Pseudomonas spp. in Canine Otitis Externa
Abstract
:1. Introduction
1.1. Canine Otitis Externa
1.2. Normal Ear Structure and Function
1.3. Normal Ear Microbiota
2. Clinical Framework
2.1. Primary Factors
2.2. Secondary Infections
2.3. Perpetuating Factors
2.4. Predisposing Factors
2.5. Prevalence
2.6. Treatment
2.7. Environmental Prevalence
2.8. Pseudomonas in the Veterinary Environment
2.9. In the Home
2.10. Isolates
3. Toxin Production/Virulence Factors
4. Antibiotic, Disinfectant, and Biological Control
Potential Alternative Treatments
5. Conclusions
Author Contributions
Funding
Data availability statement
Conflicts of Interest
References
- Bajwa, J. Canine Otitis Externa—Treatment and Complications. Can. Vet. J. 2019, 60, 97–99. [Google Scholar] [PubMed]
- Nuttall, T. Managing Recurrent Otitis Externa in Dogs: What Have We Learned and What Can We Do Better? J. Am. Vet. Med. Assoc. 2023, 261, S10–S22. [Google Scholar] [CrossRef]
- Hill, P.B.; Lo, A.; Eden, C.A.N.; Huntley, S.; Morey, V.; Ramsey, S.; Richardson, C.; Smith, D.J.; Sutton, C.; Taylor, M.D.; et al. Survey of the Prevalence, Diagnosis and Treatment of Dermatological Conditions in Small Animals in General Practice. Vet. Rec. 2006, 158, 533–539. [Google Scholar] [CrossRef] [PubMed]
- August, J.R. Otitis Externa. Vet. Clin. N. Am. Small Anim. Pract. 1988, 18, 731–742. [Google Scholar] [CrossRef]
- Smeak, D.D. Treatment of Persistent Deep Infection After Total Ear Canal Ablation and Lateral Bulla Osteotomy. Vet. Clin. N. Am. Small Anim. Pract. 2016, 46, 609–621. [Google Scholar] [CrossRef]
- Cole, L.K. Anatomy and Physiology of the Canine Ear. Vet. Dermatol. 2009, 21, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Tabacca, N.E.; Cole, L.K.; Hillier, A.; Rajala-Schultz, P.J. Epithelial Migration on the Canine Tympanic Membrane: Epithelial Migration on Canine Tympanum. Vet. Dermatol. 2011, 22, 502–510. [Google Scholar] [CrossRef]
- Huang, H.; Little, C.J.L.; McNeil, P.E. Histological Changes in the External Ear Canal of Dogs with Otitis Externa. Vet. Dermatol. 2009, 20, 422–428. [Google Scholar] [CrossRef]
- Stout-Graham, M.; Kainer, R.A.; Whalen, L.R.; Macy, D.W. Morphologic Measurements of the External Horizontal Ear Canal of Dogs. Am. J. Vet. Res. 1990, 51, 990–994. [Google Scholar]
- Grono, L.R. Studies of the Microclimate of the External Auditory Canal in the Dog. 3. Relative Humidity within the External Auditory Meatus. Res. Vet. Sci. 1970, 11, 316–319. [Google Scholar] [CrossRef]
- Panzuti, P.; Mosca, M.; Fantini, O.; Noel, G.; Cappelle, J.; Pin, D. Effect of an Ear Cleaner Instillation Containing Lipacids in a Model of Re-acidification of the External Auditory Canal in Dogs. Vet. Dermatol. 2022, 33, 402–406. [Google Scholar] [CrossRef]
- Thorp, M.A.; Kruger, J.; Oliver, S.; Nilssen, E.L.K.; Prescott, C.A.J. The Antibacterial Activity of Acetic Acid and Burow’s Solution as Topical Otological Preparations. J. Laryngol. Otol. 1998, 112, 925–928. [Google Scholar] [CrossRef]
- Swinney, A.; Fazakerley, J.; McEwan, N.; Nuttall, T. Comparative in Vitro Antimicrobial Efficacy of Commercial Ear Cleaners. Vet. Dermatol. 2008, 19, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Diamond, G.; Beckloff, N.; Weinberg, A.; Kisich, K. The Roles of Antimicrobial Peptides in Innate Host Defense. CPD 2009, 15, 2377–2392. [Google Scholar] [CrossRef]
- Santoro, D. Comparison of the Quantity and Antimicrobial Activity of Host Defence Peptides in Ear Canals between Healthy and Atopic Dogs: A Preliminary Study. Vet. Dermatol. 2023, 34, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Dickson, D.B.; Love, D.N. Bacteriology of the Horizontal Ear Canal of Dogs. J. Small Anim. Pract. 1983, 24, 413–421. [Google Scholar] [CrossRef]
- Tater, K.C.; Scott, D.W.; Miller, W.H.; Erb, H.N. The Cytology of the External Ear Canal in the Normal Dog and Cat. J. Vet. Med. Ser. A 2003, 50, 370–374. [Google Scholar] [CrossRef]
- Leonard, C.; Thiry, D.; Taminiau, B.; Daube, G.; Fontaine, J. External Ear Canal Evaluation in Dogs with Chronic Suppurative Otitis Externa: Comparison of Direct Cytology, Bacterial Culture and 16S Amplicon Profiling. Vet. Sci. 2022, 9, 366. [Google Scholar] [CrossRef]
- Shaw, S. Pathogens in Otitis Externa: Diagnostic Techniques to Identify Secondary Causes of Ear Disease. Practice 2016, 38, 12–16. [Google Scholar] [CrossRef]
- Grono, L.R.; Frost, A.J. Otitis externa in the dog: The Microbiology of the Normal and Affected External Ear Canal. Aust. Vet. J. 1969, 45, 420–422. [Google Scholar] [CrossRef]
- Yamashita, K.; Shimizu, A.; Kawano, J.; Uchida, E.; Haruna, A.; Igimi, S. Isolation and Characterization of Staphylococci from External Auditory Meatus of Dogs with or without Otitis Externa with Special Reference to Staphylococcus schleiferi Subsp. Coagulans Isolates. J. Vet. Med. Sci. 2005, 67, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Aoki-Komori, S.; Shimada, K.; Tani, K.; Katayama, M.; Saito, T.R.; Kataoka, Y. Microbial Flora in the Ears of Healthy Experimental Beagles. Exp. Anim. 2007, 56, 67–69. [Google Scholar] [CrossRef]
- Fraser, G. The Fungal Flora of the Canine Ear. J. Comp. Pathol. Ther. 1961, 71, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Lyskova, P.; Vydrzalova, M.; Mazurova, J. Identification and Antimicrobial Susceptibility of Bacteria and Yeasts Isolated from Healthy Dogs and Dogs with Otitis Externa. J. Vet. Med. Ser. A 2007, 54, 559–563. [Google Scholar] [CrossRef]
- Leonard, C.; Picavet, P.P.; Fontaine, J.; Clercx, C.; Taminiau, B.; Daube, G.; Claeys, S. The Middle Ear Microbiota in Healthy Dogs Is Similar to That of the External Ear Canal. Vet. Sci. 2023, 10, 216. [Google Scholar] [CrossRef] [PubMed]
- Igimi, S.; Takahashi, E.; Mitsuoka, T. Staphylococcus Schleiferi Subsp. Coagulans Subsp. Nov., Isolated from the External Auditory Meatus of Dogs with External Ear Otitis. Int. J. Syst. Bacteriol. 1990, 40, 409–411. [Google Scholar] [CrossRef]
