The Mechanism of Transcription Factor Swi6 in Regulating Growth and Pathogenicity of Ceratocystis fimbriata: Insights from Non-Targeted Metabolomics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strains, Sweet Potato Samples, and Culture Conditions
2.2. Vegetative Growth and Pathogenicity Assays of C. fimbriata
2.3. Ipomeamarone Detection of Sweet Potato
2.4. Ultra-High-Performance Liquid Chromatography-Mass Spectrometry Tests for C. fimbriata
2.5. Potential Metabolites Identification in C. fimbriata
2.6. Potential Differentially Accumulated Metabolites (PDAMs) Analysis
2.7. Statistical Analyses
3. Results and Discussion
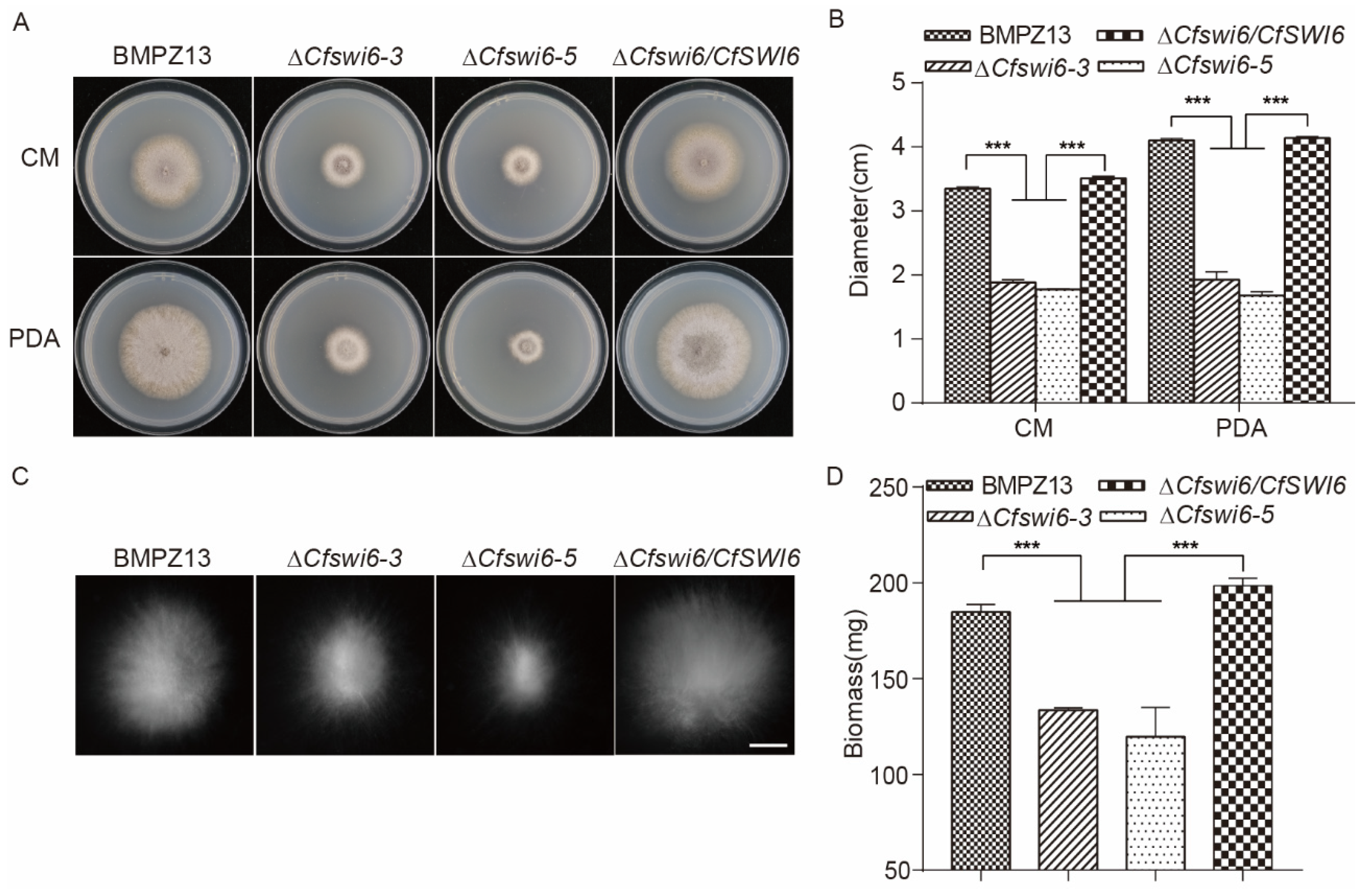
3.1. Deletion of CfSWI6 Resulted in Abnormal C. fimbriata Vegetative Growth
3.2. Deletion of CfSWI6 Gene Significantly Reduced the Virulence of C. fimbriata to Sweet Potato
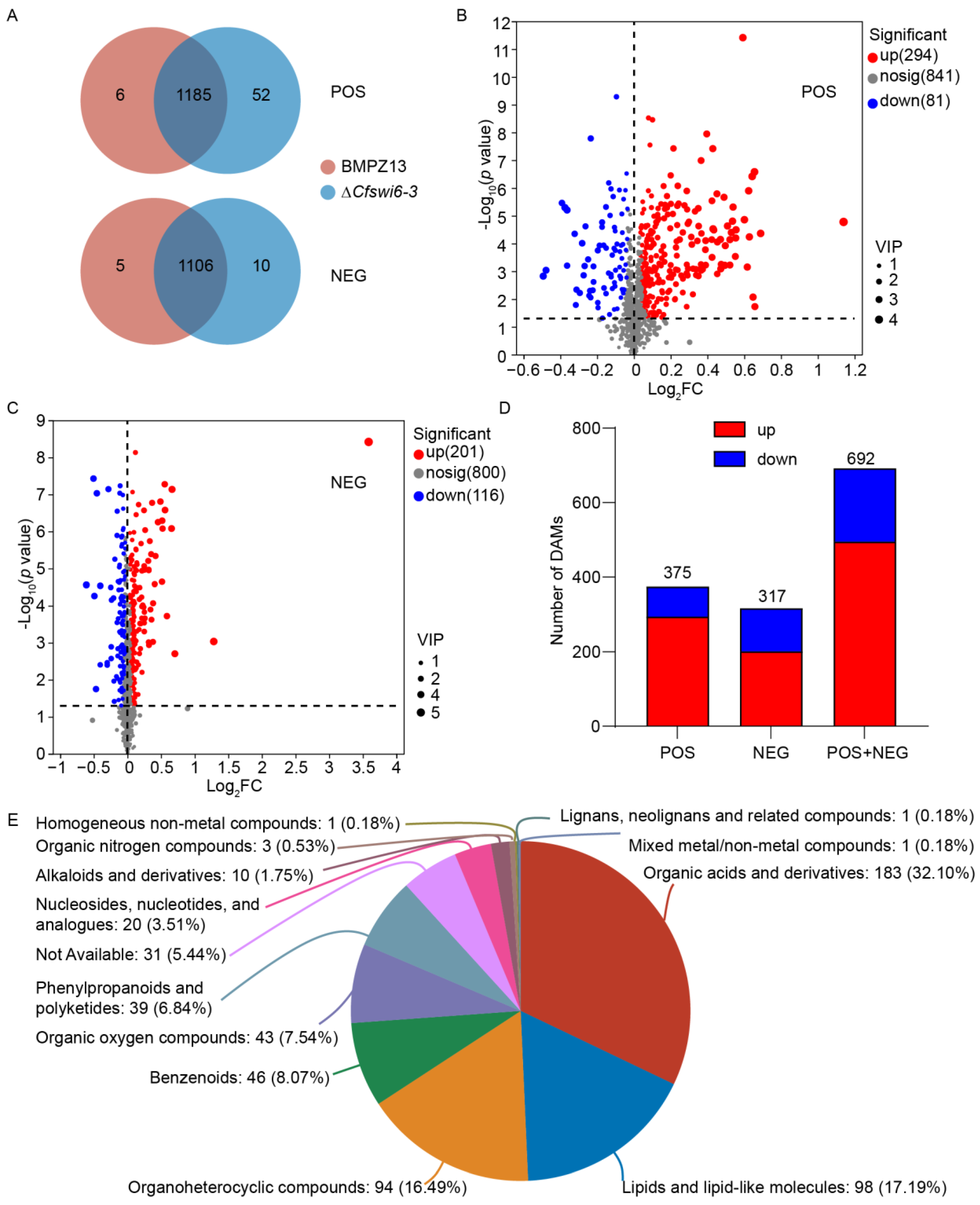
3.3. Disruption of SWI6 Gene Altered the Potential Metabolite Profile of C. fimbriata
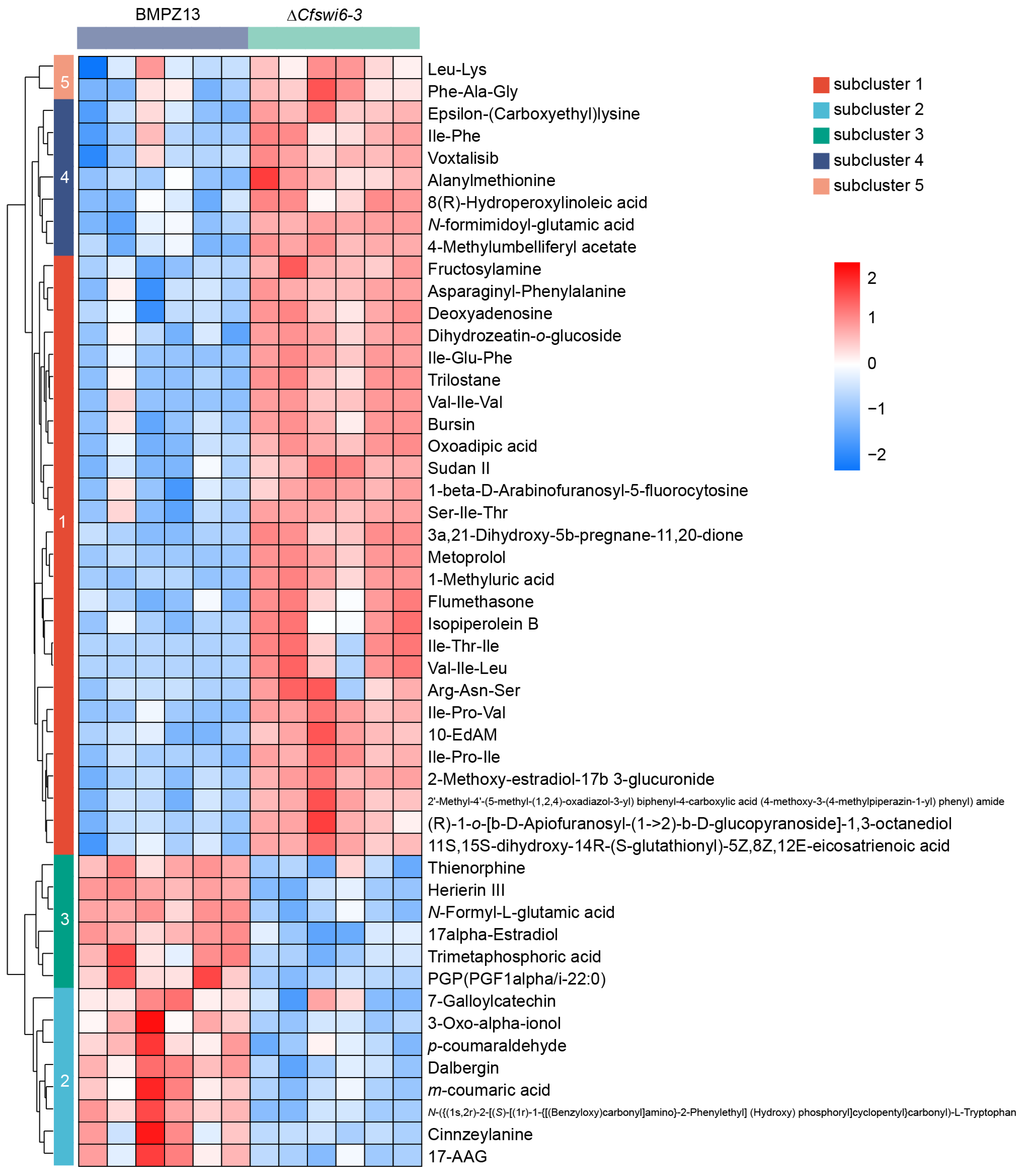
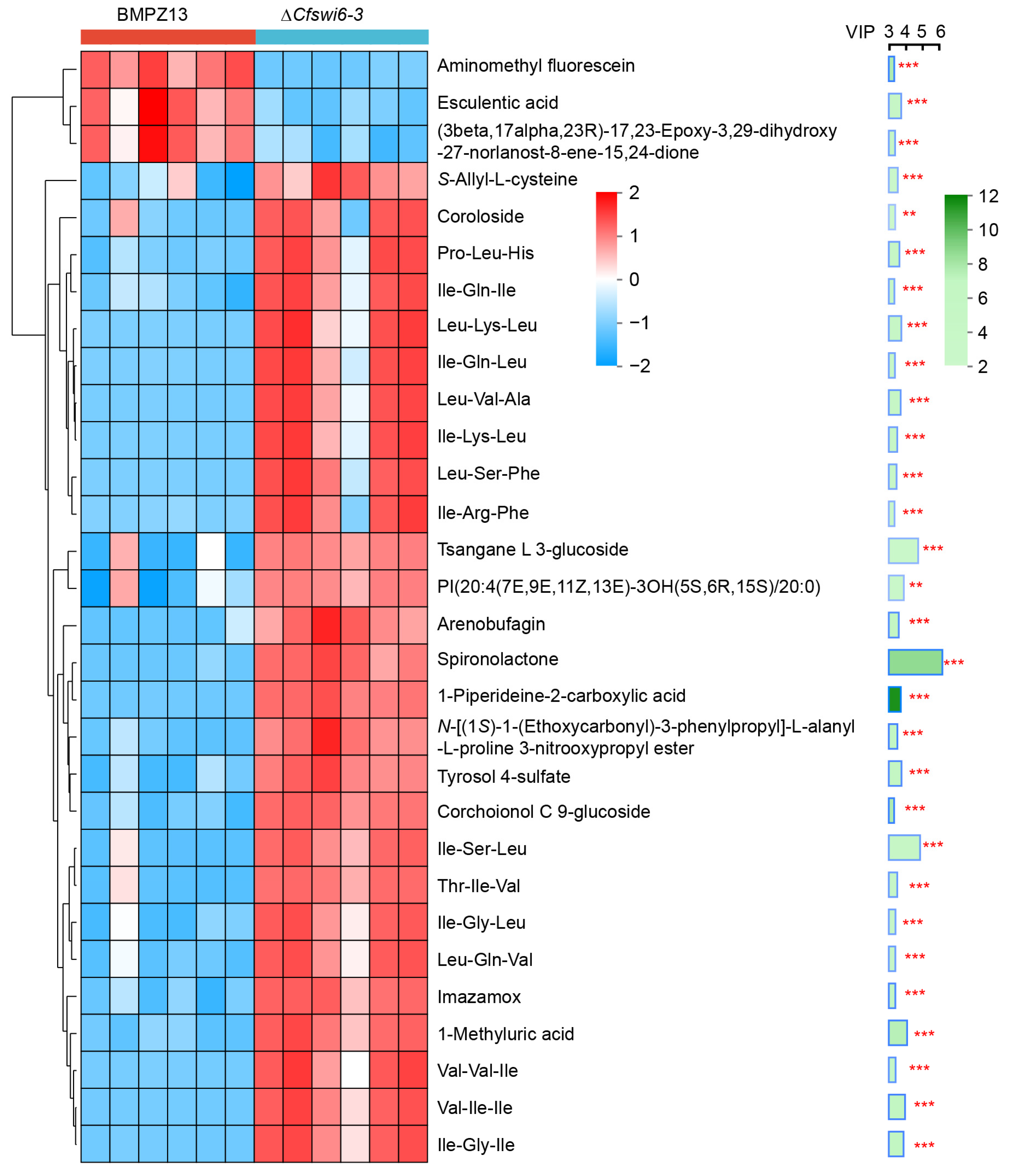
3.4. Hierarchical Cluster Analysis Revealed Potential Causes for Abnormal Growth and Pathogenicity in Mutant of C. fimbriata
3.5. Impact of Potential Key Metabolites on Growth and Pathogenicity of C. fimbriata Based on VIP
3.6. SWI6 Mediated the Potential Growth and Virulence of C. fimbriata through Key Metabolic Pathways
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, C.; Luo, Q.; Tang, W.; Ma, J.; Yang, D.; Chen, J.; Gao, F.; Sun, H.; Xie, Y. Transcriptome Characterization and Gene Changes Induced by Fusarium solani in Sweetpotato Roots. Genes 2023, 14, 969. [Google Scholar] [CrossRef]
- Stahr, M.N.; Quesada-Ocampo, L.M. Effects of Water Temperature, Inoculum Concentration and Age, and Sanitizers on Infection of Ceratocystis fimbriata, Causal Agent of Black Rot in Sweetpotato. Plant Dis. 2021, 105, 1365–1372. [Google Scholar] [CrossRef]
- Paul, N.C.; Nam, S.S.; Kachroo, A.; Kim, Y.H.; Yang, J.W. Characterization and pathogenicity of sweet potato (Ipomoea batatas) black rot caused by Ceratocystis fimbriata in Korea. Eur. J. Plant Pathol. 2018, 152, 833–840. [Google Scholar] [CrossRef]
- Liu, M.; Meng, Q.; Wang, S.; Yang, K.; Tian, J. Research progress on postharvest sweet potato spoilage fungi Ceratocystis fimbriata and control measures. Food Biosci. 2023, 53, 102627. [Google Scholar] [CrossRef]
- Sun, Y.; Li, M.; Wang, Y.; Li, L.; Wang, M.; Li, X.; Xu, M.; Loake, G.J.; Guo, M.; Jiang, J. Ceratocystis fimbriata Employs a Unique Infection Strategy Targeting Peltate Glandular Trichomes of Sweetpotato (Ipomoea batatas) Plants. Phytopathology 2020, 110, 1923–1933. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, B.; Cai, S.; Zhang, Y.; Xu, M.; Zhang, C.; Yuan, B.; Xing, K.; Qin, S. Identification of Rhizospheric Actinomycete Streptomyces lavendulae SPS-33 and the Inhibitory Effect of its Volatile Organic Compounds against Ceratocystis fmbriata in Postharvest Sweet Potato (Ipomoea Batatas (L.) Lam.). Microorganisms 2020, 8, 319. [Google Scholar] [CrossRef]
- Marincowitz, S.; Barnes, I.; de Beer, Z.W.; Wingfield, M.J. Epitypification of Ceratocystis fimbriata. Fungal Syst. Evol. 2020, 6, 289–298. [Google Scholar] [CrossRef]
- Stahr, M.N.; Quesada-Ocampo, L.M. Black Rot of Sweetpotato: A Comprehensive Diagnostic Guide. Plant Health Prog. 2019, 20, 255–260. [Google Scholar] [CrossRef]
- Mohsin, S.M.; Hasanuzzaman, M.; Parvin, K.; Morokuma, M.; Fujita, M. Effect of tebuconazole and trifloxystrobin on Ceratocystis fimbriata to control black rot of sweet potato: Processes of reactive oxygen species generation and antioxidant defense responses. World J. Microbiol. Biotechnol. 2021, 37, 148. [Google Scholar] [CrossRef]
- Wamalwa, L.N.; Cheseto, X.; Ouna, E.; Kaplan, F.; Maniania, N.K.; Machuka, J.; Torto, B.; Ghislain, M. Toxic Ipomeamarone accumulation in healthy parts of Sweetpotato (Ipomoea batatas L. Lam) storage roots upon infection by Rhizopus stolonifer. J. Agric. Food Chem. 2015, 63, 335–342. [Google Scholar] [CrossRef]
- He, D.-C.; Zhan, J.-S.; Xie, L.-H. Problems, challenges and future of plant disease management: From an ecological point of view. J. Integr. Agric. 2016, 15, 705–715. [Google Scholar] [CrossRef]
- Gong, Y.; Liu, J.Q.; Xu, M.J.; Zhang, C.M.; Gao, J.; Li, C.G.; Xing, K.; Qin, S. Antifungal Volatile Organic Compounds from Streptomyces setonii WY228 Control Black Spot Disease of Sweet Potato. Appl. Environ. Microbiol. 2022, 88, e0231721. [Google Scholar] [CrossRef] [PubMed]
