Haloalkalitolerant Fungi from Sediments of the Big Tambukan Saline Lake (Northern Caucasus): Diversity and Antimicrobial Potential
Abstract
1. Introduction
2. Materials and Methods
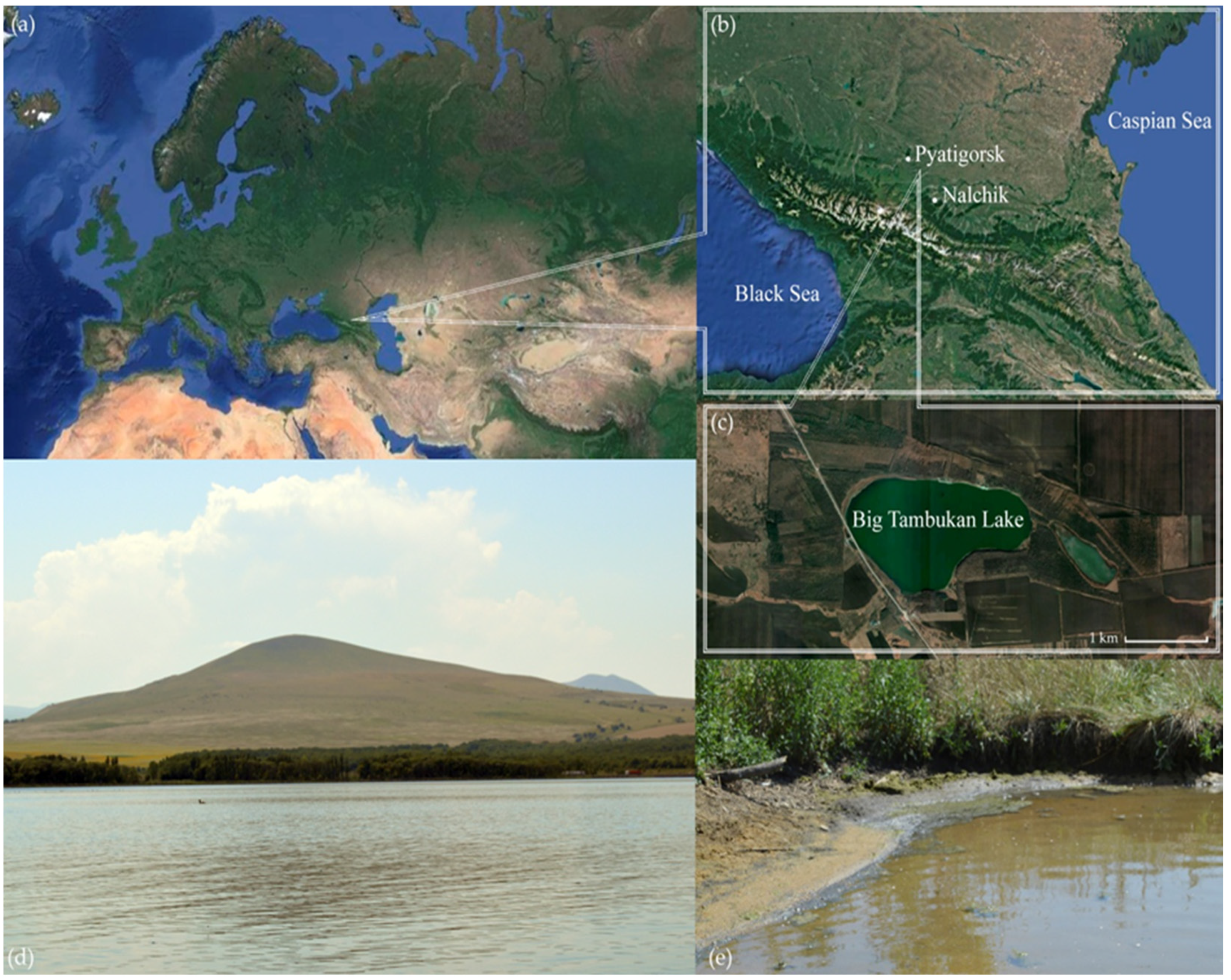
2.1. Samples
2.2. Culture Media
2.3. Isolation Techniques
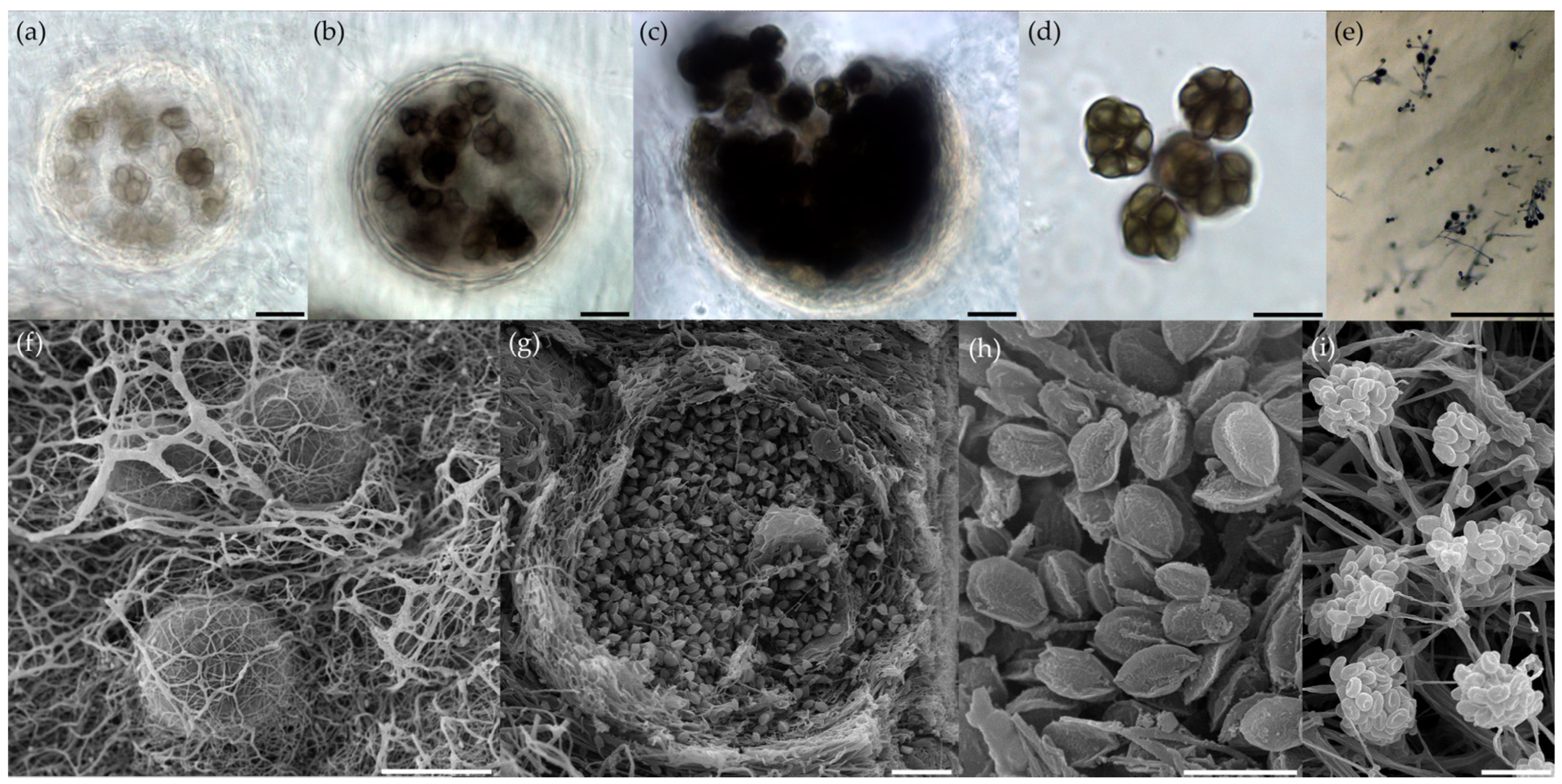
2.4. Morphological Identification of Fungal Isolates
2.5. Molecular Identification of Fungal Isolates
2.6. Phylogenetic Analysis
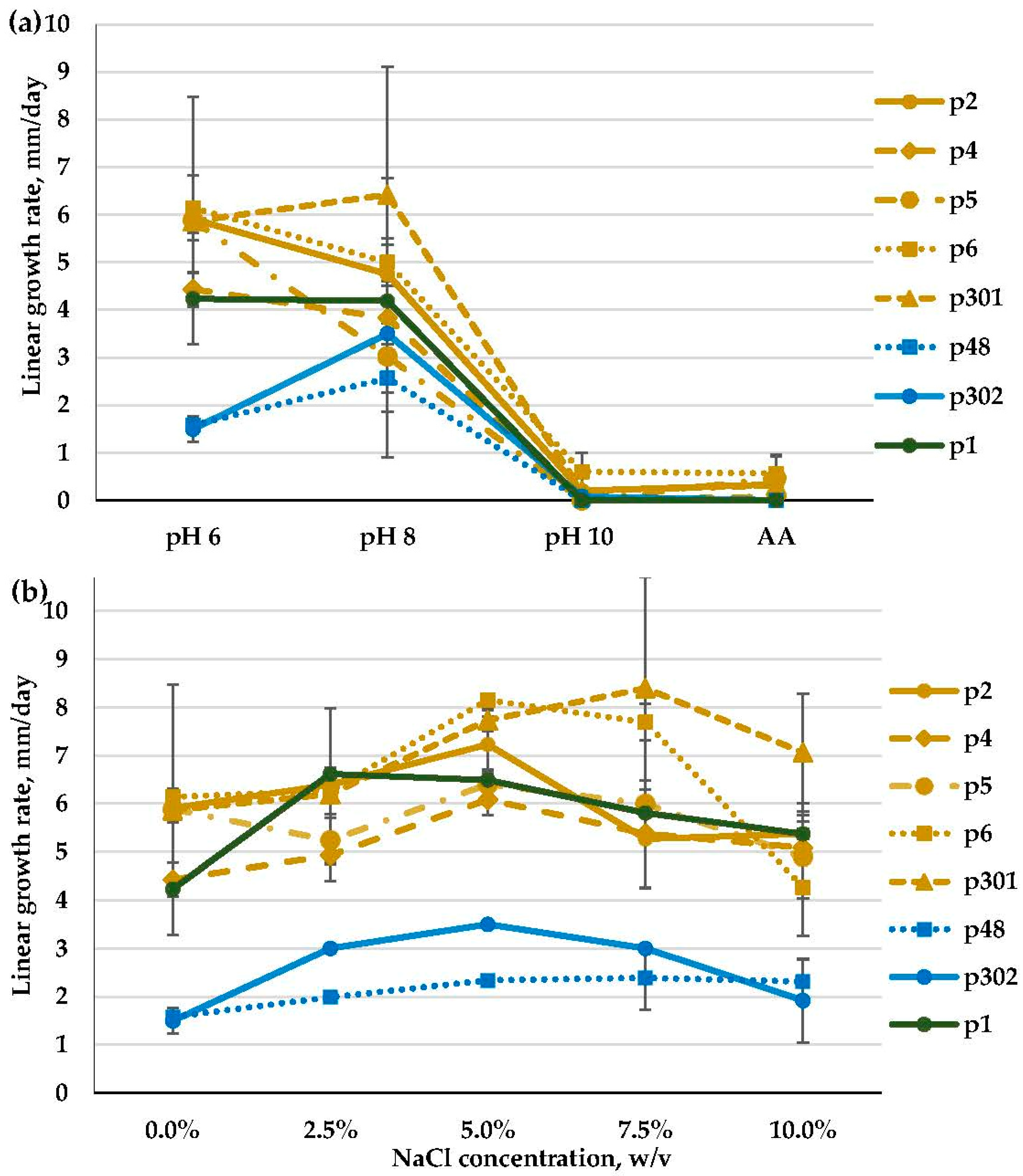
2.7. Growth at Different pH Values and Salt Concentrations
2.8. Antimicrobial Assays
3. Results
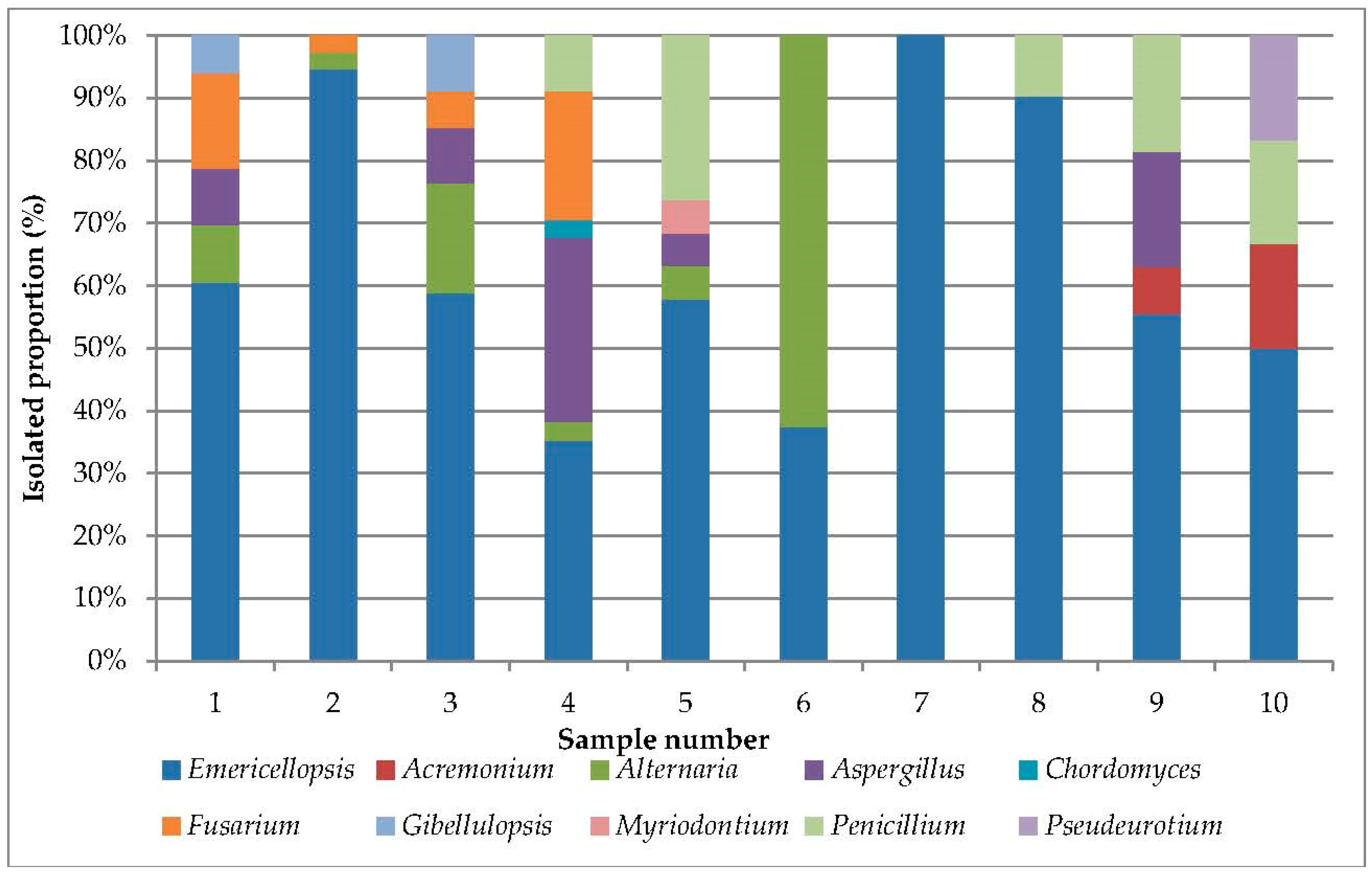
3.1. Analysis of Cultivated Haloalkalitolerant Fungi
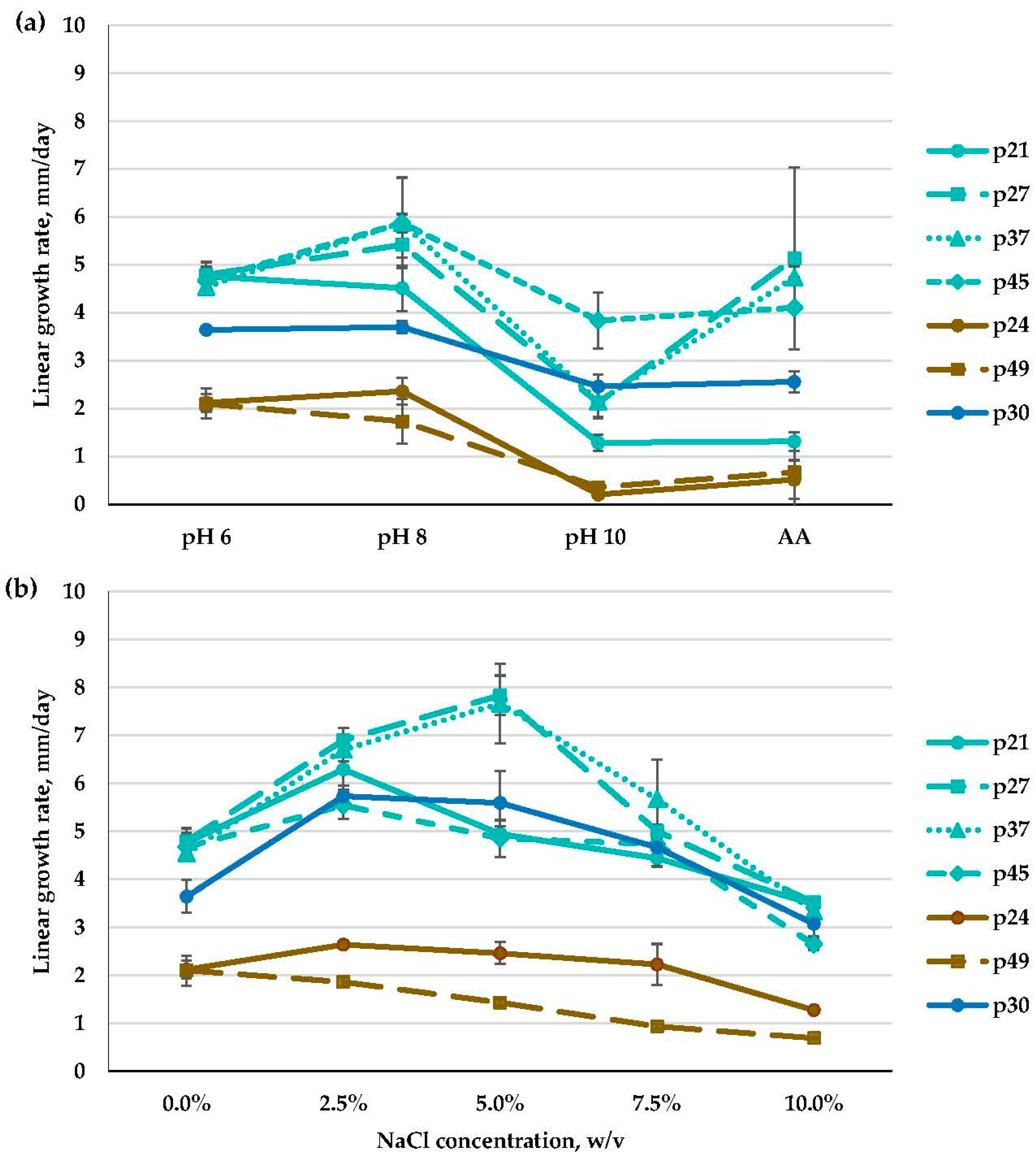
3.2. Molecular Identification, Phylogenetic Analyses, and Salt and Alkaline Preferences of the Obtained Isolates
3.2.1. Emericellopsis spp. (Bionectriaceae, Hypocreales)
3.2.2. Alternaria alternata (Pleosporaceae, Pleosporales)
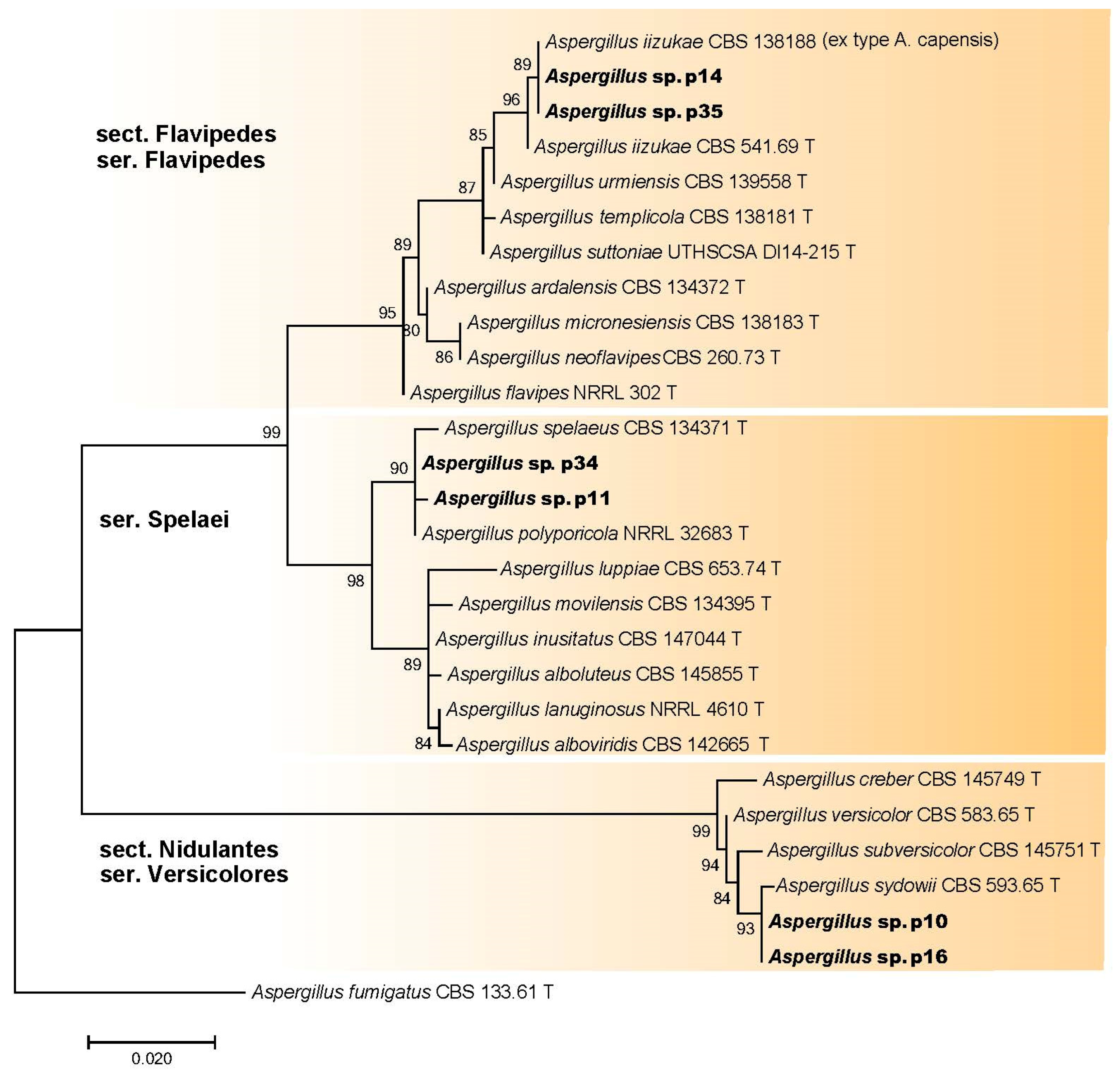
3.2.3. Aspergillus spp. (Aspergillaceae, Eurotiales)
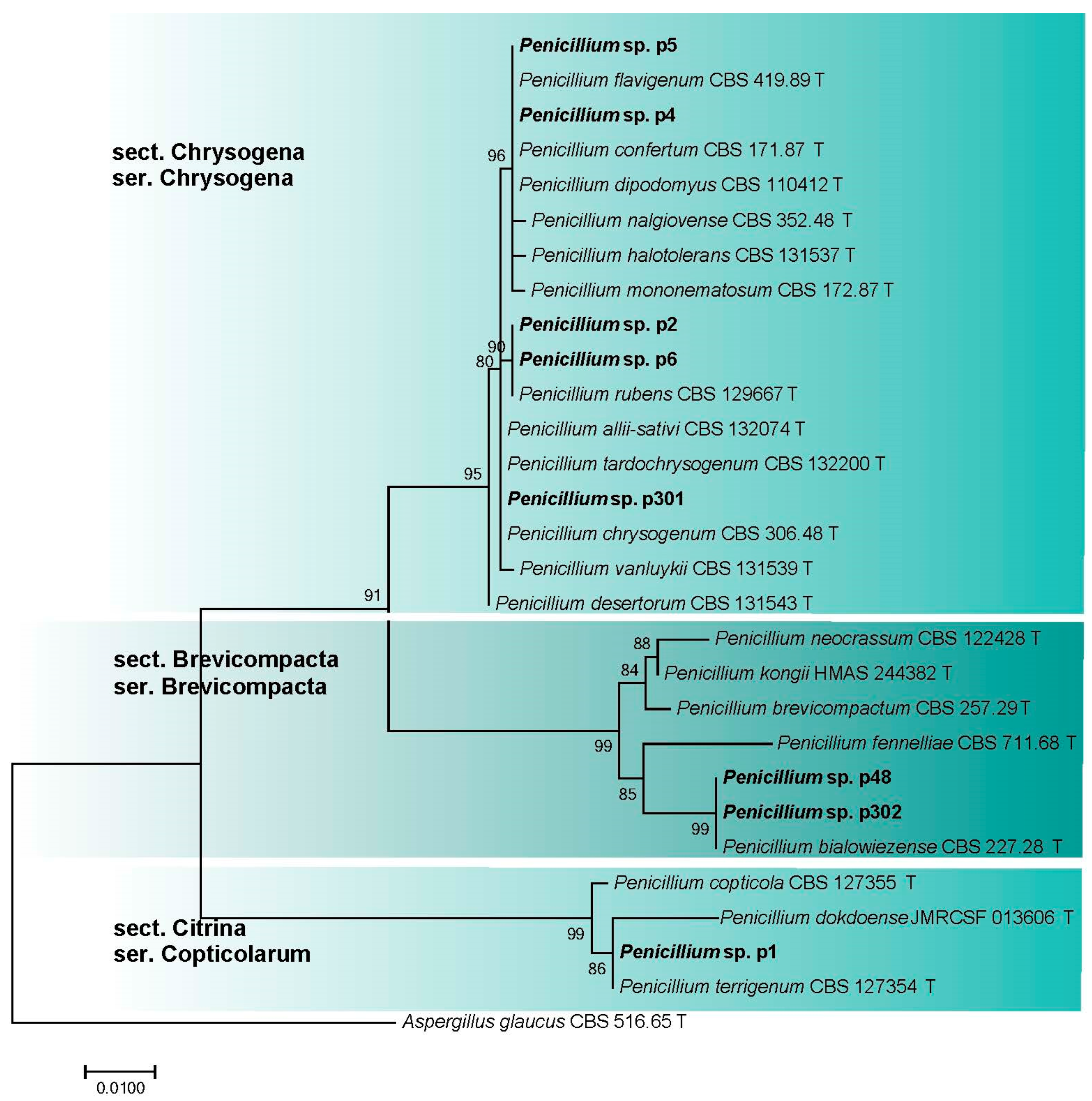
3.2.4. Penicillium spp. (Aspergillaceae, Eurotiales)
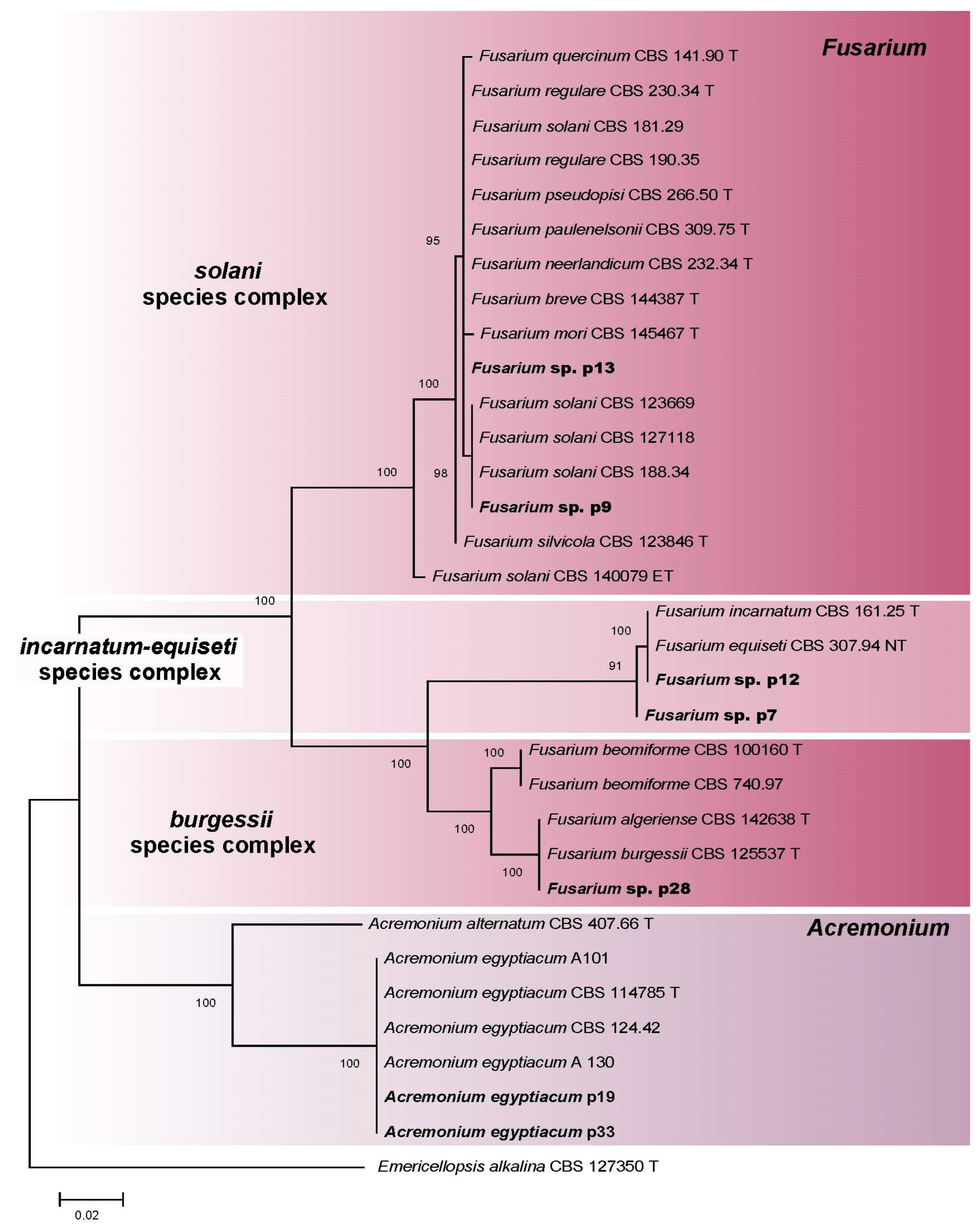
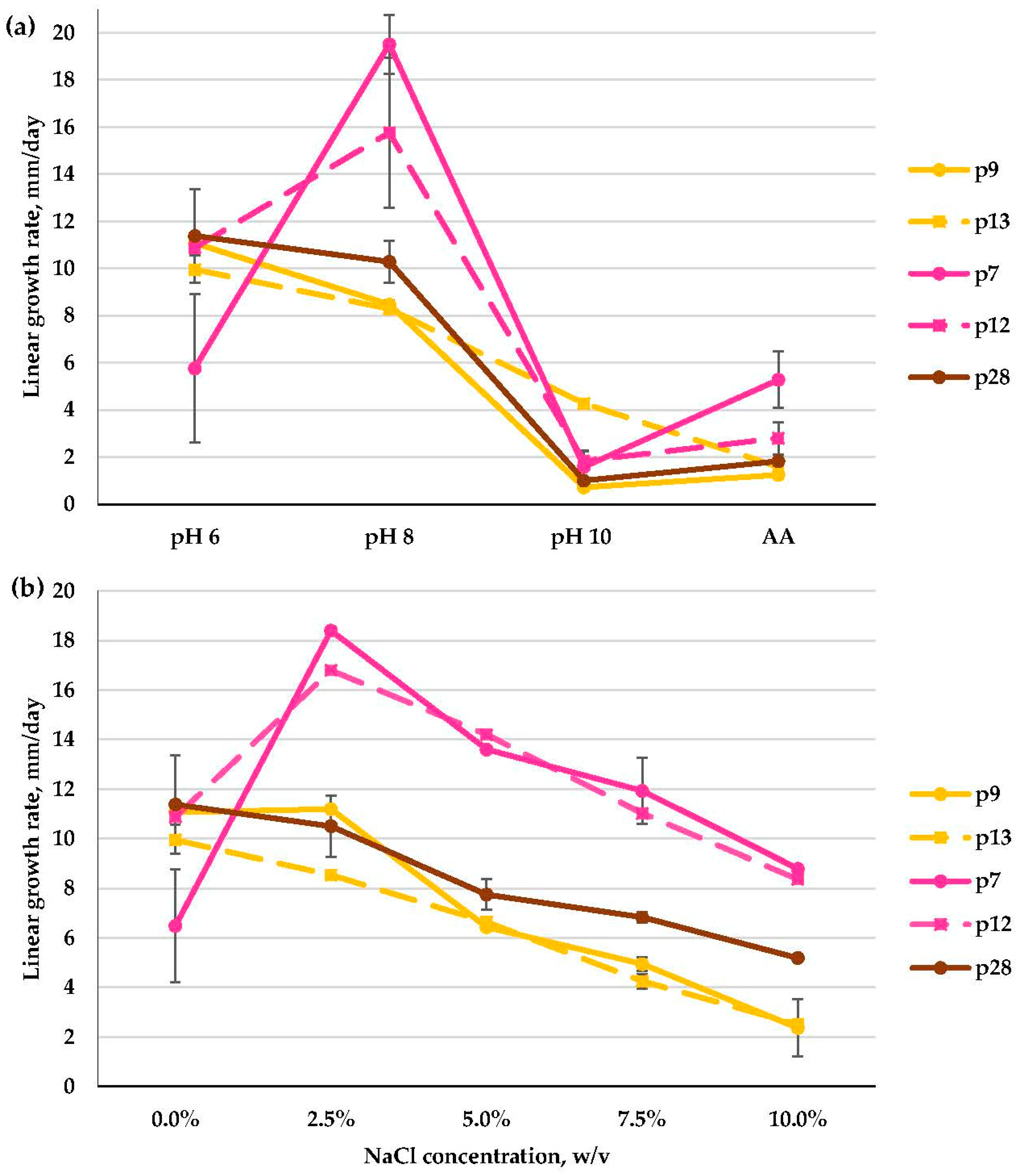
3.2.5. Fusarium spp. and Acremonium spp. (Hypocreales)
3.2.6. Low-Frequency Isolates from Sediments of Big Tambukan Lake
3.3. Antimicrobial Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gunde-Cimerman, N.; Oren, A.; Plemenitaš, A. Adaptation to Life at High Salt Concentrations in Archaea, Bacteria, and Eukarya; Springer: Dordrecht, Germany, 2005; 576p. [Google Scholar] [CrossRef]
- Mesbah, N.M.; Wiegel, J. Life under Multiple Extreme Conditions: Diversity and Physiology of the Halophilic Alkalithermophiles. Appl. Environ. Microbiol. 2012, 78, 4074–4082. [Google Scholar] [CrossRef] [PubMed]
- Gunde-Cimerman, N.; Plemenitaš, A.; Oren, A. Strategies of Adaptation of Microorganisms of the Three Domains of Life to High Salt Concentrations. FEMS Microbiol. Rev. 2018, 42, 353–375. [Google Scholar] [CrossRef]
- Samylina, O.S.; Namsaraev, Z.B.; Grouzdev, D.S.; Slobodova, N.V.; Zelenev, V.V.; Borisenko, G.V.; Sorokin, D.Y. The Patterns of Nitrogen Fixation in Haloalkaliphilic Phototrophic Communities of Kulunda Steppe Soda Lakes (Altai, Russia). FEMS Microbiol. Ecol. 2019, 95, fiz174. [Google Scholar] [CrossRef] [PubMed]
- Zgonik, V.; Mulec, J.; Eleršek, T.; Ogrinc, N.; Jamnik, P.; Ulrih, N.P. Extremophilic Microorganisms in Central Europe. Microorganisms 2021, 9, 2326. [Google Scholar] [CrossRef] [PubMed]