- Tang, S.; Prem, A.; Tjokrosurjo, J.; Sary, M.; Van Bel, M.A.; Rodrigues-Hoffmann, A.; Kavanagh, M.; Wu, G.; Van Eden, M.E.; Krumbeck, J.A. The Canine Skin and Ear Microbiome: A Comprehensive Survey of Pathogens Implicated in Canine Skin and Ear Infections Using a Novel next-Generation-Sequencing-Based Assay. Vet. Microbiol. 2020, 247, 108764. [Google Scholar] [CrossRef]
- Ngo, J.; Taminiau, B.; Fall, P.A.; Daube, G.; Fontaine, J. Ear Canal Microbiota—A Comparison between Healthy Dogs and Atopic Dogs without Clinical Signs of Otitis Externa. Vet. Dermatol. 2018, 29, 425. [Google Scholar] [CrossRef]
- Borriello, G.; Paradiso, R.; Catozzi, C.; Brunetti, R.; Roccabianca, P.; Riccardi, M.G.; Cecere, B.; Lecchi, C.; Fusco, G.; Ceciliani, F.; et al. Cerumen Microbial Community Shifts between Healthy and Otitis Affected Dogs. PLoS ONE 2020, 15, e0241447. [Google Scholar] [CrossRef]
- Rodrigues Hoffmann, A.; Patterson, A.P.; Diesel, A.; Lawhon, S.D.; Ly, H.J.; Stephenson, C.E.; Mansell, J.; Steiner, J.M.; Dowd, S.E.; Olivry, T.; et al. The Skin Microbiome in Healthy and Allergic Dogs. PLoS ONE 2014, 9, e83197. [Google Scholar] [CrossRef]
- Mauldin, E.A.; Scott, D.W.; Miller, W.H.; Smith, C.A. Malassezia Dermatitis in the Dog: A Retrospective Histopathological and Immunopathological Study of 86 Cases (1990–95). Vet. Dermatol. 1997, 8, 191–202. [Google Scholar] [CrossRef]
- Morris, D.O.; Davis, M.F.; Palmeiro, B.S.; O’Shea, K.; Rankin, S.C. Molecular and Epidemiological Characterization of Canine Pseudomonas Otitis Using a Prospective Case-Control Study Design. Vet. Dermatol. 2017, 28, 118-e25. [Google Scholar] [CrossRef]
- Newton, H.M.; Rosenkrantz, W.S.; Muse, R.; Griffin, C.E. Evaluation of Otoscope Cone Cleaning and Disinfection Procedures Commonly Used in Veterinary Medical Practices: A Pilot Study. Vet. Dermatol. 2006, 17, 147–150. [Google Scholar] [CrossRef]
- Nuttall, T.; Bensignor, E. A Pilot Study to Develop an Objective Clinical Score for Canine Otitis Externa. Vet. Dermatol. 2014, 25, 530. [Google Scholar] [CrossRef]
- Paterson, S.; Matyskiewicz, W. A Study to Evaluate the Primary Causes Associated with Pseudomonas Otitis in 60 Dogs: Primary Causes of Pseudomonas Otitis. J. Small Anim. Pract. 2018, 59, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Rosser, E.J. Diagnosis of Food Allergy in Dogs. J. Am. Vet. Med. Assoc. 1993, 203, 259–262. [Google Scholar]
- Scott, D. Observations on Canine Atopy. J. Am. Anim. Hosp. Assoc. 1981, 17, 91–100. [Google Scholar]
- Willemse, T. Atopic Skin Disease: A Review and a Reconsideration of Diagnostic Criteria. J. Small Anim. Pract. 1986, 27, 771–778. [Google Scholar] [CrossRef]
- Saridomichelakis, M.N.; Farmaki, R.; Leontides, L.S.; Koutinas, A.F. Aetiology of Canine Otitis Externa: A Retrospective Study of 100 Cases. Vet. Dermatol. 2007, 18, 341–347. [Google Scholar] [CrossRef]
- Favrot, C.; Steffan, J.; Seewald, W.; Picco, F. A prospective study on the clinical features of chronic canine atopic dermatitis and its diagnosis. Vet. Dermatol. 2010, 21, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Apostolopoulos, N.; Glaeser, S.P.; Bagwe, R.; Janssen, S.; Mayer, U.; Ewers, C.; Kämpfer, P.; Neiger, R.; Thom, N. Description and Comparison of the Skin and Ear Canal Microbiota of Non-Allergic and Allergic German Shepherd Dogs Using next Generation Sequencing. PLoS ONE 2021, 16, e0250695. [Google Scholar] [CrossRef] [PubMed]
- Zur, G.; Lifshitz, B.; Bdolah-Abram, T. The Association between the Signalment, Common Causes of Canine Otitis Externa and Pathogens. J. Small Anim. Pract. 2011, 52, 254–258. [Google Scholar] [CrossRef]
- Bergvall, K.; Ahman, S.; Mueller, R.; Boehm, T.; Chala, V.; Fournel, S.; Navarro, C. Can Topical Hydrocortisone Aceponateeffectively Control Allergic Otitis Externaand Reduce the Risk of Recurrence? Adouble-Blinded, Placebo-Controlled, Prospective Study. Abstracts of the 29th Annual Congress of the ECVD-ESVD, 7–9th September 2017, Lausanne, Switzerland. Vet. Dermatol. 2017, 28, 533–553. [Google Scholar] [CrossRef]
- Lawson, P.A.; Johnson, C.N.; Bengtsson, L.; Charalampakis, G.; Dahlén, G.; Moore, E.; Falsen, E. Peptostreptococcus Canis Sp. Nov., Isolated from Subgingival Plaque from Canine Oral Cavity. Anaerobe 2012, 18, 597–601. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.D.; Love, D.N.; Karjalainen, J.; Kanervo, A.; Forsblom, B.; Willems, A.; Stubbs, S.; Sarkiala, E.; Bailey, G.D.; Wigney, D.I.; et al. Phylogenetic Analysis of Members of the Genus Porphyromonas and Description of Porphyromonas cangingivalis Sp. Nov. and Porphyromonas cansulci Sp. Nov. Int. J. Syst. Bacteriol. 1994, 44, 674–679. [Google Scholar] [CrossRef]
- Petrov, V.; Zhelev, G.; Marutsov, P.; Koev, K.; Georgieva, S.; Toneva, I.; Urumova, V. Microbiological and Antibacterial Resistance Profile in Canine Otitis Externa—A Comparative Analysis. BJVM 2019, 22, 447–456. [Google Scholar] [CrossRef]
- Bugden, D. Identification and Antibiotic Susceptibility of Bacterial Isolates from Dogs with Otitis Externa in Australia. Aust. Vet. J. 2013, 91, 43–46. [Google Scholar] [CrossRef]
- Nuttall, T.; Cole, L.K. Evidence-Based Veterinary Dermatology: A Systematic Review of Interventions for Treatment of Pseudomonas Otitis in Dogs. Vet. Dermatol. 2007, 18, 69–77. [Google Scholar] [CrossRef]
- Paterson, S. Discovering the Causes of Otitis Externa. Practice 2016, 38, 7–11. [Google Scholar] [CrossRef]
- O’Neill, D.G.; Volk, A.V.; Soares, T.; Church, D.B.; Brodbelt, D.C.; Pegram, C. Frequency and Predisposing Factors for Canine Otitis Externa in the UK—A Primary Veterinary Care Epidemiological View. Canine Med. Genet. 2021, 8, 7. [Google Scholar] [CrossRef]
- O’Neill, D.G.; James, H.; Brodbelt, D.C.; Church, D.B.; Pegram, C. Prevalence of Commonly Diagnosed Disorders in UK Dogs under Primary Veterinary Care: Results and Applications. BMC Vet. Res. 2021, 17, 69. [Google Scholar] [CrossRef]