- Parada-Rojas, C.H.; Pecota, K.; Almeyda, C.; Yencho, G.C.; Quesada-Ocampo, L.M. Sweetpotato Root Development Influences Susceptibility to Black Rot Caused by the Fungal Pathogen Ceratocystis fimbriata. Phytopathology 2021, 111, 1660–1669. [Google Scholar] [CrossRef] [PubMed]
- Stahr, M.; Quesada-Ocampo, L.M. Assessing the Role of Temperature, Inoculum Density, and Wounding on Disease Progression of the Fungal Pathogen Ceratocystis fimbriata Causing Black Rot in Sweetpotato. Plant Dis. 2020, 104, 930–937. [Google Scholar] [CrossRef] [PubMed]
- Umer, M.J.; Zheng, J.; Yang, M.; Batool, R.; Abro, A.A.; Hou, Y.; Xu, Y.; Gebremeskel, H.; Wang, Y.; Zhou, Z.; et al. Insights to Gossypium defense response against Verticillium dahliae: The Cotton Cancer. Funct. Integr. Genom. 2023, 23, 142. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Zhao, H.; Li, J.; Gong, Y.; Li, X. The Devastating Rice Blast Airborne Pathogen Magnaporthe oryzae—A Review on Genes Studied with Mutant Analysis. Pathogens 2023, 12, 379. [Google Scholar] [CrossRef]
- Tahiri, G.; Lax, C.; Canovas-Marquez, J.T.; Carrillo-Marin, P.; Sanchis, M.; Navarro, E.; Garre, V.; Nicolas, F.E. Mucorales and Mucormycosis: Recent Insights and Future Prospects. J. Fungi 2023, 9, 335. [Google Scholar] [CrossRef]
- Suo, C.; Gao, Y.; Ding, C.; Sun, T. The function and regulation of heat shock transcription factor in Cryptococcus. Front. Cell Infect. Microbiol. 2023, 13, 1195968. [Google Scholar] [CrossRef]
- Kusch, S.; Qian, J.; Loos, A.; Kummel, F.; Spanu, P.D.; Panstruga, R. Long-term and rapid evolution in powdery mildew fungi. Mol. Ecol. 2023. early view. [Google Scholar] [CrossRef]
- Mapuranga, J.; Chang, J.; Yang, W. Combating powdery mildew: Advances in molecular interactions between Blumeria graminis f. sp. tritici and wheat. Front. Plant Sci. 2022, 13, 1102908. [Google Scholar] [CrossRef]
- Choi, Y.H.; Jun, S.C.; Lee, M.W.; Yu, J.H.; Shin, K.S. Characterization of the mbsA Gene Encoding a Putative APSES Transcription Factor in Aspergillus fumigatus. Int. J. Mol. Sci. 2021, 22, 3777. [Google Scholar] [CrossRef]
- Zhao, Y.; Su, H.; Zhou, J.; Feng, H.; Zhang, K.Q.; Yang, J. The APSES family proteins in fungi: Characterizations, evolution and functions. Fungal Genet. Biol. 2015, 81, 271–280. [Google Scholar] [CrossRef]
- Ding, J.L.; Hou, J.; Li, X.H.; Feng, M.G.; Ying, S.H. Transcription Activator Swi6 Interacts with Mbp1 in MluI Cell Cycle Box-Binding Complex and Regulates Hyphal Differentiation and Virulence in Beauveria bassiana. J. Fungi 2021, 7, 411. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.L.; Lin, H.Y.; Feng, M.G.; Ying, S.H. Mbp1, a component of the MluI cell cycle box-binding complex, contributes to morphological transition and virulence in the filamentous entomopathogenic fungus Beauveria bassiana. Environ. Microbiol. 2020, 22, 584–597. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhu, P.; Cao, B.; Ma, S.; Li, R.; Wang, X.; Zhao, A. An APSES Transcription Factor Xbp1 Is Required for Sclerotial Development, Appressoria Formation, and Pathogenicity in Ciboria shiraiana. Front. Microbiol. 2021, 12, 739686. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Ma, N.; Bai, N.; Yang, L.; Yang, X.; Zhang, K.Q.; Yang, J. PKC-SWI6 signaling regulates asexual development, cell wall integrity, stress response, and lifestyle transition in the nematode-trapping fungus Arthrobotrys oligospora. Sci. China Life Sci. 2022, 65, 2455–2471. [Google Scholar] [CrossRef] [PubMed]
- Son, M.; Lee, Y.; Kim, K.H. The Transcription Cofactor Swi6 of the Fusarium graminearum Is Involved in Fusarium Graminearum Virus 1 Infection-Induced Phenotypic Alterations. Plant Pathol. J. 2016, 32, 281–289. [Google Scholar] [CrossRef]
- Liu, N.N.; Fan, F.Y.; Qiu, D.W.; Jiang, L.H. The transcription cofactor FgSwi6 plays a role in growth and development, carbendazim sensitivity, cellulose utilization, lithium tolerance, deoxynivalenol production and virulence in the filamentous fungus Fusarium graminearum. Fungal Genet. Biol. 2013, 58–59, 42–52. [Google Scholar] [CrossRef]
- Qi, Z.Q.; Wang, Q.; Dou, X.Y.; Wang, W.; Zhao, Q.; Lv, R.L.; Zhang, H.F.; Zheng, X.B.; Wang, P.; Zhang, Z.G. MoSwi6, an APSES family transcription factor, interacts with MoMps1 and is required for hyphal and conidial morphogenesis, appressorial function and pathogenicity of Magnaporthe oryzae. Mol. Plant Pathol. 2012, 13, 677–689. [Google Scholar] [CrossRef]
- Nishimura, M.; Fukada, J.; Moriwaki, A.; Fujikawa, T.; Ohashi, M.; Hibi, T.; Hayashi, N. Mstu1, an APSES Transcription Factor, Is Required for Appressorium-Mediated Infection in Magnaporthe grisea. Biosci. Biotech. Biochem. 2009, 73, 1779–1786. [Google Scholar] [CrossRef]
