Incidence and Virulence Factor Profiling of Vibrio Species: A Study on Hospital and Community Wastewater Effluents
Abstract
1. Introduction
2. Materials and Methods
2.1. Area of Study
2.2. Sample Collection
2.3. Sample Processing, Cultivation and Identification of Vibrios
2.4. Molecular Identification
Deoxyribonucleic Acid (DNA) Extraction
2.5. Vibrio isolates Confirmation
2.6. Virulence Genes Detection
3. Results
3.1. Prevalence of Vibrio Species
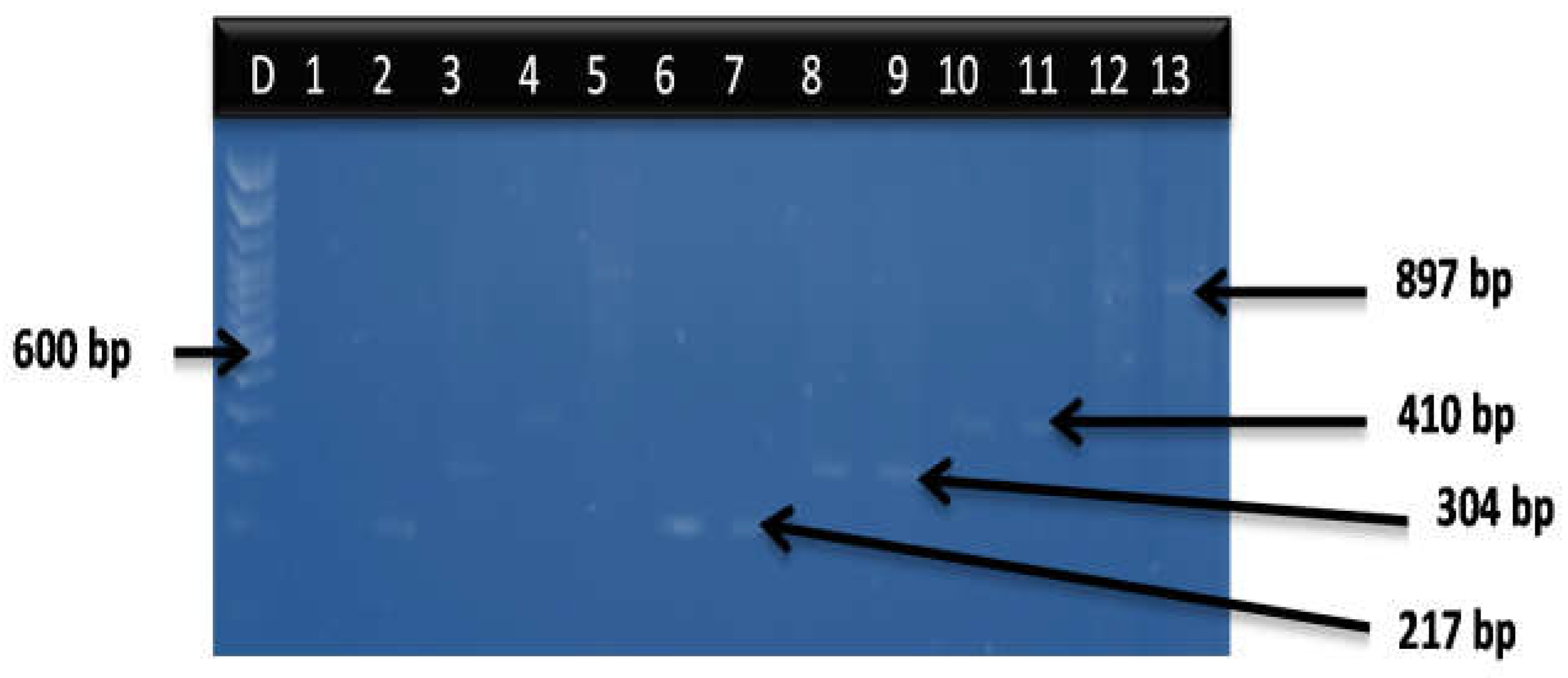
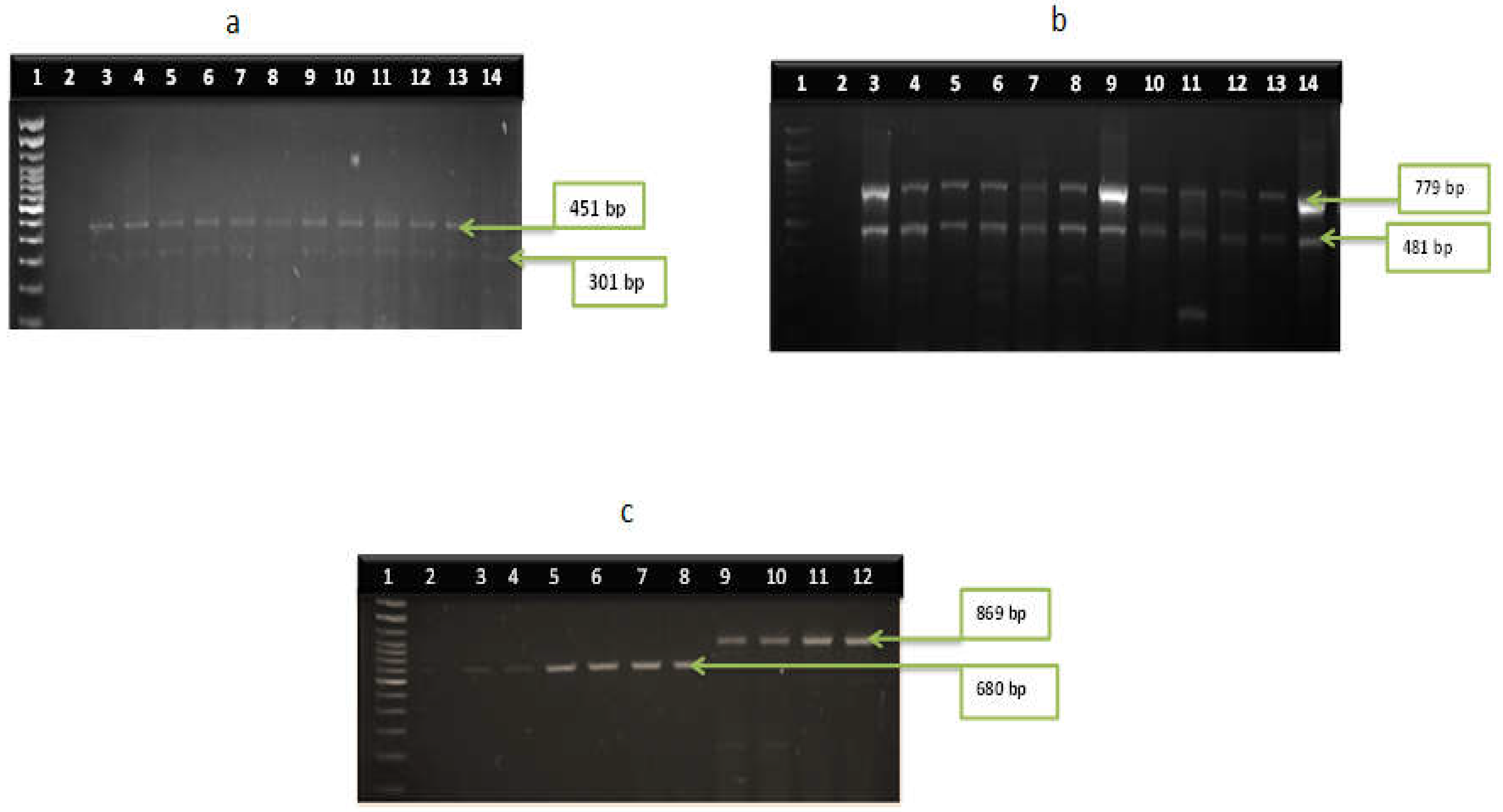
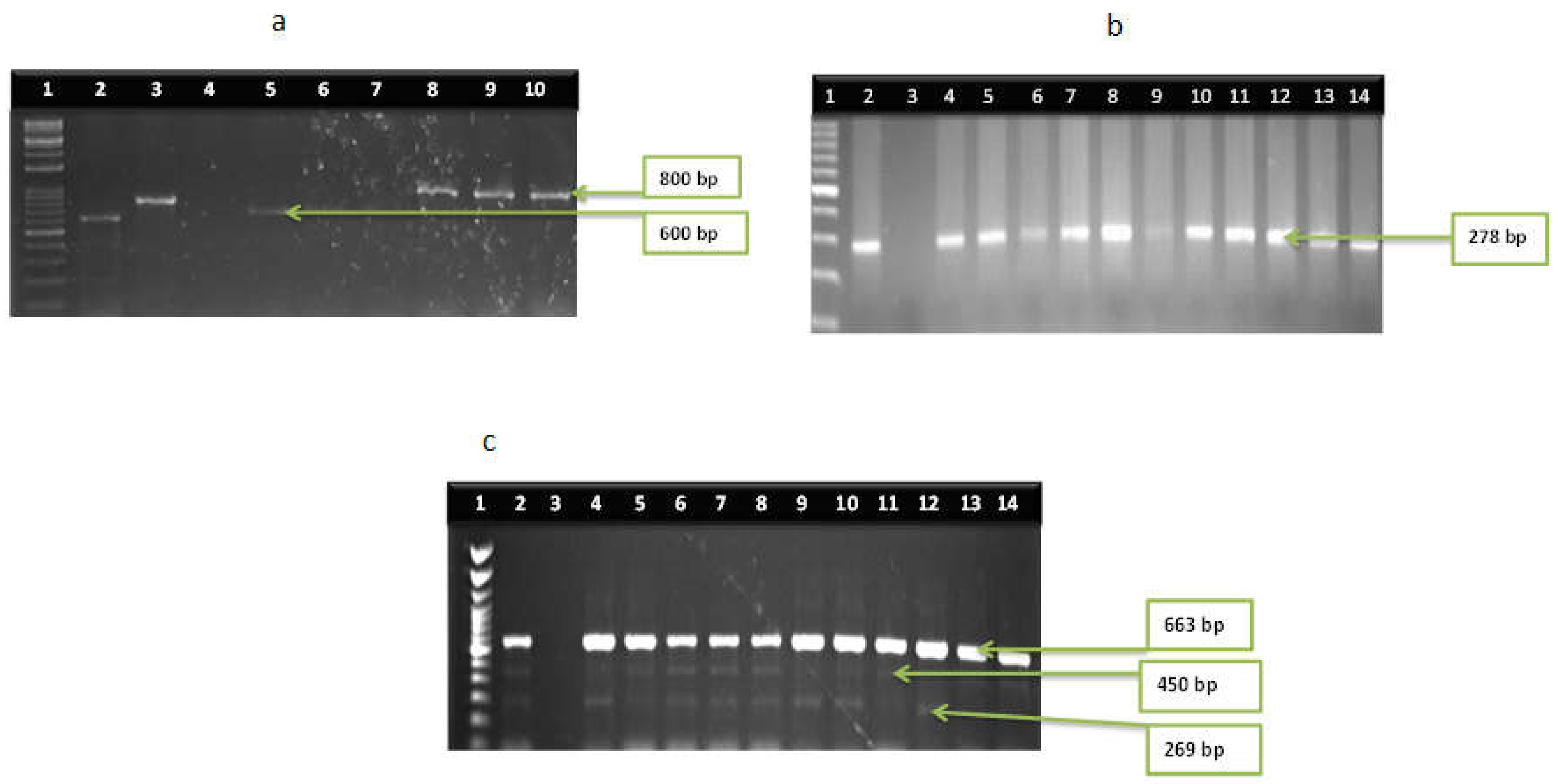
3.2. Distribution of Virulence Genes among the Vibrio Species
4. Discussion
5. Limitation of the Study
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. National Systems to Support Drinking-Water: Sanitation and Hygiene: Global Status Report 2019: UN-Water Global Analysis and Assessment of Sanitation and Drinking-Water: GLAAS 2019 Report; World Health Organization: Geneva, Switzerland, 2019.
- Bloom, D.E.; Cadarette, D. Infectious disease threats in the twenty-first century: Strengthening the global response. Front. Immunol. 2019, 10, 549. [Google Scholar] [CrossRef]
- Weiskopf, S.R.; Rubenstein, M.A.; Crozier, L.G.; Gaichas, S.; Griffis, R.; Halofsky, J.E.; Hyde, K.J.; Morelli, T.L.; Morisette, J.T.; Muñoz, R.C.; et al. Climate change effects on biodiversity, ecosystems, ecosystem services, and natural resource management in the United States. Sci. Total Environ. 2020, 733, 137782. [Google Scholar] [CrossRef]
- Watts, N.; Amann, M.; Arnell, N.; Ayeb-Karlsson, S.; Beagley, J.; Belesova, K.; Boykoff, M.; Byass, P.; Cai, W.; Campbell-Lendrum, D.; et al. The 2020 report of the Lancet Countdown on health and climate change: Responding to converging crises. Lancet 2021, 397, 129–170. [Google Scholar] [CrossRef]
- Economopoulos, A.; Chochlakis, D.; Almpan, M.A.; Sandalakis, V.; Maraki, S.; Tselentis, Y.; Psaroulaki, A. Environmental investigation for the presence of Vibrio species following a case of severe gastroenteritis in a touristic island. Environ. Sci. Pollut. Res. 2017, 24, 4835–4840. [Google Scholar] [CrossRef]
- Trinanes, J.; Martinez-Urtaza, J. Future scenarios of risk of Vibrio infections in a warming planet: A global mapping study. Lancet Planet Health 2021, 5, e426–e435. [Google Scholar] [CrossRef]