- O′Neill, D.G.; Church, D.B.; McGreevy, P.D.; Thomson, P.C.; Brodbelt, D.C. Prevalence of Disorders Recorded in Dogs Attending Primary-Care Veterinary Practices in England. PLoS ONE 2014, 9, e90501. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Choe, C.; Yoo, J.G.; Oh, S.-I.; Jung, Y.; Cho, A.; Kim, S.; Do, Y.J. Major Medical Causes by Breed and Life Stage for Dogs Presented at Veterinary Clinics in the Republic of Korea: A Survey of Electronic Medical Records. PeerJ 2018, 6, e5161. [Google Scholar] [CrossRef] [PubMed]
- Lund, E.M.; Armstrong, P.J.; Kirk, C.A.; Kolar, L.M.; Klausner, J.S. Health Status and Population Characteristics of Dogs and Cats Examined at Private Veterinary Practices in the United States. J. Am. Vet. Med. Assoc. 1999, 214, 1336–1341. [Google Scholar]
- Baxter, M.; Lawler, D.C. The Incidence and Microbiology of Otitis Externa of Dogs and Cats in New Zealand. N. Z. Vet. J. 1972, 20, 29–32. [Google Scholar] [CrossRef]
- Leonard, C.; Taminiau, B.; Ngo, J.; Fantini, O.; Daube, G.; Fontaine, J. Preventive Use of a Topical Anti-inflammatory Glucocorticoid in Atopic Dogs without Clinical Sign of Otitis Does Not Affect Ear Canal Microbiota and Mycobiota. Vet. Dermatol. 2021, 32, 355. [Google Scholar] [CrossRef] [PubMed]
- Nuttall, T. Successful Management of Otitis Externa. Practice 2016, 38, 17–21. [Google Scholar] [CrossRef]
- Morris, D.O. Medical Therapy of Otitis Externa and Otitis Media. Vet. Clin. N. Am. Small Anim. Pract. 2004, 34, 541–555. [Google Scholar] [CrossRef]
- Bateman, F.L.; Moss, S.M.; Trott, D.J.; Shipstone, M.A. Biological Efficacy and Stability of Diluted Ticarcillin–Clavulanic Acid in the Topical Treatment of Pseudomonas Aeruginosa Infections. Vet. Dermatol. 2012, 23, 97-e22. [Google Scholar] [CrossRef]
- Klinczar, A.M.; Griffies, J.D.; Bateman, F.L.; Arnold, R.D.; Jasper, S.L.; Brown, A.R. Determination of Amikacin Stability at 1% and 3% Concentrations in Four Topical Solutions over a 56-Day Period. Vet. Dermatol. 2022, 33, 23-e8. [Google Scholar] [CrossRef]
- Hoff, S.E.; Berger, D.J.; Viall, A.K.; Schrunk, D.; Noxon, J.O. Chemical and Microbiological Stability of Diluted Ceftazidime in Three Different Solutions under Three Storage Temperatures over a 28 Day Period. Vet. Dermatol. 2021, 32, 456-e124. [Google Scholar] [CrossRef]
- Metry, C.A.; Maddox, C.W.; Dirikolu, L.; Johnson, Y.J.; Campbell, K.L. Determination of Enrofloxacin Stability and in Vitro Efficacy against Staphylococcus Pseudintermedius and Pseudomonas Aeruginosa in Four Ear Cleaner Solutions over a 28 Day Period. Vet. Dermatol. 2012, 23, 23-e6. [Google Scholar] [CrossRef]
- Paterson, S. Brainstem Auditory Evoked Responses in 37 Dogs with Otitis Media before and after Topical Therapy. J. Small Anim. Pract. 2018, 59, 10–15. [Google Scholar] [CrossRef]
- Pye, C. Pseudomonas Otitis Externa in Dogs. Can. Vet. J. 2018, 59, 1231–1234. [Google Scholar] [PubMed]
- Pye, C.C.; Singh, A.; Weese, J.S. Evaluation of the Impact of Tromethamine Edetate Disodium Dihydrate on Antimicrobial Susceptibility of Pseudomonas aeruginosa in Biofilm in vitro. Vet. Dermatol. 2014, 25, 120-e34. [Google Scholar] [CrossRef]
- Blondeau, J.M. New Concepts in Antimicrobial Susceptibility Testing: The Mutant Prevention Concentration and Mutant Selection Window Approach. Vet. Dermatol. 2009, 20, 383–396. [Google Scholar] [CrossRef]
- Pasquali, F.; Manfreda, G. Mutant Prevention Concentration of Ciprofloxacin and Enrofloxacin against Escherichia Coli, Salmonella Typhimurium and Pseudomonas aeruginosa. Vet. Microbiol. 2007, 119, 304–310. [Google Scholar] [CrossRef]
- Steen, S.I.; Paterson, S. The Susceptibility of Pseudomonas Spp. Isolated from Dogs with Otitis to Topical Ear Cleaners. J. Small Anim. Pract. 2012, 53, 599–603. [Google Scholar] [CrossRef] [PubMed]
- Doyle, R.S.; Skelly, C.; Bellenger, C.R. Surgical Management of 43 Cases of Chronic Otitis Externa in the Dog. Ir. Vet. J. 2004, 57, 22. [Google Scholar] [CrossRef] [PubMed]
- Crone, S.; Vives-Flórez, M.; Kvich, L.; Saunders, A.M.; Malone, M.; Nicolaisen, M.H.; Martínez-García, E.; Rojas-Acosta, C.; Catalina Gomez-Puerto, M.; Calum, H.; et al. The Environmental Occurrence of Pseudomonas aeruginosa. APMIS 2020, 128, 220–231. [Google Scholar] [CrossRef] [PubMed]
- Van Asperen, I.A.; De Rover, C.M.; Schijven, J.F.; Oetomo, S.B.; Schellekens, J.F.P.; Van Leeuwen, N.J.; Colle, C.; Havelaar, A.H.; Kromhout, D.; Sprenger, M.W.J. Risk of Otitis Externa after Swimming in Recreational Fresh Water Lakes Containing Pseudomonas Aeruginosa. BMJ 1995, 311, 1407–1410. [Google Scholar] [CrossRef] [PubMed]
- Verdial, C.; Carneiro, C.; Machado, I.; Tavares, L.; Almeida, V.; Oliveira, M.; Gil, S. Controlling Bacteriological Contamination of Environmental Surfaces at the Biological Isolation and Containment Unit of a Veterinary Teaching Hospital. Ir. Vet. J. 2021, 74, 18. [Google Scholar] [CrossRef] [PubMed]
- Stull, J.W.; Weese, J.S. Hospital-Associated Infections in Small Animal Practice. Vet. Clin. N. Am. Small Anim. Pract. 2015, 45, 217–233. [Google Scholar] [CrossRef]
- Fraser, M.A.; Girling, S.J. Bacterial Carriage of Computer Keyboards in Veterinary Practices in Scotland. Vet. Rec. 2009, 165, 26–27. [Google Scholar] [CrossRef]
- Fine, D.M.; Tobias, A.H. Cardiovascular Device Infections in Dogs: Report of 8 Cases and Review of the Literature. J. Vet. Intern. Med. 2007, 21, 1265–1271. [Google Scholar] [CrossRef]
- Peremans, K.; Winter, F.; Janssens, L.; Dumont, F.; Bree, H.; Dierckx, R. AN INFECTED HIP PROSTHESIS IN A DOG DIAGNOSED WITH A 99mTC-CIPROFLOXACIN (INFECTON) SCAN. Vet. Radiol. Ultrasound 2002, 43, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Kirby, A.L.; Rosenkrantz, W.S.; Ghubash, R.M.; Neradilek, B.; Polissar, N.L. Evaluation of Otoscope Cone Disinfection Techniques and Contamination Level in Small Animal Private Practice. Vet. Dermatol. 2010, 21, 175–183. [Google Scholar] [CrossRef]
- Korkmaz, H.; Çetinkol, Y.; Korkmaz, M. Cross-Contamination and Cross-Infection Risk of Otoscope Heads. Eur. Arch. Otorhinolaryngol. 2013, 270, 3183–3186. [Google Scholar] [CrossRef] [PubMed]
