Abstract
Spore-forming probiotic bacteria, including Bacillus coagulans, are resilient and produce a variety of beneficial metabolites. We evaluated the immune-modulating effects of the novel probiotic strain Bacillus coagulans JBI-YZ6.3, where the germinated spores, metabolite fraction, and cell wall fraction were tested in parallel using human peripheral blood mononuclear cell cultures under both normal and lipopolysaccharide-induced inflamed culture conditions. The expression of CD25 and CD69 activation markers was evaluated via flow cytometry. Supernatants were tested for cytokines, interferons, chemokines, and growth factors using Luminex arrays. The germinated spores were highly immunogenic; both the cell wall and metabolite fractions contributed significantly. Under normal culture conditions, increased levels of immune activation were observed as increased expressions of CD25 and CD69 relative to natural killer cells, suggesting an increased ability to attack virus-infected target cells. On monocytes, a complex effect was observed, where the expression of CD25 increased under normal conditions but decreased under inflamed conditions. This, in combination with increased interleukin-10 (IL-10) and decreased monocyte chemoattractant protein-1 (MCP-1) production under inflamed conditions, points to anti-inflammatory effects. The production of the stem cell-related growth factor granulocyte colony-stimulating Factor (G-CSF) was enhanced. Further research is warranted to characterize the composition of the postbiotic metabolite fraction and document the characteristics of immunomodulating agents secreted by this probiotic strain.
1. Introduction
The gastrointestinal tract is home to a complex microbial community, and the mucosal lining and underlying tissue harbor a large proportion of the body’s innate immune cells. The cooperation of the innate immune system and the gut microbial community is required to maintain homeostasis [1]. Human physiology can be altered by the gut microbial community both with respect to health and disease. The disruption of the symbiotic relationship between the human body and its gut microbiome results in an unhealthy alteration of the composition of the microbiota, leading to a pro-inflammatory state that can adversely affect intestinal permeability, digestion, and metabolism, as well as immune responses [2,3].
Bacterial species from the spore-forming genus Bacillus, classified as Gram-positive facultative anaerobic bacteria, have gained increasing attention due to their novel probiotic potential [4]. There are 77 currently known Bacillus spp. that have shown positive health effects, including B. coagulans, B. subtilis, and B. licheniformis [5,6]. Among them, B. coagulans has gained recognition as a promising probiotic due to its status of being generally recognized as safe (GRAS) [7,8]. B. coagulans has a number of physiological characteristics that distinguish it from other probiotic Bacillus species, including growth conditions, biochemical reactions, the use of carbon sources, and a cell wall with high lipid contents compared to other Gram-positive bacteria [9,10]. Compared to non-spore-forming probiotic bacteria such as Lactobacillus spp., B. coagulans offers the advantage of improved stability during industrial processing and storage in functional foods [11,12]. The ability to withstand stomach acid and bile salts provides B. coagulans an edge for longer survival periods in the gastrointestinal tract [13,14,15,16]. The genomic analysis of B. coagulans shows no potential safety risks with regard to the transference of antibiotic resistance genes to other gut microbes [16,17,18]. In addition, B. coagulans has found wide application in the food industry because of its innate production of enzymes, vitamins, antimicrobials, amino acids, and short-chain fatty acids [19,20].
The immune-activating properties of probiotic cell walls are well documented, where the activation of host defenses includes the engagement of pattern recognition receptors (PPRs) on immune cells [21,22]. In addition, the metabolites secreted by B. coagulans have a variety of beneficial effects on the host (Table 1), including the increased production of mucin and tight junction proteins that protect the epithelial barrier [23,24,25,26], apoptosis-inducing substances that contribute to the destruction of cancer cells [27,28], antimicrobial peptides such as bacteriocin [29,30,31], and the fatty acid-mediated [32,33,34,35] and surfactants-mediated inhibition of pathogens [36,37], which prevent the formation of pathogenic biofilms. Furthermore, the metabolites stimulate the immune response by enhancing macrophage activity and modulating the secretion of immunoglobulins [38,39,40].
The secreted metabolites from probiotic bacteria are also described as postbiotics, which are defined as non-viable bacterial metabolic byproducts capable of conferring potential health benefits to the host. Different strains of B. coagulans are associated with different properties of secreted metabolites in terms of shifting the gut microbiome in favor of non-disease-related species. The production of bacteriocins by Bacillus species results in permeabilization and pore formation on target microbes, leading to the death of the target microbes. Bacteriocin secreted from B. coagulans had an inhibitory effect against Escherichia coli NCTC-10418, Pseudomonas aeruginosa NCIB-9016, Klebsiella pneumoniae NCIB-9111, B. subtilis NCTC-6346, Staphylococcus aureus NCTC7447, and fungi such as Candida albicans CBS-562 [41]. In addition, B. coagulans inhibited the growth of Bacillus cereus MTCC 430, S. aureus MTCC 3160, Enterococcus sp. MTCC 9728, Lactobacillus sp. MTCC 10093, and Micrococcus luteus MTCC 106, all of which are food-borne pathogens [42].
Previous research from our team involved another strain of B. coagulans, the B. coagulans GBI-30 6086 strain, and showed that the cell wall and metabolite fractions supported the maturation of antigen-presenting immune cells and modulated inflammatory processes in the gut by reducing proinflammatory cytokines [43,44]. Additionally, we showed that inactivated B. coagulans GBI-30 6086 cells activated human immune cells and altered the production of both immune activating and anti-inflammatory cytokines and chemokines, indicating that cell wall components remained viable and bioactive [45].
The present study investigated the effects of the germinated spores of the novel probiotic strain B. coagulans JBI-YZ6.3, in parallel with its metabolites and cell wall fractions, on immune activation and modulation under unstressed and inflamed conditions to determine whether the immunological consequences of exposure to the probiotic bacterium would differ depending on absence versus the presence of inflammation. This information is important for the development of effective probiotic formulations for specific health conditions in different populations.

Table 1.
Properties of the cell walls and postbiotic metabolites from Bacillus coagulans.
Table 1.
Properties of the cell walls and postbiotic metabolites from Bacillus coagulans.
| Features | Function | Mechanisms of Action | References |
|---|---|---|---|
| Physical | Acidophilic | Intracellular pH is kept stable in acid environments by basic amino acids, proton-efflux systems, and highly impermeable cell membranes. | [13,14,15,16] |
| Thermophilic | Thermal stability is attributed to the presence of saturated and straight-chain fatty acids, temperature-stable amino acids, and high guanine–cytosine content in DNA. | [14,15,16] | |
| Cell wall | Immune modulation | Bacterial cell wall components bind to Toll-like receptors (TLRs) and NOD-like receptors (NLRs) and activate the host immune system. | [9,43,44,45] |
| Metabolites | |||
| Bacteriocin | Anti-microbial | Target bacterial cell membranes are disrupted via the induction of cell permeabilization and pore formation. | [15,19,30] |
| Galactosidases | Improvement in carbohydrate metabolism. | Bacterial enzymes improve digestibility and carbohydrate metabolism by hydrolyzing non-digestible galactosides in food in the gut. | [18,20] |
| Fatty acids | Anti-fungal | Fungal cell membranes are disrupted using detergent-like properties of fatty acids and inhibiting the synthesis of membrane components such as ergosterol. | [9,32,33,35] |
| Regulation of inflammation | Signaling molecules and chemical messengers are involved in gut-brain communication. | [46,47,48] | |
| Biosurfactants | Anti-microbial | Pathogenic biofilm formation is inhibited on/in the gut mucosa. | [36,37] |
| Metabolites of unknown | Epithelial barrier protection | Stimulation of epithelial mucin secretion prevents microbial adhesion. | [23,24] |
| Chemical composition | Integrity of intestinal barriers is improved via the modulation of expression of tight junction proteins by host epithelial cells. | [23,24,25,26] | |
| Regulation of inflammation | Production of anti-inflammatory cytokine production by gut epithelial cells is stimulated. | [23,39,43,44,45] | |
| Anti-cancer effects | Cancer cell growth is inhibited via increased expression of pro-apoptotic genes. | [9,27,28] | |
| Antioxidant protection | Increased production of host antioxidant enzymes provides protection. | [39,49,50,51] |
2. Materials and Methods
2.1. Reagents
Roswell Park Memorial Institute 1640 medium (Gibco cat. # 11835-030), penicillin–streptomycin 100× (Gibco cat. # 15140-122), fetal bovine serum (Gibco cat. # A38401-01), Dulbecco’s phosphate-buffered saline (PBS, Gibco cat. # 141190-136), lipopolysaccharide (LPS) (Invitrogen cat. # 00-4976-93), monoclonal antibodies CD3-SB702 (clone UCHT1, Invitrogen cat. #67-0038-42), CD56-phycoerythrin (clone CMSSB Invitrogen cat. # 12-0567-42), and CD69-fluorescein isothiocyanate (clone FN50, Invitrogen cat. #11-0699-42) were purchased from Thermo Fisher Scientific (Waltham, MA, USA). CD25-Brilliant Violet 421 (clone 2A3 BD cat. # 564033) and sodium heparin vacutainer tubes (BD cat. # 367878) were purchased from Becton-Dickinson (Franklin Lakes, NJ, USA). Bio-Plex Pro™ human cytokine arrays were purchased from Bio-Rad Laboratories Inc. (Hercules, CA, USA). Interleukin-2 (IL-2) (Sigma cat. # 17908-10KU) was purchased from Sigma-Aldrich Co. (St Louis, MO, USA). Lympholyte Poly (Cedarlane cat. # CL5070) was purchased from CedarLane (Burlington, NC, USA).