- Davidson, R.C.; Blankenship, J.R.; Kraus, P.R.; Berrios, M.D.; Hull, C.M.; D’Souza, C.; Wang, P.; Heitman, J. A PCR-based strategy to generate integrative targeting alleles with large regions of homology. Microbiol.-Sgm 2002, 148, 2607–2615. [Google Scholar] [CrossRef]
- Amalamol, D.; Ashwin, N.M.R.; Lakshana, K.V.; Nirmal Bharathi, M.; Ramesh Sundar, A.; Sukumaran, R.K.; Malathi, P.; Viswanathan, R. A highly efficient stratagem for protoplast isolation and genetic transformation in filamentous fungus Colletotrichum falcatum. Folia Microbiol. 2022, 67, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.M.; Li, B.; Liu, L.; Li, Y.; Yin, S.X.; Yin, S.C.; Chen, J.; Guo, S.X. Armillaria mellea Symbiosis Drives Metabolomic and Transcriptomic Changes in Polyporus umbellatus Sclerotia. Front. Microbiol. 2021, 12, 792530. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Zavala, B.; Dominguez, A. Evolution and phylogenetic relationships of APSES proteins from Hemiascomycetes. Fems Yeast Res. 2008, 8, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Ren, A.; Shi, L.; Zhu, J.; Jiang, A.; Shi, D.; Zhao, M. Functional analysis of an APSES transcription factor (GlSwi6) involved in fungal growth, fruiting body development and ganoderic-acid biosynthesis in Ganoderma lucidum. Microbiol. Res. 2018, 207, 280–288. [Google Scholar] [CrossRef]
- Liu, M.; Gong, Y.; Sun, H.; Zhang, J.; Zhang, L.; Sun, J.; Han, Y.; Huang, J.; Wu, Q.; Zhang, C.; et al. Characterization of a Novel Chitinase from Sweet Potato and Its Fungicidal Effect against Ceratocystis fimbriata. J. Agric. Food Chem. 2020, 68, 7591–7600. [Google Scholar] [CrossRef]
- Wang, X.-Y.; Xie, J. Assessment of metabolic changes in Acinetobacter johnsonii and Pseudomonas fluorescens co-culture from bigeye tuna (Thunnus obesus) spoilage by ultra-high-performance liquid chromatography-tandem mass spectrometry. LWT 2020, 123, 109073. [Google Scholar] [CrossRef]
- Chen, L.; Zhao, X.; Wu, J.; Liu, Q.; Pang, X.; Yang, H. Metabolic characterisation of eight Escherichia coli strains including “Big Six” and acidic responses of selected strains revealed by NMR spectroscopy. Food Microbiol. 2020, 88, 103399. [Google Scholar] [CrossRef]
- Seong, K.; Hou, Z.; Tracy, M.; Kistler, H.C.; Xu, J.R. Random Insertional Mutagenesis Identifies Genes Associated with Virulence in the Wheat Scab Fungus Fusarium graminearum. Phytopathology 2005, 95, 744–750. [Google Scholar] [CrossRef]
- Aron, O.; Wang, M.; Mabeche, A.W.; Wajjiha, B.; Li, M.; Yang, S.; You, H.; Cai, Y.; Zhang, T.; Li, Y.; et al. MoCpa1-mediated arginine biosynthesis is crucial for fungal growth, conidiation, and plant infection of Magnaporthe oryzae. Appl. Microbiol. Biotechnol. 2021, 105, 5915–5929. [Google Scholar] [CrossRef]
- Du, Y.; Zhang, H.; Hong, L.; Wang, J.; Zheng, X.; Zhang, Z. Acetolactate synthases MoIlv2 and MoIlv6 are required for infection-related morphogenesis in Magnaporthe oryzae. Mol. Plant Pathol. 2013, 14, 870–884. [Google Scholar] [CrossRef]
- Zhang, H.; Ma, H.; Xie, X.; Ji, J.; Dong, Y.; Du, Y.; Tang, W.; Zheng, X.; Wang, P.; Zhang, Z. Comparative proteomic analyses reveal that the regulators of G-protein signaling proteins regulate amino acid metabolism of the rice blast fungus Magnaporthe oryzae. Proteom. 2014, 14, 2508–2522. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zuo, R.; Zhu, Q.; Sun, Y.; Li, M.; Dong, Y.; Ru, Y.; Zhang, H.; Zheng, X.; Zhang, Z. MoLys2 is necessary for growth, conidiogenesis, lysine biosynthesis, and pathogenicity in Magnaporthe oryzae. Fungal Genet. Biol. 2014, 67, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Jiang, H.; Zheng, Q.; Chen, X.; Wang, R.; Yang, S.; Zhao, G.; Liu, J.; Norvienyeku, J.; Wang, Z. Isopropylmalate isomerase MoLeu1 orchestrates leucine biosynthesis, fungal development, and pathogenicity in Magnaporthe oryzae. Appl. Microbiol. Biotechnol. 2019, 103, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Que, Y.; Wang, H.; Wang, C.; Li, Y.; Yue, X.; Ma, Z.; Talbot, N.J.; Wang, Z. The MET13 methylenetetrahydrofolate reductase gene is essential for infection-related morphogenesis in the rice blast fungus Magnaporthe oryzae. PLoS ONE 2013, 8, e76914. [Google Scholar] [CrossRef]
- Wilson, R.A.; Fernandez, J.; Quispe, C.F.; Gradnigo, J.; Seng, A.; Moriyama, E.; Wright, J.D. Towards defining nutrient conditions encountered by the rice blast fungus during host infection. PLoS ONE 2012, 7, e47392. [Google Scholar] [CrossRef]