- Remold, S.K.; Brown, C.K.; Farris, J.E.; Hundley, T.C.; Perpich, J.A.; Purdy, M.E. Differential Habitat Use and Niche Partitioning by Pseudomonas Species in Human Homes. Microb. Ecol. 2011, 62, 505–517. [Google Scholar] [CrossRef] [PubMed]
- Zupančič, J.; Turk, M.; Črnigoj, M.; Ambrožič Avguštin, J.; Gunde-Cimerman, N. The Dishwasher Rubber Seal Acts as a Reservoir of Bacteria in the Home Environment. BMC Microbiol. 2019, 19, 300. [Google Scholar] [CrossRef]
- Purdy-Gibson, M.E.; France, M.; Hundley, T.C.; Eid, N.; Remold, S.K. Pseudomonas Aeruginosa in CF and Non-CF Homes Is Found Predominantly in Drains. J. Cyst. Fibros. 2015, 14, 341–346. [Google Scholar] [CrossRef]
- Rice, S.A.; Van Den Akker, B.; Pomati, F.; Roser, D. A Risk Assessment of Pseudomonas Aeruginosa in Swimming Pools: A Review. J. Water Health 2012, 10, 181–196. [Google Scholar] [CrossRef] [PubMed]
- Perry, E.; Sutton, G.A.; Haggag, L.; Fleker, M.; Blum, S.E.; Kaufmann, R. Pseudomonas aeruginosa Isolation from Dog Grooming Products Used by Private Owners or by Professional Pet Grooming Salons: Prevalence and Risk Factors. Vet. Dermatol. 2022, 33, 316. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Oh, J.; Park, S.; Sum, S.; Song, W.; Chae, J.; Park, H. Antimicrobial Resistance and Novel Mutations Detected in the GyrA and ParC Genes of Pseudomonas Aeruginosa Strains Isolated from Companion Dogs. BMC Vet. Res. 2020, 16, 111. [Google Scholar] [CrossRef] [PubMed]
- Haenni, M.; Hocquet, D.; Ponsin, C.; Cholley, P.; Guyeux, C.; Madec, J.-Y.; Bertrand, X. Population Structure and Antimicrobial Susceptibility of Pseudomonas Aeruginosa from Animal Infections in France. BMC Vet. Res. 2015, 11, 9. [Google Scholar] [CrossRef]
- Hyun, J.-E.; Chung, T.-H.; Hwang, C.-Y. Identification of VIM-2 Metallo-β-Lactamase-Producing Pseudomonas aeruginosa Isolated from Dogs with Pyoderma and Otitis in Korea. Vet. Dermatol. 2018, 29, 186-e68. [Google Scholar] [CrossRef] [PubMed]
- Elfadadny, A.; Uchiyama, J.; Goto, K.; Imanishi, I.; Ragab, R.F.; Nageeb, W.M.; Iyori, K.; Toyoda, Y.; Tsukui, T.; Ide, K.; et al. Antimicrobial Resistance and Genotyping of Pseudomonas Aeruginosa Isolated from the Ear Canals of Dogs in Japan. Front. Vet. Sci. 2023, 10, 1074127. [Google Scholar] [CrossRef] [PubMed]
- Maatallah, M.; Cheriaa, J.; Backhrouf, A.; Iversen, A.; Grundmann, H.; Do, T.; Lanotte, P.; Mastouri, M.; Elghmati, M.S.; Rojo, F.; et al. Population Structure of Pseudomonas Aeruginosa from Five Mediterranean Countries: Evidence for Frequent Recombination and Epidemic Occurrence of CC235. PLoS ONE 2011, 6, e25617. [Google Scholar] [CrossRef]
- Santaniello, A.; Sansone, M.; Fioretti, A.; Menna, L.F. Systematic Review and Meta-Analysis of the Occurrence of ESKAPE Bacteria Group in Dogs, and the Related Zoonotic Risk in Animal-Assisted Therapy, and in Animal-Assisted Activity in the Health Context. Int. J. Environ. Res. Public Health 2020, 17, 3278. [Google Scholar] [CrossRef]
- Fernandes, M.R.; Sellera, F.P.; Moura, Q.; Carvalho, M.P.N.; Rosato, P.N.; Cerdeira, L.; Lincopan, N. Zooanthroponotic Transmission of Drug-Resistant Pseudomonas Aeruginosa, Brazil. Emerg. Infect. Dis. 2018, 24, 1160–1162. [Google Scholar] [CrossRef]
- Hattab, J.; Mosca, F.; Di Francesco, C.E.; Aste, G.; Marruchella, G.; Guardiani, P.; Tiscar, P.G. Occurrence, Antimicrobial Susceptibility, and Pathogenic Factors of Pseudomonas Aeruginosa in Canine Clinical Samples. Vet. World 2021, 14, 978–985. [Google Scholar] [CrossRef] [PubMed]
- Pye, C.C.; Yu, A.A.; Weese, J.S. Evaluation of Biofilm Production by Pseudomonas aeruginosa from Canine Ears and the Impact of Biofilm on Antimicrobial Susceptibility in vitro. Vet. Dermatol. 2013, 24, 446-e99. [Google Scholar] [CrossRef]
- Brock, M.T.; Fedderly, G.C.; Borlee, G.I.; Russell, M.M.; Filipowska, L.K.; Hyatt, D.R.; Ferris, R.A.; Borlee, B.R. Pseudomonas Aeruginosa Variants Obtained from Veterinary Clinical Samples Reveal a Role for Cyclic Di-GMP in Biofilm Formation and Colony Morphology. Microbiology 2017, 163, 1613–1625. [Google Scholar] [CrossRef]
- Boles, B.R.; Thoendel, M.; Singh, P.K. Self-Generated Diversity Produces “Insurance Effects” in Biofilm Communities. Proc. Natl. Acad. Sci. USA 2004, 101, 16630–16635. [Google Scholar] [CrossRef] [PubMed]
- Vives-Flórez, M.; Garnica, D. Comparison of Virulence between Clinical and Environmental Pseudomonas Aeruginosa Isolates. Int. Microbiol. 2006, 9, 247–252. [Google Scholar]
- Robinson, V.H.; Paterson, S.; Bennett, C.; Steen, S.I. Biofilm Production of Pseudomonas Spp. Isolates from Canine Otitis in Three Different Enrichment Broths. Vet. Dermatol. 2019, 30, 218. [Google Scholar] [CrossRef]
- Chan, W.Y.; Hickey, E.E.; Page, S.W.; Trott, D.J.; Hill, P.B. Biofilm Production by Pathogens Associated with Canine Otitis Externa, and the Antibiofilm Activity of Ionophores and Antimicrobial Adjuvants. Vet. Pharm. Ther. 2019, 42, 682–692. [Google Scholar] [CrossRef]
- Thi, M.T.T.; Wibowo, D.; Rehm, B.H.A. Pseudomonas Aeruginosa Biofilms. Int. J. Mol. Sci. 2020, 21, 8671. [Google Scholar] [CrossRef] [PubMed]
- Fusconi, M.; Petrozza, V.; Taddei, A.R.; Vinciguerra, V.; De Virgilio, A.; Chiarini, F.; Cirenza, M.; Gallinelli, C.; Conte, M.; De Vincentiis, M. Is Biofilm the Cause of Chronic Otitis Externa?: Biofilm in Chronic Otitis Externa. Laryngoscope 2011, 121, 2626–2633. [Google Scholar] [CrossRef] [PubMed]
- Sundstrom, J.; Agrup, C.; Kronvall, G.; Wretlind, B. Pseudomonas Aeruginosa Adherence to External Auditory Canal Epithelium. Arch. Otolaryngol.—Head Neck Surg. 1997, 123, 1287–1292. [Google Scholar] [CrossRef]
- Ghanem, S.M.; Abd El-Baky, R.M.; Abourehab, M.A.; Fadl, G.F.; Gamil, N.G. Prevalence of Quorum Sensing and Virulence Factor Genes Among Pseudomonas Aeruginosa Isolated from Patients Suffering from Different Infections and Their Association with Antimicrobial Resistance. IDR 2023, 16, 2371–2385. [Google Scholar] [CrossRef]