2.2. Bacillus Coagulans Germinated Spores, Metabolites, and Cell Wall Fractions
The test products, evaluated by addition to human immune cell cultures, were Bacillus coagulans germinated spores that were freshly prepared for each cell culture, and the metabolite and cell wall fractions were prepared in bulk and stored frozen in multiple aliquots so one aliquot could be thawed in preparation for each cell culture. The procedures are described below.
Bacillus coagulans spores were provided by the study sponsor, Jeneil Biotech, (Saukville, WI, USA). The strain is also available through the American Type Culture Collection, deposited as ATCC PTA-127366. The gene sequence of the bacterium is known and is available from GenBank (CP104390). The gene sequence of JBI-YZ6.3 was tested for antimicrobial resistance genes using two genome-wide screening programs, ResFinder and the Comprehensive Antibiotic Resistance Database (CARD), and screened for potentially toxigenic genes using known Bacillus toxin genes. No potential antibiotic resistance genes or toxin genes were detected in this strain.
Spores were prepared via aseptic fermentation with proprietary media and fermentation parameters to obtain a cell population comprised primarily of endospores, and they were verified via morphological observation with light microscopy. Cells were harvested from the fermentation media by centrifugation and dried under lyophilization in the absence of cryoprotectant. Enumeration was performed on dried biomass to measure spore counts (CFU/g). Dried biomass was standardized to a concentration of 1.5 × 1010 CFU/g by blending with identity-preserved maltodextrin.
To produce germinated spores, a powder sample of dry spores containing 15 billion colony-forming units (CFU)/gram was weighed into sterile PBS (40 mg/mL) and shaken until a uniform suspension was generated (Figure 1, left panel). The suspension was incubated for 30 min at room temperature to allow the spores to hydrate. The suspension was sonicated for 10 min to reduce the number of aggregated spores. The sonicated suspension was transferred to a preheated water bath at 80 °C and incubated in the water bath for 20 min. The suspension was cooled immediately to 45 °C with intermittent vigorous shaking. This suspension was diluted 10-fold to create a stock solution and used to prepare serial dilutions for addition to cell cultures (1:10, 1:40, 1:160, and 1:640).
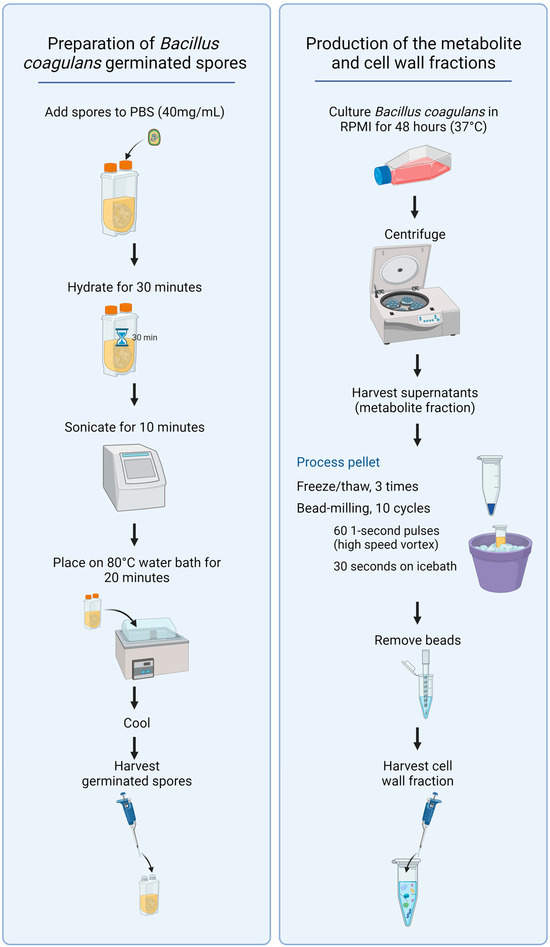
Figure 1.
Diagram showing the procedures for preparing germinated spores, the metabolite fraction, and the cell wall fraction from Bacillus coagulans.
To produce samples of fermentation metabolites and cell wall fractions, a sample of germinated spores was cultured in Roswell Park Memorial Institute 1640 (RPMI-1640) culture medium under aerobic culture conditions at 37 °C for 48 h (Figure 1, right panel). The resulting culture was used to prepare both the metabolite fraction and the cell wall fraction for testing.
The metabolite fraction was prepared from the 48 h culture via centrifugation. The supernatant containing the fermentation metabolites was decanted from the pellet and harvested.
The pellet was used to prepare the cell wall fraction. The pelleted bacteria were washed three times in PBS, followed by 3 freeze–thaw cycles and subsequent bead-milling using low-protein binding 100-micron zirconium beads. The bead-milling consisted of 10 cycles, where each cycle involved 60 1 s pulse-vortexing to grind up the bacterial particles. Each 1 s of pulse-vortexing was followed by immediate immersion into an ice bath for 30 s to avoid unnecessary heating during the bead-milling cycles. The beads were allowed to settle, and the suspension of bacterial cell wall material was transferred to a clean tube, pelleted by centrifugation, the supernatant discarded, and the cell wall fraction resuspended in sterile PBS.
For both the cell wall and metabolite fractions, multiple aliquots were prepared in PBS + penicillin (100 units/mL) and streptomycin (100 units/mL). The aliquots were frozen at −30 °C, and 1 aliquot was thawed on each lab testing day. After thawing, samples were vortexed and used for testing in immune cell cultures.
2.3. Immune Cell Activation
Peripheral venous blood was drawn from three healthy human donors upon written informed consent, as approved by the Sky Lakes Medical Center Institutional Review Board, Federalwide Assurance 2603. The blood was drawn into heparin vacutainer vials, and peripheral blood mononuclear cells (PBMCs) were isolated using Lympholyte Poly by centrifugation for 35 min at 400× g. PBMCs were washed twice in PBS and counted, and density was adjusted to establish cultures with a cell density of 106/mL using Roswell Park Memorial Institute 1640 medium containing 10% heat-inactivated fetal calf serum and penicillin–streptomycin.
Serial dilutions of test products were added to PBMC cultures in U-bottom 96-well cell culture plates (NUNClon Delta Surface cat# 163320) at a density of 106 cells/mL and a volume of 0.2 mL. Two parallel sets of cultures were prepared: (1) normal (un-stressed) culture conditions and (2) inflamed culture conditions, where the cells were treated with test products for 10 min, after which inflammatory conditions were triggered by the addition of 0.01 mL LPS per well for a final dose of 5 µg/mL LPS in cell cultures. Cultures were incubated at 37 °C and 5% CO2 for 16 h.
