Insect Microbial Symbionts: Ecology, Interactions, and Biological Significance
Abstract
1. Introduction
2. Microbial Symbionts in Insects
3. Types of Insects–Bacterial Interaction
4. Habitat of Microorganisms within Insect Gut
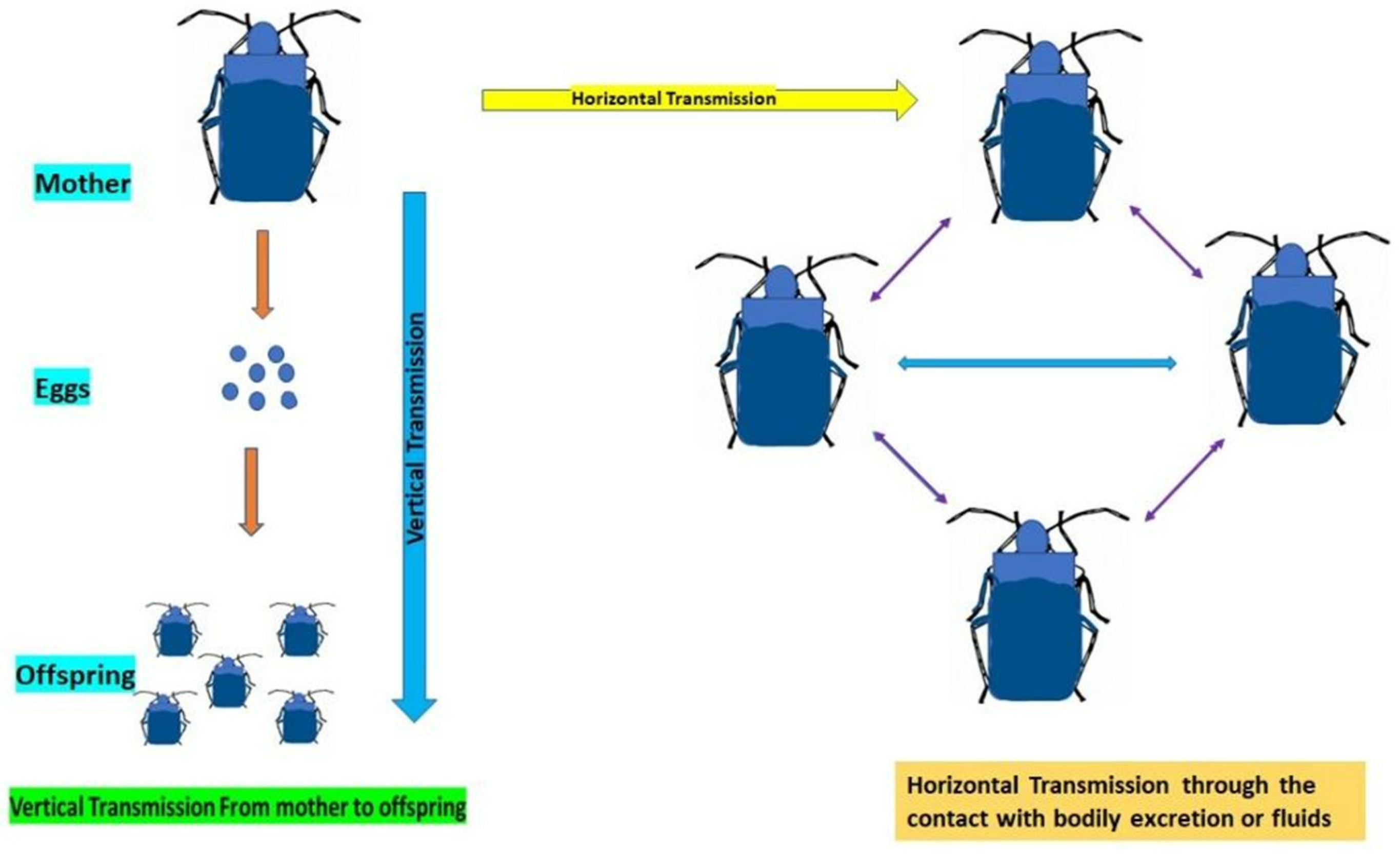
5. Mechanism of Transmission of Gut Symbionts
6. Composition of Microbiome in Insect Gut
7. Detection and Diagnosis of Gut Bacteria
8. Influence of Gut Bacteria on the Activity of Pesticides
9. Role of Gut Bacteria in Acquisition of Tolerance and Resistance
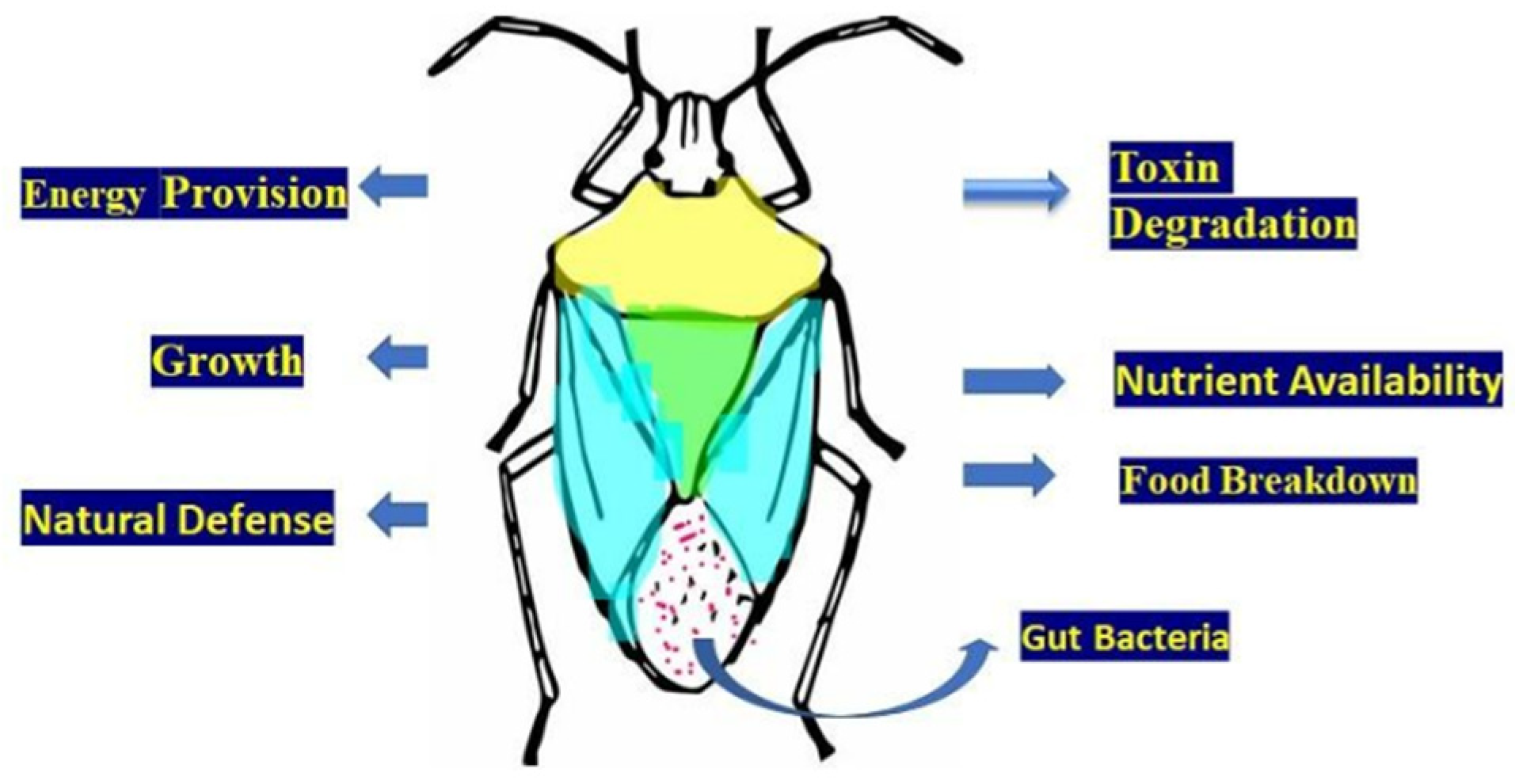
10. Biological Significance of Gut Symbiotic Microfauna
10.1. Symbiont-Mediated Detoxification of Phytotoxin
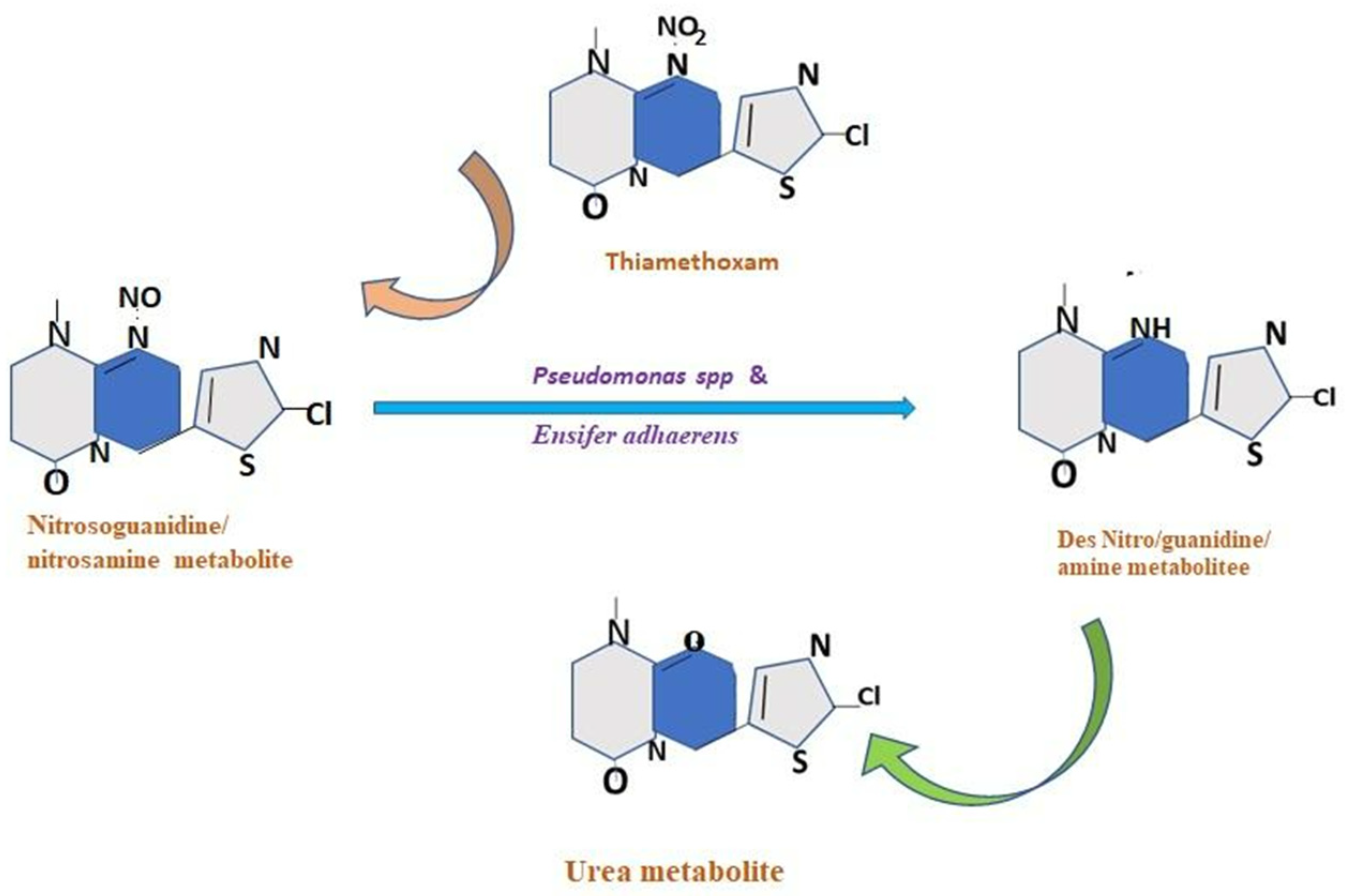
10.2. Symbiont-Mediated Detoxification of Insecticides
10.3. Molecular Mechanism of Enzyme-Mediated Insecticide Detoxification
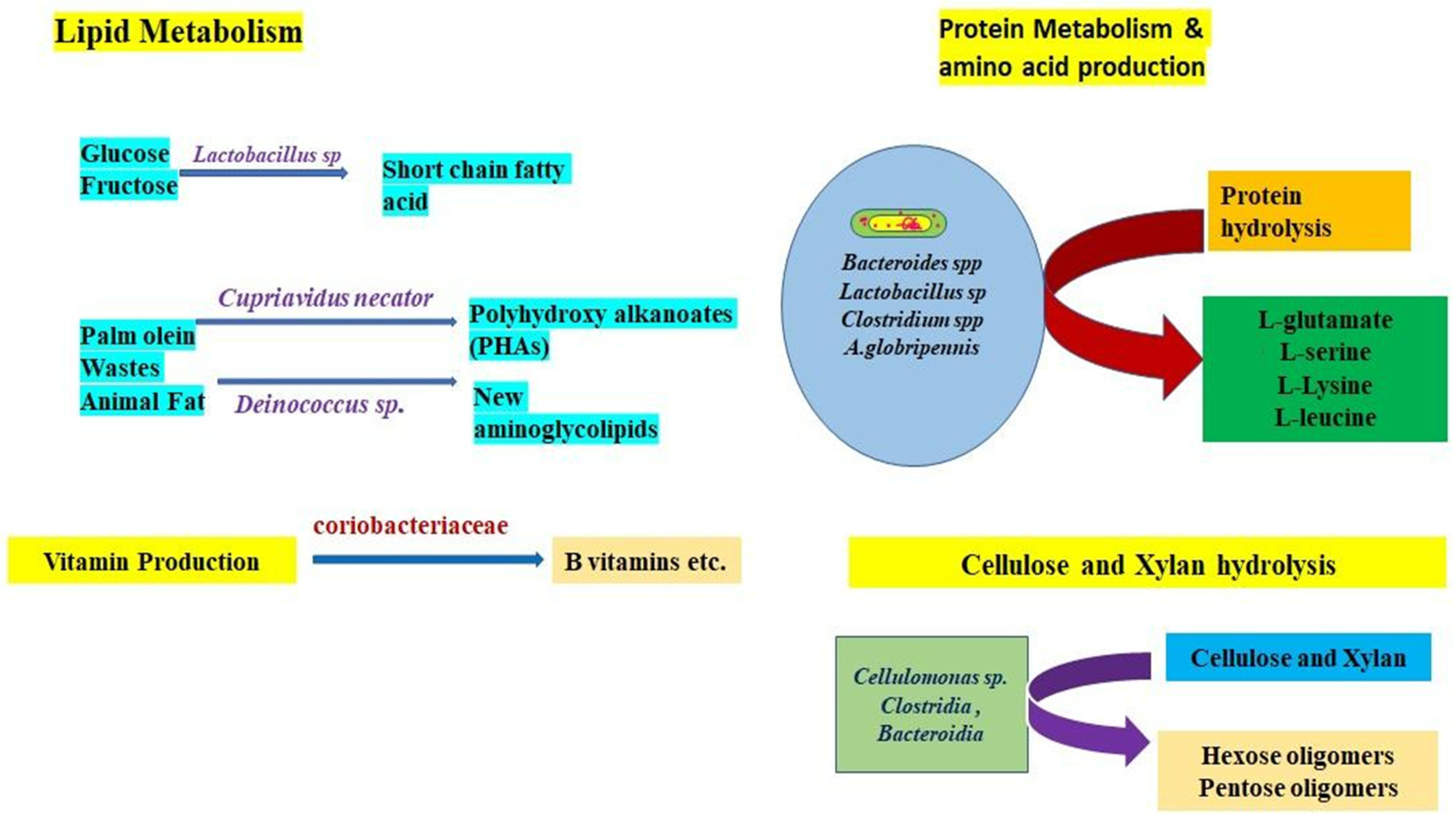
10.4. Gut Microbe-Mediated Nutrient Metabolism
10.4.1. Protein Metabolism
10.4.2. Sugar Fermentation
10.4.3. Nitrogen Fixation
10.4.4. Cellulose Digestion
10.4.5. Lipid Metabolism
10.4.6. Vitamin Production
10.5. Insect Gut Bacteria-Mediated Plastic Degradation
10.6. Gut Microbiota-Mediated Lignocellulose Digestion
11. Potential of Gut Microbes in Pest Management
12. Role of Gut Symbiont in Insecticide Resistance and Possible Management Strategies
13. Biotechnological Applications Based on Insect–Microbe Interactions
13.1. Industrial Application
13.2. Clinical Applications
13.3. Environmental Applications
14. Conclusions and Future Perspective
Funding
Acknowledgments
Conflicts of Interest
References
- Sanchez-Contreras, M.; Vlisidou, I. The diversity of insect-bacteria interactions and its applications for disease control. Biotechnol. Genet. Eng. Rev. 2008, 25, 203–244. [Google Scholar] [CrossRef]
- Gupta, A.; Nair, S. Dynamics of Insect–Microbiome Interaction Influence Host and Microbial Symbiont. Front. Microbiol. 2020, 11, 1357. [Google Scholar] [CrossRef] [PubMed]
- Arora, A.K.; Douglas, A.E. Hype or Opportunity? Using Microbial Symbionts in Novel Strategies for Insect Pest Control. J. Insect Physiol. 2017, 103, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Dillon, R.J.; Dillon, V.M. The Gut Bacteria of Insects: Nonpathogenic Interactions. Annu. Rev. Entomol. 2004, 49, 71–92. [Google Scholar] [CrossRef] [PubMed]
- Crotti, E.; Balloi, A.; Hamdi, C.; Sansonno, L.; Marzorati, M.; Gonella, E.; Favia, G.; Cherif, A.; Bandi, C.; Alma, A.; et al. Microbial symbionts: A resource for the management of insect-related problems. Microb. Biotechnol. 2012, 5, 307–317. [Google Scholar] [CrossRef]
- Ramadhar, T.R.; Beemelmanns, C.; Currie, C.R.; Clardy, J. Bacterial symbionts in agricultural systems provide a strategic source for antibiotic discovery. J. Antibiot. 2014, 67, 53. [Google Scholar] [CrossRef]
- Berasategui, A.; Salem, H.; Paetz, C.; Santoro, M.; Gerhenzon, J.; Kaltenpoth, M.; Schmidt, A. Gut microbiota of the pine weevil degrades conifer diterpenes and increases insect fitness. Mol. Ecol. 2017, 26, 15. [Google Scholar] [CrossRef]
- Kikuchi, Y. Detoxifying symbiosis: Microbe-mediated detoxification of phytotoxins and pesticides in insects. Nat. Prod. Rep. 2018, 35, 434–454. [Google Scholar] [CrossRef]
- Sloan, D.B.; Moran, N.A. Genome reduction and co-evolution between the primary and secondary bacterial symbionts of psyllids. Mol. Biol. Evol. 2012, 29, 3781–3792. [Google Scholar] [CrossRef]
- Shigenobu, S.; Watanabe, H.; Hattori, M.; Sakaki, Y.; Ishikawa, H. Genome sequence of the endocellular bacterial symbiont of aphids Buchnera sp. APS. Nature 2000, 407, 81–86. [Google Scholar] [CrossRef]
- van den Bosch, T.J.M.; Welte, C.U. Detoxifying symbionts in agriculturally important pest insects. Microb. Biotechnol. 2017, 10, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Himler, A.G.; Adachi-Hagimori, T.; Bergen, J.E.; Kozuch, A.; Kelly, S.E.; Tabashnik, B.E.; Chiel, E.; Duckworth, V.E.; Dennehy, T.J.; Zchori-Fein, E.; et al. Rapid spread of a bacterial symbiont in an invasive whitefly is driven by fitness benefits and female bias. Science 2011, 332, 254–256. [Google Scholar] [CrossRef] [PubMed]
- Hendry, T.A.; Hunter, M.S.; Baltrus, D.A. The facultative symbiont Rickettsia protects an invasive whitefly against entomopathogenic Pseudomonas syringae strains. Appl. Environ. Microbiol. 2014, 80, 7161–7168. [Google Scholar] [CrossRef] [PubMed]
- Shan, H.W.; Luan, J.B.; Liu, Y.Q.; Douglas, A.E.; Liu, S.S. The inherited bacterial symbiont Hamiltonella influences the sex ratio of an insect host. Proc. R. Soc. B Biol. Sci. 2019, 286, 1677. [Google Scholar] [CrossRef] [PubMed]
- Moya, A.; Peretó, J.; Gil, R.; Latorre, A. Learning how to live together: Genomic insights into prokaryote-animal symbioses. Nat. Rev. Genet. 2008, 9, 218–229. [Google Scholar] [CrossRef]
- Stephen, F.M.; Berisford, C.W.; Dahlsten, D.L.; Fenn, P.; Moser, J.C. Invertebrate and microbial associates. In Beetle-Pathogen Interactions in Conifer Forests; Schowalter, T.D., Filip, G.R., Eds.; Academic Press: San Diego, CA, USA, 1993; pp. 129–153. [Google Scholar]
- Bayen, S.; Roy, S.; Chakraborti, D.; Mukhopadhyay, A.; Kanta, L. Mutualistic relation of termites with associated microbes for their harmonious survival. Symbiosis 2021, 85, 145–161. [Google Scholar] [CrossRef]
- Pandey, A.K.; Deka, B.; Varshney, R.; Cheramgoi, E.C.; Babu, A. Do the beneficial fungi manage phytosanitary problems in the tea agro-ecosystem? BioControl 2021, 66, 445–462. [Google Scholar] [CrossRef]
- Simpson, S.J.; Clissold, F.J.; Lihoreau, M.; Ponton, F.; Wilder, S.M.; Raubenheimer, D. Recent advances in the integrative nutrition of arthropods. Annu. Rev. Entomol. 2015, 60, 293–311. [Google Scholar] [CrossRef]
- Cho, Y.L.; Huang, P.; Zhang, C. Randomized-controlled trial of anti-scarring effectiveness on filtrating surgery combined with amniotic membrane. Zhonghua Shiyan Yanke Zazhi Chin. J. Exp. Ophthalmol. 2013, 31, 265–269. [Google Scholar] [CrossRef]