- Liu, X.; Cai, Y.; Zhang, X.; Zhang, H.; Zheng, X.; Zhang, Z. Carbamoyl Phosphate Synthetase Subunit MoCpa2 Affects Development and Pathogenicity by Modulating Arginine Biosynthesis in Magnaporthe oryzae. Front. Microbiol. 2016, 7, 2023. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, H.; Liang, S.; Ning, G.; Xu, N.; Lu, J.; Liu, X.; Lin, F. MoARG1, MoARG5,6 and MoARG7 involved in arginine biosynthesis are essential for growth, conidiogenesis, sexual reproduction, and pathogenicity in Magnaporthe oryzae. Microbiol. Res. 2015, 180, 11–22. [Google Scholar] [CrossRef]
- Kingsbury, J.M.; Yang, Z.; Ganous, T.M.; Cox, G.M.; McCusker, J.H. Cryptococcus neoformans Ilv2p confers resistance to sulfometuron methyl and is required for survival at 37 degrees C and in vivo. Microbiology 2004, 150, 1547–1558. [Google Scholar] [CrossRef]
- Kingsbury, J.M.; McCusker, J.H. Cytocidal amino acid starvation of Saccharomyces cerevisiae and Candida albicans acetolactate synthase (ilv2Delta) mutants is influenced by the carbon source and rapamycin. Microbiology 2010, 156, 929–939. [Google Scholar] [CrossRef]
- Roche, C.M.; Blanch, H.W.; Clark, D.S.; Glass, N.L. Physiological role of Acyl coenzyme A synthetase homologs in lipid metabolism in Neurospora crassa. Eukaryot. Cell 2013, 12, 1244–1257. [Google Scholar] [CrossRef]
- Liu, Q.; Li, D.; Bai, N.; Zhu, Y.; Yang, J. Peroxin Pex14/17 Is Required for Trap Formation, and Plays Pleiotropic Roles in Mycelial Development, Stress Response, and Secondary Metabolism in Arthrobotrys oligospora. Msphere 2023, 8, e0001223. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Li, Y.N.; Li, X.; Gu, W.; Moeketsi, E.K.; Zhou, R.; Zheng, X.; Zhang, Z.; Zhang, H. The Peroxisomal-CoA Synthetase MoPcs60 Is Important for Fatty Acid Metabolism and Infectious Growth of the Rice Blast Fungus. Front. Plant Sci. 2021, 12, 811041. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Zhang, Z.; Shao, W.; Yang, Y.; Zhou, M.; Chen, C. The autophagy-related gene BcATG1 is involved in fungal development and pathogenesis in Botrytis cinerea. Mol. Plant Pathol. 2017, 18, 238–248. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Soanes, D.M.; Kershaw, M.J.; Talbot, N.J. Functional analysis of lipid metabolism in Magnaporthe grisea reveals a requirement for peroxisomal fatty acid beta-oxidation during appressorium-mediated plant infection. Mol. Plant Microbe Interact. 2007, 20, 475–491. [Google Scholar] [CrossRef] [PubMed]
- Bhambra, G.K.; Wang, Z.Y.; Soanes, D.M.; Wakley, G.E.; Talbot, N.J. Peroxisomal carnitine acetyl transferase is required for elaboration of penetration hyphae during plant infection by Magnaporthe grisea. Mol. Microbiol. 2006, 61, 46–60. [Google Scholar] [CrossRef]
- Motoyama, T. Secondary Metabolites of the Rice Blast Fungus Pyricularia oryzae: Biosynthesis and Biological Function. Int. J. Mol. Sci. 2020, 21, 8698. [Google Scholar] [CrossRef]
- Jacob, S.; Grötsch, T.; Foster, A.J.; Schüffler, A.; Rieger, P.H.; Sandjo, L.P.; Liermann, J.C.; Opatz, T.; Thines, E. Unravelling the biosynthesis of pyriculol in the rice blast fungus Magnaporthe oryzae. Microbiology 2017, 163, 541–553. [Google Scholar] [CrossRef]
- Matis, M.; Zakelj-Mavric, M.; Peter-Katalinic, J. Global Analysis of the Hortaea werneckii proteome: Studying steroid response in yeast. J. Proteome Res. 2005, 4, 2043–2051. [Google Scholar] [CrossRef]
- Jeraj, N.; Lenasi, H.; Breskvar, K. The involvement of cAMP in the growth inhibition of filamentous fungus Rhizopus nigricans by steroids. Fems Microbiol. Lett. 2005, 242, 147–154. [Google Scholar] [CrossRef]
- Dlugonski, J.; Wilmanska, D. Deleterious effects of androstenedione on growth and cell morphology of Schizosaccharomyces pombe. Antonie Van. Leeuwenhoek 1998, 73, 189–194. [Google Scholar] [CrossRef]
- Breskvar, K.; Ferencak, Z.; Hudnik-Plevnik, T. The role of cytochrome P450(11 alpha) in detoxification of steroids in the filamentous fungus Rhizopus nigricans. J. Steroid Biochem. Mol. Biol. 1995, 52, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Borges-Walmsley, M.I.; Chen, D.; Shu, X.; Walmsley, A.R. The pathobiology of Paracoccidioides brasiliensis. Trends Microbiol. 2002, 10, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Gooday, G.W.; Adams, D.J. Sex hormones and fungi. Adv. Microb. Physiol. 1993, 34, 69–145. [Google Scholar] [CrossRef] [PubMed]
- Bataineh, M.T.A.; Cacciatore, S.; Semreen, M.H.; Dash, N.R.; Soares, N.C.; Zhu, X.; Mousa, M.K.; Salam, J.S.A.; Zerbini, L.F.; Hajjo, R.; et al. Exploring the effect of estrogen on Candida albicans hyphal cell wall glycans and ergosterol synthesis. Front. Cell Infect. Microbiol. 2022, 12, 977157. [Google Scholar] [CrossRef]