LPS from Escherichia coli was used as a positive control for immune-cell activation. In parallel, interleukin-2 (IL-2) was used as a positive control for natural killer (NK) cell activation at a concentration of 100 IU/mL in cell culture. Untreated negative control cultures consisted of PBMCs exposed to phosphate-buffered saline in the absence of test products. All treatments, including each dose of the test product and each positive and negative control, were tested in triplicate. After 16 h, blood cells were isolated from each culture well and stained for 15 min with fluorochrome-labeled monoclonal antibodies at doses predetermined by titration. PBMC were then fixed in 0.5% formalin and acquired by flow cytometry using an Attune acoustic-focusing flow cytometer (Thermo Fisher Scientific, Waltham, MA, USA). Data analysis utilized gating on forward/side scatter to characterize lymphocyte and monocyte populations. In the lymphocyte population, 4 subsets were evaluated for expression of the CD25 and CD69 activation markers: CD3+ CD56− T cells, CD3+ CD56+ NKT cells, CD3− CD56+ NK cells, and the CD3-CD56− non-NK non-T cells.
2.4. Production of Cytokines, Chemokines, and Growth Factors
After 16 h of incubation, the supernatants were harvested from the PBMC cultures described above. The levels of 27 cytokines and chemokines were quantified using Bio-Plex protein arrays (Bio-Rad Laboratories Inc., Hercules, CA, USA) and utilizing xMAP technology (Luminex, Austin, TX, USA). The cytokine array included the following: IL-1β, IL-1ra, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12 (p70), IL-13, IL-15, IL-17, Eotaxin, basic FGF, G-CSF, GM-CSF, IFN-γ, IP-10, MCP-1 (MCAF), MIP-1α, MIP-1β, PDGF-BB, RANTES, TNF-α, and VEGF.
2.5. Statistical Analysis
The average and standard deviation for each data set were calculated using Microsoft Excel for Microsoft 365 MSO (Version 2309) (Microsoft Corporation, Redmond, WA, USA). Statistical analysis of in vitro data was performed using the 2-tailed, independent t-test. Statistical significance was set at p < 0.05, and a high level of significance was set at p < 0.01.
3. Results
3.1. Induction of the CD25 and CD69 Activation Markers on Immune Cell Subsets
The CD25 activation marker, also known as the alpha-chain of the heterotrimer IL-2 receptor, was induced by the germinated spores and the cell wall fraction under both normal and inflamed culture conditions (Figure 2). Compared to untreated control cultures, increased levels of CD25 expression were observed in natural killer (NK) cells; T cells; the non-NK non-T cell fraction, which contains dendritic cells; and monocytes. The increased levels of CD25 on all four cell subsets reached a high level of statistical significance for the cell wall fraction when compared to untreated control cultures (p < 0.01).
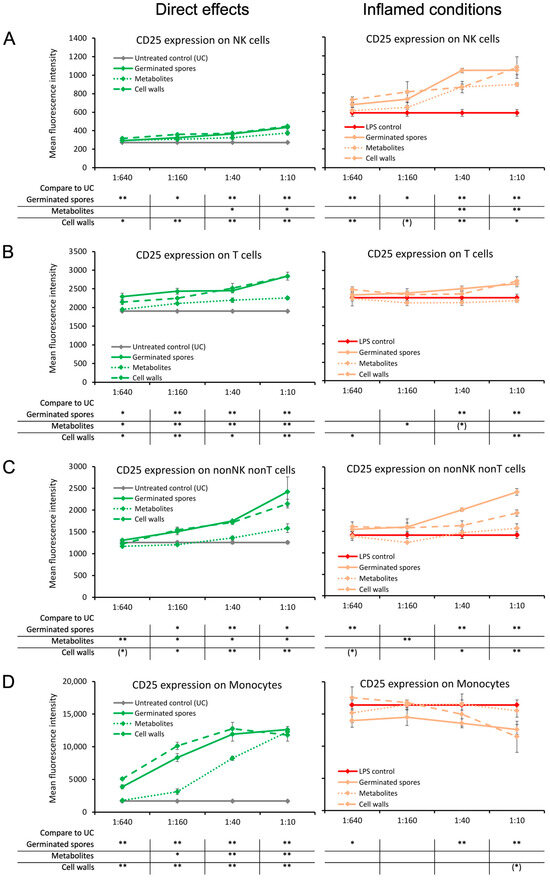
Figure 2.
Expression levels of the CD25 activation marker on subsets of human peripheral blood mononuclear cells. The left panel (green lines) shows the direct effects of the germinated spores, metabolites, and cell walls; untreated control cultures (UCs) were used as negative controls (grey lines). The right panel (orange lines) shows the effects of the test products under inflamed culture conditions, where inflammation was induced by lipopolysaccharides (LPSs); LPS alone was used as a positive control (red lines). (A) Expression of CD25 on natural killer (NK) cells was mildly induced by all 3 products; under inflamed culture conditions, the products triggered an increase in LPS-induced levels of CD25. (B) Expression of CD25 on T cells was induced by all 3 products; under inflamed culture conditions, the germinated spores and the cell walls triggered a mild increase in LPS-induced levels of CD25, whereas the metabolites had no effect. (C) Expression of CD25 on nonNK nonT cells was induced by all 3 products; under inflamed culture conditions, the germinated spores triggered a robust increase, and the cell walls triggered a mild increase in the LPS-induced levels of CD25, whereas the effects of the metabolites were minimal. (D) Expression of CD25 on monocytes was induced by all 3 products; under inflamed culture conditions, the germinated spores triggered a robust decrease, and the cell walls triggered a mild decrease in the LPS-induced levels of CD25, whereas the metabolites had no effect. Statistical comparisons are shown in the tables below each graph. For the graphs on the left side, statistical comparisons are carried out on the untreated control cultures (UCx). For the graphs on the right side, statistical comparisons are carried out on the LPS-treated (LPS) control cultures. The statistical significance when comparing the test products to controls is indicated with (*) for p < 0.1, * for p < 0.05, and ** for p < 0.01.
The increased levels of CD25 expression were milder in cultures treated with the metabolite fraction, for NK cells, T cells, and the non-NK non-T cell population (Figure 2A–C). In contrast, the two highest doses of the metabolite fraction induced highly significant increases in CD25 expression in the monocyte population compared to untreated control cultures (p < 0.01; Figure 2D).
Under inflamed conditions, CD25 expression was increased in NK cells by all three test products, reaching a high level of statistical significance at the second dose compared to the LPS control (p < 0.01; Figure 2A). CD25 expression levels were increased by both the germinated spores and cell wall fraction with respect to T cells and non-NK non-T cells, reaching a high level of statistical significance at the highest dose (p < 0.01; Figure 2B,C). The germinated spores and the cell wall fraction triggered the down-regulation of CD25 expression on monocytes under inflamed conditions (Figure 2D). In contrast, the metabolite fraction had no effect on CD25 expression levels on T cells, non-NK non-T cells, or monocytes under inflamed conditions (Figure 2B–D).
The CD69 activation marker, a transmembrane C-Type lectin protein known to be upregulated by NF-κB signaling at the onset of an immune response, was induced on lymphocytes by all three test products under normal culture conditions (Figure 3). Compared to the untreated control cultures, CD69 expression was strongest on NK cells and non-NK non-T cells and almost absent on T cells (Figure 3A–C). The levels of CD69 expression on monocytes under normal conditions were increased by the germinated spores (p < 0.05) and the cell wall fraction (p < 0.01) but were not affected by the metabolite fraction when compared to untreated control cultures (Figure 3D).
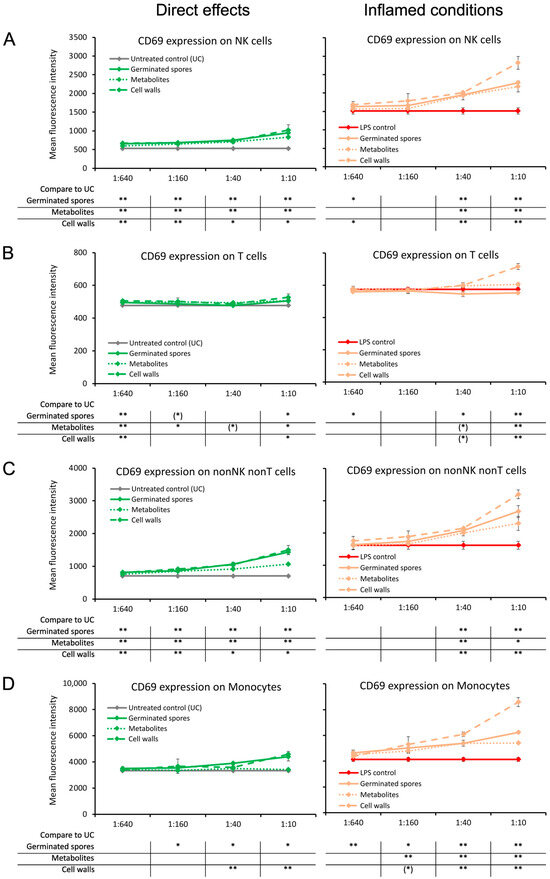
Figure 3.
