The Molecular Genetic Epidemiology and Antimicrobial Resistance of Neisseria gonorrhoeae Strains Obtained from Clinical Isolates in Central Panama
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Antibiotic Susceptibility Test
2.3. Molecular Typing Analysis and Molecular Identification of β-Lactamase
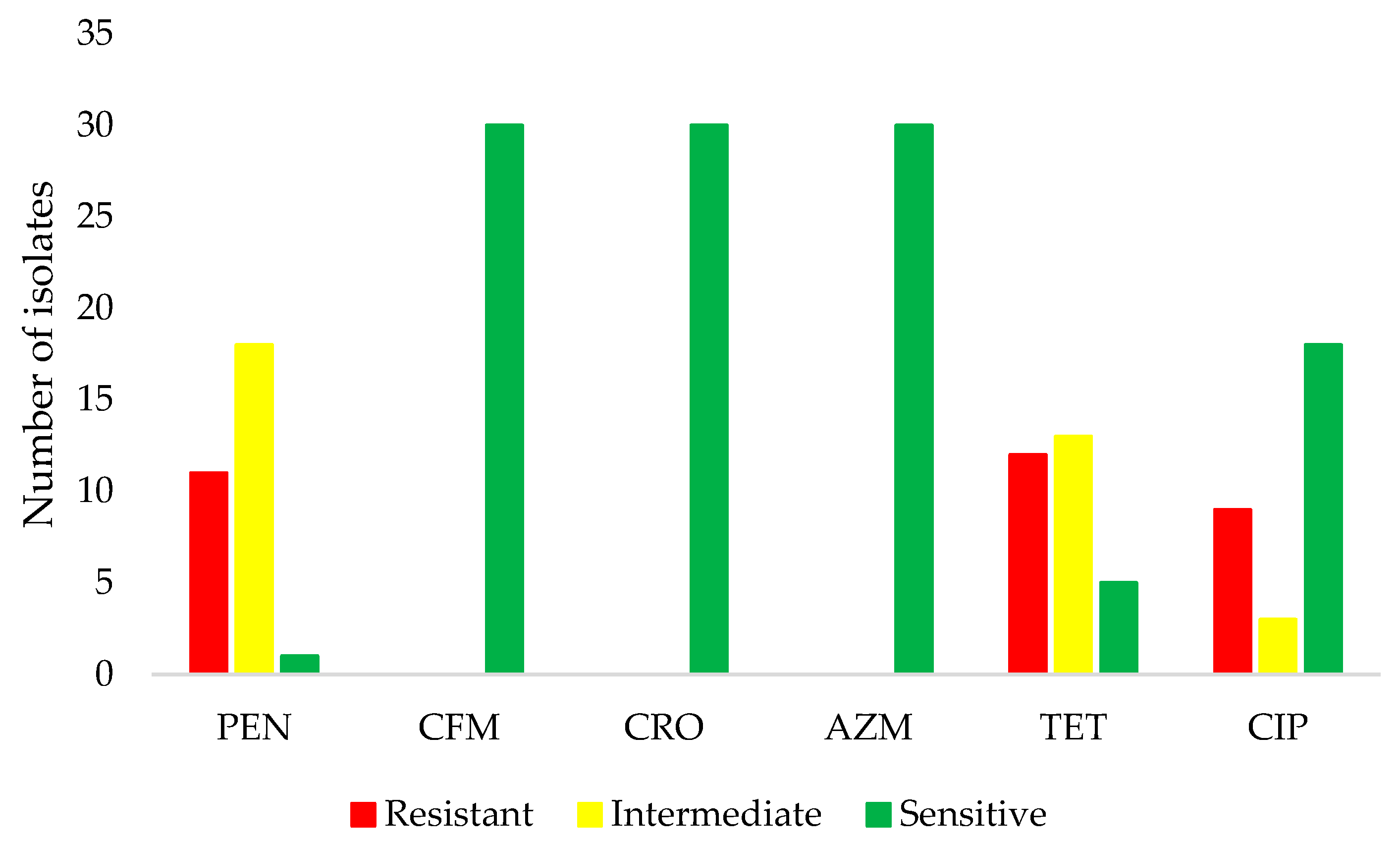
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Quillin, S.J.; Seifert, H.S. Neisseria gonorrhoeae host adaptation and pathogenesis. Nat. Rev. Microbiol. 2018, 16, 226–240. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Progress Report on HIV, Viral Hepatitis and Sexually Transmitted Infections. Available online: https://www.who.int/publications/i/item/9789240027077 (accessed on 30 August 2023).