- Qin, S.; Xiao, W.; Zhou, C.; Pu, Q.; Deng, X.; Lan, L.; Liang, H.; Song, X.; Wu, M. Pseudomonas aeruginosa: Pathogenesis, virulence factors, antibiotic resistance, interaction with host, technology advances and emerging therapeutics. Sig. Transduct. Target Ther. 2022, 7, 199. [Google Scholar] [CrossRef] [PubMed]
- Galdino, A.C.M.; Branquinha, M.H.; Santos, A.L.S.; Viganor, L. Pseudomonas Aeruginosa and Its Arsenal of Proteases: Weapons to Battle the Host. In Pathophysiological Aspects of Proteases; Chakraborti, S., Dhalla, N.S., Eds.; Springer: Singapore, 2017; pp. 381–397. ISBN 978-981-10-6140-0. [Google Scholar]
- Avidano, M.A.; Cotter, C.S.; Stringer, S.P.; Schultz, G.S. Analysis of Protease Activity in Human Otitis Media. Otolaryngol.—Head Neck Surg. 1998, 119, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Petermann, S.R.; Doetkott, C.; Rust, L. Elastase Deficiency Phenotype of Pseudomonas aeruginosa Canine Otitis Externa Isolates. Clin. Diagn. Lab. Immunol. 2001, 8, 632–636. [Google Scholar] [CrossRef]
- Cotter, C.S.; Avidano, M.A.; Stringer, S.P.; Schultz, G.S. Inhibition of Proteases in Pseudomonas otitis Media in Chinchillas. Otolaryngol.—Head Neck Surg. 1996, 115, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Tron, E.A.M.; Wilke, H.L.; Petermann, S.R.; Rust, L. Pseudomonas Aeruginosa from Canine Otitis Externa Exhibit a Quorum Sensing Deficiency. Vet. Microbiol. 2004, 99, 121–129. [Google Scholar] [CrossRef]
- Shoriridge, V.D.; Lazdunski, A.; Vasil, M.L. Osmoprotectants and Phosphate Regulate Expression of Phospholipase C in Pseudomonas Aeruginosa. Mol. Microbiol. 1992, 6, 863–871. [Google Scholar] [CrossRef]
- Berka, R.M.; Vasil, M.L. Phospholipase C (Heat-Labile Hemolysin) of Pseudomonas Aeruginosa: Purification and Preliminary Characterization. J. Bacteriol. 1982, 152, 239–245. [Google Scholar] [CrossRef]
- Berk, R.S.; Brown, D.; Coutinho, I.; Meyers, D. In Vivo Studies with Two Phospholipase C Fractions from Pseudomonas Aeruginosa. Infect. Immun. 1987, 55, 1728–1730. [Google Scholar] [CrossRef]
- Michalska, M.; Wolf, P. Pseudomonas Exotoxin A: Optimized by Evolution for Effective Killing. Front. Microbiol. 2015, 6, 963. [Google Scholar] [CrossRef]
- Kounnas, M.Z.; Morris, R.E.; Thompson, M.R.; FitzGerald, D.J.; Strickland, D.K.; Saelinger, C.B. The Alpha 2-Macroglobulin Receptor/Low Density Lipoprotein Receptor-Related Protein Binds and Internalizes Pseudomonas Exotoxin A. J. Biol. Chem. 1992, 267, 12420–12423. [Google Scholar] [CrossRef]
- Matar, G.M.; Ramlawi, F.; Hijazi, N.; Khneisser, I.; Abdelnoor, A.M. Transcription Levels of Pseudomonas Aeruginosa Exotoxin A Gene and Severity of Symptoms in Patients with Otitis Externa. Curr. Microbiol. 2002, 45, 350–354. [Google Scholar] [CrossRef] [PubMed]
- Lundman, L.; Harada, T.; Santi, P.A.; Juhn, S.K.; Morizono, T.; Bagger-Sjöbäck, D. Inner Ear Damage and Passage through the Round Window Membrane of Pseudomonas aeruginosa Exotoxin a in a Chinchilla Model. Ann. Otol. Rhinol. Laryngol. 1992, 101, 437–444. [Google Scholar] [CrossRef]
- Stenqvist, M.; Anniko, M.; Rask-Andersen, H. Middle Ear Mucosa Changes Following Exposure to Pseudomonas aeruginosa Exotoxin A. Eur. Arch. Oto-Rhino-Laryngol. 1999, 256, 484–490. [Google Scholar] [CrossRef]
- Stenqvist, M.; Anniko, M.; Pettersson, Å. Effect of Pseudomonas Aeruginosa Exotoxin A on Inner Ear Function. Acta Oto-Laryngol. 1997, 117, 73–79. [Google Scholar] [CrossRef]
- Horna, G.; Ruiz, J. Type 3 Secretion System of Pseudomonas Aeruginosa. Microbiol. Res. 2021, 246, 126719. [Google Scholar] [CrossRef] [PubMed]
- Feltman, H.; Schulert, G.; Khan, S.; Jain, M.; Peterson, L.; Hauser, A.R. Prevalence of Type III Secretion Genes in Clinical and Environmental Isolates of Pseudomonas Aeruginosa. Microbiology 2001, 147, 2659–2669. [Google Scholar] [CrossRef] [PubMed]
- Rutherford, V.; Yom, K.; Ozer, E.A.; Pura, O.; Hughes, A.; Murphy, K.R.; Cudzilo, L.; Mitchell, D.; Hauser, A.R. Environmental Reservoirs for ExoS+ and ExoU+ Strains of Pseudomonas Aeruginosa. Environ. Microbiol. Rep. 2018, 10, 485–492. [Google Scholar] [CrossRef]
- Park, M.-H.; Kim, S.Y.; Roh, E.Y.; Lee, H.S. Difference of Type 3 Secretion System (T3SS) Effector Gene Genotypes (ExoU and ExoS) and Its Implication to Antibiotics Resistances in Isolates of Pseudomonas Aeruginosa from Chronic Otitis Media. Auris Nasus Larynx 2017, 44, 258–265. [Google Scholar] [CrossRef]
- Cox, G.; Wright, G.D. Intrinsic Antibiotic Resistance: Mechanisms, Origins, Challenges and Solutions. Int. J. Med. Microbiol. 2013, 303, 287–292. [Google Scholar] [CrossRef]
- Guedeja-Marrón, J.; Blanco, J.L.; Ruperez, C.; Garcia, M.-E. Susceptibility of Bacterial Isolates from Chronic Canine Otitis Externa to Twenty Antibiotics. J. Vet. Med. Ser. B 1998, 45, 507–512. [Google Scholar] [CrossRef] [PubMed]
- KuKanich, K.S.; Bagladi-Swanson, M.; KuKanich, B. Pseudomonas aeruginosa Susceptibility, Antibiogram and Clinical Interpretation, and Antimicrobial Prescribing Behaviors for Dogs with Otitis in the Midwestern United States. Vet. Pharm. Ther. 2022, 45, 440–449. [Google Scholar] [CrossRef]
- Mekić, S.; Matanović, K.; Šeol, B. Antimicrobial Susceptibility of Pseudomonas aeruginosa Isolates from Dogs with Otitis Externa. Vet. Rec. 2011, 169, 125. [Google Scholar] [CrossRef] [PubMed]
- Bourély, C.; Cazeau, G.; Jarrige, N.; Leblond, A.; Madec, J.Y.; Haenni, M.; Gay, E. Antimicrobial Resistance Patterns of Bacteria Isolated from Dogs with Otitis. Epidemiol. Infect. 2019, 147, e121. [Google Scholar] [CrossRef]
- Sweeney, M.T.; Lubbers, B.V.; Schwarz, S.; Watts, J.L. Applying Definitions for Multidrug Resistance, Extensive Drug Resistance and Pandrug Resistance to Clinically Significant Livestock and Companion Animal Bacterial Pathogens. J. Antimicrob. Chemother. 2018, 73, 1460–1463. [Google Scholar] [CrossRef] [PubMed]
- WHO Publishes List of Bacteria for Which New Antibiotics Are Urgently Needed. Available online: https://www.who.int/news/item/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed (accessed on 21 September 2023).