- Zhang, X.; Essmann, M.; Burt, E.T.; Larsen, B. Estrogen effects on Candida albicans: A potential virulence-regulating mechanism. J. Infect. Dis. 2000, 181, 1441–1446. [Google Scholar] [CrossRef]
- Cresnar, B.; Zakelj-Mavric, M. Aspects of the steroid response in fungi. Chem. Biol. Interact. 2009, 178, 303–309. [Google Scholar] [CrossRef]
- Levin, D.E. Cell wall integrity signaling in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 2005, 69, 262–291. [Google Scholar] [CrossRef]
- Wilkinson, B.M.; Purswani, J.; Stirling, C.J. Yeast GTB1 encodes a subunit of glucosidase II required for glycoprotein processing in the endoplasmic reticulum. J. Biol. Chem. 2006, 281, 6325–6333. [Google Scholar] [CrossRef]
- Fernandez-Alvarez, A.; Elias-Villalobos, A.; Jimenez-Martin, A.; Marin-Menguiano, M.; Ibeas, J.I. Endoplasmic reticulum glucosidases and protein quality control factors cooperate to establish biotrophy in Ustilago maydis. Plant Cell 2013, 25, 4676–4690. [Google Scholar] [CrossRef]
- Mora-Montes, H.M.; Bates, S.; Netea, M.G.; Diaz-Jimenez, D.F.; Lopez-Romero, E.; Zinker, S.; Ponce-Noyola, P.; Kullberg, B.J.; Brown, A.J.; Odds, F.C.; et al. Endoplasmic reticulum alpha-glycosidases of Candida albicans are required for N glycosylation, cell wall integrity, and normal host-fungus interaction. Eukaryot. Cell 2007, 6, 2184–2193. [Google Scholar] [CrossRef]
- Geysens, S.; Pakula, T.; Uusitalo, J.; Dewerte, I.; Penttila, M.; Contreras, R. Cloning and characterization of the glucosidase II alpha subunit gene of Trichoderma reesei: A frameshift mutation results in the aberrant glycosylation profile of the hypercellulolytic strain Rut-C30. Appl. Environ. Microbiol. 2005, 71, 2910–2924. [Google Scholar] [CrossRef] [PubMed]
- D’Alessio, C.; Fernandez, F.; Trombetta, E.S.; Parodi, A.J. Genetic evidence for the heterodimeric structure of glucosidase II. The effect of disrupting the subunit-encoding genes on glycoprotein folding. J. Biol. Chem. 1999, 274, 25899–25905. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Liu, X.; Liu, Z.; Sun, Y.; Liu, M.; Wang, X.; Zhang, H.; Zheng, X.; Zhang, Z. Glycoside Hydrolase MoGls2 Controls Asexual/Sexual Development, Cell Wall Integrity and Infectious Growth in the Rice Blast Fungus. PLoS ONE 2016, 11, e0162243. [Google Scholar] [CrossRef] [PubMed]
- Levin, D.E. Regulation of cell wall biogenesis in Saccharomyces cerevisiae: The cell wall integrity signaling pathway. Genetics 2011, 189, 1145–1175. [Google Scholar] [CrossRef] [PubMed]
- Sanz, A.B.; Garcia, R.; Pavon-Verges, M.; Rodriguez-Pena, J.M.; Arroyo, J. Control of Gene Expression via the Yeast CWI Pathway. Int. J. Mol. Sci. 2022, 23, 1791. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.Y.; Truman, A.W.; Levin, D.E. Yeast Mpk1 mitogen-activated protein kinase activates transcription through Swi4/Swi6 by a noncatalytic mechanism that requires upstream signal. Mol. Cell Biol. 2008, 28, 2579–2589. [Google Scholar] [CrossRef]
- Lian, L.D.; Zhang, G.; Zhu, J.; Wang, Y.X.; Wang, L.S.; Liu, R.; Shi, L.; Ren, A.; Zhao, M.W. Swi6B, an alternative splicing isoform of Swi6, mediates the cell wall integrity of Ganoderma lucidum. Environ. Microbiol. 2021, 23, 4405–4417. [Google Scholar] [CrossRef]
- Fujioka, T.; Mizutani, O.; Furukawa, K.; Sato, N.; Yoshimi, A.; Yamagata, Y.; Nakajima, T.; Abe, K. MpkA-Dependent and -independent cell wall integrity signaling in Aspergillus nidulans. Eukaryot. Cell 2007, 6, 1497–1510. [Google Scholar] [CrossRef]
- Kim, K.Y.; Truman, A.W.; Caesar, S.; Schlenstedt, G.; Levin, D.E. Yeast Mpk1 cell wall integrity mitogen-activated protein kinase regulates nucleocytoplasmic shuttling of the Swi6 transcriptional regulator. Mol. Biol. Cell 2010, 21, 1609–1619. [Google Scholar] [CrossRef]
- Zhang, Z.; Hao, Z.; Chai, R.; Qiu, H.; Wang, Y.; Wang, J.; Sun, G. Adenylsuccinate Synthetase MoADE12 Plays Important Roles in the Development and Pathogenicity of the Rice Blast Fungus. J. Fungi 2022, 8, 780. [Google Scholar] [CrossRef]
- Aron, O.; Otieno, F.J.; Tijjani, I.; Yang, Z.; Xu, H.; Weng, S.; Guo, J.; Lu, S.; Wang, Z.; Tang, W. De novo purine nucleotide biosynthesis mediated by MoAde4 is required for conidiation, host colonization and pathogenicity in Magnaporthe oryzae. Appl. Microbiol. Biotechnol. 2022, 106, 5587–5602. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Zhang, X.; Li, X.; Liu, M.; Liu, X.; Wang, X.; Zhang, H.; Zheng, X.; Zhang, Z. The Atypical Guanylate Kinase MoGuk2 Plays Important Roles in Asexual/Sexual Development, Conidial Septation, and Pathogenicity in the Rice Blast Fungus. Front. Microbiol. 2017, 8, 2467. [Google Scholar] [CrossRef] [PubMed]