- Du, M.; Yan, W.; Jing, W.; Qin, C.; Liu, Q.; Liu, M.; Liu, J. Increasing incidence rates of sexually transmitted infections from 2010 to 2019: An analysis of temporal trends by geographical regions and age groups from the 2019 Global Burden of Disease Study. BMC Infect. Dis. 2022, 22, 574. [Google Scholar] [CrossRef]
- Radovanovic, M.; Kekic, D.; Jovicevic, M.; Kabic, J.; Gajic, I.; Opavski, N.; Ranin, L. Current susceptibility surveillance and distribution of antimicrobial resistance in N. gonorrheae within WHO regions. Pathogens 2022, 11, 1230. [Google Scholar] [CrossRef]
- Workowski, K.A.; Bachmann, L.H.; Chan, P.A.; Johnston, C.M.; Muzny, C.A.; Park, I.; Reno, H.; Zenilman, J.M.; Bolan, G.A. Sexually Transmitted Infections Treatment Guidelines, 2021. MMWR Recomm. Rep. 2021, 70, 1–187. [Google Scholar] [CrossRef] [PubMed]
- Unemo, M.; Ross, J.; Serwin, A.B.; Gomberg, M.; Cusini, M.; Jensen, J.S. Background review for the ‘2020 European guideline for the diagnosis and treatment of gonorrhoea in adults’. Int. J. STD AIDS 2021, 32, 108–126. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics. Available online: https://www.who.int/news/item/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed (accessed on 30 August 2023).
- Unemo, M.; Lahra, M.M.; Escher, M.; Eremin, S.; Cole, M.J.; Galarza, P.; Ndowa, F.; Martin, I.; Dillon, J.-A.R.; Galas, M.; et al. WHO global antimicrobial resistance surveillance for Neisseria gonorrhoeae 2017–18: A retrospective observational study. Lancet Microbe 2021, 2, e627–e636. [Google Scholar] [CrossRef] [PubMed]
- Organización Panamericana de la Salud. Magnitud y Tendencias de la Resistencia a los Antimicrobianos en Latinoamérica. Available online: https://www.paho.org/es/documentos/magnitud-tendencias-resistencia-antimicrobianos-latinoamerica-relavra-2014-2015-2016 (accessed on 30 August 2023).
- Gianecini, R.A.; Poklepovich, T.; Golparian, D.; Cuenca, N.; Tuduri, E.; Unemo, M.; Campos, J.; Galarza, P. Genomic epidemiology of azithromycin-nonsusceptible Neisseria gonorrhoeae, Argentina, 2005–2019. Emerg. Infect. Dis. 2021, 27, 2369–2378. [Google Scholar] [CrossRef] [PubMed]
- Golparian, D.; Bazzo, M.L.; Golfetto, L.; Gaspar, P.C.; Schörner, M.A.; Schwartz Benzaken, A.; Ramos, M.C.; Ferreira, W.A.; Alonso Neto, J.B.; Mendes Pereira, G.F.; et al. Genomic epidemiology of Neisseria gonorrhoeae elucidating the gonococcal antimicrobial resistance and lineages/sublineages across Brazil, 2015–16. J. Antimicrob. Chemother. 2020, 75, 3163–3172. [Google Scholar] [CrossRef]
- Starnino, S.; Galarza, P.; Carvallo, M.E.; Benzaken, A.S.; Ballesteros, A.M.; Cruz, O.M.; Hernandez, A.L.; Carbajal, J.L.; Borthagaray, G.; GASP-LAC Working Group; et al. Retrospective analysis of antimicrobial susceptibility trends (2000–2009) in Neisseria gonorrhoeae isolates from countries in Latin America and the Caribbean shows evolving resistance to ciprofloxacin, azithromycin and decreased susceptibility to ceftriaxone. Sex. Transm. Dis. 2012, 39, 813–821. [Google Scholar] [CrossRef]