Expression levels of the CD69 activation marker on subsets of human peripheral blood mononuclear cells. The left panel (green lines) shows the direct effects of the germinated spores, metabolites, and cell walls; untreated control cultures (UCs) were used as negative controls (grey lines). The right panel (orange lines) shows the effects of the test products under inflamed culture conditions, where inflammation was induced by lipopolysaccharide (LPS); LPS alone was used as a positive control (red lines). (A) Expression of CD69 on natural killer (NK) cells was mildly induced by all 3 products; under inflamed culture conditions, the products triggered an increase in LPS-induced levels of CD69, and the effect was the strongest for the cell walls. (B) Expression of CD69 on T cells was not affected by the 3 products; under inflamed culture conditions, the highest dose of the cell walls triggered a mild increase in the LPS-induced levels of CD69. (C) Expression of CD69 on non-NK non-T cells was induced by all 3 products; under inflamed culture conditions, the cell walls triggered a robust increase, with milder effects from the germinated spores and metabolites. (D) Expression of CD69 on monocytes was induced by the germinated spores and the cell walls but not affected by the metabolites; under inflamed culture conditions, the cell walls triggered a robust increase, and the germinated spores and metabolites triggered a milder increase in LPS-induced levels of CD69. Statistical comparisons are shown in the tables below each graph. For the graphs on the left side, the statistical comparisons are for the untreated control cultures (UCs). For the graphs on the right side, the statistical comparisons are for the LPS-treated (LPS) control cultures. The statistical significance when comparing the test products to controls is indicated with (*) for p < 0.1, * for p < 0.05, and ** for p < 0.01.
Under inflamed conditions, CD69 expression was increased on all cell types by the cell wall fraction (p < 0.01; Figure 3A–D). The germinated spores and the metabolite fraction also triggered increased CD69 expressions under inflamed conditions on NK cells, non-NK non-T cells, and monocytes but had no effect on CD69 expression in T cells (Figure 3A–D).
3.2. Increased Pro-Activating Cytokine Production
The germinated spores and the cell wall fraction had similar effects under normal unstressed culture conditions, inducing a dose-dependent upregulation of the following immune-activating cytokines: interleukin-1β (IL-1β), interleukin-6 (IL-6), interleukin-17A (IL-17A), and tumor necrosis factor-alpha (TNF-α) (Figure 4). The metabolite fraction also induced the expression of these four cytokines, but the effect was milder (Figure 4).
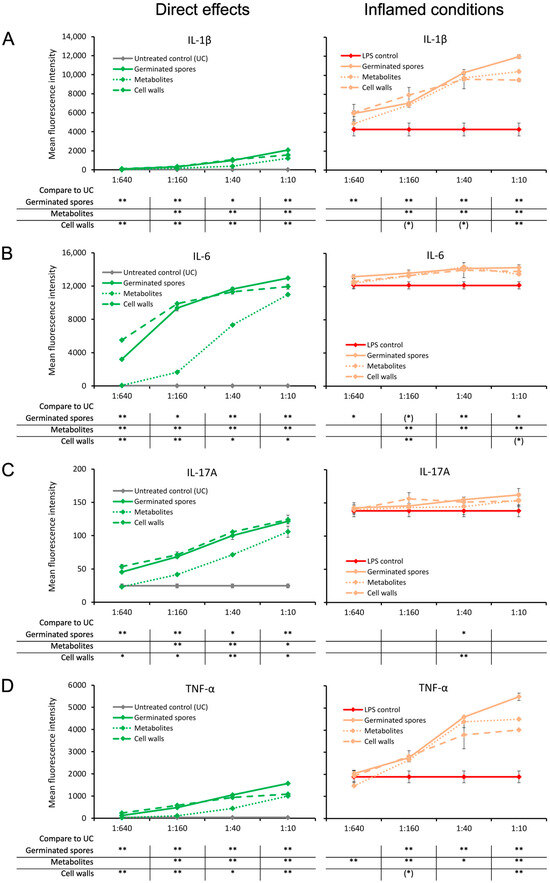
Figure 4.
Cytokine production in cell cultures of human peripheral blood mononuclear cells. The left panel (green lines) shows the direct effects of the germinated spores, metabolites, and cell walls; untreated control cultures (UCs) were used as negative controls (grey lines). The right panel (orange lines) shows the effects of the test products under inflamed culture conditions, where inflammation was induced by lipopolysaccharides (LPSs); LPS alone was used as a positive control (red lines). (A) Interleukin-1-beta (IL-1β) was mildly induced by all 3 products, and under inflamed culture conditions, it triggered a robust increase in the LPS-induced levels of IL-1β. (B) Interleukin-6 (IL-6) was robustly induced by all 3 products, and under inflamed culture conditions, it triggered a mild but highly significant increase in the LPS-induced levels of IL-6. (C) Interleukin-17A (IL-17A) was robustly induced by all 3 products, and under inflamed culture conditions, it triggered a mild increase in the LPS-induced levels of IL-17A. (D) Tumor necrosis factor-alpha (TNF-α) was mildly induced by all 3 products, and under inflamed culture conditions, it triggered a robust increase in the LPS-induced levels of TNF-α. Statistical comparisons are shown in the tables below each graph. For the graphs on the left side, the statistical comparisons are for the untreated control cultures (UCs). For the graphs on the right side, the statistical comparisons are for the LPS-treated (LPS) control cultures. The statistical significance when comparing the test products to controls is indicated with (*) for p < 0.1, * for p < 0.05, and ** for p < 0.01.
When the test products were added to PBMC cultures in the context of an inflammatory challenge, the levels of these four cytokines were significantly higher than for the LPS control, but they were most robust for IL-1β and TNF-α. When comparing the TNF-α levels induced by the three test products, the germinated spores induced the highest level of TNF-α, and the cell wall fraction induced the lowest TNF-α levels compared to the LPS control (Figure 4D). The metabolite fraction induced higher levels of TNF-α under inflamed culture conditions than the cell wall fraction (Figure 4D).
3.3. Increased Interferon and Chemokine Production
The germinated spores and the cell wall fraction had similar effects under normal unstressed culture conditions, inducing a dose-dependent upregulation of interferon-gamma (IFN-γ) and three chemokines: monocyte chemoattractant protein-1 (MCP-1), macrophage inflammatory protein-beta (MIP-1β), and regulated upon activation, normal T cell expressed and secreted (RANTES) (Figure 5). The metabolite fraction also induced these four cytokines, but the effect was milder (Figure 5).
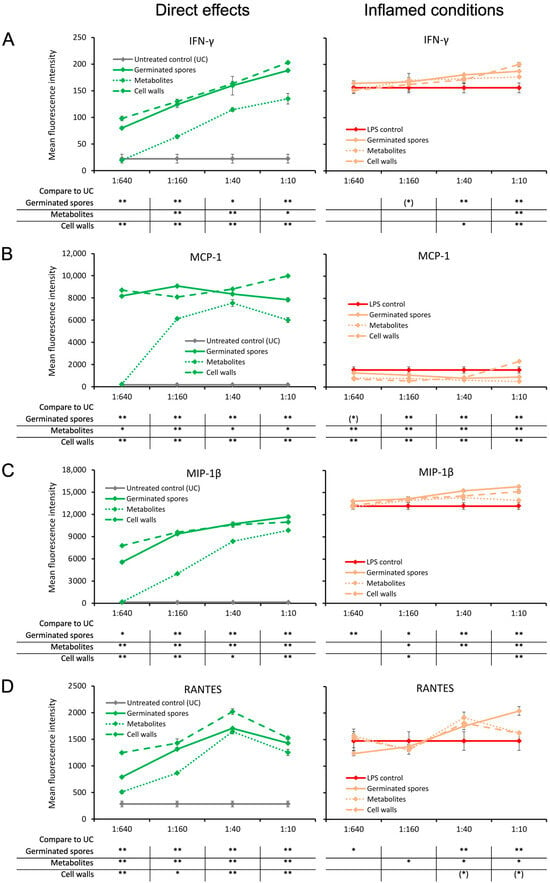
Figure 5.