- Das, T.; Al-Tawaha, A.R.; Pandey, D.K.; Nongdam, P.; Shekhawat, M.S.; Dey, A.; Choudhary, K.; Sahay, S. Halophilic, Acidophilic, Alkaliphilic, Metallophilic, and Radioresistant Fungi: Habitats and Their Living Strategies. In Extremophilic Fungi: Ecology, Physiology and Applications; Sahay, S., Ed.; Springer Nature: Singapore, 2022; pp. 171–193. [Google Scholar]
- Cantrell, S.A.; Tkavc, R.; Gunde-Cimerman, N.; Zalar, P.; Acevedo, M.; Báez-Félix, C. Fungal Communities of Young and Mature Hypersaline Microbial Mats. Mycologia 2013, 105, 827–836. [Google Scholar] [CrossRef]
- Carreira, C.; Lønborg, C.; Kühl, M.; Lillebø, A.I.; Sandaa, R.-A.; Villanueva, L.; Cruz, S. Fungi and Viruses as Important Players in Microbial Mats. FEMS Microbiol. Ecol. 2020, 96, fiaa187. [Google Scholar] [CrossRef]
- Coleine, C.; Stajich, J.E.; Selbmann, L. Fungi Are Key Players in Extreme Ecosystems. Trends Ecol. Evol. 2022, 37, 517–528. [Google Scholar] [CrossRef]
- Orwa, P.; Mugambi, G.; Wekesa, V.; Mwirichia, R. Isolation of Haloalkaliphilic Fungi from Lake Magadi in Kenya. Heliyon 2020, 6, e02823. [Google Scholar] [CrossRef]
- Andrei, A.-Ş.; Baricz, A.; Robeson, M.S.; Păuşan, M.R.; Tămaş, T.; Chiriac, C.; Szekeres, E.; Barbu-Tudoran, L.; Levei, E.A.; Coman, C.; et al. Hypersaline Sapropels Act as Hotspots for Microbial Dark Matter. Sci. Rep. 2017, 7, 6150. [Google Scholar] [CrossRef]
- Heo, Y.M.; Lee, H.; Kim, K.; Kwon, S.L.; Park, M.Y.; Kang, J.E.; Kim, G.-H.; Kim, B.S.; Kim, J.-J. Fungal Diversity in Intertidal Mudflats and Abandoned Solar Salterns as a Source for Biological Resources. Mar. Drugs 2019, 17, 601. [Google Scholar] [CrossRef]
- Martínez, G.M.; Pire, C.; Martínez-Espinosa, R.M. Hypersaline Environments as Natural Sources of Microbes with Potential Applications in Biotechnology: The Case of Solar Evaporation Systems to Produce Salt in Alicante County (Spain). Curr. Res. Microb. Sci. 2022, 3, 100136. [Google Scholar] [CrossRef]
- Fernández-López, M.G.; Batista-García, R.A.; Aréchiga-Carvajal, E.T. Alkaliphilic/Alkali-Tolerant Fungi: Molecular, Biochemical, and Biotechnological Aspects. J. Fungi 2023, 9, 652. [Google Scholar] [CrossRef] [PubMed]
- Oren, A. Diversity of Halophilic Microorganisms: Environments, Phylogeny, Physiology, and Applications. J. Ind. Microbiol. Biotechnol. 2002, 28, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Grum-Grzhimaylo, A.A.; Georgieva, M.L.; Bondarenko, S.A.; Debets, A.J.M.; Bilanenko, E.N. On the Diversity of Fungi from Soda Soils. Fungal Divers. 2016, 76, 27–74. [Google Scholar] [CrossRef]