- Nordmann, P.; Poirel, L. Epidemiology and Diagnostics of Carbapenem Resistance in Gram-Negative Bacteria. Clin. Infect. Dis. 2019, 69, S521–S528. [Google Scholar] [CrossRef] [PubMed]
- Nuttall, T.J. Use of Ticarcillin in the Management of Canine Otitis Externa Complicated by Pseudomonas aeruginosa. J. Small Anim. Pract. 1998, 39, 165–168. [Google Scholar] [CrossRef]
- Pietschmann, S.; Meyer, M.; Voget, M.; Cieslicki, M. The Joint in Vitro Action of Polymyxin B and Miconazole against Pathogens Associated with Canine Otitis Externa from Three E Uropean Countries. Vet. Dermatol. 2013, 24, 439. [Google Scholar] [CrossRef]
- Jerzsele, Á.; Pásztiné-Gere, E. Evaluating Synergy between Marbofloxacin and Gentamicin in Pseudomonas Aeruginosa Strains Isolated from Dogs with Otitis Externa. Acta Microbiol. Immunol. Hung. 2015, 62, 45–55. [Google Scholar] [CrossRef]
- Wooley, R.E.; Jones, M.S. Action of EDTA-Tris and Antimicrobial Agent Combinations on Selected Pathogenic Bacteria. Vet. Microbiol. 1983, 8, 271–280. [Google Scholar] [CrossRef]
- Sparks, T.A.; Kemp, D.T.; Wooley, R.E.; Gibbs, P.S. Antimicrobial Effect of Combinations of EDTA-Tris and Amikacin or Neomycin on the Microorganisms Associated with Otitis Externa in Dogs. Vet. Res. Commun. 1994, 18, 241–249. [Google Scholar] [CrossRef]
- Wooley, R.E.; Jones, M.S.; Gilbert, J.P.; Shotts, E.B. In Vitro Action of Combinations of Antimicrobial Agents and EDTA-Tromethamine on Pseudomonas Aeruginosa. Am. J. Vet. Res. 1983, 44, 1521–1524. [Google Scholar]
- Buckley, L.M.; McEwan, N.A.; Nuttall, T. Tris-EDTA Significantly Enhances Antibiotic Efficacy against Multidrug-Resistant Pseudomonas Aeruginosa in Vitro. Vet. Dermatol. 2013, 24, 519-e122. [Google Scholar] [CrossRef]
- GBADAMOSIS, S.; GOTTHELF, L. Abstracts from the American Academy of Veterinary Dermatology and American College of Veterinary Dermatology Annual Meeting. Monterey, CA, 9–13 April 2003. Vet. Dermatol. 2003, 14, 210–236. [Google Scholar] [CrossRef]
- May, E.R.; Ratliff, B.E.; Bemis, D.A. Antibacterial Effect of N-acetylcysteine in Combination with Antimicrobials on Common Canine Otitis Externa Bacterial Isolates. Vet. Dermatol. 2019, 30, 531. [Google Scholar] [CrossRef]
- Chan, W.Y.; Khazandi, M.; Hickey, E.E.; Page, S.W.; Trott, D.J.; Hill, P.B. In Vitro Antimicrobial Activity of Seven Adjuvants against Common Pathogens Associated with Canine Otitis Externa. Vet. Dermatol. 2019, 30, 133. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.Y.; Hickey, E.E.; Khazandi, M.; Page, S.W.; Trott, D.J.; Hill, P.B. In Vitro Antimicrobial Activity of Narasin and Monensin in Combination with Adjuvants against Pathogens Associated with Canine Otitis Externa. Vet. Dermatol. 2020, 31, 138. [Google Scholar] [CrossRef]
- Chan, W.Y.; Hickey, E.E.; Khazandi, M.; Page, S.W.; Trott, D.J.; Hill, P.B. In Vitro Antimicrobial Activity of Narasin against Common Clinical Isolates Associated with Canine Otitis Externa. Vet. Dermatol. 2018, 29, 149. [Google Scholar] [CrossRef] [PubMed]
- Trott, D.; Moss, S.; See, A.; Rees, R. Evaluation of Disc Diffusion and MIC Testing for Determining Susceptibility of Pseudomonas Aeruginosa Isolates to Topical Enrofloxacin/Silver Sulfadiazine. Aust. Vet. J. 2007, 85, 464–466. [Google Scholar] [CrossRef]