- Handique, G.; Phukan, A.; Bhattacharyya, B.; Baruah, A.A.L.H.; Rahman, S.W.; Baruah, R. Characterization of cellulose degrading bacteria from the larval gut of the white grub beetle Lepidiota mansueta (Coleoptera: Scarabaeidae). Arch. Insect Biochem. Physiol. 2017, 94, e21370. [Google Scholar] [CrossRef]
- Jang, S.; Mergaert, P.; Ohbayashi, T.; Ishigami, K.; Shigenobu, S.; Itoh, H.; Kikuchi, Y. Dual oxidase enables insect gut symbiosis by mediating respiratory network formation. Proc. Natl. Acad. Sci. USA 2021, 118, e922. [Google Scholar] [CrossRef] [PubMed]
- Douglas, A.E. Symbiotic microorganisms: Untapped resources for insect pest control. Trends Biotechnol. 2007, 25, 338–342. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Guo, Z.; Riegler, M.; Xi, Z.; Liang, G.; Xu, Y. Gut symbiont enhances insecticide resistance in a significant pest, the oriental fruit fly Bactrocera dorsalis (Hendel). Microbiome 2017, 5, 13. [Google Scholar] [CrossRef]
- Sampson, T.R.; Mazmanian, S.K. Control of brain development, function, and behaviour by the microbiome. Cell Host Microbe 2015, 17, 565–576. [Google Scholar] [CrossRef]
- Siddiqui, J.A.; Khan, M.M.; Bamisile, B.S.; Hafeez, M.; Qasim, M.; Rasheed, M.T.; Rasheed, M.A.; Ahmad, S.; Shahid, M.I.; Xu, Y. Role of Insect Gut Microbiota in Pesticide Degradation: A Review. Front. Microbiol. 2022, 13, 870462. [Google Scholar] [CrossRef] [PubMed]
- Takeshita, K.; Matsuura, Y.; Itoh, H. Burkholderia of Plant-Beneficial Group are Symbiotically Associated with Bordered Plant Bugs (Heteroptera: Pyrrhocoroidea: Largidae). Microbes Environ. 2015, 30, 321–329. [Google Scholar] [CrossRef]
- Nászai, M.; Carroll, L.R.; Cordero, J.B. Intestinal stem cell proliferation and epithelial homeostasis in the adult Drosophila midgut. Insect Biochem. Mol. Biol. 2015, 67, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Bonfini, A.; Liu, X.; Buchon, N. From pathogen to microbiota: How Drosophila intestinal stem cells react to gut microbes. Dev. Comp. Immunol. 2016, 64, 22–38. [Google Scholar] [CrossRef]
- Jiang, H.; Tian, A.; Jing, J. Intestinal stem cell response to injury: Lessons from Drosophila. Cell Mol. Life Sci. 2016, 73, 3337–3349. [Google Scholar] [CrossRef]
- Janeh, M.; Osman, D.; Kambris, Z. Damage-induced cell regeneration in the midgut of aedes albopictus mosquitoes. Sci. Rep. 2017, 7, 44594. [Google Scholar] [CrossRef]
- Jang, S.; Kikuchi, Y. Impact of the insect gut microbiota on ecology, evolution, and industry. Curr. Opin. Insect Sci. 2020, 41, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Oliver, K.M.; Russel, J.A.; Moran, N.A.; Hunter, M.S. Facultative bacterial symbionts in aphids confer resistance to parasitic wasps. Proc. Natl. Acad. Sci. USA 2003, 100, 1803–1807. [Google Scholar] [CrossRef] [PubMed]
- Nakabachi, A.; Yamashita, A.; Toh, H.; Ishikwa, H.; Dunbar, H.E.; Moran, N.A.; Hattori, M. The 160-Kilobase Genome of the Bacterial Endosymbiont Carsonella. Science 2003, 314, 267. [Google Scholar] [CrossRef] [PubMed]
- Baumann, P. Biology of bacteriocyte-associated endosymbionts of plant sap-sucking insects. Annu. Rev. Microbiol. 2005, 59, 155–189. [Google Scholar] [CrossRef]
- Tao, K.; Long, Z.; Liu, Y.; Liu, S. Purification and Properties of a Novel Insecticidal Protein from the Locust Pathogen Serratia marcescens HR-3. Curr. Micribiol. 2006, 52, 45–49. [Google Scholar] [CrossRef]
- Andersson, S.G.; Kurland, C.G. Reductive evolution of resident ge- nomes. Trends Microbiol. 1998, 6, 263–268. [Google Scholar] [CrossRef]
- Heddi, A.; Charles, H.; Khatchadourian, C.; Bonnot, G.; Nardon, P. Molecular Characterization of the Principal Symbiotic Bacteria of the Weevil Sitophilus oryzae: A PeculiarG+C Content of an Endocytobiotic DNA. J. Mol. Evol. 1998, 47, 52–61. [Google Scholar] [CrossRef]
- Nishiwaki, H.; Ito, K.; Shimomura, M.; Nakashima, K.; Matsuda, K. Insecticidal bacteria isolated from predatory larvae of the antlion species Myrmeleon bore (Neuroptera: Myrmeleontidae). J. Invertebr. Pathol. 2007, 96, 80–88. [Google Scholar] [CrossRef]
- Perry, R.D.; Fertherston, J.D. Yersinia pestis—Etiologic Agent of Plague. Clin. Microbiol. Rev. 1997, 10, 35–66. [Google Scholar] [CrossRef]
- Stouthamer, R.; Breeuwer, J.A.J.; Hurst, G.D.D. Wolbachia pipientis: Microbial manipulator of arthropod reproduction. Annu. Rev. Microbiol. 1999, 53, 71–102. [Google Scholar] [CrossRef]
- Jaenike, J.; Unckless, R.; Cockburn, S.N.; Boelio, L.M.; Perlman, S.J. Adaptation via symbiosis: Recent spread of a Drosophila defensive symbiont. Science 2010, 329, 212–215. [Google Scholar] [CrossRef]
- Ffrench-constant, R.; Waterfield, N.; Daborn, P.; Joyee, S.; Bennett, H.; Boundy, S.; Reynolds, S.; Clarke, D. Photorhabdus: Towards a functional genomic analysis of a symbiont and pathogen. FEMS Microbiol. Rev. 2003, 26, 433–456. [Google Scholar] [CrossRef] [PubMed]
- Herbert, E.E.; Goodricj-Blair, H. Friend and foe: The two faces of Xenorhabdus nematophila. Nat. Rev. Microbiol. 2007, 5, 634–646. [Google Scholar] [CrossRef] [PubMed]
- Zientz, E.; Beyaert, I.; Gross, R.; Feldhaar, H. Relevance of the endosymbiosis of Blochmannia floridanus and carpenter ants at different stages of the life cycle of the host. Appl. Environ. Microbiol. 2006, 72, 6027–6033. [Google Scholar] [CrossRef] [PubMed]
- Chapman, R.F.; Simpson, S.J.; Douglas, A.E. The Insects: Structure and Function, 5th ed.; Cambridge University Press: Cambridge, UK, 1998. [Google Scholar]
- Huang, S.W.; Zhang, H.Y.; Marshall, S.; Jackson, T.A. The scarab gut: A potential bioreactor for bio-fuel production. Insect Sci. 2010, 17, 175–183. [Google Scholar] [CrossRef]
- Engel, P.; Moran, N.A. The gut microbiota of insects—Diversity in structure and function. FEMS Microbiol. Rev. 2013, 37, 699–735. [Google Scholar] [CrossRef] [PubMed]