- Hoefler, F.; Pouget-Abadie, X.; Roncato-Saberan, M.; Lemarié, R.; Takoudju, E.M.; Raffi, F.; Corvec, S.; Le Bras, M.; Cazanave, C.; Lehours, P.; et al. Clinical and Epidemiologic Characteristics and Therapeutic Management of Patients with Vibrio Infections, Bay of Biscay, France, 2001–2019. Emerg. Infec. Dis. 2022, 28, 2367. [Google Scholar] [CrossRef]
- Ali, M.; Nelson, A.R.; Lopez, A.L.; Sack, D.A. Updated global burden of cholera in endemic countries. PLoS Negl. Trop. Dis. 2015, 9, e0003832. [Google Scholar] [CrossRef]
- Brumfield, K.D.; Cotruvo, J.A.; Shanks, O.C.; Sivaganesan, M.; Hey, J.; Hasan, N.A.; Huq, A.; Colwell, R.R.; Leddy, M.B. Metagenomic sequencing and quantitative real-time PCR for faecal pollution assessment in an urban watershed. Front. Water 2021, 3, 626849. [Google Scholar] [CrossRef]
- Mbanga, J.; Abia, A.L.K.; Amoako, D.G.; Essack, S. Quantitative microbial risk assessment for waterborne pathogens in a wastewater treatment plant and its receiving surface water body. BMC Microbiol. 2020, 20, 346. [Google Scholar] [CrossRef]
- Bonnin-Jusserand, M.; Copin, S.; Le Bris, C.; Brauge, T.; Gay, M.; Brisabois, A.; Grard, T.; Midelet-Bourdin, G. Vibrio species involved in seafood-borne outbreaks (Vibrio cholerae, V. parahaemolyticus and V. vulnificus): Review of microbiological versus recent molecular detection methods in seafood products. Crit. Rev. Food Sci. Nutr. 2019, 59, 597–610. [Google Scholar] [CrossRef]
- Baker-Austin, C.; Oliver, J.D.; Alam, M.; Ali, A.; Waldor, M.K.; Qadri, F.; Martinez-Urtaza, J. Vibrio spp. infections. Nat. Rev. Dis. Primers 2018, 4, 8. [Google Scholar]
- Brehm, T.T.; Berneking, L.; Rohde, H.; Chistner, M.; Schlickewei, C.; Martins, M.S.; Schmiedel, S. Wound infection with Vibrio harveyi following a traumatic leg amputation after a motorboat propeller injury in Mallorca, Spain: A case report and review of literature. BMC Infect. Dis. 2020, 20, 104. [Google Scholar] [CrossRef]
- Costa, W.F.; Giambiagi-deMarval, M.; Laport, M.S. Antibiotic and Heavy Metal Susceptibility of Non-Cholera Vibrio Isolated from Marine Sponges and Sea Urchins: Could They Pose a Potential Risk to Public Health? Antibiotics 2021, 10, 1561. [Google Scholar] [CrossRef]
- Zeaiter, Z.; Mapelli, F.; Crotti, E.; Borin, S. Methods for the genetic manipulation of marine bacteria. Electron. J. Biotechnol. 2018, 33, 17–28. [Google Scholar] [CrossRef]