- Kevbrin, V.V. Isolation and Cultivation of Alkaliphiles. In Alkaliphiles in Biotechnology. Advances in Biochemical Engineering/Biotechnology; Mamo, G., Mattiasson, B., Eds.; Springer International Publishing: Cham, Switzerland, 2019; Volume 172, pp. 53–84. [Google Scholar] [CrossRef]
- Gostinčar, C.; Stajich, J.E.; Kejžar, A.; Sinha, S.; Nislow, C.; Lenassi, M.; Gunde-Cimerman, N. Seven Years at High Salinity—Experimental Evolution of the Extremely Halotolerant Black Yeast Hortaea werneckii. J. Fungi 2021, 7, 723. [Google Scholar] [CrossRef] [PubMed]
- Bondarenko, S.A.; Georgieva, M.L.; Bilanenko, E.N. Alkalitolerant Micromycetes in Acidic and Neutral Soils of the Temperate Zone. Microbiology 2016, 85, 737–744. [Google Scholar] [CrossRef]
- Ayva, F.; Demirel, R.; İlhan, S.; Kyzy, L.U.; Çiğdem, U.; Zorluer, N.C.; İrdem, E.; Özbiçen, E.; Ocak, E.; Tunca, G. Haloalkalitolerant and Haloalkaliphilic Fungal Diversity of Acıgöl/Turkey. Mantar Derg. 2021, 12, 33–41. [Google Scholar]
- Bondarenko, S.A.; Georgieva, M.L.; Kokaeva, L.Y.; Bilanenko, E.N. The First Discovery of Alkali-Resistant Fungi on the Coast of Chloride Lake Baskunchak. Mosc. Univ. Biol. Sci. Bull. 2019, 74, 57–62. [Google Scholar] [CrossRef]
- Mamo, G. Challenges and Adaptations of Life in Alkaline Habitats. In Alkaliphiles in Biotechnology. Advances in Biochemical Engineering/Biotechnology; Mamo, G., Mattiasson, B., Eds.; Springer International Publishing: Cham, Switzerland, 2020; Volume 172, pp. 85–133. [Google Scholar] [CrossRef]
- Gostinčar, C.; Lenassi, M.; Gunde-Cimerman, N.; Plemenitaš, A. Fungal Adaptation to Extremely High Salt Concentrations. In Advances in Applied Microbiology; Laskin, A.I., Sariaslani, S., Gadd, G.M., Eds.; Academic Press: Burlington, VT, USA, 2011; Volume 77, pp. 71–96. [Google Scholar] [CrossRef]
- Kuvarina, A.E.; Gavryushina, I.A.; Kulko, A.B.; Ivanov, I.A.; Rogozhin, E.A.; Georgieva, M.L.; Sadykova, V.S. The Emericellipsins A–E from an Alkalophilic Fungus Emericellopsis alkalina Show Potent Activity against Multidrug-Resistant Pathogenic Fungi. J. Fungi 2021, 7, 153. [Google Scholar] [CrossRef] [PubMed]