- Klowden, M.J. Physiological Systems in Insects, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Shao, L.; Devenport, M.; Jacobs-Lorena, M. The peritrophic matrix of hematophagous insects. Arch. Insect Biochem. Physiol. 2001, 47, 119–125. [Google Scholar] [CrossRef]
- Hosokawa, T.; Kikuchi, Y.; Nikoh, N.; Fukatsu, T. Polyphyly of gut symbionts in stinkbugs of the family Cydnidae. Appl. Env. Microbiol. 2012, 78, 4758–4761. [Google Scholar] [CrossRef]
- Perreau, J.; Patel, D.J.; Maeda, G.P.; Elston, K.M.; Barrick, J.E.; Moran, N.A. Vertical transmission at the pathogen-symbiont Interface: Serratia Symbiotica and Aphids. mBio 2021, 20, 12. [Google Scholar] [CrossRef]
- Duron, O.; Wilkes, T.E.; Hurst, G.D.D. Interspecific transmission of a male-killing bacterium on an ecological timescale. Ecol. Lett. 2010, 3, 1139–1148. [Google Scholar] [CrossRef]
- Bright, M.; Bulgheresi, S.A. complex journey: Transmission of microbial symbionts. Nat. Rev. Microbiol. 2010, 8, 218–230. [Google Scholar] [CrossRef] [PubMed]
- Douglas, A.E. Mycetocyte symbiosis in insects. Biol. Rev. 1989, 64, 409–434. [Google Scholar] [CrossRef] [PubMed]
- Hosokawa, T.; Kikuchi, Y.; Xien, Y.M.; Fukatsu, T. The making of symbiont capsule in the plataspid stinkbug Megacopta punctatissima. FEMS Microbiol. Ecol. 2005, 54, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Hosokawa, T.; Kikuchi, Y.; Nikoh, N.; Shimada, M.; Fukatsu, T. Strict host-symbiont cospeciation and reductive genome evolution in insect gut bacteria. PLoS Biol. 2006, 4, 1841–1851. [Google Scholar] [CrossRef]
- Prado, S.S.; Rubinoff, D.; Almeida, R.P.P. Vertical transmission of a Pentatomid caeca-associated symbiont. Ann. Entomol. Soc. Am. 2006, 99, 577–585. [Google Scholar] [CrossRef]
- Attardo, G.M.; Lohs, C.; Heddi, A.; Alam, U.H.; Yildirim, S.; Aksoy, S. Analysis of milk gland structure and function in Glossina morsitans: Milk protein production, symbiont populations and fecundity. J. Insect Physiol. 2008, 54, 1236–1242. [Google Scholar] [CrossRef]
- Martinson, V.G.; Moy, J.; Moran, N.A. Establishment of characteristic gut bacteria during development of the honeybee worker. Appl. Environ. Microbiol. 2012, 78, 2830–2840. [Google Scholar] [CrossRef]
- Köhler, T.; Dietrich, C.; Scheffrahn, R.H.; Brune, A. High-resolution analysis of gut environment and bacterial microbiota reveals functional compartmentation of the gut in wood-feeding higher termites (Nasutitermes spp.). Appl. Environ. Microbiol. 2012, 78, 4691–4701. [Google Scholar] [CrossRef]
- Woodbury, N.; Gries, G. Firebrats, Thermobia domestica, aggregate in response to the microbes Enterobacter cloacae and Mycotypha Microspora. Entomol. Exp. Appl. 2013, 147, 154–159. [Google Scholar] [CrossRef]
- Woodbury, N.; Moore, M.; Gries, G. Horizontal transmission of the microbial symbionts Enterobacter cloacae and Mycotypha microspora to their firebrat host. Entomol. Exp. Appl. 2013, 147, 160–166. [Google Scholar] [CrossRef]
- Colman, D.R.; Toolson, E.C.; Takacs-Vesbach, C.D. Do diet and taxonomy influence insect gut bacterial communities? Mol. Ecol. 2012, 21, 5124–5137. [Google Scholar] [CrossRef] [PubMed]
- Hongoh, Y. Diversity and genomes of uncultured microbial symbionts in the termite gut. Biosci. Biotechnol. Biochem. 2010, 74, 1145–1151. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, L.; Fan, X.; Yu, C.; Feng, L.; Yi, L. An Insight into Diversity and Functionalities of Gut Microbiota in Insects. Curr. Microbiol. 2020, 77, 1976–1986. [Google Scholar] [CrossRef] [PubMed]
- Lemke, T.; Sting, U.; Egert, M.; Friedrich, M.W.; Brune, A. Physicochemical Conditions and Microbial Activities in the Highly Alkaline Gut of the Humus-Feeding Larva of Pachnoda ephippiata (Coleoptera: Scarabaeidae). Appl. Environ. Microbiol. 2003, 69, 6650–6658. [Google Scholar] [CrossRef] [PubMed]
- Eichler, S.; Schaub, G.A. Development of symbionts in triatomine bugs and the effects of infections with trypanosomatids. Exp. Parasitol. 2002, 100, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Webster, P.; Finkel, S.E.; Tower, J. Increased Internal and External Bacterial Load during Drosophila Aging without Life-Span Trade-Off. Cell Metab. 2007, 6, 144–152. [Google Scholar] [CrossRef]
- Ryu, J.H.; Ha, E.M.; Lee, W.J. Innate immunity and gut-microbe mutualism in Drosophila. Dev. Comp. Immunol. 2010, 34, 369–376. [Google Scholar] [CrossRef]
- McFall-Ngai, M.; Hadfield, M.G.; Bosch, T.C.G.; Carey, H.V.; Domazet-Lošo, T.; Douglas, A.E.; Dubilier, N.; Eberl, G.; Fukami, T.; Gilbert, S.F.; et al. Animals in a bacterial world, a new imperative for the life sciences. Proc. Natl. Acad. Sci. USA 2013, 110, 3229–3236. [Google Scholar] [CrossRef]
- Wier, A.M.; Nyholm, S.V.; Mandel, M.J.; Massengo-Tiassé, R.P.; Schaefer, A.L.; Koroleva, I.; Splinter-BonDurant, S.; Brown, B.; Manzella, L.; Snir, E.; et al. Transcriptional patterns in both host and bacterium underlie a daily rhythm of anatomical and metabolic change in a beneficial symbiosis. Proc. Natl. Acad. Sci. USA 2010, 107, 2259–2264. [Google Scholar] [CrossRef]
- Lloyd, D. Biochemistry of cell cycle. Boichem. J. 1987, 242, 313–321. [Google Scholar] [CrossRef]
- Naaz, N.; Choudhary, J.S.; Prabhakar, C.S. Identification and evaluation of cultivable gut bacteria associated with peach fruit fly, Bactrocera zonata (Diptera: Tephritidae). Phytoparasitica 2016, 44, 165–176. [Google Scholar] [CrossRef]
- Sneath, P.H.A.; Holt, J.G.; Krieg, N.R.; Staley, J.T.; Williams, S.T. Lactic acid bacteria. In Bergey’s Manual of Systematic Bacteriology; Bergey, D.H., Ed.; Lippincott William and Wilkins: New York, NY, USA, 1986; 721p. [Google Scholar]