- Mlynarczyk-Bonikowska, B.; Kowalewski, C.; Krolak-Ulinska, A.; Marusza, W. Molecular mechanisms of drug resistance and epidemiology of multidrug-resistant variants of Neisseria gonorrhoeae. Int. J. Mol. Sci. 2022, 23, 10499. [Google Scholar] [CrossRef]
- Muhammad, I.; Golparian, D.; Dillon, J.A.; Johansson, A.; Ohnishi, M.; Sethi, S.; Chen, S.C.; Nakayama, S.; Sundqvist, M.; Bala, M.; et al. Characterisation of blaTEM genes and types of β-lactamase plasmids in Neisseria gonorrhoeae—The prevalent and conserved blaTEM-135 has not recently evolved and existed in the Toronto plasmid from the origin. BMC Infect. Dis. 2014, 14, 454. [Google Scholar] [CrossRef]
- Unemo, M.; Shafer, W.M. Antimicrobial resistance in Neisseria gonorrhoeae in the 21st century: Past, evolution, and future. Clin. Microbiol. Rev. 2014, 27, 587–613. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing, 30th ed.; CLSI supplement M100; CLSI: Wayne, PA, USA, 2020. [Google Scholar]
- Bennett, J.S.; Jolley, K.A.; Sparling, P.F.; Saunders, N.J.; Hart, C.A.; Feavers, I.M.; Maiden, M.C. Species status of Neisseria gonorrhoeae: Evolutionary and epidemiological inferences from multilocus sequence typing. BMC Biol. 2007, 5, 35. [Google Scholar] [CrossRef] [PubMed]
- Olesen, I.; Hasman, H.; Aarestrup, F.M. Prevalence of beta-lactamases among ampicillin-resistant Escherichia coli and Salmonella isolated from food animals in Denmark. Microb. Drug Resist. 2004, 10, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Antignac, A.; Alonso, J.M.; Taha, M.K. Nonculture prediction of Neisseria meningitidis susceptibility to penicillin. Antimicrob. Agents Chemother. 2001, 45, 3625–3628. [Google Scholar] [CrossRef]
- Demczuk, W.; Sidhu, S.; Unemo, M.; Whiley, D.M.; Allen, V.G.; Dillon, J.R.; Cole, M.; Seah, C.; Trembizki, E.; Trees, D.L.; et al. Neisseria gonorrhoeae sequence typing for antimicrobial resistance, a novel antimicrobial resistance multilocus typing scheme for tracking global dissemination of N. gonorrhoeae strains. J. Clin. Microbiol. 2017, 55, 1454–1468. [Google Scholar] [CrossRef]
- Whiley, D.M.; Limnios, E.A.; Ray, S.; Sloots, T.P.; Tapsall, J.W. Diversity of penA alterations and subtypes in Neisseria gonorrhoeae strains from Sydney, Australia, that are less susceptible to ceftriaxone. Antimicrob. Agents Chemother. 2007, 51, 3111–3116. [Google Scholar] [CrossRef]
- Sandoval, M.M.; Bardach, A.; Rojas-Roque, C.; Alconada, T.; Gomez, J.A.; Pinto, T.; Palermo, C.; Ciapponi, A. Antimicrobial resistance of Neisseria gonorrhoeae in Latin American countries: A systematic review. J. Antimicrob. Chemother. 2023, 78, 1322–1336. [Google Scholar] [CrossRef]