- Kokina, A.; Kibilds, J.; Liepins, J. Adenine auxotrophy--be aware: Some effects of adenine auxotrophy in Saccharomyces cerevisiae strain W303-1A. FEMS Yeast Res. 2014, 14, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, J.; Yang, K.T.; Cornwell, K.M.; Wright, J.D.; Wilson, R.A. Growth in rice cells requires de novo purine biosynthesis by the blast fungus Magnaporthe oryzae. Sci. Rep. 2013, 3, 2398. [Google Scholar] [CrossRef]
- Zaworski, J.; Bouderlique, E.; Anglicheau, D.; Duong Van Huyen, J.P.; Gnemmi, V.; Gibier, J.B.; Neugebauer, Y.; Haymann, J.P.; Bazin, D.; Frochot, V.; et al. 1-Methyluric Acid Nephropathy. Kidney Int. Rep. 2020, 5, 737–741. [Google Scholar] [CrossRef]
- Davies, P.M.; Fairbanks, L.D.; Safranow, K.; Bending, M.R.; Simmonds, H.A. An unusual patient with kidney stones composed of 1-methyluric acid. Urol. Res. 2006, 34, 58–60. [Google Scholar] [CrossRef]
- Balasubramanian, T. Uric acid or 1-methyl uric acid in the urinary bladder increases serum glucose, insulin, true triglyceride, and total cholesterol levels in Wistar rats. Sci. World J. 2003, 3, 930–936. [Google Scholar] [CrossRef]
- Cohen, B.A.; Amsellem, Z.; Maor, R.; Sharon, A.; Gressel, J. Transgenically enhanced expression of indole-3-acetic Acid confers hypervirulence to plant pathogens. Phytopathology 2002, 92, 590–596. [Google Scholar] [CrossRef]
- Luo, K.; DesRoches, C.L.; Johnston, A.; Harris, L.J.; Zhao, H.Y.; Ouellet, T. Multiple metabolic pathways for metabolism of l-tryptophan in Fusarium graminearum. Can. J. Microbiol. 2017, 63, 921–927. [Google Scholar] [CrossRef]
- Cui, G.; Huang, C.; Bi, X.; Wang, Y.; Yin, K.; Zhu, L.; Jiang, Z.; Chen, B.; Deng, Y.Z. Aminotransferase SsAro8 Regulates Tryptophan Metabolism Essential for Filamentous Growth of Sugarcane Smut Fungus Sporisorium scitamineum. Microbiol. Spectr. 2022, 10, e0057022. [Google Scholar] [CrossRef]
- Tan, Q.; Zhao, X.; He, H.; Zhang, J.; Yi, T. Carbamoyl phosphate synthetase subunit Cpa1 interacting with Dut1, controls development, arginine biosynthesis, and pathogenicity of Colletotrichum gloeosporioides. Fungal Biol. 2021, 125, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, A.; Tariq, M.; Ahmed, M.; Zhou, Z.; Ali, I.; Mahmood, R.T. Carbamoyl Phosphate Synthase Subunit CgCPS1 Is Necessary for Virulence and to Regulate Stress Tolerance in Colletotrichum gloeosporioides. Plant Pathol. J. 2021, 37, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, K.; Wagener, J.; Ames, R.M.; Christou, S.; MacCallum, D.M.; Bates, S.; Gow, N.A.R. Three Related Enzymes in Candida albicans Achieve Arginine- and Agmatine-Dependent Metabolism That Is Essential for Growth and Fungal Virulence. mBio 2020, 11, e0184520. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, S.; Kang, L.; Xu, F.; Lan, X.; He, M.; Jin, K.; Xia, Y. Arginine metabolism governs microcycle conidiation by changing nitric oxide content in Metarhizium acridum. Appl. Microbiol. Biotechnol. 2023, 107, 1257–1268. [Google Scholar] [CrossRef]
- Cruz, C.; Egsgaard, H.; Trujillo, C.; Ambus, P.; Requena, N.; Martins-Loucao, M.A.; Jakobsen, I. Enzymatic evidence for the key role of arginine in nitrogen translocation by Arbuscular mycorrhizal fungi. Plant Physiol. 2007, 144, 782–792. [Google Scholar] [CrossRef]
- Touhami, N.; Buhl, K.; Schmidt-Heydt, M.; Geisen, R. Arginine acts as an inhibitor of the biosynthesis of several mycotoxins. Int. J. Food Microbiol. 2016, 235, 46–52. [Google Scholar] [CrossRef]
- Zulkifli, M.; Neff, J.K.; Timbalia, S.A.; Garza, N.M.; Chen, Y.; Watrous, J.D.; Murgia, M.; Trivedi, P.P.; Anderson, S.K.; Tomar, D.; et al. Yeast homologs of human MCUR1 regulate mitochondrial proline metabolism. Nat. Commun. 2020, 11, 4866. [Google Scholar] [CrossRef]
- Takagi, H.; Taguchi, J.; Kaino, T. Proline accumulation protects Saccharomyces cerevisiae cells in stationary phase from ethanol stress by reducing reactive oxygen species levels. Yeast 2016, 33, 355–363. [Google Scholar] [CrossRef]
- Liang, X.; Dickman, M.B.; Becker, D.F. Proline biosynthesis is required for endoplasmic reticulum stress tolerance in Saccharomyces cerevisiae. J. Biol. Chem. 2014, 289, 27794–27806. [Google Scholar] [CrossRef]
- Nishimura, A.; Nasuno, R.; Takagi, H. The proline metabolism intermediate Delta1-pyrroline-5-carboxylate directly inhibits the mitochondrial respiration in budding yeast. Febs Lett. 2012, 586, 2411–2416. [Google Scholar] [CrossRef]