- Ramya, S.L.; Venkatesan, T.; Srinivasa Murthy, K.S.; Jalali, S.K.; Verghese, A. Detection of carboxylesterase and esterase activity in culturable gut bacterial flora isolated from diamondback moth, Plutella xylostella (Linnaeus), from India and its possible role in indoxacarb degradation. Braz. J. Microbiol. 2016, 47, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Walker, T.M.; Kohl, T.A.; Omar, S.V. Whole-genome sequencing for prediction of Mycobacterium tuberculosis drug susceptibility and resistance: A retrospective cohort study. Lancet Infect 2015, 18, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, Y.; Hu, L.; Wang, Y. The metaverse in education: Definition, framework, features, potential applications, challenges, and future research topics. Front. Microbiol. 2022, 13, 1016300. [Google Scholar] [CrossRef] [PubMed]
- Akman Gündüz, E.; Douglas, A.E. Symbiotic bacteria enable insect to use a nutritionally inadequate diet. Proc. R. Soc. B Biol. Sci. 2012, 276, 987–991. [Google Scholar] [CrossRef]
- Gomes, A.F.F.; Omoto, C.; Cônsoli, F.L. Gut bacteria of field-collected larvae of Spodoptera frugiperda undergo selection and are more diverse and active in metabolizing multiple insecticides than laboratory-selected resistant strains. J. Pest Sci. 2020, 93, 833–851. [Google Scholar] [CrossRef]
- Boush, M.G.; Matsumura, F. Insecticidal Degradation by Pseudomonas melophthora, the Bacterial Symbiote of the Apple Maggot. J. Econ. Entomol. 1967, 60, 918–920. [Google Scholar] [CrossRef]
- Prabhakar, C.S.; Sood, P.; Kanwar, S.S. Isolation and characterization of gut bacteria of fruit fly, Bactrocera tau (Walker). Phytoparasitica 2013, 41, 193–201. [Google Scholar] [CrossRef]
- Lauzon, C.R.; Sjogren, R.E.; Prokopy, R.J. Enzymatic capabilities of bacteria associated with apple maggot flies: A postulated role in attraction. J. Chem. Ecol. 2000, 26, 953–967. [Google Scholar] [CrossRef]
- Narit, T.; Anuchit, C. Attraction of Bactrocera cucurbitae and B. papayae (Diptera: Tephritidae) to the odor of the bacterium enterobacter cloacae. Philipp. Agric. Sci. 2011, 94, 83–87. [Google Scholar]
- Kikuchi, Y.; Hayatsu, M.; Hosokawa, T.; Nagayama, A.; Tago, K.; Fukatsu, T. Symbiont-mediated insecticide resistance. Proc. Natl. Acad. Sci. USA 2012, 109, 8618–8622. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, Y.; Yumoto, I. Efficient colonization of the bean bug Riptortus pedestris by an environmentally transmitted Burkholderia symbiont. Appl. Environ. Microbiol. 2013, 79, 2088–2091. [Google Scholar] [CrossRef]
- Russell, R.J.; Scott Jackson, C.J.; Pandey, R.; Pandey, G.; Taylor, M.C.; Coppin, C.W.; Liu, J.W.; Oakeshott, J.G. The evolution of new enzyme function: Lessons from xenobiotic metabolizing bacteria versus insecticide-resistant insects. Evol. Appl. 2011, 4, 225–248. [Google Scholar] [CrossRef] [PubMed]
- Itoh, H.; Hori, T.; Sato, Y.; Nagayama, A.; Tago, K.; Hayatsu, M.; Kikuchi, Y. Infection dynamics of insecticide-degrading symbionts from soil to insects in response to insecticide spraying. ISME J. 2018, 12, 909–920. [Google Scholar] [CrossRef] [PubMed]
- Pang, S.; Lin, Z.; Zhang, Y.; Chen, S. Inshight into the toxicity and degradation mechanism of imidacloprid via physicochemical and microbial approaches. Res. Gate. Sep. 2020, 8, 65. [Google Scholar] [CrossRef]
- Pang, R.; Chen, M.; Yue, L.; Li, T.; Kang, K.; Liang, Z.; Yuan, Z.; Zhang, W. A distinct strain of Arsenophonus symbiont decreases insecticide resistance resistance in its insect host. PLoS Genet. 2018, 14, e1007725. [Google Scholar] [CrossRef]
- Hussain, M.B.; Zhang, H.B.; Xu, J.L.; Liu, Q.; Jiang, Z.; Zhang, L.H. The acyl-homoserine lactone-type quorum-sensing system modulates cell mortility and virulence of Erwinia chrysanthemi pv. Zeae. J. Bacteriol. 2008, 190, 1045–1053. [Google Scholar] [CrossRef]
- Kirkman, M.S.; Mahmud, H.; Korytkowski, M.T. Intensive Blood Glucose Control and Vascular Outcomes in Patients with Type 2 Diabetes Mellitus. Endocrinol. Metab. Clin. N. Am. 2018, 47, 81–96. [Google Scholar] [CrossRef]
- Schneider, D.S.; Ayres, J.S. Two ways to survive infection: What resistance and tolerance can teach us about treating infectious diseases. Nat. Rev. Immunol. 2008, 8, 889–895. [Google Scholar] [CrossRef]
- Muthukrishnan, S.; Merzendorfer, H.; Arakane, Y.; Kramer, K.J. Chitin Metabolism in Insects. In Insect Molecular Biology and Biochemistry; Academic Press: Cambridge, MA, USA, 2012. [Google Scholar] [CrossRef]
- Terra, W.R.; Ferreira, C. Biochemistry and Molecular Biology of Digestion. In Insect Molecular Biology and Biochemistry; Academic Press: Cambridge, MA, USA, 2012. [Google Scholar] [CrossRef]
- Moreno-García, M.; Condé, R.; Bello-Bedoy, R.; Lanz-Mendoza, H. The damage threshold hypothesis and the immune strategies of insects. Infect. Genet. Evol. 2014, 24, 25–33. [Google Scholar] [CrossRef]
- Cirimotich, C.M.; Ramirez, J.L.; Dimopoulos, G. Native microbiota shape insect vector competence for human pathogens. Cell Host Microbe 2011, 10, 307–310. [Google Scholar] [CrossRef] [PubMed]
- Mason, C.J. Complex Relationships at the Intersection of Insect Gut Microbiomes and Plant Defenses. J. Chem. Ecol. 2020, 46, 793–807. [Google Scholar] [CrossRef] [PubMed]
- Erb, M.; Kliebenstein, D.J. Plant Secondary Metabolites as Defenses Regulators and Primary Metabolites: The Blurred Functional Trichotomy. Plant Physiol. 2020, 184, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Nishida, A.; Kwong, W.K.; Koch, H.; Engel, P.; Steele, M.I.; Moran, N.A. Metabolism of toxic sugars by strains of the bee gut symbiont Gilliamella apicola. mBio 2016, 7, e01326-16. [Google Scholar] [CrossRef]
- Engel, P.; Kwong, W.K.; McFrederick, Q.; Anderson, K.E.; Barribeau, S.M.; Chandler, J.A.; Cornman, R.S.; Dainat, J.; De Miranda, J.R.; Doublet, V.; et al. The bee microbiome: Impact on bee health and model for evolution and ecology of host-microbe interactions. mBio 2016, 7, e02164-15. [Google Scholar] [CrossRef]
- Hammer, T.J.; Bowers, M.D. Gut microbes may facilitate insect herbivory of chemically defended plants. Oecologia 2015, 179, 1–14. [Google Scholar] [CrossRef]
- Vorburger, C.; Gehrer, L.; Rodriguez, P. A strain of the bacterial symbiont Regiella insecticola protects aphids against parasitoids. Biol. Lett. 2010, 6, 109–111. [Google Scholar] [CrossRef]