Cytokine production in cell cultures of human peripheral blood mononuclear cells. The left panel (green lines) shows the direct effects of the germinated spores, metabolites, and cell walls; untreated control cultures (UCs) were used as negative controls (grey lines). The right panel (orange lines) shows the effects of the test products under inflamed culture conditions, where inflammation was induced by lipopolysaccharides (LPSs); LPS alone was used as a positive control (red lines). (A) Interferon-gamma (IFN-γ) was robustly induced by all 3 products, and under inflamed culture conditions, they triggered a very mild increase in the LPS-induced levels of IFN-γ. (B) Monocyte chemoattractant protein-1 (MCP-1) was robustly induced by all 3 products; however, under inflamed culture conditions, the products triggered a decrease in the LPS-induced levels of MCP-1. (C) Macrophage inflammatory protein-1 beta (MIP-1β) was robustly induced by all 3 products, and under inflamed culture conditions, it triggered a mild increase in the LPS-induced levels of MIP-1β. (D) The regulated upon activation, normal T cell expressed and secreted (RANTES) chemokine was robustly induced by all 3 products; under inflamed culture conditions, the products triggered a mild increase in the LPS-induced levels of RANTES, which was the strongest for the germinated spores. Statistical comparisons are shown in the tables below each graph. For the graphs on the left side, the statistical comparisons are for the untreated control cultures (UCs). For the graphs on the right side, the statistical comparisons are for the LPS-treated (LPS) control cultures. The statistical significance when comparing the test products to controls is indicated with (*) for p < 0.1, * for p < 0.05, and ** for p < 0.01.
Under inflamed culture conditions, the levels of IFN-γ and MIP-1β were slightly higher in the cultures that were pre-treated with test products prior to inflammation. The mild increase was highly significant for the highest dose of all three test products compared to the LPS-treated control (p < 0.01; Figure 5A,C).
In addition, the test products increased the level of RANTES under inflamed culture conditions compared to the LPS-treated control cultures at the two higher doses (Figure 5D). This increase in RANTES production was highly significant for the germinated spores (p < 0.01) and significant for the cell wall fraction (p < 0.05); moreover, a statistical trend for the metabolite fraction was observed, suggesting that both the cell walls and metabolites contributed to the effects observed in the germinated spores (Figure 5D).
In contrast, the level of MCP-1 was reduced in inflamed cultures treated with the test products compared to LPS-treated control cultures (Figure 5B). The reduction in MCP-1 under inflamed culture conditions was highly significant for all three test products at the two middle doses (p < 0.01; Figure 5B).
3.4. Cytokines Involved in the Return to Homeostasis
The germinated spores and the cell wall fraction, and to a lesser extent the metabolite fraction, triggered a dose-dependent upregulation of the anti-inflammatory cytokine IL-10 under normal culture conditions (Figure 6A). The increase was highly significant for the germinated spores at the highest dose (p < 0.01) and significant for the cell walls and metabolites (p < 0.05) when compared to the untreated control cultures (Figure 6A). Under inflamed culture conditions, an increase in the LPS-induced production of IL-10 was observed, reaching a high level of statistical significance for the second-highest dose of germinated spores and for the highest dose of the cell walls and metabolites (p < 0.01; Figure 6A).
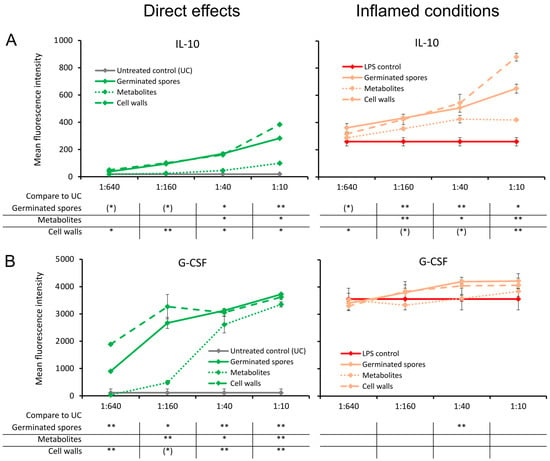
Figure 6.
Cytokine production in cell cultures of human peripheral blood mononuclear cells. The left panel (green lines) shows the direct effects of the germinated spores, metabolites, and cell walls; untreated control cultures (UCs) were used as negative controls (grey lines). The right panel (orange lines) shows the effects of the test products under inflamed culture conditions, where inflammation was induced by lipopolysaccharides (LPSs); LPS alone was used as a positive control (red lines). (A) Interleukin-10 (IL-10) was induced by all 3 products; under inflamed culture conditions, a robust increase was observed in the LPS-induced levels of IL-10. (B) Granulocyte colony-stimulating factor (G-CSF) was robustly induced by all 3 products; under inflamed culture conditions, the germinated spores and the cell walls triggered an increase in the LPS-induced levels of G-CSF. Statistical comparisons are shown in the tables below each graph. For the graphs on the left side, the statistical comparisons are for the untreated control cultures (UCs). For the graphs on the right side, the statistical comparisons are for the LPS-treated control cultures (LPS). The statistical significance when comparing the test products to controls is indicated with (*) for p < 0.1, * for p < 0.05, and ** for p < 0.01.
Compared to the untreated control cultures, the germinated spores and the cell wall fraction, and to a lesser extent the metabolite fraction, also triggered a dose-dependent upregulation of the growth factor G-CSF, which is known to affect restorative functions via the effects on stem cell migration (Figure 6B). This was also observed under inflamed conditions of the germinated spores and the cell wall fraction. The increase in G-CSF levels was highly significant for the second dose of germinated spores (p < 0.01) compared to the LPS control. In contrast, the metabolite fraction did not affect G-CSF levels under inflamed culture conditions (Figure 6B).
4. Discussion
Gut health has a definitive effect on physical and mental wellbeing. It requires a beneficial microbiome along the gut mucosal surfaces, where immune cells residing in the gut epithelium interact with the cell walls of commensal probiotic organisms and where secreted bacterial metabolites affect many aspects of health in the gut lumen and the underlying mucosal tissue. In order to maintain host health, gut bacteria and the human gut epithelial tissue must communicate. The immune cells inhabiting the gut mucosal tissue are an integral part of this crosstalk, and the postbiotic secreted metabolites are essential in this process [49].
This study examined the effects of the germinated spores of the novel probiotic strain Bacillus coagulans JBI-YZ6.3, alongside the metabolite and cell wall fractions, on immune activation and modulation in an unstressed versus inflamed environment. The goal was to test whether the immunological consequences of exposure to this probiotic bacterial strain would differ depending on the absence versus presence of inflammation.
The results reported here have shown that in the absence of inflammation, both the cell wall and metabolite fractions from B. coagulans JBI-YZ6.3 triggered direct immune-activating effects. This was observed due to the up-regulation of both CD25 and CD69 activation markers and via the up-regulation of multiple immune-activating pro-inflammatory cytokines, including IL-1β, IL-6, IL-17, and TNF-α. For the cell wall fraction, immune-activating effects were expected based on the known engagement of cell wall peptidoglycans with Toll-Like receptor-2, which triggers the activation of immune cells via the NF-kβ and MAPK signaling pathways. The direct immune-activating properties of the metabolite fraction secreted by the germinated spores are more complex. The effects are likely mediated by multiple, distinct, non-overlapping mechanisms, some involving the modulation of inflammatory cell signaling pathways and others involving gene regulation via the induction of antioxidant response elements [46,50,51]. Consequently, the immune-activating effects caused by the secreted metabolites highlight the observation that various components of the metabolite fraction act synergistically to activate the immune system [47,48,52].