- Jorge-Berrocal, A.; Vargas-Herrera, N.; Benites, C.; Salazar-Quispe, F.; Mayta-Barrios, M.; Barrios-Cárdenas, Y.J.; Melano, R.G.; Yagui, M. Antimicrobial susceptibility of Neisseria gonorrhoeae isolates from Peru, 2018 and 2019. Sex. Transm. Dis. 2022, 49, 682–686. [Google Scholar] [CrossRef]
- Jorge-Berrocal, A.; Mayta-Barrios, M.; Fiestas-Solórzano, V. Resistencia antimicrobiana de Neisseria gonorrhoeae en Perú. Rev. Peru. Med. Exp. Salud Publica 2018, 35, 155–156. [Google Scholar] [CrossRef]
- Wi, T.; Lahra, M.M.; Ndowa, F.; Bala, M.; Dillon, J.-A.R.; Ramon-Pardo, P.; Eremin, S.R.; Bolan, G.; Unemo, M. Antimicrobial resistance in Neisseria gonorrhoeae: Global surveillance and a call for international collaborative action. PLoS Med. 2017, 14, e1002344. [Google Scholar] [CrossRef] [PubMed]
- Reimche, J.L.; Clemons, A.A.; Chivukula, V.L.; Joseph, S.J.; Schmerer, M.W.; Pham, C.D.; Schlanger, K.; Cyr, S.B.S.; Kersh, E.N.; Gernert, K.M.; et al. Genomic analysis of 1710 surveillance-based Neisseria gonorrhoeae isolates from the USA in 2019 identifies predominant strain types and chromosomal antimicrobial-resistance determinants. Microb. Genom. 2023, 9, mgen001006. [Google Scholar] [CrossRef]
- Costa-Lourenço, A.P.R.D.; Abrams, A.J.; Dos Santos, K.T.B.; Argentino, I.C.V.; Coelho-Souza, T.; Caniné, M.C.A.; Ferreira, A.L.P.; Moreira, B.M.; Fracalanzza, S.E.L.; Trees, D.L.; et al. Phylogeny and antimicrobial resistance in Neisseria gonorrhoeae isolates from Rio de Janeiro, Brazil. Infect. Genet. Evol. 2018, 58, 157–163. [Google Scholar] [CrossRef]
- Sánchez-Busó, L.; Cole, M.J.; Spiteri, G.; Day, M.; Jacobsson, S.; Golparian, D.; Sajedi, N.; Yeats, C.A.; Abudahab, K.; Underwood, A.; et al. Europe-wide expansion and eradication of multidrug-resistant Neisseria gonorrhoeae lineages: A genomic surveillance study. Lancet Microbe 2022, 3, e452–e463. [Google Scholar] [CrossRef] [PubMed]
- Harris, S.R.; Cole, M.J.; Spiteri, G.; Sánchez-Busó, L.; Golparian, D.; Jacobsson, S.; Goater, R.; Abudahab, K.; Yeats, C.A.; Bercot, B.; et al. Public health surveillance of multidrug-resistant clones of Neisseria gonorrhoeae in Europe: A genomic survey. Lancet Infect. Dis. 2018, 18, 758–768. [Google Scholar] [CrossRef] [PubMed]
- Yahara, K.; Ma, K.C.; Mortimer, T.D.; Shimuta, K.; Nakayama, S.-I.; Hirabayashi, A.; Suzuki, M.; Jinnai, M.; Ohya, H.; Kuroki, T.; et al. Emergence and evolution of antimicrobial resistance genes and mutations in Neisseria gonorrhoeae. Genome Med. 2021, 13, 51. [Google Scholar] [CrossRef]
- Ministerio de Salud de Panamá. Normativa Nacional para el Abordaje Integral de las Infecciones de Trasmisión Sexual en Panamá. Available online: https://www.minsa.gob.pa/sites/default/files/programas/normas_its_panama.pdf (accessed on 30 August 2023).