- Lessler, J.; Moore, S.M.; Luquero, F.J.; McKay, H.S.; Grais, R.; Henkens, M.; Mengel, M.; Dunoyer, J.; M’bangombe, M.; Lee, E.C.; et al. Mapping the burden of cholera in sub-Saharan Africa and implications for control: An analysis of data across geographical scales. Lancet 2018, 391, 1908–1915. [Google Scholar] [CrossRef]
- Tsai, Y.H.; Hsu, R.W.W.; Huang, K.C.; Chen, C.H.; Cheng, C.C.; Peng, K.T.; Huang, T.J. Systemic Vibrio infection presenting as necrotizing fasciitis and sepsis: A series of thirteen cases. JBJS 2004, 86, 2497–2502. [Google Scholar] [CrossRef]
- Yamazaki, K.; Kashimoto, T.; Morita, M.; Kado, T.; Matsuda, K.; Yamasaki, M.; Ueno, S. Identification of in vivo essential genes of Vibrio vulnificus for establishment of wound infection by signature-tagged mutagenesis. Front. Microbiol. 2019, 10, 123. [Google Scholar] [CrossRef]
- Froelich, B.A.; Daines, D.A. In hot water: Effects of climate change on Vibrio–human interactions. Environ. Microbiol. 2020, 22, 4101–4111. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, C.; Zhang, G.; Tian, J.; Liu, Y.; Shen, X.; Feng, J. IS CR 2 is associated with the dissemination of multiple resistance genes among Vibrio spp. and Pseudoalteromonas spp. isolated from farmed fish. Arch. Microbiol. 2017, 199, 891–896. [Google Scholar] [CrossRef]
- Schwartz, K.; Hammerl, J.A.; Göllner, C.; Strauch, E. Environmental and clinical strains of Vibrio cholerae Non-O1, Non-O139 from Germany possess similar virulence gene profiles. Front. Microbiol. 2019, 10, 733. [Google Scholar] [CrossRef]
- Okada, K.; Wongboot, W.; Chantaroj, S.; Natakuathung, W.; Roobthaisong, A.; Kamjumphol, W.; Maruyama, F.; Takemura, T.; Nakagawa, I.; Ohnishi, M.; et al. Vibrio cholerae embraces two major evolutionary traits as revealed by targeted gene sequencing. Sci. Rep. 2018, 8, 1631. [Google Scholar] [CrossRef]
- Feglo, P.K.; Sewurah, M. Characterization of highly virulent multidrug resistant Vibrio cholerae isolated from a large cholera outbreak in Ghana. BMC Res. Notes 2018, 11, 45. [Google Scholar] [CrossRef]
- Gxalo, O.; Digban, T.O.; Igere, B.E.; Olapade, O.A.; Okoh, A.I.; Nwodo, U.U. Virulence and Antibiotic Resistance Characteristics of Vibrio Isolates from Rustic Environmental Freshwaters. Front. Cell. Infect. Microbiol. 2021, 765, 732001. [Google Scholar] [CrossRef]