- Kuvarina, A.E.; Rogozhin, E.A.; Sykonnikov, M.A.; Timofeeva, A.V.; Serebryakova, M.V.; Fedorova, N.V.; Kokaeva, L.Y.; Efimenko, T.A.; Georgieva, M.L.; Sadykova, V.S. Isolation and Characterization of a Novel Hydrophobin, Sa-HFB1, with Antifungal Activity from an Alkaliphilic Fungus, Sodiomyces alkalinus. J. Fungi 2022, 8, 659. [Google Scholar] [CrossRef]
- Kazankin, A.P.; Florinskiy, O.S. Hydrological Regime of Lake Bol’shoi Tambukan. Water Resour. 2007, 34, 132–139. [Google Scholar] [CrossRef]
- Efimenko, N.V.; Romanova, E.V.; Kochieva, D.P.; Lobzhanidze, P.B.; Karagulov, H.G. Balneohomeopathic Drugs Based on the Silt Sulphide Mud of Big Tambukan Lake. Meditsinskiy Vestn. Yuga Ross. 2012, 70–73. [Google Scholar]
- Fedorov, Y.A. Hydrological and hydrochemical study of the sulphide lake Bolshoy Tambukan. Izv. Vuzov. North Cauc. Region. Nat. Sci. 2013, 81–88. [Google Scholar]
- Bondareva, G.L.; Derkacheva, M.G. Formation conditions, the current state and action for preservation of the field of therapeutic mud of the Lake Big Tambukan. Razved. I Okhrana Nedr. 2017, 51–56. [Google Scholar]
- Fedorov, Y.A.; Ruban, D.A. Peloids as Important Resource for Regional Sustainable Development: Conceptual Considerations. Rev. Espac. 2018, 39, 21. [Google Scholar]
- Gomes, C.; Carretero, M.I.; Pozo, M.; Maraver, F.; Cantista, P.; Armijo, F.; Legido, J.L.; Teixeira, F.; Rautureau, M.; Delgado, R. Peloids and Pelotherapy: Historical Evolution, Classification and Glossary. Appl. Clay Sci. 2013, 75–76, 28–38. [Google Scholar] [CrossRef]
- Carbajo, J.M.; Maraver, F. Sulphurous Mineral Waters: New Applications for Health. Evid. Based Complement. Alternat. Med. 2017, 2017, e8034084. [Google Scholar] [CrossRef]
- Gálvez, I.; Torres-Piles, S.; Ortega-Rincón, E. Balneotherapy, Immune System, and Stress Response: A Hormetic Strategy? Int. J. Mol. Sci. 2018, 19, 1687. [Google Scholar] [CrossRef]
- Maraver, F.; Armijo, F.; Fernandez-Toran, M.A.; Armijo, O.; Ejeda, J.M.; Vazquez, I.; Corvillo, I.; Torres-Piles, S. Peloids as Thermotherapeutic Agents. Int. J. Environ. Res. Public Health 2021, 18, 1965. [Google Scholar] [CrossRef]
- Mourelle, M.L.; Gómez, C.P.; Legido, J.L. Microalgal Peloids for Cosmetic and Wellness Uses. Mar. Drugs 2021, 19, 666. [Google Scholar] [CrossRef]
- Malkhazova, S.; Orlov, D.; Shartova, N.; Starikov, S.; Puzanova, T. Mud Springs (Peloids). In Healing Springs of Russia; Malkhazova, S., Orlov, D., Shartova, N., Starikov, S., Puzanova, T., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 71–80. [Google Scholar] [CrossRef]
