg/L) | 0.94 (1.20) | 2.38 (4.90) | 0.005 |
| HbA1c (%) | 5.88 (1.52) | 5.93 (1.85) | 0.551 |
| FBG (mmol/L) | 5.45 (1.79) | 5.11 (1.71) | 0.593 |
| Hcy (μmol/L) | 9.20 (4.90) | 11.75 (5.70) | 0.098 |
| TG (mmol/L) | 1.70 (1.38) | 1.52 (0.82) | 0.551 |
| HDL (mmol/L) | 1.04 (0.39) | 0.99 (0.28) | 0.349 |
| TC (mmol/L) | 4.67 (0.98) | 4.62 (1.01) | 0.849 |
| LDL (mmol/L) | 3.07 (0.96) | 3.04 (0.96) | 0.899 |
| Folate (ng/mL) | 10.5 (6.6) | 8.9 (4.5) | 0.181 |
| Vitamin B12 (pg/mL) | 350 (152) | 339 (313) | 0.967 |
| Uric Acid (μmol/L) | 313 (81) | 320 (83) | 0.733 |
| FT3 (pg/mL) | 2.99 (0.43) | 2.97 (0.44) | 0.809 |
| FT4 (ng/dL) | 1.17 (0.19) | 1.21 (0.18) | 0.481 |
| TSH (μIU/mL) | 1.906 (1.941) | 1.906 (1.079) | 0.311 |
| Scale scores | |||
| NIHSS score | 2 (2.0) | 3 (4.5) | 0.001 |
| HAMA score | 7 (4.0) | 11 (8.5) | 0.019 |
| HAMD score | 5.0 (4.0) | 10.5 (8.0) | 0.001 |
| PSQI score | 3.0 (2.0) | 3.5 (8.0) | 0.058 |
| MMSE score | 24 (6.0) | 24 (8.3) | 0.319 |
| Parameters | B (SE) | p-Value | OR | 95% CI |
|---|---|---|---|---|
| Hcy | 0.260 (0.119) | 0.029 | 1.297 | 1.026–1.639 |
| NIHSS score | 0.536 (0.270) | 0.047 | 1.710 | 1.007–2.901 |
| HAMD score | 0.553 (0.277) | 0.046 | 1.738 | 9.010–2.990 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, J.; Zhao, Y.; Chen, Q.; Liao, X.; Chen, J.; Xie, H.; Liu, J.; Sun, J.; Chen, S. Association Analysis of Gut Microbiota and Prognosis of Patients with Acute Ischemic Stroke in Basal Ganglia Region. Microorganisms 2023, 11, 2667. https://doi.org/10.3390/microorganisms11112667
Shi J, Zhao Y, Chen Q, Liao X, Chen J, Xie H, Liu J, Sun J, Chen S. Association Analysis of Gut Microbiota and Prognosis of Patients with Acute Ischemic Stroke in Basal Ganglia Region. Microorganisms. 2023; 11(11):2667. https://doi.org/10.3390/microorganisms11112667
Chicago/Turabian StyleShi, Jiayu, Yiting Zhao, Qionglei Chen, Xiaolan Liao, Jiaxin Chen, Huijia Xie, Jiaming Liu, Jing Sun, and Songfang Chen. 2023. "Association Analysis of Gut Microbiota and Prognosis of Patients with Acute Ischemic Stroke in Basal Ganglia Region" Microorganisms 11, no. 11: 2667. https://doi.org/10.3390/microorganisms11112667
APA StyleShi, J., Zhao, Y., Chen, Q., Liao, X., Chen, J., Xie, H., Liu, J., Sun, J., & Chen, S. (2023). Association Analysis of Gut Microbiota and Prognosis of Patients with Acute Ischemic Stroke in Basal Ganglia Region. Microorganisms, 11(11), 2667. https://doi.org/10.3390/microorganisms11112667