- Akoachere, J.F.T.K.; Masalla, T.N.; Njom, H.A. Multi-drug resistant toxigenic Vibrio cholerae O1 is persistent in water sources in New Bell-Douala, Cameroon. BMC Infec. Dis. 2013, 13, 366. [Google Scholar] [CrossRef]
- Kalule, J.B.; Smith, A.M.; Vulindhlu, M.; Tau, N.P.; Nicol, M.P.; Keddy, K.H.; Robberts, L. Prevalence and antibiotic susceptibility patterns of enteric bacterial pathogens in human and non-human sources in an urban informal settlement in Cape Town, South Africa. BMC Microbiol. 2019, 19, 244. [Google Scholar] [CrossRef]
- Edokpayi, J.N.; Odiyo, J.O.; Durowoju, O.S. Impact of wastewater on surface water quality in developing countries: A case study of South Africa. Water Qual. 2017, 10, 10–5772. [Google Scholar]
- Lépesová, K.; Olejníková, P.; Mackuľak, T.; Cverenkárová, K.; Krahulcová, M.; Bírošová, L. Hospital Wastewater—Important Source of Multidrug Resistant Coliform Bacteria with ESBL-Production. Int. J. Environ. Res. Public Health 2020, 17, 7827. [Google Scholar] [CrossRef]
- American Public Health Association. Standard Methods for the Examination of Water and Wastewater Analysis; American Public Health Association: Washington, DC, USA, 1998. [Google Scholar]
- Mapipa, Q.; Digban, T.O.; Nnolim, N.E.; Nwodo, U.U. Antibiogram profile and virulence signatures of Pseudomonas aeruginosa isolates recovered from selected agrestic hospital effluents. Sci. Rep. 2021, 11, 11800. [Google Scholar] [CrossRef]
- Kwok, A.Y.; Wilson, J.T.; Coulthart, M.; Ng, L.K.; Mutharia, L.; Chow, A.W. Phylogenetic study and identification of human pathogenic Vibrio species based on partial hsp 60 gene sequences. Can. J. Microbiol. 2002, 48, 903–910. [Google Scholar] [CrossRef]
- Alam, M.; Sultana, M.; Nair, G.B.; Sack, R.B.; Sack, D.A.; Siddique, A.K.; Ali, A.; Huq, A.; Colwell, R.R. Toxigenic Vibrio cholerae in the aquatic environment of Mathbaria, Bangladesh. Appl. Environ. Microbiol. 2006, 72, 2849–2855. [Google Scholar] [CrossRef]
- Tarr, C.L.; Patel, J.S.; Puhr, N.D.; Sowers, E.G.; Bopp, C.A.; Strockbine, N.A. Identification of Vibrio isolates by a multiplex PCR assay and rpoB sequence determination. J. Clin. Microbiol. 2007, 45, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, R.; Sinha, S.; Mukhopadhyay, A.K.; Asakura, M.; Yamasaki, S.; Bhattacharya, S.K.; Nair, G.B.; Ramamurthy, T. Species-specific identification of Vibrio fluvialis by PCR targeted to the conserved transcriptional activation and variable membrane tether regions of the toxR gene. J. Med. Microbiol. 2006, 55, 805–808. [Google Scholar] [CrossRef] [PubMed]