- Carretero, M.I. Clays in Pelotherapy. A Review. Part I: Mineralogy, Chemistry, Physical and Physicochemical Properties. Appl. Clay Sci. 2020, 189, 105526. [Google Scholar] [CrossRef]
- Morer, C.; Roques, C.-F.; Françon, A.; Forestier, R.; Maraver, F. The Role of Mineral Elements and Other Chemical Compounds Used in Balneology: Data from Double-Blind Randomized Clinical Trials. Int. J. Biometeorol. 2017, 61, 2159–2173. [Google Scholar] [CrossRef]
- Fedorov, Y.A.; Ruban, D.A. Geoheritage Resource of Small Mud Lakes in the Semi-Arid Environments of the Russian South. Resources 2019, 8, 75. [Google Scholar] [CrossRef]
- Vadlja, D.; Bujak, M.; Čož-Rakovac, R.; Roje, M.; Čižmek, L.; Horvatić, A.; Svetličić, E.; Diminić, J.; Rakovac, S.; Oros, D.; et al. Bioprospecting for Microorganisms in Peloids—Extreme Environment Known for Its Healing Properties. Front. Mar. Sci. 2022, 9, 822139. [Google Scholar] [CrossRef]
- Carretero, M.I. Clays in Pelotherapy. A Review. Part II: Organic Compounds, Microbiology and Medical Applications. Appl. Clay Sci. 2020, 189, 105531. [Google Scholar] [CrossRef]
- Shadrin, N.V.; Anufriieva, E.V.; Shadrina, S.N. Brief Review of Phototrophs in the Crimean Hypersaline Lakesand Lagoons: Diversity, Ecological Role, the Possibility of Using. Mar. Biol. J. 2017, 2, 80–85. [Google Scholar] [CrossRef]
- Pesciaroli, C.; Viseras, C.; Aguzzi, C.; Rodelas, B.; González-López, J. Study of Bacterial Community Structure and Diversity during the Maturation Process of a Therapeutic Peloid. Appl. Clay Sci. 2016, 132–133, 59–67. [Google Scholar] [CrossRef]
- Isachenko, B.L. Mikrobiologicheskie Issledovaniya nad Gryazevymi Ozerami (Microbiological Analysis over Mud Lakes); Selected Works; Akad. Nauk SSSR: Moscow, Russia, 1951; Volume 2, pp. 26–142. [Google Scholar]
- Kolotilova, N.N. Vertical movements of a phototrophic bacteria population in a laboratory microcosm. Life Earth 2022, 44, 377–382. [Google Scholar] [CrossRef]
- Potapov, E.G.; Sibukaev, E.S. The current state and problems of Tambukan field of therapeutic mud. Kurortnaya Meditsina 2021, 31–44. [Google Scholar] [CrossRef]
- Grum-Grzhimaylo, A.A.; Debets, A.J.M.; van Diepeningen, A.D.; Georgieva, M.L.; Bilanenko, E.N. Sodiomyces alkalinus, a New Holomorphic Alkaliphilic Ascomycete within the Plectosphaerellaceae. Persoonia-Mol. Phylogeny Evol. Fungi 2013, 31, 147–158. [Google Scholar] [CrossRef]
- Mycobank. Available online: https://www.mycobank.org/ (accessed on 8 September 2023).