The changes to the expression of the immune cell activation markers CD25 and CD69 showed complexity and pointed to the intricate regulation of immune activity by B. coagulans JBI-YZ6.3. On NK cells, the expression levels of both CD25 and CD69 were increased significantly. The target cells that are recognized and killed by NK cells are not limited to cancer cells but also include virus-infected cells; thus, the effects of B. coagulans JBI-YZ6.3 on the NK cell activation status are clinically important. CD69 is rapidly induced in NK cells shortly after activation [53], and its direct role in NK cytotoxic activity has been demonstrated [54]. When human NK cells are co-cultured with K562 target cells, CD69 expression is upregulated, and the increase significantly correlated with NK cell activity, as measured by today’s gold standard CD107 mobilization assay [55]. CD69 has the capacity to activate the NK cytolytic machinery in the absence of other NK–target cell adhesion molecule interactions. Most importantly, a direct and highly significant correlation between CD69 levels and NK cell activity was demonstrated by Clausen’s team in a study involving 14 breast cancer patients tested repeatedly during chemotherapy [56].
On monocytes, CD25 expression levels were upregulated under normal culture conditions but downregulated under inflamed conditions, where the reduction was highly significant for germinated spores. Antigen-presenting cells of myeloid origin express CD25, the alpha chain of the IL-2 receptor, but they do not express the beta chain and hence are unresponsive to IL-2. However, they may compete for IL-2 and regulate T cell proliferation under inflamed conditions [57]. In contrast, CD69 was upregulated on monocytes both under normal and inflamed conditions. CD69 expression on monocytes has been functionally linked to the 5-lipoxygenase pathway, in which leukotrienes are produced [58]. Leukotrienes are important chemoattraction mediators, they are responsible for the rapid recruitment of immune cells relative to affected tissue [59], and the expression of CD69 on monocytes by B. coagulans JBI-YZ6.3 suggests that, in tissues, this would be associated with the recruitment of additional immune cells to the area.
Under inflamed culture conditions, the metabolite fraction showed immunomodulatory effects by stimulating the production of anti-inflammatory cytokines, such as IL-10, while also triggering a decrease in the pro-inflammatory chemokine MCP-1, suggesting that under inflamed conditions, there may be a reduced migration of inflammatory cells to tissue exposed to the bacterium and its metabolites. The metabolite fraction may offer a novel treatment strategy for immune support in patients with gut dysbiosis and a hostile gut microbiome. In some patients, consuming probiotics to change the composition of the gut microbiome may take time or be unsuccessful. Consuming the metabolite fraction, which is manufactured on a commercial production scale, may provide immediate health benefits independent of changes to the gut microbiome.
In previous studies on another strain of B. coagulans GBI-30 6086, the cell wall and secreted metabolite fractions demonstrated immunomodulatory effects by increasing the production of anti-inflammatory cytokines [43]. In another study, Benson et al. (2012) showed that the secreted metabolite fraction in the same strain supported the maturation of mononuclear phagocytes towards both macrophages and dendritic cells [44], highlighting their importance in protecting against foreign antigens.
Additionally, the upregulation of the stem cell-mobilizing growth factor G-CSF by germinated spores and cell wall components points to an effect on reparative functions. This is further supported by Jensen et al. (2017) [45], which showed that inactivated B. coagulans GBI-30 6086 induced a selective upregulation of G-CSF, which is known to be involved in post-injury and post-inflammation repair and regeneration. This suggests a beneficial role for B. coagulans JBI-YZ6.3 in the restoration of gut health and is the topic for ongoing studies in our lab.
From a methods perspective, we are presenting the details for isolating and testing the cell wall fraction and the postbiotic secreted metabolite fraction in parallel. This is due to an urgent need for standardized protocols and descriptive naming on this research topic [60]. One team compared the effects of the secreted metabolites to the bacterial lysate from B. coagulans and found significant differences between the two fractions on bone mass in ovariectomized rats [61]. Another team characterized the intracellular content of the B. coagulans GBI-30 strain, i.e., the bacterial lysate from which the cell walls were removed, but they did not compare the intracellular content to secreted metabolites [62]. While there is likely overlap between what the bacterium contains and what it is secreting, the two fractions are not identical, and we suggest that the intracellular content should not be defined as a postbiotic.
Notably, the results presented here demonstrated the superior immune-activating properties of the metabolically active, intact germinated spores when compared to the isolated cell wall and metabolite fractions. This was specifically observed for the upregulation of IL-1β and TNF-α under inflamed conditions and is likely due to synergistic actions of cell wall and metabolite components, which are present in cultures of the germinated spores. This work presents one method for testing the metabolically active bacterium in co-cultures with various cell types from the host.
The work presented here serves as a proof-of-concept study. Work is ongoing to evaluate the effects of cell walls and secreted metabolites on gut epithelial cells. It is of utmost importance to expand on the metabolomics analysis of secreted metabolites in order to better understand the potential for the support of gut health by this fraction. Further work should also characterize the immune-modulating effects on isolated immune cell types, including monocytes, NK cells, and T cells. It is also of interest to evaluate the effects on monocyte/macrophage polarization and dendritic cell maturation. Clinical studies on acute effects associated with consuming the metabolite fraction are also warranted.
5. Conclusions
The metabolically active probiotic bacterium Bacillus coagulans JBI-YZ6.31 showed direct immune-activating properties, and under inflamed conditions, it showed anti-inflammatory properties. Both the secreted metabolites and cell walls contributed to the immune-activating and anti-inflammatory properties of the novel probiotic strain B. coagulans JBI-YZ6.31. The cell wall fraction enhanced immune cell activation under inflamed conditions, while at the same time, it reduced the production of inflammatory cytokine TNF-α and increased the production of anti-inflammatory cytokine IL-10. This is of clinical importance, with the health-protective benefit of activating the immune defense along mucosal linings while at the same time modulating inflammatory reactions in a milieu widely exposed to potential pathogens.
Author Contributions
Conceptualization, G.S.J.; methodology, G.S.J.; formal analysis, L.Y. and G.S.J.; original draft preparation, S.V.M., I.I., L.Y., D.C. and G.S.J.; writing—review and editing, I.I., S.V.M., L.Y., D.C. and G.S.J.; visualization, L.Y. and D.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Jeneil Biotech, Inc., a producer of commercially available fermented foods and probiotic bacteria.
Data Availability Statement
The data presented in this study are available upon reasonable request from the corresponding author.
Acknowledgments
We are grateful for the excellent technical support from Krista Sanchez.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; or in the writing of the manuscript. The funders supported the decision to publish the results.