- Takagi, H. Proline as a stress protectant in yeast: Physiological functions, metabolic regulations, and biotechnological applications. Appl. Microbiol. Biotechnol. 2008, 81, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Garbe, E.; Miramon, P.; Gerwien, F.; Ueberschaar, N.; Hansske-Braun, L.; Brandt, P.; Bottcher, B.; Lorenz, M.; Vylkova, S. GNP2 Encodes a High-Specificity Proline Permease in Candida albicans. mBio 2022, 13, e0314221. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Hoshida, H.; Akada, R. Inhibition of Distinct Proline- or N-Acetylglucosamine-Induced Hyphal Formation Pathways by Proline Analogs in Candida albicans. BioMed Res. Int. 2020, 2020, 7245782. [Google Scholar] [CrossRef] [PubMed]
- Okada, U.; Murakami, S. Structural and functional characteristics of the tripartite ABC transporter. Microbiology 2022, 168. [Google Scholar] [CrossRef]
- Yin, Y.; Wang, Z.; Cheng, D.; Chen, X.; Chen, Y.; Ma, Z. The ATP-binding protein FgArb1 is essential for penetration, infectious and normal growth of Fusarium graminearum. New Phytol. 2018, 219, 1447–1466. [Google Scholar] [CrossRef]
- Dean, M.; Rzhetsky, A.; Allikmets, R. The human ATP-binding cassette (ABC) transporter superfamily. Genome Res. 2001, 11, 1156–1166. [Google Scholar] [CrossRef]
- Perlin, M.H.; Andrews, J.; Toh, S.S. Essential letters in the fungal alphabet: ABC and MFS transporters and their roles in survival and pathogenicity. Adv. Genet. 2014, 85, 201–253. [Google Scholar] [CrossRef]
- Klein, C.; Kuchler, K.; Valachovic, M. ABC proteins in yeast and fungal pathogens. Essays Biochem. 2011, 50, 101–119. [Google Scholar] [CrossRef]
- Patkar, R.N.; Xue, Y.K.; Shui, G.; Wenk, M.R.; Naqvi, N.I. Abc3-mediated efflux of an endogenous digoxin-like steroidal glycoside by Magnaporthe oryzae is necessary for host invasion during blast disease. PLoS Pathog. 2012, 8, e1002888. [Google Scholar] [CrossRef]
- Sun, C.B.; Suresh, A.; Deng, Y.Z.; Naqvi, N.I. A multidrug resistance transporter in Magnaporthe is required for host penetration and for survival during oxidative stress. Plant Cell 2006, 18, 3686–3705. [Google Scholar] [CrossRef]
- Gardiner, D.M.; Stephens, A.E.; Munn, A.L.; Manners, J.M. An ABC pleiotropic drug resistance transporter of Fusarium graminearum with a role in crown and root diseases of wheat. Fems Microbiol. Lett. 2013, 348, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Abou Ammar, G.; Tryono, R.; Doll, K.; Karlovsky, P.; Deising, H.B.; Wirsel, S.G. Identification of ABC transporter genes of Fusarium graminearum with roles in azole tolerance and/or virulence. PLoS ONE 2013, 8, e79042. [Google Scholar] [CrossRef]
- Zhang, X.W.; Jia, L.J.; Zhang, Y.; Jiang, G.; Li, X.; Zhang, D.; Tang, W.H. In planta stage-specific fungal gene profiling elucidates the molecular strategies of Fusarium graminearum growing inside wheat coleoptiles. Plant Cell 2012, 24, 5159–5176. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Han, S.; Li, S.; Zhu, H.; Li, S.; Yan, J.; Zhu, T. Tandem Mass Tags Quantitative Proteome Identification and Function Analysis of ABC Transporters in Neofusicoccum parvum. Int. J. Mol. Sci. 2022, 23, 9908. [Google Scholar] [CrossRef] [PubMed]
- Zwiers, L.H.; Roohparvar, R.; de Waard, M.A. MgAtr7, a new type of ABC transporter from Mycosphaerella graminicola involved in iron homeostasis. Fungal Genet. Biol. 2007, 44, 853–863. [Google Scholar] [CrossRef] [PubMed]
- Stergiopoulos, I.; Zwiers, L.H.; De Waard, M.A. The ABC transporter MgAtr4 is a virulence factor of Mycosphaerella graminicola that affects colonization of substomatal cavities in wheat leaves. Mol. Plant Microbe Interact. 2003, 16, 689–698. [Google Scholar] [CrossRef]
- Oliveira, M.C.; Dos Santos, G.Q.; Teixeira, J.A.; Correia, H.L.N.; da Silva, L.L.; de Araujo, E.F.; de Queiroz, M.V. The AbcCl1 transporter of Colletotrichum lindemuthianum acts as a virulence factor involved in fungal detoxification during common bean (Phaseolus vulgaris) infection. Braz. J. Microbiol. 2022, 53, 1121–1132. [Google Scholar] [CrossRef]
- Khandelwal, N.K.; Kaemmer, P.; Forster, T.M.; Singh, A.; Coste, A.T.; Andes, D.R.; Hube, B.; Sanglard, D.; Chauhan, N.; Kaur, R.; et al. Pleiotropic effects of the vacuolar ABC transporter MLT1 of Candida albicans on cell function and virulence. Biochem. J. 2016, 473, 1537–1552. [Google Scholar] [CrossRef]
- Theiss, S.; Kretschmar, M.; Nichterlein, T.; Hof, H.; Agabian, N.; Hacker, J.; Kohler, G.A. Functional analysis of a vacuolar ABC transporter in wild-type Candida albicans reveals its involvement in virulence. Mol. Microbiol. 2002, 43, 571–584. [Google Scholar] [CrossRef]