- Id, J.E.P.; Tiffany, C.; Liang, D. Disruption of the microbiota affects physiological and evolutionary aspects of insecticide resistance in the German cockroach, an important urban pest. PLoS ONE 2018, 13, e0207985. [Google Scholar]
- Mitsumori, M.; Xu, L.; Kajikawa, H.; Kurihara, M.; Tajima, K.; Hai, J.; Takenaka, A. Possible quorum sensing in the rumen microbial community: Detection of quorum-sensing signal molecules from rumen bacteria. FEMS Microbiol. Lett. 2003, 219, 47–52. [Google Scholar] [CrossRef]
- Chimel, J.A.; Daisley, B.A.; Burton, J.P.; Reid, G. Deleterious Effects of Neonicotinoid Pesticides on Drosophila melanogaster Immune Pathways. mBio 2019, 10, e01395-19. [Google Scholar] [CrossRef]
- Giambò, F.; Teodoro, M.; Costa, C.; Fenga, C. Toxicology and Microbiota: How Do Pesticides Influence Gut Microbiota? A Review. Int. J. Environ. Res. Public Health 2021, 18, 5510. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, L.G.; De Moraes, L.A.B.; Trigo, J.R.; Omoto, C.; Cônsoli, F.L. The gut microbiota of insecticide-resistant insects houses insecticide-degrading bacteria: A potential source for biotechnological exploitation. PLoS ONE 2017, 12, e0174754. [Google Scholar] [CrossRef] [PubMed]
- Ceja-Navarro, J.A.; Vega, F.E.; Karaoz, U.; Hao, Z.; Jenkins, S.; Lim, H.C.; Kosina, P.; Infante, F.; Northen, T.R.; Brodie, E.L. Gut microbiota mediate caffeine detoxification in the primary insect pest of coffee. Nat. Commun. 2015, 6, 7618. [Google Scholar] [CrossRef] [PubMed]
- Welte, C.U.; Rosengarten, J.F.; de Graaf, R.M.; Jetten, M.S.M. SaxA-mediated isothiocyanate metabolism in phytopathogenic pectobacteria. Appl. Environ. Microbiol. 2016, 82, 2372–2379. [Google Scholar] [CrossRef] [PubMed]
- Martin, V.; Yu, Z.; Mohn, W.W. Recent advances in understanding resin acid biodegradation: Microbial diversity and metabolism. Arch. Microbiol. 1999, 172, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Gurr, G.M.; Vasseur, L.; Zheng, D.; Zhong, H.; Qin, B. Metagenomic sequencing of diamondback moth gut microbiome unveils key holobiont adaptations for herbivory. Front. Microbiol. 2017, 8, 663. [Google Scholar] [CrossRef]
- Cordeiro, R.P.; Doria, J.H.; Zhanel, G.G.; Sparling, R.; Holley, R.A. Role of glycoside hydrolase genes in sinigrin degradation by E. coli O157: H7. Int. J. 2015, 205, 105–111. [Google Scholar] [CrossRef]
- Tomasek, P.H.; Karns, J.S. Cloning of a carbofuran hydrolase gene from Achromobacter sp. strain WM111 and its expression in gram-negative bacteria. J. Bacteriol. 1989, 171, 4038–4044. [Google Scholar] [CrossRef]
- Nagata, Y.; Ohtsubo, Y.; Endo, R.; Ichikawa, N.; Ankai, A.; Oguchi, A.; Fukui, S.; Fujita, N.; Tsuda, M. Complete Genome Sequence of the Representative γ-Hexachlorocyclohexane-Degrading Bacterium Sphingobium japonicum UT26. J. Bacteriol. 2010, 192, 5852–5853. [Google Scholar] [CrossRef]
- Sun, S.-L.; Yang, W.; Guo, J.-J.; Zhou, Y.-N.; Rui, X.; Chen, C.; Ge, F.; Dai, Y.-J. Biodegradation of the neonicotinoid insecticide acetamiprid in surface water by the bacterium Variovorax boronicumulans CGMCC 4969 and its enzymatic mechanism. R. Soc. Chem. 2017, 7, 25387–25397. [Google Scholar] [CrossRef]
- Ranson, H.; Claudianos, C.; Ortelli, F.; Abgrall, C.; Hemingway, J.; Sharakhova, M.V.; Unger, M.F.; Collins, F.H.; Feyereisen, R. Evolution of supergene families associated with insecticide resistance. Science 2002, 298, 179–181. [Google Scholar] [CrossRef] [PubMed]
- Nikoh, N.; Hosokawa, T.; Oshima, K.; Hattori, M.; Fukatsu, T. Reductive evolution of bacterial genome in insect gut environment. Genome Biol. Evol. 2011, 3, 702–714. [Google Scholar] [CrossRef] [PubMed]
- Hosokawa, T.; Fukatsu, T. Relevance of microbial symbiosis to insect behavior. Curr. Opin. Insect Sci. 2020, 39, 91–100. [Google Scholar] [CrossRef]
- Franceschi, V.R.; Nakata, P.A. Calcium oxalate in plants: Formation and function. Annu. Rev. Plant Biol. 2005, 56, 41. [Google Scholar] [CrossRef]
- Fan, J.; Doerner, P.; Lamb, C. Pseudomonas sax Genes Overcome Non-Host Resistance in Arabidopsis. Science 2011, 331, 1185–1188. [Google Scholar] [CrossRef] [PubMed]
- Summers, R.M.; Louie, T.M.; Yu, C.L.; Gakhar, L.; Louie, K.C.; Subramanian, M. Novel, highly specific N-demethylases enable bacteria to live on caffeine and related purine alkaloids. J. Bacteriol. 2012, 194, 2041–2049. [Google Scholar] [CrossRef]
- Devine, G. Global Pesticide Resistance in Arthropods—By M. E. Whalon, D. Mota-Sanchez & R. M. Hollingworth. Entomol. Exp. Appl. 2009, 131, 106. [Google Scholar] [CrossRef]
- Hsu, J.C.; Haymer, D.S.; Wu, W.J.; Feng, H.T. Mutations in the acetylcholinesterase gene of Bactrocera dorsalis associated with resistance to organophosphorus insecticides. Insect Biochem. Mol. Biol. 2006, 36, 396–402. [Google Scholar] [CrossRef]
- Itoh, H.; Navarro, R.; Takeshita, K.; Tago, K.; Hayatsu, M. Bacterial population succession and adaptation affected by insecticide application and soil spraying history. Front. Microbiol. 2014, 5, 457. [Google Scholar] [CrossRef]
- Lin, T.; Cai, Z.; Wu, H. Transcriptome analysis of the Japanese pine sawyer beetle, Monochamus alternatus (Coleoptera: Cerambycidae) by high-throughput Illumina sequencing. J. Asia-Pac. Entomol. 2015, 18, 439–445. [Google Scholar] [CrossRef]
- Hu, Z.; Di Feng, X.; Lin, Q.S.; Chen, H.Y.; Li, Z.Y.; Yin, F.; Liang, P.; Gao, X.W. Biochemical mechanism of chlorantraniliprole resistance in the diamondback moth, Plutella xylostella Linnaeus. J. Integr. Agric. 2014, 13, 2452–2459. [Google Scholar] [CrossRef]
- Ismail, S.M. Effect of sublethal doses of some insecticides and their role on detoxication enzymes and protein-content of Spodoptera littoralis (Boisd.) (Lepidoptera: Noctuidae). Bull. Natl. Res. Cent. 2020, 44, 35. [Google Scholar] [CrossRef]