- Westerdijk Institute. Available online: https://wi.knaw.nl/ (accessed on 8 September 2023).
- National Center for Biotechnology Information. Available online: https://www.ncbi.nlm.nih.gov/ (accessed on 8 September 2023).
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J.; Innis, M.A.; Gelfand, D.H.; Sninsky, J.J. Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogenetics. In PCR Protocols; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar]
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT Online Service: Multiple Sequence Alignment, Interactive Sequence Choice and Visualization. Brief. Bioinform. 2019, 20, 1160–1166. [Google Scholar] [CrossRef]
- Kozlov, A.M.; Darriba, D.; Flouri, T.; Morel, B.; Stamatakis, A. RAxML-NG: A Fast, Scalable and User-Friendly Tool for Maximum Likelihood Phylogenetic Inference. Bioinformatics 2019, 35, 4453–4455. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.-T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A Fast and Effective Stochastic Algorithm for Estimating Maximum-Likelihood Phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Baranova, A.A.; Georgieva, M.L.; Bilanenko, E.N.; Andreev, Y.A.; Rogozhin, E.A.; Sadykova, V.S. Antimicrobial Potential of Alkalophilic Micromycetes Emericellopsis alkalina. Appl. Biochem. Microbiol. 2017, 53, 703–710. [Google Scholar] [CrossRef]
- Kuvarina, A.E.; Gavryushina, I.A.; Sykonnikov, M.A.; Efimenko, T.A.; Markelova, N.N.; Bilanenko, E.N.; Bondarenko, S.A.; Kokaeva, L.Y.; Timofeeva, A.V.; Serebryakova, M.V.; et al. Exploring Peptaibol’s Profile, Antifungal, and Antitumor Activity of Emericellipsin A of Emericellopsis Species from Soda and Saline Soils. Molecules 2022, 27, 1736. [Google Scholar] [CrossRef] [PubMed]
- Kuvarina, A.E.; Sukonnikov, M.A.; Timofeeva, A.V.; Serebryakova, M.V.; Baratova, L.A.; Buzurnyuk, M.N.; Golyshkin, A.V.; Georgieva, M.L.; Sadykova, V.S. Uncovering the Effects of the Cultivation Condition on Different Forms of Peptaibol’s Emericellipsins Production from an Alkaliphilic Fungus, Emericellopsis alkalina. Fermentation 2023, 9, 422. [Google Scholar] [CrossRef]
- Rogozhin, E.A.; Sadykova, V.S.; Baranova, A.A.; Vasilchenko, A.S.; Lushpa, V.A.; Mineev, K.S.; Georgieva, M.L.; Kul’ko, A.B.; Krasheninnikov, M.E.; Lyundup, A.V.; et al. A Novel Lipopeptaibol Emericellipsin A with Antimicrobial and Antitumor Activity Produced by the Extremophilic Fungus Emericellopsis alkalina. Molecules 2018, 23, 2785. [Google Scholar] [CrossRef]
- Zuccaro, A.; Summerbell, R.C.; Gams, W.; Schroers, H.-J.; Mitchell, J.I. A New Acremonium Species Associated with Fucus spp., and Its Affinity with a Phylogenetically Distinct Marine Emericellopsis Clade. Stud. Mycol. 2004, 50, 283–297. [Google Scholar]
- Grum-Grzhimaylo, A.A.; Georgieva, M.L.; Debets, A.J.M.; Bilanenko, E.N. Are Alkalitolerant Fungi of the Emericellopsis Lineage (Bionectriaceae) of Marine Origin? IMA Fungus 2013, 4, 213–228. [Google Scholar] [CrossRef]
- Hagestad, O.C.; Hou, L.; Andersen, J.H.; Hansen, E.H.; Altermark, B.; Li, C.; Kuhnert, E.; Cox, R.J.; Crous, P.W.; Spatafora, J.W.; et al. Genomic Characterization of Three Marine Fungi, Including Emericellopsis atlantica sp. nov. with Signatures of a Generalist Lifestyle and Marine Biomass Degradation. IMA Fungus 2021, 12, 21. [Google Scholar] [CrossRef]
- Gostinčar, C.; Zalar, P.; Gunde-Cimerman, N. No Need for Speed: Slow Development of Fungi in Extreme Environments. Fungal Biol. Rev. 2022, 39, 1–14. [Google Scholar] [CrossRef]
- Bondarenko, S.A.; Georgieva, M.L.; Bilanenko, E.N. Fungi Inhabiting the Coastal Zone of Lake Magadi. Contemp. Probl. Ecol. 2018, 11, 439–448. [Google Scholar] [CrossRef]
- Georgieva, M.L.; Bondarenko, S.A.; Markelova, N.N.; Bilanenko, E.N. Alkali-Resistant Filamentous Fungi of the Coastal Zone of the Dauria Saline Lakes. Contemp. Probl. Ecol. 2023, 16, 391–402. [Google Scholar] [CrossRef]
- Domsch, K.H.; Gams, W.; Anderson, T.-H. Compendium of Soil Fungi, 2nd ed.; IHW-Verlag: Eching, Germany, 2007; 672p. [Google Scholar]
- Hou, L.W.; Giraldo, A.; Groenewald, J.Z.; Rämä, T.; Summerbell, R.C.; Huang, G.Z.; Cai, L.; Crous, P.W. Redisposition of Acremonium-like Fungi in Hypocreales. Stud. Mycol. 2023, 105, 23–203. [Google Scholar] [CrossRef]
- Tubaki, K. Aquatic Sediment as a Habitat of Emericellopsis, with a Description of an Undescribed Species of Cephalosporium. Mycologia 1973, 65, 938–941. [Google Scholar] [CrossRef]
- Gonçalves, M.F.M.; Vicente, T.F.L.; Esteves, A.C.; Alves, A. Novel Halotolerant Species of Emericellopsis and Parasarocladium Associated with Macroalgae in an Estuarine Environment. Mycologia 2020, 112, 154–171. [Google Scholar] [CrossRef]
- Phookamsak, R.; Hyde, K.D.; Jeewon, R.; Bhat, D.J.; Jones, E.B.G.; Maharachchikumbura, S.S.N.; Raspé, O.; Karunarathna, S.C.; Wanasinghe, D.N.; Hongsanan, S.; et al. Fungal Diversity Notes 929–1035: Taxonomic and Phylogenetic Contributions on Genera and Species of Fungi. Fungal Divers. 2019, 95, 1–273. [Google Scholar] [CrossRef]
- Hyde, K.D.; Hongsanan, S.; Jeewon, R.; Bhat, D.J.; McKenzie, E.H.C.; Jones, E.B.G.; Phookamsak, R.; Ariyawansa, H.A.; Boonmee, S.; Zhao, Q.; et al. Fungal Diversity Notes 367–490: Taxonomic and Phylogenetic Contributions to Fungal Taxa. Fungal Divers. 2016, 80, 1–270. [Google Scholar] [CrossRef]
- Sigler, L.; Zuccaro, A.; Summerbell, R.; Mitchell, J.I.; Paré, J. Acremonium exuviarum sp. nov., a lizard-associated fungus with affinity to Emericellopsis. Stud. Mycol. 2004, 50, 409–413. [Google Scholar]
- Giraldo, A.; Gené, J.; Sutton, D.A.; Wiederhold, N.; Guarro, J. New Acremonium-like Species in the Bionectriaceae and Plectosphaerellaceae. Mycol. Prog. 2017, 16, 349–368. [Google Scholar] [CrossRef]
- Gonçalves, M.F.M.; Hilário, S.; Van de Peer, Y.; Esteves, A.C.; Alves, A. Genomic and Metabolomic Analyses of the Marine Fungus Emericellopsis cladophorae: Insights into Saltwater Adaptability Mechanisms and Its Biosynthetic Potential. J. Fungi 2022, 8, 31. [Google Scholar] [CrossRef] [PubMed]
- Carreira, C.; Staal, M.; Falkoski, D.; de Vries, R.P.; Middelboe, M.; Brussaard, C.P.D. Disruption of Photoautotrophic Intertidal Mats by Filamentous Fungi. Environ. Microbiol. 2015, 17, 2910–2921. [Google Scholar] [CrossRef]
- Houbraken, J.; Kocsubé, S.; Visagie, C.M.; Yilmaz, N.; Wang, X.-C.; Meijer, M.; Kraak, B.; Hubka, V.; Bensch, K.; Samson, R.A.; et al. Classification of Aspergillus, Penicillium, Talaromyces and Related Genera (Eurotiales): An Overview of Families, Genera, Subgenera, Sections, Series and Species. Stud. Mycol. 2020, 95, 5–169. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Andrade, E.; Stchigel, A.M.; Cano-Lira, J.F. New Xerophilic Species of Penicillium from Soil. J. Fungi 2021, 7, 126. [Google Scholar] [CrossRef] [PubMed]