- Von Silva-Tarouca, M.S.E.; Wolf, G.; Mueller, R.S. Determination of Minimum Inhibitory Concentrations for Silver Sulfadiazine and Other Topical Antimicrobial Agents against Strains of Pseudomonas aeruginosa Isolated from Canine Otitis Externa. Vet. Dermatol. 2019, 30, 145. [Google Scholar] [CrossRef] [PubMed]
- Sim, J.X.F.; Khazandi, M.; Pi, H.; Venter, H.; Trott, D.J.; Deo, P. Antimicrobial Effects of Cinnamon Essential Oil and Cinnamaldehyde Combined with EDTA against Canine Otitis Externa Pathogens. J. Appl. Microbiol. 2019, 127, 99–108. [Google Scholar] [CrossRef]
- Sim, J.X.F.; Khazandi, M.; Chan, W.Y.; Trott, D.J.; Deo, P. Antimicrobial Activity of Thyme Oil, Oregano Oil, Thymol and Carvacrol against Sensitive and Resistant Microbial Isolates from Dogs with Otitis Externa. Vet. Dermatol. 2019, 30, 524. [Google Scholar] [CrossRef]
- Ebani, V.; Nardoni, S.; Bertelloni, F.; Najar, B.; Pistelli, L.; Mancianti, F. Antibacterial and Antifungal Activity of Essential Oils against Pathogens Responsible for Otitis Externa in Dogs and Cats. Medicines 2017, 4, 21. [Google Scholar] [CrossRef]
- Moulari, B.; Pellequer, Y.; Chaumont, J.; Guillaume, Y.; Millet, J. In Vitro Antimicrobial Activity of the Leaf Extract of Harungana Madagascariensis Lam. Ex Poir. (Hypericaceae) against Strains Causing Otitis Externa in Dogs and Cats. Acta Vet. Hung. 2007, 55, 97–105. [Google Scholar] [CrossRef]
- Song, S.; Hyun, J.; Kang, J.-H.; Hwang, C. In Vitro Antibacterial Activity of the Manuka Essential Oil from Leptospermum scoparium Combined with Tris-EDTA against Gram-negative Bacterial Isolates from Dogs with Otitis Externa. Vet. Dermatol. 2020, 31, 81. [Google Scholar] [CrossRef] [PubMed]
- Vercelli, C.; Pasquetti, M.; Giovannetti, G.; Visioni, S.; Re, G.; Giorgi, M.; Gambino, G.; Peano, A. In Vitro and in Vivo Evaluation of a New Phytotherapic Blend to Treat Acute Externa Otitis in Dogs. Vet. Pharm. Ther. 2021, 44, 910–918. [Google Scholar] [CrossRef]
- Seeger, M.G.; Ries, A.S.; Gressler, L.T.; Botton, S.A.; Iglesias, B.A.; Cargnelutti, J.F. In Vitro Antimicrobial Photodynamic Therapy Using Tetra-Cationic Porphyrins against Multidrug-Resistant Bacteria Isolated from Canine Otitis. Photodiagnosis Photodyn. Ther. 2020, 32, 101982. [Google Scholar] [CrossRef]
- Machado, C.S.; Seeger, M.G.; Moreira, K.S.; Burgo, T.A.L.; Iglesias, B.A.; Vogel, F.S.F.; Cargnelutti, J.F. In Vitro Porphyrin-Based Photodynamic Therapy against Mono and Polyculture of Multidrug-Resistant Bacteria Isolated from Integumentary Infections in Animals. Photodiagnosis Photodyn. Ther. 2022, 40, 103179. [Google Scholar] [CrossRef] [PubMed]
- Sellera, F.P.; Fernandes, M.R.; Sabino, C.P.; De Freitas, L.M.; Da Silva, L.C.B.A.; Pogliani, F.C.; Ribeiro, M.S.; Hamblin, M.R.; Lincopan, N. Effective Treatment and Decolonization of a Dog Infected with Carbapenemase (VIM-2)-producing Pseudomonas Aeruginosa Using Probiotic and Photodynamic Therapies. Vet. Dermatol. 2019, 30, 170. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Hwang, C.; Kang, J.; Baek, S.; Hyun, J. In Vitro Antimicrobial Activity of Cold Atmospheric Microwave Plasma against Bacteria Causing Canine Skin and Ear Infections. Vet. Dermatol. 2021, 32, 462. [Google Scholar] [CrossRef]
- Wright, A.; Hawkins, C.H.; Änggård, E.E.; Harper, D.R. A Controlled Clinical Trial of a Therapeutic Bacteriophage Preparation in Chronic Otitis Due to Antibiotic-Resistant Pseudomonas aeruginosa; a Preliminary Report of Efficacy. Clin. Otolaryngol. 2009, 34, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, C.; Harper, D.; Burch, D.; Änggård, E.; Soothill, J. Topical Treatment of Pseudomonas Aeruginosa Otitis of Dogs with a Bacteriophage Mixture: A before/after Clinical Trial. Vet. Microbiol. 2010, 146, 309–313. [Google Scholar] [CrossRef] [PubMed]