| Isolate | ST MLST | Year | Origin | PEN | CFM | CRO | AZM | TET | CIP | β-Lactamase/Test | Substitution * | penA Pattern |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 11516 | 2017 | V | I | S | S | S | I | S | − | LVG | II |
| 2 | 1584 | 2017 | O | R | S | S | S | I | S | TEM/+ | LVGN | XIV |
| 3 | 1893 | 2017 | U | I | S | S | S | I | R | − | LVG | II |
| 4 | 11516 | 2018 | U | I | S | S | S | S | S | − | VLVGS | VII |
| 5 | 11516 | 2017 | V | I | S | S | S | S | S | − | LVG | II |
| 6 | 10932 | 2018 | U | I | S | S | S | I | S | − | LVG | II |
| 7 | 8145 | 2016 | O | R | S | S | S | I | S | TEM/+ | LVG | II |
| 8 | 8145 | 2016 | V | R | S | S | S | I | S | TEM/+ | LVG | II |
| 9 | ND | 2017 | U | I | S | S | S | R | I | − | LVG | II |
| 10 | 11516 | 2017 | U | I | S | S | S | I | S | − | LVG | II |
| 11 | 1901 | 2017 | U | I | S | S | S | R | R | TEM/+ | LVGS | IV |
| 12 | 1893 | 2015 | U | I | S | S | S | R | S | TEM/+ | LVG | II |
| 13 | ND | 2017 | U | I | S | S | S | I | R | + | LVGN | XIV |
| 14 | 8145 | 2017 | U | R | S | S | S | R | S | TEM/+ | LVGN | XIV |
| 15 | ND | 2014 | U | R | S | S | S | R | R | TEM/+ | LVGNVNV | XIX |
| 16 | 1901 | 2016 | U | I | S | S | S | R | R | TEM/+ | LVGS | IV |
| 17 | 1893 | 2014 | U | I | S | S | S | R | I | TEM/+ | LVG | II |
| 18 | 11148 | 2018 | U | I | S | S | S | R | S | − | LVG | II |
| 19 | 7367 | 2018 | U | I | S | S | S | I | I | − | N | XV |
| 20 | 7367 | 2016 | U | I | S | S | S | R | R | − | TLVG | 44 ** |
| 21 | 8145 | 2018 | U | R | S | S | S | I | S | TEM/+ | LVGN | XIV |
| 22 | 1584 | 2017 | V | R | S | S | S | R | R | + | LVGN | XIV |
| 23 | 11516 | 2018 | U | S | S | S | S | S | S | − | LVG | II |
| 24 | ND | 2017 | U | R | S | S | S | I | R | TEM/+ | LVGN | XIV |
| 25 | 1901 | 2017 | U | I | S | S | S | R | R | TEM/− | LVG | II |
| 26 | 1902 | 2013 | U | R | S | S | S | I | S | TEM/+ | LVGS | IV |
| 27 | 10932 | 2015 | U | I | S | S | S | R | S | − | LVGNVQVVNV | XXII |
| 28 | 1905 | 2014 | U | I | S | S | S | I | S | − | LVG | II |
| 29 | 1584 | 2013 | U | R | S | S | S | S | S | TEM/+ | LVGN | XIV |
| 30 | 11516 | 2014 | U | R | S | S | S | S | S | + | LVG | II |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Núñez-Samudio, V.; Herrera, M.; Herrera, G.; Pimentel-Peralta, G.; Landires, I. The Molecular Genetic Epidemiology and Antimicrobial Resistance of Neisseria gonorrhoeae Strains Obtained from Clinical Isolates in Central Panama. Microorganisms 2023, 11, 2572. https://doi.org/10.3390/microorganisms11102572
Núñez-Samudio V, Herrera M, Herrera G, Pimentel-Peralta G, Landires I. The Molecular Genetic Epidemiology and Antimicrobial Resistance of Neisseria gonorrhoeae Strains Obtained from Clinical Isolates in Central Panama. Microorganisms. 2023; 11(10):2572. https://doi.org/10.3390/microorganisms11102572
Chicago/Turabian StyleNúñez-Samudio, Virginia, Mellissa Herrera, Genarino Herrera, Gumercindo Pimentel-Peralta, and Iván Landires. 2023. "The Molecular Genetic Epidemiology and Antimicrobial Resistance of Neisseria gonorrhoeae Strains Obtained from Clinical Isolates in Central Panama" Microorganisms 11, no. 10: 2572. https://doi.org/10.3390/microorganisms11102572
APA StyleNúñez-Samudio, V., Herrera, M., Herrera, G., Pimentel-Peralta, G., & Landires, I. (2023). The Molecular Genetic Epidemiology and Antimicrobial Resistance of Neisseria gonorrhoeae Strains Obtained from Clinical Isolates in Central Panama. Microorganisms, 11(10), 2572. https://doi.org/10.3390/microorganisms11102572