- Shinoda, S.; Nakagawa, T.; Shi, L.; Bi, K.; Kanoh, Y.; Tomochika, K.I.; Miyoshi, S.I.; Shimada, T. Distribution of virulence-associated genes in Vibrio mimicus isolates from clinical and environmental origins. Microbiol. Immunol. 2004, 48, 547–551. [Google Scholar] [CrossRef] [PubMed]
- Mantri, C.K.; Mohapatra, S.S.; Ramamurthy, T.; Ghosh, R.; Colwell, R.R.; Singh, D.V. Septaplex PCR assay for rapid identification of Vibrio cholerae including detection of virulence and int SXT genes. FEMS Microbiol. Lett. 2006, 265, 208–214. [Google Scholar] [CrossRef][Green Version]
- Singh, D.V.; Isac, S.R.; Colwell, R.R. Development of a hexaplex PCR assay for rapid detection of virulence and regulatory genes in Vibrio cholerae and Vibrio mimicus. J. Clin. Microbiol. 2002, 40, 4321–4324. [Google Scholar] [CrossRef]
- Bi, K.; Miyoshi, S.I.; Tomochika, K.I.; Shinoda, S. Detection of virulence associated genes in clinical strains of Vibrio mimicus. Microbiol. Immunol. 2001, 45, 613–616. [Google Scholar] [CrossRef]
- Xie, Z.Y.; Hu, C.Q.; Chen, C.; Zhang, L.P.; Ren, C.H. Investigation of seven Vibrio virulence genes among Vibrio alginolyticus and Vibrio parahaemolyticus strains from the coastal mariculture systems in Guangdong, China. Lett. Appl. Microbiol. 2005, 41, 202–207. [Google Scholar] [CrossRef]
- Rivera, I.N.; Chun, J.; Huq, A.; Sack, R.B.; Colwell, R.R. Genotypes associated with virulence in environmental isolates of Vibrio cholerae. Appl. Environ. Microbiol. 2001, 67, 2421–2429. [Google Scholar] [CrossRef]
- Rosche, T.M.; Yano, Y.; Oliver, J.D. A rapid and simple PCR analysis indicates there are two subgroups of Vibrio vulnificus which correlate with clinical or environmental isolation. Microbiol. Immunol. 2005, 49, 381–389. [Google Scholar] [CrossRef]
- Liang, P.; Cui, X.; Du, X.; Kan, B.; Liang, W. The virulence phenotypes and molecular epidemiological characteristics of Vibrio fluvialis in China. Gut Pathog. 2013, 5, 6. [Google Scholar] [CrossRef]