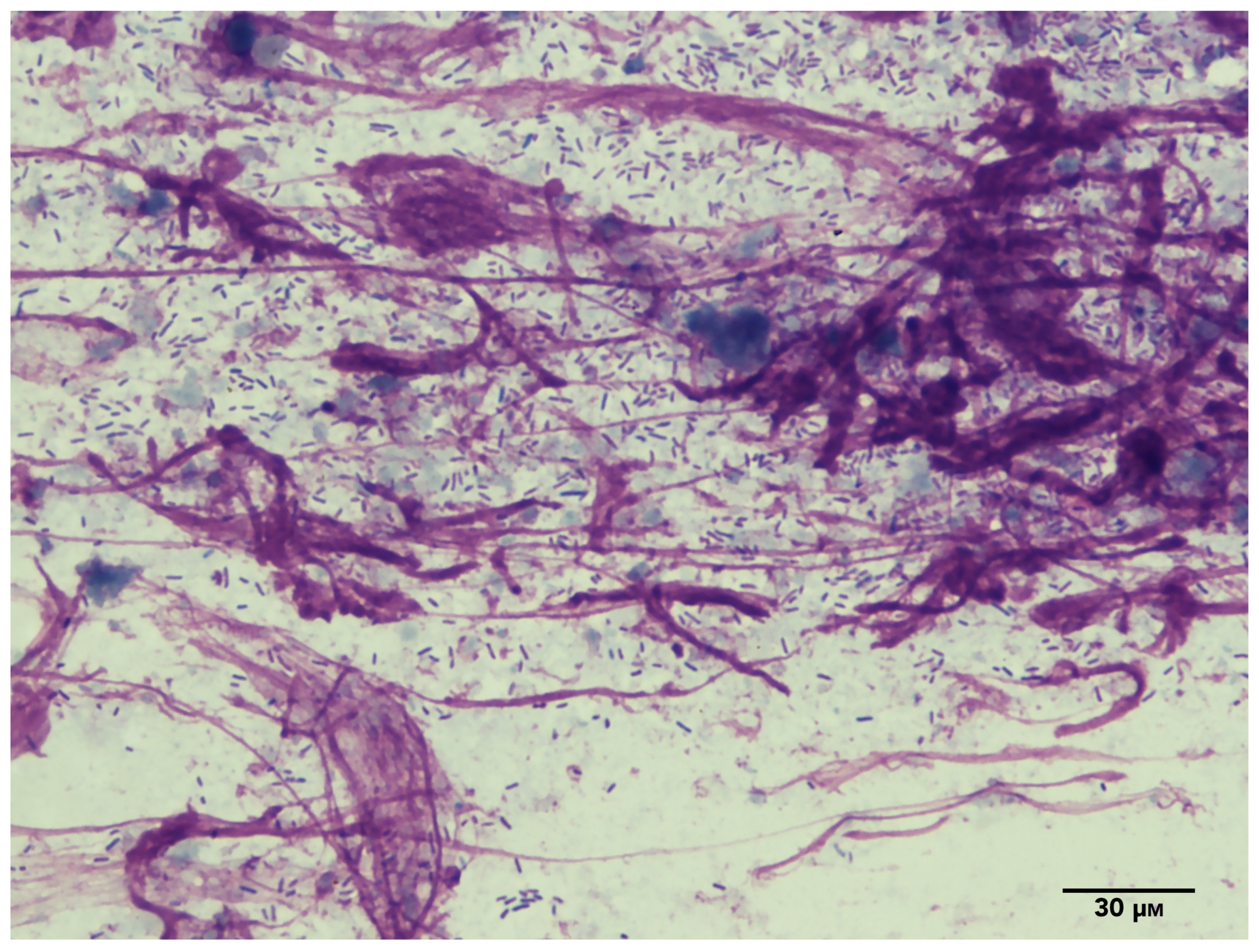
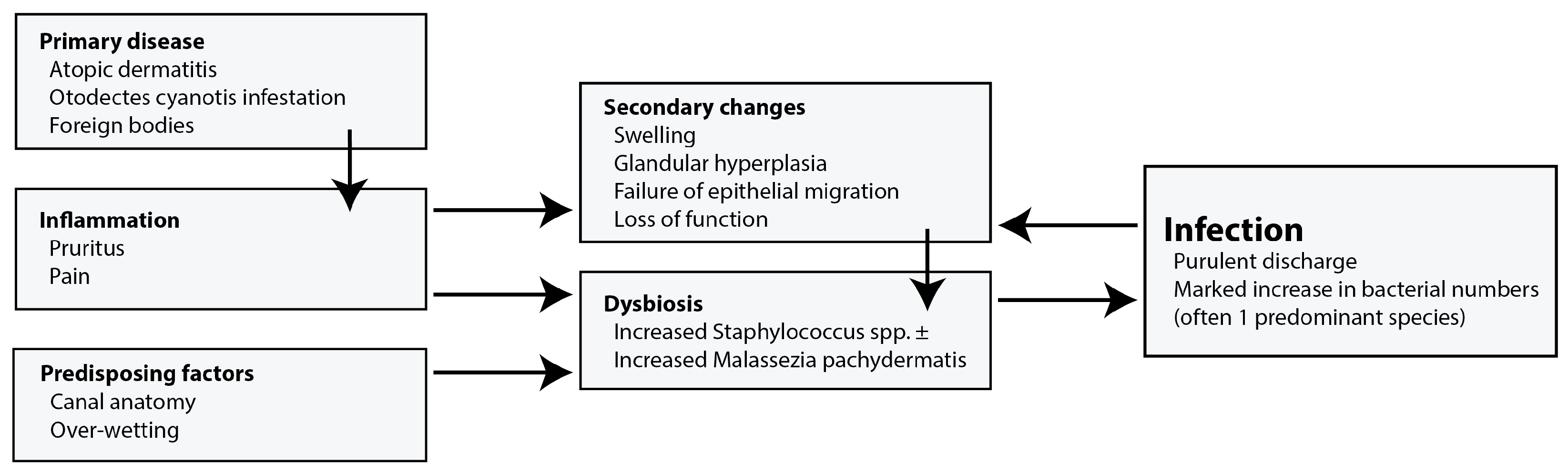
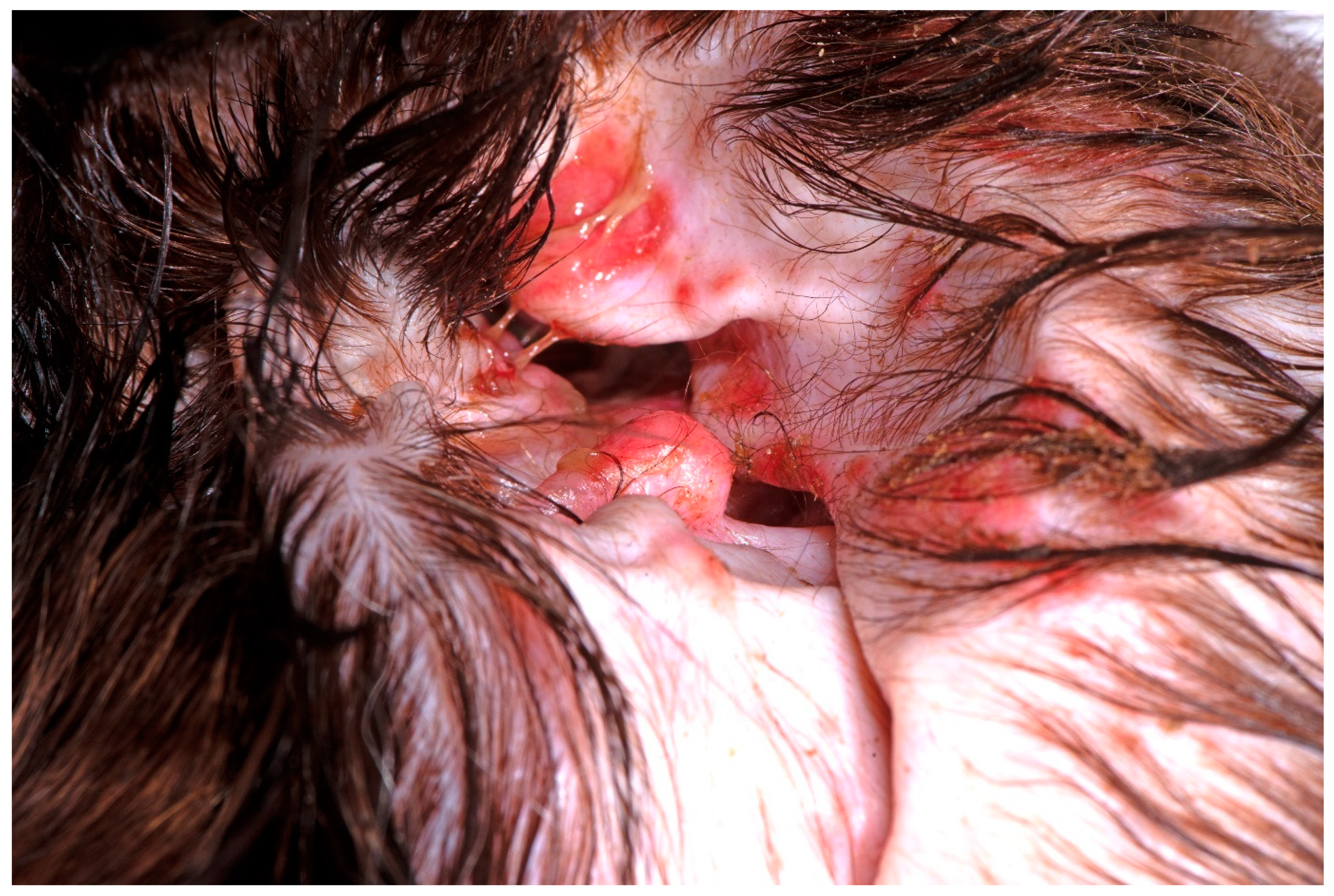
| Genus | Species | Prevalence Using Traditional Culture | Prevalence Using Metagenomics |
|---|---|---|---|
| Staphylococcus | S. intermedius, coagulase-positive Staphylococci, S. pseudintermedius, S. schleiferi, S. schleiferi spp. Coagulans, coagulase-negative Staphylococci | 58.5% + 6.2% [24]; 36.83% + 2.97% [46]; 24.30% [47] | 22% [29]; 11.25% + 8.54% [27] |
| Pseudomonas | P. aeruginosa | 35.5% [47]; 16.24% [46]; 7.2% [24] | 18.6% [29]; 5.83% [27] |
| Malassezia | M. pachydermatis | 30.9% [24]; 30.01% [46] | 8.75% [27] |
| Streptococcus | S. canis, β-haemolytic Streptococci, non-haemolytic Streptococci, S. halichoeri, S. agalactiae | 29.9% + 4.1% [24]; 6.2% [47]; 2.97% [46] | 5.42% + 3.80% + 0.83% [27]; 2.2% [29] |
| Proteus | P. spp., P. mirabilis | 14.4% [24]; 6.8% [47]; 3.56% [46] | 5.60% [29]; 2.29% [27] |
| Escherichia | E. coli | 10.30% [24]; 4.2% [47]; 3.17% [46] | |
| Corynebacterium | C. auriscanis, C. freneyi, C. spp. | 0.79% [46] | 7.08% + 4.38% [27]; 5.4% [29] |
| Finegoldia | F. magna | 5.83% [27] | |
| Peptostreptococcus | P. canis | 5.52% [27] | |
| Lactobacillus | 5.5% [29] | ||
| Enterococcus | E. faecium, E. faecalis | 5.2% [24] | 2.30% [29]; 1.04% [27] |
| Enterobacteriaceae (Unknown genus) | 4.9% [29] | ||
| Porphyrimonas | P. cangingivalis | 4.5% [29]; 4.38% [27] | |
| Arcanobacterium | A. canis | 4% [27] | |
| Peptoniphilus | P. harei | 2.29% [27] | |
| Candida | C. spp. | 2.38% [46] | |
| Bacillus | B. spp. | 0.99% [46] | |
| 14 Others | 2.08–0.63% [27] |
| Steroid | Antibiotic | Antifungal |
|---|---|---|
| Dexamethasone (as acetate) 0.9 mg/mL | Marbofloxacin 3 mg/mL | Clotrimazole 10 mg/mL |
| Prednisolone 2.5 mg/mL | Diethanolamine fusidate 5.0 mg/mL Framycetin sulphate 5.0 mg/mL | Nystatin 100,000 iu/mL |
| Hydrocortisone aceponate 1.11 mg/mL | Gentamicin sulfate 1505 iu/mL | Miconazole nitrate 15.1 mg/mL |
| Betamethasone valerate 0.88 mg/mL | Gentamicin sulfate 2640 iu/mL | Clotrimazole 8.80 mg/mL |
| Mometasone furoate 0.9 mg/mL | Orbifloxacin 8.5 mg/mL | Posaconazole 0.9 mg/mL |
| Prednisolone acetate 5 mg/mL | Polymyxin B sulfate 0.5293 mg/mL | Miconazole nitrate 23 mg/mL |
| Mometasone furoate 2.2 mg/dose | Florfenicol 16.7 mg/dose | Terbinafine 14.9 mg/dose |
| Betamethasone acetate 1 mg/dose | Florfenicol 10 mg/dose | Terbinifine 10 mg/dose |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Secker, B.; Shaw, S.; Atterbury, R.J. Pseudomonas spp. in Canine Otitis Externa. Microorganisms 2023, 11, 2650. https://doi.org/10.3390/microorganisms11112650
Secker B, Shaw S, Atterbury RJ. Pseudomonas spp. in Canine Otitis Externa. Microorganisms. 2023; 11(11):2650. https://doi.org/10.3390/microorganisms11112650
Chicago/Turabian StyleSecker, Bailey, Stephen Shaw, and Robert J. Atterbury. 2023. "Pseudomonas spp. in Canine Otitis Externa" Microorganisms 11, no. 11: 2650. https://doi.org/10.3390/microorganisms11112650
APA StyleSecker, B., Shaw, S., & Atterbury, R. J. (2023). Pseudomonas spp. in Canine Otitis Externa. Microorganisms, 11(11), 2650. https://doi.org/10.3390/microorganisms11112650