- Khan, M.M.; Khan, A.H.; Ali, M.W.; Hafeez, M.; Ali, S.; Du, C.; Fan, Z.; Sattar, M.; Hua, H. Emamectin benzoate induced enzymatic and transcriptional alternation in detoxification mechanism of predatory beetle Paederus fuscipes (Coleoptera: Staphylinidae) at the sublethal concentration. Ecotoxicology 2021, 30, 1227–1241. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhang, L.; Cui, R.; Zeng, X.; Gao, X. Cloning and expression of multiple cytochrome P450 genes: Induction by Fipronil in Workers of the Red Imported Fire Ant (Solenopsis invicta Buren). PLoS ONE 2016, 11, e0150915. [Google Scholar] [CrossRef]
- Bao, H.; Gao, H.; Zhang, Y.; Fan, D.; Fang, J.; Liu, Z. The roles of CYP6AY1 and CYP6ER1 in imidacloprid resistance in the brown planthopper: Expression levels and detoxification efficiency. Pestic. Biochem. Physiol. 2016, 129, 70–74. [Google Scholar] [CrossRef]
- Pang, R.; Chen, M.; Liang, Z.; Yue, X.; Ge, H.; Zhang, W. Functional analysis of CYP6ER1, a P450 gene associated with imidacloprid resistance in Nilaparvata lugens. Sci. Rep. 2016, 6, 34992. [Google Scholar] [CrossRef]
- Mohammadi, M.; Shadnoush, M.; Sohrabvandi, S.; Yousefi, M.; Khorshidian, N.; Mortazavian, A.M. Probiotics as potential detoxification tools for mitigation of pesticides: A mini review. Int. J. Food Sci. Technol. 2021, 56, 2078–2087. [Google Scholar] [CrossRef]
- Savijoki, K.; Ingmer, H.; Varmanen, P. Proteolytic systems of lactic acid bacteria. Appl. Microbiol. Biotechnol. 2006, 71, 394–406. [Google Scholar] [CrossRef]
- Liu, M.; Bayjanov, J.R.; Renckens, B.; Nauta, A.; Siezen, R.J. The proteolytic system of lactic acid bacteria revisited: A genomic comparison. BMC Genom. 2010, 11, 5–8. [Google Scholar] [CrossRef]
- Breznak, J.A.; Brune, A. Role of microorganisms in the digestion of lignocellulose by termites. Annu. Rev. Entomol 1994, 39, 453–487. [Google Scholar] [CrossRef]
- Sabree, Z.L.; Kambhampati, S.; Moran, N.A. Nitrogen recycling and nutritional provisioning by Blattabacterium, the cockroach endosymbiont. Proc. Natl. Acad. Sci. USA 2009, 106, 19521–19526. [Google Scholar] [CrossRef]
- Bridges, J.R. Nitrogen-fixing bacteria associated with bark beetles. Microbial Ecology. Microb. Ecol. 1981, 7, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Vera-Ponce de Leon, A.; Orrillo, E.O.; Puebla, S.T.R.; Romero, E.M. Candidatus Dactylopiibacterium craminicum, a Nitrogen-Fixing symbiont of Dactylopius cochineal insects. (Hemiptera: Coccoidea: Dactylopiidae). Res. Gate 2017, 9, 2237–2250. [Google Scholar]
- Anand, A.A.P.; Vennison, S.J.; Sankar, S.G.; Prabhu, D.I.G.; Vasan, P.T.; Raghuraman, T.; Geoffrey, C.J.; Vendan, S.E. Isolation and characterization of bacteria from the gut of Bombyx mori that degrade cellulose, xylan, pectin and starch and their impact on digestion. J. Insect Sci. 2010, 10, 107. [Google Scholar] [CrossRef] [PubMed]
- Ong, S.Y.; Kho, H.P.; Riedel, S.L.; Kim, S.W.; Gan, C.Y.; Taylor, T.D.; Sudesh, K. An integrative study on biologically recovered polyhydroxyalkanoates (PHAs) and simultaneous assessment of gut microbiome in yellow mealworm. J. Biotechnol. 2018, 265, 31–39. [Google Scholar] [CrossRef]
- Kokoza, V.; Ahmed, A.; Shin, S.W.; Okafor, N.; Zou, Z.; Raikhel, A.S. Blocking of Plasmodium transmission by cooperative action of Cecropin a and Defensin a in transgenic Aedes aegypti mosquitoes. Proc. Natl. Acad. Sci. USA 2010, 107, 8111–8116. [Google Scholar] [CrossRef]
- Voirol, L.R.P.; Frago, E.; Kaltenpoth, M.; Hilker, M.; Fatouros, N.E. Bacterial symbionts in lepidoptera: Their diversity, transmission, and impact on the host. Front. Microbiol. 2018, 9, 556. [Google Scholar] [CrossRef]
- Geyer, R.; Jambeck, J.; Law, K.L. Production, use and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef]
- Yang, J.; Yang, Y.; Wu, W.M.; Zhao, J.; Jiang, L. Evidence of polyethylene biodegradation by bacterial strains from the guts of plastic-eating wax worms. Environ. Sci. Technol. 2014, 48, 13776–13784. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, J.; Wu, W.; Zhao, J.; Song, Y.; Gao, L.; Yang, R.; Jiang, L. Biodegradation and Mineralization of Polystyrene by Plastic-Eating Mealworms: Part 1. Chemical and Physical Characterization and Isotopic Tests. Environ. Sci. Technol. 2015, 49, 12080–12086. [Google Scholar] [CrossRef]
- Yoshida, S.; Hiraga, K.; Takehana, T.; Taniguchi, I.; Yamaji, H.; Maeda, Y.; Toyohara, K.; Miyamoto, K.; Kimura, Y.; Oda, K. A bacterium that degrades and assimilates poly (ethylene terephthalate). Science 2016, 353, 759. [Google Scholar] [CrossRef] [PubMed]
- Palm, G.J.; Reisky, L.; Böttcher, D.; Müller, H.; Michels, E.A.P.; Walczak, M.C.; Berndt, L.; Weiss, M.S.; Bornscheuer, U.T.; Weber, G. Structure of the plastic-degrading Ideonella sakaiensis MHETase bound to a substrate. Nat. Commun. 2019, 10, 1717. [Google Scholar] [CrossRef] [PubMed]
- Albertsson, A.; Andersson, S.O.; Karlsson, S. The Mechanism of Biodegradation of Polyethylene. Polym. Degrad. Stab. 1987, 18, 73–87. [Google Scholar] [CrossRef]
- Ni, J.; Tokuda, G. Lignocellulose-degrading enzymes from termites and their symbiotic microbiota. Biotechnol. Adv. 2013, 31, 838–850. [Google Scholar] [CrossRef]
- Yongqi, S.; Xie, S.; Lan, Y.; Sun, C. Insect microbial Symbionts as a novel Source for Biotechnology. World J. Microbiol. Biotechnol. 2019, 35, 25. [Google Scholar] [CrossRef]
- Zhong, J.; Jasinskas, A.; Barbour, A.G. Antibiotic treatment of the tick vector Amblyomma americanum reduced reproductive fitness. PLoS ONE 2007, 2, e405. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Patočka, J.; Kuča, K. Insect antimicrobial peptides, a mini review. Toxins 2018, 10, 461. [Google Scholar] [CrossRef] [PubMed]
- Fieck, A.; Hurwitz, I.; Kang, A.S.; Durvasula, R. Trypanosoma cruzi: Synergistic cytotoxicity of multiple amphipathic anti-microbial peptides to T. cruzi and potential bacterial hosts. Exp. Parasitol. 2010, 125, 342–347. [Google Scholar] [CrossRef]