- Rojas, M.V.R.; Matté, M.H.; Dropa, M.; Silva, M.L.D.; Matté, G.R. Characterization of Vibrio parahaemolyticus isolated from oysters and mussels in São Paulo, Brazil. Rev. Inst. Med. Trop. Sao 2011, 53, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, N.; Amal, M.N.A.; Saad, M.Z.; Yasin, I.S.M.; Zulkiply, N.A.; Mustafa, M.; Nasruddin, N.S. Virulence-associated genes and antibiotic resistance patterns of Vibrio spp. isolated from cultured marine fishes in Malaysia. BMC Vet. Res. 2019, 15, 176. [Google Scholar] [CrossRef] [PubMed]
- Hoekstra, A.Y.; Mekonnen, M.M.; Chapagain, A.K.; Mathews, R.E.; Richter, B.D. Global monthly water scarcity: Blue water footprints versus blue water availability. PLoS ONE 2012, 7, e32688. [Google Scholar] [CrossRef] [PubMed]
- Chahal, C.; Van Den Akker, B.; Young, F.; Franco, C.; Blackbeard, J.; Monis, P. Pathogen and particle associations in wastewater: Significance and implications for treatment and disinfection processes. Adv. Appl. Microbiol. 2016, 97, 63–119. [Google Scholar] [PubMed]
- Zagui, G.S.; Tonani, K.A.A.; Fregonesi, B.M.; Machado, G.P.; Silva, T.V.; Andrade, L.N.; Andrade, D.; Segura-Muñoz, S.I. Tertiary hospital sewage as reservoir of bacteria expressing MDR phenotype in Brazil. Braz. J. Biol. 2021, 82, e234471. [Google Scholar] [CrossRef]
- Maje, M.D.; Kaptchouang Tchatchouang, C.D.; Manganyi, M.C.; Fri, J.; Ateba, C.N. Characterisation of Vibrio species from surface and drinking water sources and assessment of biocontrol potentials of their bacteriophages. Int. J. Microbiol. 2020, 2020, 8863370. [Google Scholar] [CrossRef]
- Bakhshi, B.; Barzelighi, H.M.; Adabi, M.; Lari, A.R.; Pourshafie, M.R. A molecular survey on virulence associated genotypes of non-O1 non-O139 Vibrio cholerae in aquatic environment of Tehran, Iran. Water Res. 2009, 43, 1441–1447. [Google Scholar] [CrossRef]
- Canigral, I.; Moreno, Y.; Alonso, J.L.; González, A.; Ferrús, M.A. Detection of Vibrio vulnificus in seafood, seawater and wastewater samples from a Mediterranean coastal area. Microbiol. Res. 2010, 165, 657–664. [Google Scholar] [CrossRef]
- Kokashvili, T.; Whitehouse, C.A.; Tskhvediani, A.; Grim, C.J.; Elbakidze, T.; Mitaishvili, N.; Janelidze, N.; Jaiani, E.; Haley, B.J.; Lashkhi, N.; et al. Occurrence and diversity of clinically important Vibrio species in the aquatic environment of Georgia. Front. Public Health 2015, 3, 232. [Google Scholar] [CrossRef]
- CDC. Vibrio Species Causing Vibriosis—Symptoms. 2019. Available online: https://www.cdc.gov/vibrio/symptoms.html (accessed on 2 September 2023).