References
- McDermott, A.J.; Huffnagle, G.B. The Microbiome and Regulation of Mucosa Immunity. Immunology 2014, 142, 24–31. [Google Scholar] [CrossRef]
- Gomaa, E.Z. Human gut microbiota/microbiome in health and diseases: A review. Antonie Van Leeuwenhoek 2020, 113, 2019–2040. [Google Scholar] [CrossRef]
- Kristensen, N.B.; Bryrup, T.; Allin, K.H.; Nielsen, T.; Hansen, T.H.; Pedersen, O. Alterations in fecal microbiota composition by probiotic supplementation in healthy adults: A systematic review of randomized controlled trials. Genome Med. 2016, 8, 52. [Google Scholar] [CrossRef]
- Talebi, S.; Makhdoumi, A.; Bahreini, M.; Matin, M.M.; Moradi, H.S. Three novel Bacillus strains from a traditional lacto-fermented pickle as potential probiotics. J. Appl. Microbiol. 2018, 125, 888–896. [Google Scholar] [CrossRef] [PubMed]
- Upadrasta, A.; Pitta, S.; Madempudi, R.S. Draft Genome Sequence of the Spore-Forming Probiotic Strain Bacillus coagulans Unique IS-2. Genome Announc. 2016, 4, e00225-16. [Google Scholar] [CrossRef] [PubMed]
- Elshaghabee, F.M.F.; Rokana, N.; Gulhane, R.D.; Sharma, C.; Panwar, H. Bacillus as Potential Probiotics: Status, Concerns, and Future Perspectives. Front. Microbiol. 2017, 8, 1490. [Google Scholar] [CrossRef] [PubMed]
- Konuray, G.; Erginkaya, Z. Potential Use of Bacillus coagulans in the Food Industry. Foods 2018, 7, 92. [Google Scholar] [CrossRef] [PubMed]
- Saroj, D.B.; Gupta, A.K. Genome based safety assessment for Bacillus coagulans strain LBSC (DSM 17654) for probiotic application. Int. J. Food Microbiol. 2020, 318, 108523. [Google Scholar] [CrossRef]
- Cao, J.; Yu, Z.; Liu, W.; Zhao, J.; Zhang, H.; Zhai, Q.; Chen, W. Probiotic characteristics of Bacillus coagulans and associated implications for human health and diseases. J. Funct. Foods 2020, 64, 103643. [Google Scholar] [CrossRef]
- Forrester, I.T.; Wicken, A.J. The chemical composition of the cell walls of some thermophilic bacilli. J. Gen. Microbiol. 1966, 42, 147–154. [Google Scholar] [CrossRef]
- Bader, J.; Albin, A.; Stahl, U. Spore-forming bacteria and their utilisation as probiotics. Benef. Microbes 2012, 3, 67–75. [Google Scholar] [CrossRef]
- Majeed, M.; Majeed, S.; Nagabhushanam, K.; Natarajan, S.; Sivakumar, A.; Ali, F. Evaluation of the Stability of Bacillus coazgulans MTCC 5856 during Processing and Storage of Functional Foods. Int. J. Food Sci. Technol. 2016, 51, 894–901. [Google Scholar] [CrossRef]
- Bora, P.S.; Puri, V.; Bansal, A.K. Physicochemical Properties and Excipient Compatibility studies of Probiotic Bacillus coagulans Spores. Sci. Pharm. 2009, 77, 625–638. [Google Scholar] [CrossRef]
- Patel, M.A.; Ou, M.S.; Harbrucker, R.; Aldrich, H.C.; Buszko, M.L.; Ingram, L.O.; Shanmugam, K.T. Isolation and characterization of acid-tolerant, thermophilic bacteria for effective fermentation of biomass-derived sugars to lactic acid. Appl. Environ. Microbiol. 2006, 72, 3228–3235. [Google Scholar] [CrossRef] [PubMed]
- Hyronimus, B.; Le Marrec, C.; Sassi, A.H.; Deschamps, A. Acid and bile tolerance of spore-forming lactic acid bacteria. Int. J. Food Microbiol. 2000, 61, 193–197. [Google Scholar] [CrossRef]
- Endres, J.R.; Clewell, A.; Jade, K.A.; Farber, T.; Hauswirth, J.; Schauss, A.G. Safety assessment of a proprietary preparation of a novel Probiotic, Bacillus coagulans, as a food ingredient. Food Chem. Toxicol. 2009, 47, 1231–1238. [Google Scholar] [CrossRef] [PubMed]
- Salvetti, E.; Orrù, L.; Capozzi, V.; Martina, A.; Lamontanara, A.; Keller, D.; Cash, H.; Felis, G.E.; Cattivelli, L.; Torriani, S.; et al. Integrate genome-based assessment of safety for probiotic strains: Bacillus coagulans GBI-30, 6086 as a case study. Appl. Microbiol. Biotechnol. 2016, 100, 4595–4605. [Google Scholar] [CrossRef] [PubMed]
- Bang, W.Y.; Ban, O.H.; Lee, B.S.; Oh, S.; Park, C.; Park, M.K.; Jung, S.K.; Yang, J.; Jung, Y.H. Genomic-, phenotypic-, and toxicity-based safety assessment and probiotic potency of Bacillus coagulans IDCC 1201 isolated from green malt. J. Ind. Microbiol. Biotechnol. 2021, 48, kuab026. [Google Scholar] [CrossRef]
- Adibpour, N.; Hosseininezhad, M.; Pahlevanlo, A.; Hussain, M. A review on Bacillus coagulans as a Spore-Forming Probiotic. Appl. Food Biotechnol. 2019, 6, 91–100. [Google Scholar]
- Aulitto, M.; Strazzulli, A.; Sansone, F.; Cozzolino, F.; Monti, M.; Moracci, M.; Fiorentino, G.; Limauro, D.; Bartolucci, S.; Contursi, P. Prebiotic properties of Bacillus coagulans MA-13: Production of galactoside hydrolyzing enzymes and characterization of the transglycosylation properties of a GH42 β-galactosidase. Microb. Cell Factories 2021, 20, 71. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity 2011, 34, 637–650. [Google Scholar] [CrossRef]
- Fernandez, E.M.; Valenti, V.; Rockel, C.; Hermann, C.; Pot, B.; Boneca, I.G.; Grangette, C. Anti-inflammatory capacity of selected lactobacilli in experimental colitis is driven by NOD2-mediated recognition of a specific peptidoglycan-derived muropeptide. Gut 2011, 60, 1050–1059. [Google Scholar] [CrossRef]
- Liu, Z.; Jiang, Z.; Zhang, Z.; Liu, T.; Fan, Y.; Liu, T.; Peng, N. Bacillus coagulans in Combination with Chitooligosaccharides Regulates Gut Microbiota and Ameliorates the DSS-Induced Colitis in Mice. Microbiol. Spectr. 2022, 10, e00641-22. [Google Scholar] [CrossRef]
- Jung, S.M.; Ha, A.W.; Choi, S.J.; Kim, S.Y.; Kim, W.K. Effect of Bacillus coagulans SNZ 1969 on the Improvement of Bowel Movement in Loperamide-Treated SD Rats. Nutrients 2022, 14, 3710. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Liu, B.; Wang, N.; Yang, J.; Zhou, Q.; Sun, C.; Zhao, Y. Low fish meal diet supplemented with probiotics ameliorates intestinal barrier and immunological function of Macrobrachium rosenbergii via the targeted modulation of gut microbes and derived secondary metabolites. Front. Immunol. 2022, 13, 1074399. [Google Scholar] [CrossRef]
- Zheng, W.; Zhao, Z.; Yang, Y.; Ding, L.; Yao, W. The synbiotic mixture of lactulose and Bacillus coagulans protects intestinal barrier dysfunction and apoptosis in weaned piglets challenged with lipopolysaccharide. J. Anim. Sci. Biotechnol. 2023, 14, 80. [Google Scholar] [CrossRef] [PubMed]
- Dolati, M.; Tafvizi, F.; Salehipour, M.; Komeili Movahed, T.; Jafari, P. Biogenic copper oxide nanoparticles from Bacillus coagulans induced reactive oxygen species generation and apoptotic and anti-metastatic activities in breast cancer cells. Sci. Rep. 2023, 13, 3256. [Google Scholar] [CrossRef] [PubMed]
- Dolati, M.; Tafvizi, F.; Salehipour, M.; Movahed, T.K.; Jafari, P. Inhibitory effects of probiotic Bacillus coagulans against MCF7 breast cancer cells. Iran. J. Microbiol. 2021, 13, 839–847. [Google Scholar] [CrossRef]
- Kumariya, R.; Garsa, A.K.; Rajput, Y.S.; Sood, S.K.; Akhtar, N.; Patel, S. Bacteriocins: Classification, synthesis, mechanism of action and resistance development in food spoilage causing bacteria. Microb. Pathog. 2019, 128, 171–177. [Google Scholar] [CrossRef]
- Moll, G.N.; Konings, W.N.; Driessen, A.J. Bacteriocins: Mechanism of membrane insertion and pore formation. Antonie Van Leeuwenhoek 1999, 76, 185–198. [Google Scholar] [CrossRef]