- Stefanato, F.L.; Abou-Mansour, E.; Buchala, A.; Kretschmer, M.; Mosbach, A.; Hahn, M.; Bochet, C.G.; Metraux, J.P.; Schoonbeek, H.J. The ABC transporter BcatrB from Botrytis cinerea exports camalexin and is a virulence factor on Arabidopsis thaliana. Plant J. 2009, 58, 499–510. [Google Scholar] [CrossRef]
- Liu, Y.S.; Wang, Y.; Zhou, X.; Zhang, L.; Yang, G.; Gao, X.D.; Murakami, Y.; Fujita, M.; Kinoshita, T. Accumulated precursors of specific GPI-anchored proteins upregulate GPI biosynthesis with ARV1. J. Cell Biol. 2023, 222, e202208159. [Google Scholar] [CrossRef] [PubMed]
- Richard, M.L.; Plaine, A. Comprehensive analysis of glycosylphosphatidylinositol-anchored proteins in Candida albicans. Eukaryot. Cell 2007, 6, 119–133. [Google Scholar] [CrossRef] [PubMed]
- Muller, G.A.; Muller, T.D. (Patho)Physiology of Glycosylphosphatidylinositol-Anchored Proteins I: Localization at Plasma Membranes and Extracellular Compartments. Biomolecules 2023, 13, 855. [Google Scholar] [CrossRef] [PubMed]
- Orlean, P.; Menon, A.K. Thematic review series: Lipid posttranslational modifications. GPI anchoring of protein in yeast and mammalian cells, or: How we learned to stop worrying and love glycophospholipids. J. Lipid Res. 2007, 48, 993–1011. [Google Scholar] [CrossRef] [PubMed]
- Osset-Trenor, P.; Pascual-Ahuir, A.; Proft, M. Fungal Drug Response and Antimicrobial Resistance. J. Fungi 2023, 9, 565. [Google Scholar] [CrossRef] [PubMed]
- Umemura, M.; Okamoto, M.; Nakayama, K.; Sagane, K.; Tsukahara, K.; Hata, K.; Jigami, Y. GWT1 gene is required for inositol acylation of glycosylphosphatidylinositol anchors in yeast. J. Biol. Chem. 2003, 278, 23639–23647. [Google Scholar] [CrossRef]
- Klis, F.M.; Sosinska, G.J.; de Groot, P.W.; Brul, S. Covalently linked cell wall proteins of Candida albicans and their role in fitness and virulence. FEMS Yeast Res. 2009, 9, 1013–1028. [Google Scholar] [CrossRef]
- Komath, S.S.; Singh, S.L.; Pratyusha, V.A.; Sah, S.K. Generating anchors only to lose them: The unusual story of glycosylphosphatidylinositol anchor biosynthesis and remodeling in yeast and fungi. Iubmb Life 2018, 70, 355–383. [Google Scholar] [CrossRef]
- Fu, Y.; Estoppey, D.; Roggo, S.; Pistorius, D.; Fuchs, F.; Studer, C.; Ibrahim, A.S.; Aust, T.; Grandjean, F.; Mihalic, M.; et al. Jawsamycin exhibits in vivo antifungal properties by inhibiting Spt14/Gpi3-mediated biosynthesis of glycosylphosphatidylinositol. Nat. Commun. 2020, 11, 3387. [Google Scholar] [CrossRef]
- Yadav, U.; Khan, M.A. Targeting the GPI biosynthetic pathway. Pathog. Glob. Health 2018, 112, 115–122. [Google Scholar] [CrossRef]
- Mann, P.A.; McLellan, C.A.; Koseoglu, S.; Si, Q.; Kuzmin, E.; Flattery, A.; Harris, G.; Sher, X.; Murgolo, N.; Wang, H.; et al. Chemical Genomics-Based Antifungal Drug Discovery: Targeting Glycosylphosphatidylinositol (GPI) Precursor Biosynthesis. ACS Infect. Dis. 2015, 1, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Pittet, M.; Conzelmann, A. Biosynthesis and function of GPI proteins in the yeast Saccharomyces cerevisiae. Biochim. Biophys. Acta 2007, 1771, 405–420. [Google Scholar] [CrossRef] [PubMed]


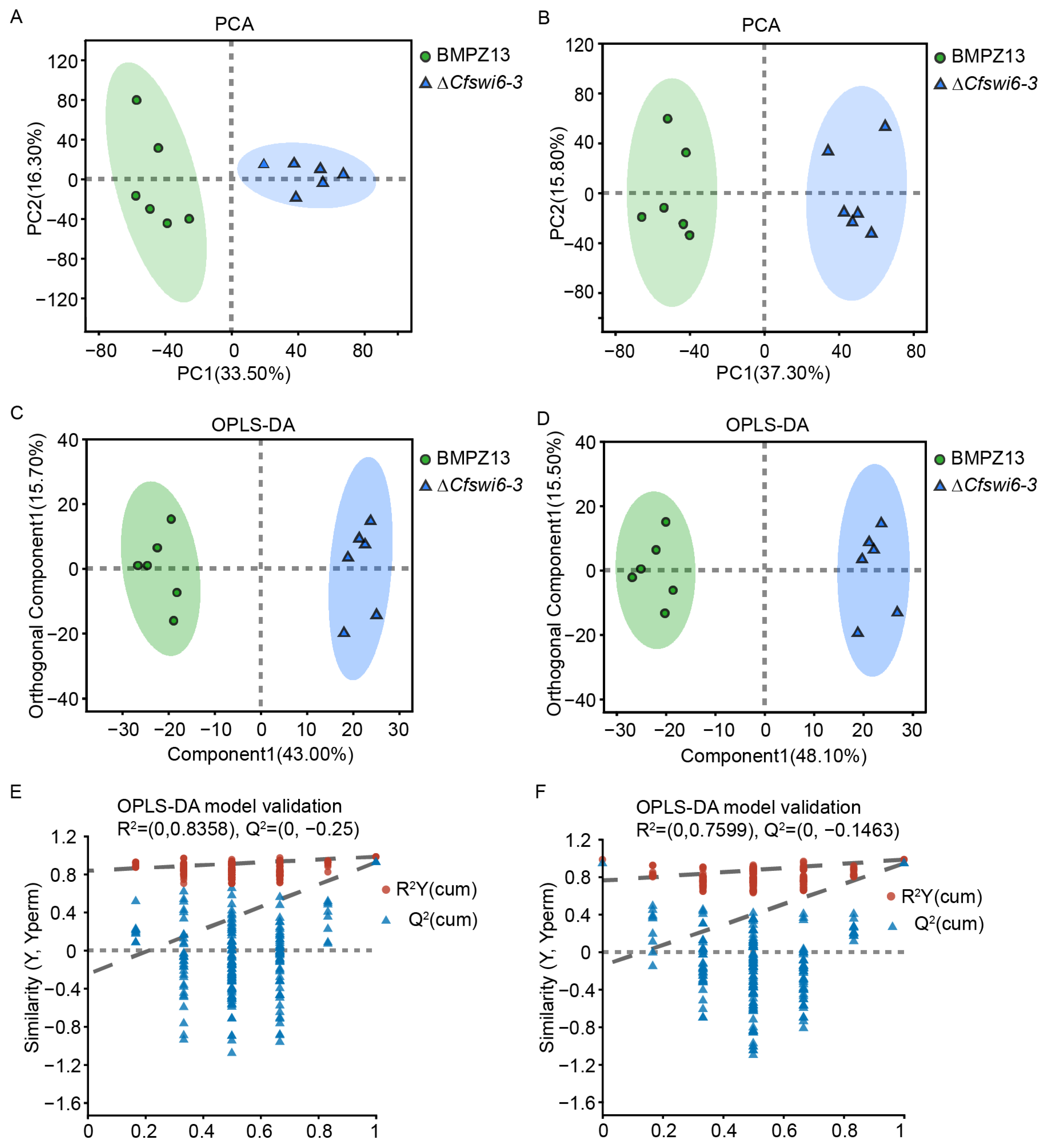


| Ion Mode | PCA | OPLS-DA | |||
|---|---|---|---|---|---|
| R2X (cum) | R2Y (cum) | Q2 (cum) | R2 | Q2 | |
| POS | 0.608 | 0.983 | 0.925 | 0.8358 | −0.25 |
| NEG | 0.644 | 0.984 | 0.943 | 0.7599 | −0.1463 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cong, H.; Li, C.; Wang, Y.; Zhang, Y.; Ma, D.; Li, L.; Jiang, J. The Mechanism of Transcription Factor Swi6 in Regulating Growth and Pathogenicity of Ceratocystis fimbriata: Insights from Non-Targeted Metabolomics. Microorganisms 2023, 11, 2666. https://doi.org/10.3390/microorganisms11112666
Cong H, Li C, Wang Y, Zhang Y, Ma D, Li L, Jiang J. The Mechanism of Transcription Factor Swi6 in Regulating Growth and Pathogenicity of Ceratocystis fimbriata: Insights from Non-Targeted Metabolomics. Microorganisms. 2023; 11(11):2666. https://doi.org/10.3390/microorganisms11112666
Chicago/Turabian StyleCong, Hao, Changgen Li, Yiming Wang, Yongjing Zhang, Daifu Ma, Lianwei Li, and Jihong Jiang. 2023. "The Mechanism of Transcription Factor Swi6 in Regulating Growth and Pathogenicity of Ceratocystis fimbriata: Insights from Non-Targeted Metabolomics" Microorganisms 11, no. 11: 2666. https://doi.org/10.3390/microorganisms11112666
APA StyleCong, H., Li, C., Wang, Y., Zhang, Y., Ma, D., Li, L., & Jiang, J. (2023). The Mechanism of Transcription Factor Swi6 in Regulating Growth and Pathogenicity of Ceratocystis fimbriata: Insights from Non-Targeted Metabolomics. Microorganisms, 11(11), 2666. https://doi.org/10.3390/microorganisms11112666