- Sharma, A.K.; Dhasmana, N.; Dubey, N.; Kumar, N.; Gangwal, A.; Gupta, M.; Singh, Y. Bacterial virulence factors: Secreted for survival. Indian J. Microbiol. 2017, 57, 1–10. [Google Scholar] [CrossRef]
- Abd El-Baky, R.M.; Ibrahim, R.A.; Mohamed, D.S.; Ahmed, E.F.; Hashem, Z.S. Prevalence of virulence genes and their association with antimicrobial resistance among pathogenic E. coli isolated from Egyptian patients with different clinical infections. Infec. Drug Resist. 2020, 13, 1221. [Google Scholar] [CrossRef] [PubMed]
- Kirkup, B.C.; Chang, L.; Chang, S.; Gevers, D.; Polz, M.F. Vibrio chromosomes share common history. BMC Microbiol. 2010, 10, 137. [Google Scholar] [CrossRef] [PubMed]
- Gennari, M.; Ghidini, V.; Caburlotto, G.; Lleo, M.M. Virulence genes and pathogenicity islands in environmental Vibrio strains non-pathogenic to humans. FEMS Microbiol. Ecol. 2012, 82, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, E.; Ochi, S.; Mizuno, T.; Morita, D.; Morita, M.; Ohnishi, M.; Koley, H.; Dutta, M.; Chowdhury, G.; Mukhopadhyay, A.K.; et al. Virulence of Cholera Toxin Gene-Positive Vibrio cholerae Non-O1/non-O139 Strains Isolated From Environmental Water in Kolkata, India. Front. Microbiol. 2021, 12, 2439. [Google Scholar] [CrossRef] [PubMed]
- Hounmanou, Y.M.; Mdegela, R.H.; Dougnon, T.V.; Mhongole, O.J.; Mayila, E.S.; Malakalinga, J.; Makingi, G.; Dalsgaard, A. Toxigenic Vibrio cholerae O1 in vegetables and fish raised in wastewater irrigated fields and stabilization ponds during a non-cholera outbreak period in Morogoro, Tanzania: An environmental health study. BMC Res. Notes 2016, 9, 466. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Zhang, J.; Lou, J.; Li, J.; Qin, Q.; Shi, Q.; Zhang, Y.; Kan, B. Direct Binding and Regulation by Fur and HapR of the Intermediate Regulator and Virulence Factor Genes within the ToxR Virulence Regulon in Vibrio cholerae. Front. Microbiol. 2020, 11, 709. [Google Scholar] [CrossRef]
- Alishahi, A.; Fooladi, A.I.; Mehrabadi, J.F.; Hosseini, H.M. Facile and rapid detection of Vibrio cholerae by Multiplex PCR based on ompU, ctxA, and toxR Genes. Jundishapur J. Microbiol. 2013, 6, 5. [Google Scholar] [CrossRef]
- Matson, J.S.; Withey, J.H.; DiRita, V.J. Regulatory networks controlling Vibrio cholerae virulence gene expression. Infect. Immun. 2007, 75, 5542–5549. [Google Scholar] [CrossRef]
- Marashi, S.M.A.; Bakhshi, B.; Fooladi, A.A.I.; Tavakoli, A.; Sharifnia, A.; Pourshafie, M.R. Quantitative expression of cholera toxin mRNA in Vibrio cholerae isolates with different CTX cassette arrangements. J. Med. Microbiol. 2012, 61, 1071–1073. [Google Scholar] [CrossRef][Green Version]
- Kumar, A.; Das, B.; Kumar, N. Vibrio pathogenicity island-1: The master determinant of cholera pathogenesis. Front. Cell. Infect. Microbiol. 2020, 10, 561296. [Google Scholar] [CrossRef]
- Castillo, D.; Kauffman, K.; Hussain, F.; Kalatzis, P.; Rørbo, N.; Polz, M.F.; Middelboe, M. Widespread distribution of prophage-encoded virulence factors in marine Vibrio communities. Sci. Rep. 2018, 8, 9973. [Google Scholar] [CrossRef] [PubMed]