- Kim, W.; Koo, H.; Richman, A.M.; Seeley, D.; Vezzoli, J.; Klocko, A.D.; O’Brochta, D.A. Ectopic expression of a cecropin transgene in the human malaria vector mosquito Anopheles gambiae (Diptera: Culicidae): Effects on susceptibility to Plasmodium. J. Med. Entomol. 2004, 41, 447–455. [Google Scholar] [CrossRef]
- Moran, N.A.; Yun, Y. Experimental replacement of an obligate insect symbiont. Proc. Natl. Acad. Sci. USA 2015, 112, 2093–2096. [Google Scholar] [CrossRef]
- Hilgenboecker, K.; Hammerstein, P.; Schlattmann, P.; Telschow, A.; Werren, J.H. How many species are infected with Wolbachia?—A statistical analysis of current data. FEMS Microbiol. Lett. 2008, 281, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Zabalou, S.; Riegler, M.; Theodorakopoulou, M.; Stauffer, C.; Savakis, C.; Bourtzis, K. Wolbachia-induced cytoplasmic incompatibility as a means for insect pest population control. Proc. Natl. Acad. Sci. USA 2004, 101, 15042–15045. [Google Scholar] [CrossRef] [PubMed]
- Jaffar, S.; Ahmad, S.; Lu, Y. Contribution of insect gut microbiota and their associated enzymes in insect Physiology and biodegradation of Pesticides. Front. Microbiol. 2022, 13, 97938. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, Y.; Hosokawa, T.; Fukatsu, T. An ancient but promiscuous host-symbiont association between Burkholderia gut symbionts and their heteropteran hosts. ISME J. 2011, 5, 446–460. [Google Scholar] [CrossRef]
- Tago, K.; Sekiya, E.; Kiho, A.; Katsuyama, C.; Hoshito, Y.; Yamada, N.; Hirano, K.; Sawada, H.; Hayatsu, M. Diversity of Fenitrothion-Degrading Bacteria in soils from Distant Geographical Areas. Microbes Environ. 2006, 21, 58–64. [Google Scholar] [CrossRef][Green Version]
- Chinnarajan, A.M.; Jayaraj, S.; Narayanan, K. Destruction of endosymbionts with oxytetracycline and sulphanilamid in the gourd fruit fly, Dacus cucurbitae Coq. (Dipter: Tephritidae). Hindustan Antibiot. Bull. 1972, 15, 16–22. [Google Scholar]
- Baker, A.C.; Ston, W.E.; Plummer, C.C.; McPhail, M. A review of studies on Mexican fruit fly and releated Mexican species. USDA Misc. Publ. 1944, 531, 155. [Google Scholar]
- Jamnongluk, W.; Kittayapong, P.; Baimai, V.; O’Neill, S.L. Wolbachia infections in tephritid fruit flies: Molecular evidence for five distinct strains in a single host species. Curr. Microbiol. 2002, 45, 255–260. [Google Scholar] [CrossRef]
- Guo, Z.J.; Lu, Y.Y.; Yang, F.; Zeng, L.; Liang, G.W.; Xu, Y.J. Transmission modes of a pesticide-degrading symbiont of the oriental fruit fly Bactrocera dorsalis (Hendel). Appl. Microbiol. Biotechnol. 2017, 101, 8543–8556. [Google Scholar] [CrossRef]
- Sood, P.; Nath, A. Colonization of marker strains of bacteria in fruit fly, Bactrocera tau. Indian J. Agric. Res. 2005, 39, 103–109. [Google Scholar]
- Mika, N.; Zorn, H.; Rühl, M. Insect-derived enzymes: A treasure for industrial biotechnology and food biotechnology. In Advances in Biochemical Engineering/Biotechnology; Springer: Berlin/Heidelberg, Germany, 2013; Volume 136, pp. 1–17. [Google Scholar] [CrossRef]
- Liang, X.; Sun, C.; Chen, B.; Du, K.; Yu, T.; Luang-In, V.; Lu, X.; Shao, Y. Insect symbionts as valuable grist for the biotechnological mill: An alkaliphilic silkworm gut bacterium for efficient lactic acid production. Appl. Microbiol. Biotechnol. 2018, 102, 4951–4962. [Google Scholar] [CrossRef] [PubMed]
- Matsui, T.; Tanaka, J.; Namihira, T.; Shinzato, N. Antibiotics production by an actinomycete isolated from the termite gut. J. Basic Microbiol. 2012, 52, 731–735. [Google Scholar] [CrossRef] [PubMed]
- Haeder, S.; Wirth, R.; Herz, H.; Spiteller, D. Candicidin-producing Streptomyces support leaf-cutting ants to protect their fungus garden against the pathogenic fungus Escovopsis. Proc. Natl. Acad. Sci. USA 2009, 106, 4742–4746. [Google Scholar] [CrossRef]
- Piel, J. A polyketide synthase-peptide synthetase gene cluster from an uncultured bacterial symbiont of Paederus beetles. Proc. Natl. Acad. Sci. USA 2002, 99, 14002–14007. [Google Scholar] [CrossRef] [PubMed]
- Werren, J.H. Biology of Wolbachia. Annu. Rev. Entomol. 1997, 42, 587–609. [Google Scholar] [CrossRef] [PubMed]
- Bian, G.; Xu, Y.; Lu, P.; Xie, Y.; Xi, Z. The endosymbiotic bacterium Wolbachia induces resistance to dengue virus in Aedes aegypti. PLoS Pathog. 2010, 6, e1000833. [Google Scholar] [CrossRef]
- van den Hurk, A.F.; Hall-Mendelin, S.; Pyke, A.T.; Frentiu, F.D.; McElroy, K.; Day, A.; Higgs, S.; O’Neill, S.L. Impact of Wolbachia on Infection with Chikungunya and Yellow Fever Viruses in the Mosquito Vector Aedes aegypti. PLoS Negl. Trop. Dis. 2012, 6, e1892. [Google Scholar] [CrossRef]
- Durvasula, R.V.; Kroger, A.; Goodwin, M.; Panackal, A.; Kruglov, O.; Taneja, J.; Gumbs, A.; Richards, F.F.; Beard, C.B.; Cordon-Rosales, C. Strategy for introduction of foreign genes into field populations of Chagas disease vectors. Ann. Entomol. Soc. Am. 1992, 92, 937–943. [Google Scholar] [CrossRef]
- Bisi, D.C.; Lampe, D.J. Secretion of anti-Plasmodium effector proteins from a natural Pantoea agglomerans isolate by using PelB and HlyA secretion signals. Appl. Environ. 2011, 77, 4669–4675. [Google Scholar] [CrossRef]
- Bongio, N.J.; Lampe, D.J. Inhibition of Plasmodium berghei development in mosquitoes by effector proteins secreted from Asaia sp. Bacteria using a novel native secretion signal. PLoS ONE 2015, 10, e0143541. [Google Scholar] [CrossRef]
| Insect Order, Common Name, Species Name | Bacterial Species | Type of Interaction | Phenotype | References |
|---|---|---|---|---|
| Hemiptera | ||||
| Blood sucking bug: Rhodnius prolixus Stal et al. (Reduviidae) | Rhodococcus rhodnii Tsukamura et al. | Gut symbiont/Commensal | Amino acid synthesis | [23] |
| Sap-sucking insects: Aphids, Acyrthosiphon pisum Harris, Schiaphis graminum Rondani et al. | Buchnera aphidicola Munson et al. | P-endosymbiont | Confers host defense against natural enemies, parasitic wasps | [33] |
| Aphids, Acyrthosiphon pisum Harris (Aphididae) | Hamiltonella defensa Moran et al. | S-symbiont | Confers host defense against natural enemies, parasitic wasps | [33] |