- Le Marrec, C.; Hyronimus, B.; Bressollier, P.; Verneuil, B.; Urdaci, M.C. Biochemical and genetic characterization of coagulin, a new antilisterial bacteriocin in the pediocin family of bacteriocins, produced by Bacillus coagulans I(4). Appl. Environ. Microbiol. 2000, 66, 5213–5220. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, Y.G.; Khadke, S.K.; Lee, J. Antibiofilm and antifungal activities of medium-chain fatty acids against Candida albicans via mimicking of the quorum-sensing molecule farnesol. Microb. Biotechnol. 2021, 14, 1353–1366. [Google Scholar] [CrossRef]
- Crowley, S.; Mahony, J.; van Sinderen, D. Current Perspectives on Antifungal Lactic Acid Bacteria as Natural Bio-Preservatives. Trends Food Sci. Technol. 2013, 33, 93–109. [Google Scholar] [CrossRef]
- Sjögren, J.; Magnusson, J.; Broberg, A.; Schnürer, J.; Kenne, L. Antifungal 3-hydroxy fatty acids from Lactobacillus plantarum MiLAB 14. Appl. Environ. Microbiol. 2003, 69, 7554–7557. [Google Scholar] [CrossRef]
- Riazi, S.; Wirawan, R.E.; Badmaev, V.; Chikindas, M.L. Characterization of lactosporin, a novel antimicrobial protein produced by Bacillus coagulans ATCC 7050. J. Appl. Microbiol. 2009, 106, 1370–1377. [Google Scholar] [CrossRef]
- Vazquez-Munoz, R.; Dongari-Bagtzoglou, A. Anticandidal Activities by Lactobacillus Species: An Update on Mechanisms of Action. Front. Oral Health 2021, 2, 689382. [Google Scholar] [CrossRef] [PubMed]
- Huszcza, E.; Burczyk, B. Surfactin isoforms from Bacillus coagulans. Z. Für Naturforschung C 2006, 61, 727–733. [Google Scholar] [CrossRef]
- La Fata, G.; Weber, P.; Mohajeri, M.H. Probiotics and the Gut Immune System: Indirect Regulation. Probiotics Antimicrob Proteins 2018, 10, 11–21. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, J.; Cheng, Z.; Wang, T.; Chen, J.; Long, M. Bacillus coagulans TL3 Inhibits LPS-Induced Caecum Damage in Rat by Regulating the TLR4/MyD88/NF-κB and Nrf2 Signal Pathways and Modulating Intestinal Microflora. Oxidative Med. Cell. Longev. 2022, 2022, 5463290. [Google Scholar] [CrossRef] [PubMed]
- Macnaughtan, J.; Figorilli, F.; García-López, E.; Lu, H.; Jones, H.; Sawhney, R.; Suzuki, K.; Fairclough, S.; Marsden, J.; Moratella, A.; et al. A Double-Blind, Randomized Placebo-Controlled Trial of Probiotic Lactobacillus casei Shirota in Stable Cirrhotic Patients. Nutrients 2020, 12, 1651. [Google Scholar] [CrossRef] [PubMed]
- Abada, E.A.E. Isolation and characterization of a antimicrobial compound from Bacillus coagulans. Anim. Cells Syst. 2008, 12, 41–46. [Google Scholar] [CrossRef]
- Abdhul, K.; Ganesh, M.; Shanmughapriya, S.; Vanithamani, S.; Kanagavel, M.; Anbarasu, K.; Natarajaseenivasan, K. Bacteriocinogenic potential of a probiotic strain Bacillus coagulans [BDU3] from Ngari. Int. J. Biol. Macromol. 2015, 79, 800–806. [Google Scholar] [CrossRef] [PubMed]
- Jensen, G.S.; Benson, K.F.; Carter, S.G.; Endres, J.R. GanedenBC30™ cell wall and metabolites: Anti-inflammatory and immune modulating effects in vitro. BMC Immunol. 2010, 11, 15. [Google Scholar] [CrossRef] [PubMed]
- Benson, K.F.; Redman, K.A.; Carter, S.G.; Keller, D.; Farmer, S.; Endres, J.R.; Jensen, G.S. Probiotic metabolites from Bacillus coagulans GanedenBC30™ support maturation of antigen-presenting cells in vitro. World J. Gastroenterol. 2012, 18, 1875–1883. [Google Scholar] [CrossRef] [PubMed]
- Jensen, G.S.; Cash, H.A.; Farmer, S.; Keller, D. Inactivated probiotic Bacillus coagulans GBI-30 induces complex immune activating, anti-inflammatory, and regenerative markers in vitro. J. Inflamm. Res. 2017, 10, 107–117. [Google Scholar] [CrossRef]
- Gao, Y.; Yu, T.; Wu, Y.; Huang, X.; Teng, J.; Zhao, N.; Zheng, X.; Yan, F. Bacillus coagulans (Weizmannia coagulans) XY2 attenuates Cu-induced oxidative stress via DAF-16/FoxO and SKN-1/Nrf2 pathways and gut microbiota regulation. J. Hazard. Mater. 2023, 457, 131741. [Google Scholar] [CrossRef]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids from Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef]
- Kaur, H.; Kaur, G.; Ali, S.A. Postbiotics Implication in the Microbiota-Host Intestinal Epithelial Cells Mutualism. Probiotics Antimicrob. Proteins 2023, 1–16. [Google Scholar] [CrossRef]
- Chen, J.; Cai, J.; Lin, J.; Cheng, Z.; Long, M. Inhibitory Effects of Bacillus coagulans TL3 on the Ileal Oxidative Stress and Inflammation Induced by Lipopolysaccharide in Rats. Curr. Microbiol. 2023, 80, 84. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, Y.; Mu, G.; Xu, Y.; Wang, X.; Tuo, Y.; Qian, F. Protecting Effect of Bacillus coagulans T242 on HT-29 Cells Against AAPH-Induced Oxidative Damage. Probiotics Antimicrob. Proteins 2022, 14, 741–750. [Google Scholar] [CrossRef]
- Vinolo, M.A.; Rodrigues, H.G.; Nachbar, R.T.; Curi, R. Regulation of inflammation by short chain fatty acids. Nutrients 2011, 3, 858–876. [Google Scholar] [CrossRef] [PubMed]
- Borrego, F.; Peña, J.; Solana, R. Regulation of CD69 expression on human natural killer cells: Differential involvement of protein kinase C and protein tyrosine kinases. Eur. J. Immunol. 1993, 23, 1039–1043. [Google Scholar] [CrossRef] [PubMed]
- Moretta, A.; Poggi, A.; Pende, D.; Tripodi, G.; Orengo, A.M.; Pella, N.; Augugliaro, R.; Bottino, C.; Ciccone, E.; Moretta, L. CD69-mediated pathway of lymphocyte activation: Anti-CD69 monoclonal antibodies trigger the cytolytic activity of different lymphoid effector cells with the exception of cytolytic T lymphocytes expressing T cell receptor alpha/beta. J. Exp. Med. 1991, 174, 1393–1398. [Google Scholar] [CrossRef] [PubMed]
- Dons’koi, B.V.; Chernyshov, V.P.; Osypchuk, D.V. Measurement of NK activity in whole blood by the CD69 up-regulation after co-incubation with K562, comparison with NK cytotoxicity assays and CD107a degranulation assay. J. Immunol. Methods 2011, 372, 187–195. [Google Scholar] [CrossRef]
- Clausen, J.; Vergeiner, B.; Enk, M.; Petzer, A.L.; Gastl, G.; Gunsilius, E. Functional significance of the activation-associated receptors CD25 and CD69 on human NK-cells and NK-like T-cells. Immunobiology 2003, 207, 85–93. [Google Scholar] [CrossRef]
- Driesen, J.; Popov, A.; Schultze, J.L. CD25 as an immune regulatory molecule expressed on myeloid dendritic cells. Immunobiology 2008, 213, 849–858. [Google Scholar] [CrossRef]
- Wöbke, T.K.; von Knethen, A.; Steinhilber, D.; Sorg, B.L. CD69 is a TGF-β/1α,25-dihydroxyvitamin D3 target gene in monocytes. PLoS ONE 2013, 8, e64635. [Google Scholar] [CrossRef]
- Dannull, J.; Schneider, T.; Lee, W.T.; de Rosa, N.; Tyler, D.S.; Pruitt, S.K. Leukotriene C4 induces migration of human monocyte-derived dendritic cells without loss of immunostimulatory function. Blood 2012, 119, 3113–3122. [Google Scholar] [CrossRef]
- Suárez, N.; Ferrara, F.; Rial, A.; Dee, V.; Chabalgoity, J.A. Bacterial Lysates as Immunotherapies for Respiratory Infections: Methods of Preparation. Front. Bioeng. Biotechnol. 2020, 8, 545. [Google Scholar] [CrossRef]
- Montazeri-Najafabady, N.; Ghasemi, Y.; Dabbaghmanesh, M.H.; Ashoori, Y.; Talezadeh, P.; Koohpeyma, F.; Abootalebi, S.N.; Gholami, A. Exploring the bone sparing effects of postbiotics in the post-menopausal rat model. BMC Complement. Med. Ther. 2021, 21, 155. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Toalá, J.E.; Hall, F.G.; Urbizo-Reyes, U.C.; Garcia, H.S.; Vallejo-Cordoba, B.; González-Córdova, A.F.; Hernández-Mendoza, A.; Liceaga, A.M. In Silico Prediction and In Vitro Assessment of Multifunctional Properties of Postbiotics Obtained from Two Probiotic Bacteria. Probiotics Antimicrob. Proteins 2020, 12, 608–622. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).