- Jurjević, Ž.; Houbraken, J.; Sklenář, F.; Kolařík, M.; Arendrup, M.C.; Jørgensen, K.M.; Siqueira, J.P.Z.; Gené, J.; Yaguchi, T.; Ezekiel, C.N.; et al. Re-Examination of Species Limits in Aspergillus Section Flavipedes Using Advanced Species Delimitation Methods and Description of Four New Species. Stud. Mycol. 2021, 99, 100120. [Google Scholar] [CrossRef]
- Sklenář, F.; Glässnerová, K.; Jurjević, Ž.; Houbraken, J.; Samson, R.A.; Visagie, C.M.; Yilmaz, N.; Gené, J.; Cano, J.; Chen, A.J.; et al. Taxonomy of Aspergillus Series Versicolores: Species Reduction and Lessons Learned about Intraspecific Variability. Stud. Mycol. 2022, 102, 53–93. [Google Scholar] [CrossRef]
- Giraldo, A.; Hernández-Restrepo, M.; Crous, P.W. New Plectosphaerellaceous Species from Dutch Garden Soil. Mycol. Prog. 2019, 18, 1135–1154. [Google Scholar] [CrossRef]
- Kochhar, S.; Gupta, V.S.; Sethi, H.S.; Capoor, M.R. A Rare Case of Keratomycosis Due to Myriodontium keratinophilum. Delhi. J. Ophthalmol. 2018, 29, 54–56. [Google Scholar] [CrossRef]
- Kochkina, G.A.; Ozerskaya, S.M.; Ivanushkina, N.E.; Chigineva, N.I.; Vasilenko, O.V.; Spirina, E.V.; Gilichinskii, D.A. Fungal Diversity in the Antarctic Active Layer. Microbiology 2014, 83, 94–101. [Google Scholar] [CrossRef]
- Kohlmeyer, J.; Volkmann-Kohlmeyer, B. Fungi from Coral Reefs: A Commentary. Mycol. Res. 2003, 107, 386–387. [Google Scholar] [CrossRef]
- Maza-Márquez, P.; Lee, M.D.; Bebout, B.M. The Abundance and Diversity of Fungi in a Hypersaline Microbial Mat from Guerrero Negro, Baja California, México. J. Fungi 2021, 7, 210. [Google Scholar] [CrossRef]
- Jeilu, O.; Gessesse, A.; Simachew, A.; Johansson, E.; Alexandersson, E. Prokaryotic and Eukaryotic Microbial Diversity from Three Soda Lakes in the East African Rift Valley Determined by Amplicon Sequencing. Front. Microbiol. 2022, 13, 999876. [Google Scholar] [CrossRef]
- Abraham, E.P.; Newton, G.G.F. Penicillins and Cephalosporins. In Biosynthesis; Gottlieb, D., Shaw, P.D., Eds.; Springer: Berlin/Heidelberg, Germany, 1967; pp. 1–16. [Google Scholar]
- Hou, X.; Sun, R.; Feng, Y.; Zhang, R.; Zhu, T.; Che, Q.; Zhang, G.; Li, D. Peptaibols: Diversity, Bioactivity, and Biosynthesis. Eng. Microbiol. 2022, 2, 100026. [Google Scholar] [CrossRef]
- Rima, M.; Rima, M.; Fajloun, Z.; Sabatier, J.-M.; Bechinger, B.; Naas, T. Antimicrobial Peptides: A Potent Alternative to Antibiotics. Antibiotics 2021, 10, 1095. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.-Y.; Yan, Z.-B.; Meng, Y.-M.; Hong, X.-Y.; Shao, G.; Ma, J.-J.; Cheng, X.-R.; Liu, J.; Kang, J.; Fu, C.-Y. Antimicrobial Peptides: Mechanism of Action, Activity and Clinical Potential. Mil. Med. Res. 2021, 8, 48. [Google Scholar] [CrossRef] [PubMed]
- Gavryushina, I.A.; Georgieva, M.L.; Kuvarina, A.E.; Sadykova, V.S. Peptaibols as Potential Antifungal and Anticancer Antibiotics: Current and Foreseeable Development (Review). Appl. Biochem. Microbiol. 2021, 57, 556–563. [Google Scholar] [CrossRef]
- Stoppacher, N.; Neumann, N.K.N.; Burgstaller, L.; Zeilinger, S.; Degenkolb, T.; Brückner, H.; Schuhmacher, R. The Comprehensive Peptaibiotics Database. Chem. Biodivers. 2013, 10, 734–743. [Google Scholar] [CrossRef]
- Flissi, A.; Ricart, E.; Campart, C.; Chevalier, M.; Dufresne, Y.; Michalik, J.; Jacques, P.; Flahaut, C.; Lisacek, F.; Leclère, V.; et al. Norine: Update of the Nonribosomal Peptide Resource. Nucleic Acids Res. 2020, 48, D465–D469. [Google Scholar] [CrossRef] [PubMed]
- Ovchinnikova, T.V.; Levitskayab, N.G.; Voskresenskayab, O.G.; Kamenskiib, A. Neuroleptic Properties of the Ion-Channel-Forming Peptaibol Zervamicin: Locomotor Activity and Behavioral Effects. Chem. Biodivers. 2007, 4, 1374. [Google Scholar] [CrossRef]
- Balashova, T.A.; Shenkarev, Z.O.; Tagaev, A.A.; Ovchinnikova, T.V.; Raap, J.; Arseniev, A.S. NMR Structure of the Channel-Former Zervamicin IIB in Isotropic Solvents. FEBS Lett. 2000, 466, 333–336. [Google Scholar] [CrossRef]
- Gessmann, R.; Axford, D.; Brückner, H.; Berg, A.; Petratos, K. A Natural, Single-Residue Substitution Yields a Less Active Peptaibiotic: The Structure of Bergofungin A at Atomic Resolution. Acta Crystallogr. Sect. F Struct. Biol. Commun. 2017, 73, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Berg, A.; Ritzau, M.; Ihn, W.; Schlegel, B.; Fleck, W.F.; Heinze, S.; Gräfe, U. Isolation and Structure of Bergofungin, a New Antifungal Peptaibol from Emericellopsis donezkii HKI 0059. J. Antibiot. 1996, 49, 817–820. [Google Scholar] [CrossRef][Green Version]
- Berg, A.; Schlegel, B.; Ihn, W.; Demuth, U.; Gräfe, U. Isolation and Structural Elucidation of New Peptaibols, Bergofungins B, C and D, from Emericellopsis donezkii HKI 0059. J. Antibiot. 1999, 52, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Ishiyama, D.; Satou, T.; Senda, H.; Fujimaki, T.; Honda, R.; Kanazawa, S. Heptaibin, a Novel Antifungal Peptaibol Antibiotic from Emericellopsis Sp. BAUA8289. J. Antibiot. 2000, 53, 728–732. [Google Scholar] [CrossRef]
- De Zotti, M.; Biondi, B.; Peggion, C.; Park, Y.; Hahm, K.-S.; Formaggio, F.; Toniolo, C. Synthesis, Preferred Conformation, Protease Stability, and Membrane Activity of Heptaibin, a Medium-Length Peptaibiotic. J. Pept. Sci. 2011, 17, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Inostroza, A.; Lara, L.; Paz, C.; Perez, A.; Galleguillos, F.; Hernandez, V.; Becerra, J.; González-Rocha, G.; Silva, M. Antibiotic Activity of Emerimicin IV Isolated from Emericellopsis Minima from Talcahuano Bay, Chile. Nat. Prod. Res. 2018, 32, 1361–1364. [Google Scholar] [CrossRef]




| Species | Isolate No. | GenBank Accession No. | |
|---|---|---|---|
| ITS | Beta-Tubulin | ||
| Acremonium egyptiacum | p19 */SLF 0218.0913 ** | OR335864 | - |
| p33/SLF 0218.1001 | OR335865 | - | |
| Alternaria alternata | p18/SLF 0218.0604 | OR335862 | - |
| p44/SLF 0218.0306 | OR335861 | - | |
| Aspergillus sp. sect. Flavipedes ser. Flavipedes | p14/SLF 0218.0503 | OR335848 | - |
| Aspergillus sp. sect. Flavipedes ser. Flavipedes | p35/SLF 0218.0110 | OR335851 | - |
| Aspergillus sp. sect. Flavipedes ser. Spelaei | p11/SLF 0218.0402 | OR335847 | - |
| Aspergillus sp. sect. Flavipedes ser. Spelaei | p34/SLF 0218.0911 | OR335850 | - |
| Aspergillus sp. sect. Nidulantes ser. Versicolores | p10/SLF 0218.0315 | OR335846 | - |
| Aspergillus sp. sect. Nidulantes ser. Versicolores | p16/SLF 0218.0914 | OR335849 | - |
| Chordomyces sp. | p42/SLF 0218.0408 | OR335860 | - |
| Emericellopsis alkalina | p30/SLF 0218.0608 | OR335874 | OR287057 |
| p36/SLF 0218.0313 | OR335876 | OR287059 | |
| p43/SLF 0218.1002 | OR335882 | OR287065 | |
| Emericellopsis fimetaria | p24/SLF 0218.0620 | OR335869 | OR287052 |
| p26/SLF 0218.0308 | OR335871 | OR287054 | |
| p29/SLF 0218.0609 | OR335873 | OR287056 | |
| Emericellopsis sp. | p20/SLF 0218.0117 | OR335866 | OR287049 |
| Emericellopsis sp. | p21/SLF 0218.0708 | OR335867 | OR287050 |
| Emericellopsis sp. | p22/SLF 0218.0101 | OR335868 | OR287051 |
| Emericellopsis sp. | p25/SLF 0218.0601 | OR335870 | OR287053 |