| Sap-sucking insects: Psyllids, Pachypsylla venusta Thomas et al. (Psyllidae) | Carsonella ruddii Thao et al. (y-proteobacteria) | Endosymbiont | Essential nutrients, possibly amino acids | [34] |
| Sap-sucking insects: mealybugs, Planococcus citri Risso et al. | Tremblaya princeps (β-proteobacteria) | Endosymbiont | Probably amino acid | [35] |
| Orthoptera | ||||
| Grassland locusts, Myrmeleotettix palpalis Zubovski, 1900 | Serratia marcescens strain HR-3 (y-proteobacteria) | Pathogen | Paralysis induced by insecticidal metalloprotease. | [36] |
| Anoplura | ||||
| Human body louse, Pediculus humanus L. (Pediculidae) | Rickttsia prowazekii da Rocha-Lima et al. | Obligate Intracellular | [37] | |
| Coleoptera | ||||
| Rice weevil, Sitophilus oryzae L. (Curculionidae) | P-endosymbiont SOPE (y-proteobacteria) | P-endosymbiont | Vitamin synthesis and in fluence mitochondrial respiration in the host | [38] |
| Neuroptera | ||||
| Antlion Myrmeleon bore ( Myrmeleontide) | Enterobacter aerogenes, Bacillus cereus, B. sphaericus, Morganella morganii | Temporal association | Pathogens for other insect species prey of the antlion. | [39] |
| Siphonaptera | ||||
| Human North America Flea, Oropsylla montana (Ceratophyllida) | Yersinia pestis Lehmann and Neumann, (γ-proteobacteria) | Vector | Transmission of mammalian and human pathogen | [40] |
| Diptera | ||||
| Tsetse fly, Glossina spp. (Glossinidae) | Wigglesworthia glossinidia Dale et al. (γ-proteobacteria) | Symbiont | Cytoplasmic incompatibility | [24] |
| Fruit fly, Drosophila melanogaster Meigen (Drosophilidae) | Sodalis glossinidius Dale and Maudlin (γ-proteobacteria) | Symbiont | Cytoplasmic incompatibility | [41] |
| Tsetse fly, Glossinia brevipalpis Newstead (Glossinidae) | Wolbachia pipientis Hertig and Wolbach (α-proteobacteria) | P-endosymbiont | Essential for fly fertility | [42] |
| Lepidoptera | ||||
| Tobacco horn worm, Manduca Sexta L. (Sphingidae) | Photorhabdus luminescens Thomas et al. | Pathogen | Several toxins with oral and injectable toxicity | [43] |
| Wax moth, Galleria mellonella L. (Pyralidae) | (γ-proteobacteria) Xenorhabdus nematophilus Thomas and Poinar. | Pathogen | Xpt and Xax Toxins | [44] |
| Hymenoptera | ||||
| Carpenter ant, Camponotus floridanus Buckley (Formicidae) | Blochmannia floridanus Blochmann (γ-proteobacteria) | Nonessential endosymbiont | Improves viability of host pupae | [45] |
| Insect Pests | Gut Microbiota | Insecticides | Reference |
|---|---|---|---|
| Drosophila melanogaster Meigen (Drosophilidae) | Acetobacter spp. Beijerinck et al., Lactobacillus acidophilus, L. plantarum Orla-Jensen | Neonicotinoid | [106] |
| Rhagoletis pomonella Walsh (Tephritidae) Anopheles stephensi Liston (Culicidae) | Exiguobacterium sp. Collins et al., Aeromonas spp. Stanier et al., P. putida Migula, Citrobacter freundii Werkman and Gillen | Organochloride, Organophosphates | [90] |
| Rhagoletis pomonella Walsh Aedes spp. and Anopheles gambiae Meigen (Culicidae) | Lysinibacillus spp., Meyer and Neide, Staphylococcus spp. Rosenbach et al., P. melophthora, Clostridium botulinum Van Eminem | Carbamate, Methoprene. | [107] |
| Spodoptera frugiperda Smith (Noctuidae) | Microbacterium arborescens Imai et al., Staphylococcus sciuri, Enterococcus mundtii Collins et al. | Benzoylurea | [108] |
| Insect Species | Gut Bacteria Present | Action on Phytotoxin | Enzyme Involved in Degradation | Name of the Gene | Reference |
|---|---|---|---|---|---|
| Drosophila melanogaster Meigen (Diptera: Drosophilidae) | Pseudomonas fulva Iizuka and Komagata | Caffeine | Methylxanthine N1-demethylase | GST, P450 | [109] |
| Trichoplusia ni Hübner (Lepidoptera: Noctuidae) | Pectobacterium sp. Jones et al. | Isothiocyanates | Metal-dependent beta-lactamase | GST | [110] |
| Dendroctonus ponderosae Hopkins (Coleoptera: Curculionidae) | Pseudomonas sp. Migula et al. | Terpenes | Diterpene acid degradation pathway | GST | [111] |
| Drosophila melanogaster Meigen | Enterobacter asburiae Farmer et al. | Phenols | Oxygenase, Isomerase, Transferase | GST | [112] |
| Myzus persicae Sulzer (Hemiptera: Aphididae) | Escherichia coli O157 | Glycosides | 6-Phospho-beta-glucosidase | GST | [113] |
| Myzus persicae Sulzer | Achromobactor sp. Yabuuchi and Yano | Carbamates | N-methylcarbamate hydrolase | Carboxylesterase | [114] |
| Drosophila melanogaster Meigen | Burkholderia cepacia | Organophosphates | Organophosphate hydrolase | GST | [109] |
| Anopheles gambiae Meigen (Diptera:Culicidae) | Sphingobium japonicum UT26. Pal et al. | Organochlorines | Lin pathway | GST, P450 | [115] |
| Cimex lectularius Linnaeus (Hemiptera: Cimicidae) | Variovorax boronicumulans Miwa et al. | Neonicotinoids | Nitrile hydratase | Esterase, GST, P450 | [116] |
| Anopheles gambiae Meigen, Musca domestica L. (Diptera: Muscidae) | Sphingobium sp. Pal et al. | Pyrethroids | Carboxylesterase | P450 | [66] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mondal, S.; Somani, J.; Roy, S.; Babu, A.; Pandey, A.K. Insect Microbial Symbionts: Ecology, Interactions, and Biological Significance. Microorganisms 2023, 11, 2665. https://doi.org/10.3390/microorganisms11112665
Mondal S, Somani J, Roy S, Babu A, Pandey AK. Insect Microbial Symbionts: Ecology, Interactions, and Biological Significance. Microorganisms. 2023; 11(11):2665. https://doi.org/10.3390/microorganisms11112665
Chicago/Turabian StyleMondal, Sankhadeep, Jigyasa Somani, Somnath Roy, Azariah Babu, and Abhay K. Pandey. 2023. "Insect Microbial Symbionts: Ecology, Interactions, and Biological Significance" Microorganisms 11, no. 11: 2665. https://doi.org/10.3390/microorganisms11112665
APA StyleMondal, S., Somani, J., Roy, S., Babu, A., & Pandey, A. K. (2023). Insect Microbial Symbionts: Ecology, Interactions, and Biological Significance. Microorganisms, 11(11), 2665. https://doi.org/10.3390/microorganisms11112665