| Emericellopsis sp. | p27/SLF 0218.0504 | OR335872 | OR287055 |
| Emericellopsis sp. | p32/SLF 0218.0908 | OR335875 | OR287058 |
| Emericellopsis sp. | p37/SLF 0218.0401 | OR335877 | OR287060 |
| Emericellopsis sp. | p38/SLF 0218.0203 | OR335878 | OR287061 |
| Emericellopsis sp. | p39/SLF 0218.0701 | OR335879 | OR287062 |
| Emericellopsis sp. | p40/SLF 0218.0702 | OR335880 | OR287063 |
| Emericellopsis sp. | p41/SLF 0218.0312 | OR335881 | OR287064 |
| Emericellopsis sp. | p45/SLF 0218.0201 | OR335883 | OR287066 |
| Emericellopsis sp. | p46/SLF 0218.0801 | OR335884 | OR287067 |
| Emericellopsis sp. | p49/SLF 0218.1006 | OR335885 | OR287068 |
| Fusarium sp. complex Burgessii | p28/SLF 0218.0204 | OR335857 | - |
| Fusarium sp. complex Incarnatum-Equiseti | p7/SLF 0218.0106 | OR335853 | - |
| Fusarium sp. complex Incarnatum-Equiseti | p12/SLF 0218.0404 | OR335855 | - |
| Fusarium sp. complex Solani | p9/SLF 0218.0309 | OR335854 | - |
| Fusarium sp. complex Solani | p13/SLF 0218.0405 | OR335856 | - |
| Gibellulopsis nigrescens | p17/SLF 0218.0103 | OR335859 | - |
| Gibellulopsis serrae | p8/SLF 0218.0302 | OR335858 | - |
| Myriodontium keratinophilum | p15/SLF 0218.0510 | OR335852 | - |
| Penicillium sp. sect. Brevicompacta ser. Brevicompacta | p302/SLF 0218.0562 | OR335841 | - |
| Penicillium sp. sect. Brevicompacta ser. Brevicompacta | p48/SLF 0218.1005 | OR335845 | - |
| Penicillium sp. sect. Chrysogena ser. Chrysogena | p2/SLF 0218.0902 | OR335839 | - |
| Penicillium sp. sect. Chrysogena ser. Chrysogena | p301/SLF 0218.0561 | OR335840 | - |
| Penicillium sp. sect. Chrysogena ser. Chrysogena | p4/SLF 0218.0915 | OR335842 | - |
| Penicillium sp. sect. Chrysogena ser. Chrysogena | p5/SLF 0218.0903 | OR335843 | - |
| Penicillium sp. sect. Chrysogena ser. Chrysogena | p6/SLF 0218.0802 | OR335844 | - |
| Penicillium sp. sect. Citrina ser. Copticolarum | p1/SLF 0218.0407 | OR335838 | - |
| Pseudeurotium bakeri | p47/SLF 0218.1004 | OR335863 | - |
| Taxon | Frequency of Occurrence, % | Abundance, % |
|---|---|---|
| SORDARIOMYCETES, Hypocreales, Bionectriaceae | ||
| Acremonium egyptiacum | 20 | 1.1 |
| Emericellopsis alkalina | 50 | 3.3 |
| Emericellopsis fimetaria | 20 | 1.1 |
| Emericellopsis spp. Marine clade | 100 | 61.4 |
| Emericellopsis sp. Terrestrial clade | 10 | 0.4 |
| SORDARIOMYCETES, Hypocreales, Nectriaceae | ||
| Fusarium sp. complex Burgessii | 10 | 0.4 |
| Fusarium sp. complex Incarnatum-Equiseti | 20 | 2.9 |
| Fusarium sp. complex Solani | 20 | 2.2 |
| SORDARIOMYCETES, Glomerellales, Plectosphaerellaceae | ||
| Chordomyces sp. | 10 | 0.4 |
| Gibellulopsis nigrescens | 10 | 0.7 |
| Gibellulopsis serrae | 10 | 1.1 |
| DOTHIDEOMYCETES, Pleosporales, Pleosporaceae | ||
| Alternaria alternata | 60 | 9.9 |
| EUROTIOMYCETES, Eurotiales, Aspergillaceae | ||
| Aspergillus sp. sect. Flavipedes ser. Flavipedes | 30 | 1.8 |
| Aspergillus sp. sect. Flavipedes ser. Spelaei | 20 | 4.0 |
| Aspergillus sp. sect. Nidulantes ser. Versicolores | 20 | 2.2 |
| Penicillium sp. sect. Brevicompacta ser. Brevicompacta | 20 | 1.1 |
| Penicillium sp. sect. Chrysogena ser. Chrysogena | 30 | 4.0 |
| Penicillium sp. sect. Citrina ser. Copticolarum | 10 | 1.1 |
| EUROTIOMYCETES, Onygenales, Incertae sedis | ||
| Myriodontium keratinophilum | 10 | 0.4 |
| LEOTIOMYCETES, Thelebolales, Pseudeurotiaceae | ||
| Pseudeurotium bakeri | 10 | 0.4 |
| Genera | Number of Strains | Test Organisms | |||
|---|---|---|---|---|---|
| Escherichia coli ATCC 25922 | Bacillus subtilis ATCC 6633 | Aspergillius niger INA 00760 | Candida albicans ATCC 2091 | ||
| Emericellopsis | 20 | 13 (65% *) | 19 (95%) | 18 (90%) | 16 (80%) |
| Penicillium | 8 | 2 (25%) | 6 (75%) | 3 (37.5%) | 2 (25%) |
| Aspergillus | 6 | 2 (33.3%) | 5 (83.3%) | 3 (50%) | 4 (66.7%) |
| Fusarium | 5 | 0 (0%) | 4 (80%) | 3 (60%) | 2 (40%) |
| Gibellulopsis | 2 | 1 (50%) | 1 (50%) | 1 (50%) | 0 (0%) |
| Alternaria | 2 | 0 (0%) | 2 (100%) | 2 (100%) | 2 (100%) |
| Acremonium | 2 | 1 (50%) | 2 (100%) | 1 (50%) | 1 (50%) |
| Myriodontinum | 1 | 0 (0%) | 1 (100%) | 0 (0%) | 1 (100%) |
| Pseudeurotium | 1 | 0 (0%) | 1 (100%) | 0 (0%) | 1 (100%) |
| Chordomyces | 1 | 0 (0%) | 1 (100%) | 1 (100%) | 1 (100%) |
| Total number of strains showing activity | 48 | 19 (39.6% **) | 42 (87.5%) | 32 (66.7%) | 30 (62.5%) |
| Genera | Total Strains | Inactive | Weakly Active | Moderately Active | Highly Active | ||||
|---|---|---|---|---|---|---|---|---|---|
| Escherichia coli | Bacillus subtilis | Escherichia coli | Bacillus subtilis | Escherichia coli | Bacillus subtilis | Escherichia coli | Bacillus subtilis | ||
| Emericellopsis | 20 | 7 | 1 | 3 | 0 | 9 | 11 | 1 | 8 |
| Penicillium | 8 | 6 | 2 | 0 | 2 | 2 | 4 | 0 | 0 |
| Aspergillus | 6 | 4 | 1 | 1 | 1 | 1 | 4 | 0 | 0 |
| Fusarium | 5 | 5 | 1 | 0 | 1 | 0 | 3 | 0 | 0 |
| Genera | Total Strains | Inactive | Weakly Active | Moderately Active | Highly Active | ||||
|---|---|---|---|---|---|---|---|---|---|
| Aspergillius niger | Candida albicans | Aspergillius niger | Candida albicans | Aspergillius niger | Candida albicans | Aspergillius niger | Candida albicans | ||
| Emericellopsis | 20 | 2 | 4 | 1 | 0 | 4 | 5 | 13 | 11 |
| Penicillium | 8 | 5 | 6 | 2 | 2 | 1 | 0 | 0 | 0 |
| Aspergillus | 6 | 3 | 2 | 0 | 1 | 3 | 3 | 0 | 0 |
| Fusarium | 5 | 2 | 3 | 0 | 0 | 3 | 2 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Georgieva, M.L.; Bilanenko, E.N.; Ponizovskaya, V.B.; Kokaeva, L.Y.; Georgiev, A.A.; Efimenko, T.A.; Markelova, N.N.; Kuvarina, A.E.; Sadykova, V.S. Haloalkalitolerant Fungi from Sediments of the Big Tambukan Saline Lake (Northern Caucasus): Diversity and Antimicrobial Potential. Microorganisms 2023, 11, 2587. https://doi.org/10.3390/microorganisms11102587
Georgieva ML, Bilanenko EN, Ponizovskaya VB, Kokaeva LY, Georgiev AA, Efimenko TA, Markelova NN, Kuvarina AE, Sadykova VS. Haloalkalitolerant Fungi from Sediments of the Big Tambukan Saline Lake (Northern Caucasus): Diversity and Antimicrobial Potential. Microorganisms. 2023; 11(10):2587. https://doi.org/10.3390/microorganisms11102587
Chicago/Turabian StyleGeorgieva, Marina L., Elena N. Bilanenko, Valeria B. Ponizovskaya, Lyudmila Y. Kokaeva, Anton A. Georgiev, Tatiana A. Efimenko, Natalia N. Markelova, Anastasia E. Kuvarina, and Vera S. Sadykova. 2023. "Haloalkalitolerant Fungi from Sediments of the Big Tambukan Saline Lake (Northern Caucasus): Diversity and Antimicrobial Potential" Microorganisms 11, no. 10: 2587. https://doi.org/10.3390/microorganisms11102587
APA StyleGeorgieva, M. L., Bilanenko, E. N., Ponizovskaya, V. B., Kokaeva, L. Y., Georgiev, A. A., Efimenko, T. A., Markelova, N. N., Kuvarina, A. E., & Sadykova, V. S. (2023). Haloalkalitolerant Fungi from Sediments of the Big Tambukan Saline Lake (Northern Caucasus): Diversity and Antimicrobial Potential. Microorganisms, 11(10), 2587. https://doi.org/10.3390/microorganisms11102587