- Mauritzen, J.J.; Castillo, D.; Tan, D.; Svenningsen, S.L.; Middelboe, M. Beyond cholera: Characterization of zot-encoding filamentous phages in the marine fish pathogen Vibrio anguillarum. Viruses 2020, 12, 730. [Google Scholar] [CrossRef] [PubMed]
- Chomvarin, C.; Jumroenjit, W.; Tangkanakul, W.; Hasan, N.A.; Chaicumpar, K.; Faksri, K.; Huq, A. Genotype and drug resistance of clinical and environmental Vibrio cholerae non-O1/non-O139 in Northeastern Thailand. Southeast Asian J. Trop. Med. Public Health 2014, 45, 1354–1364. [Google Scholar] [PubMed]
- Danso, E.K.; Asare, P.; Otchere, I.D.; Akyeh, L.M.; Asante-Poku, A.; Aboagye, S.Y.; Osei-Wusu, S.; Opare, D.; Ntoumi, F.; Zumla, A.; et al. A molecular and epidemiological study of Vibrio cholerae isolates from cholera outbreaks in southern Ghana. PLoS ONE 2020, 15, e0236016. [Google Scholar] [CrossRef]
- Ramamurthy, T.; Chowdhury, G.; Pazhani, G.P.; Shinoda, S. Vibrio fluvialis: An emerging human pathogen. Front. Microbiol. 2014, 5, 91. [Google Scholar] [CrossRef]
- D’Souza, C.; Prithvisagar, K.S.; Deekshit, V.K.; Karunasagar, I.; Karunasagar, I.; Kumar, B.K. Exploring the Pathogenic Potential of Vibrio vulnificus isolated from Seafood Harvested along the Mangaluru Coast, India. Microorganisms 2020, 8, 999. [Google Scholar] [CrossRef]
- Fri, J.; Ndip, R.N.; Njom, H.A.; Clarke, A.M. Occurrence of virulence genes associated with human pathogenic vibrios isolated from two commercial dusky kob (Argyrosmus japonicus) farms and Kareiga estuary in the Eastern Cape Province, South Africa. Int. J. Environ. Res. Public Health 2017, 14, 1111. [Google Scholar] [CrossRef]
- Nagarajan, U.M.; Prantner, D.; Sikes, J.D.; Andrews Jr, C.W.; Goodwin, A.M.; Nagarajan, S.; Darville, T. Type I interferon signalling exacerbates Chlamydia muridarum genital infection in a murine model. Infec. Immun. 2008, 76, 4642–4648. [Google Scholar] [CrossRef]
- Lu, X.; Liang, W.; Wang, Y.; Xu, J.; Zhu, J.; Kan, B. Identification of genetic bases of Vibrio fluvialis species-specific biochemical pathways and potential virulence factors by comparative genomic analysis. Appl. Environ. Microbiol. 2014, 80, 2029–2037. [Google Scholar] [CrossRef]
- Song, L.; Huang, Y.; Zhao, M.; Wang, Z.; Sun, H.; Wang, S.; Kan, B.; Meng, G.; Liang, W.; Ren, Z. A critical role for haemolysin in Vibrio fluvialis-induced IL-1β secretion mediated by the NLRP3 inflammasome in macrophages. Front. Microbiol. 2015, 6, 510. [Google Scholar] [CrossRef]
- Chowdhury, G.; Pazhani, G.P.; Dutta, D.; Guin, S.; Dutta, S.; Ghosh, S.; Izumiya, H.; Asakura, M.; Yamasaki, S.; Takeda, Y.; et al. Vibrio fluvialis in patients with diarrhea, Kolkata, India. Emerg. Infect. Dis. 2012, 18, 1868. [Google Scholar] [CrossRef] [PubMed]
- Ralston, E.P.; Kite-Powell, H.; Beet, A. An estimate of the cost of acute health effects from food-and water-borne marine pathogens and toxins in the USA. J. Water Health 2011, 9, 680–694. [Google Scholar] [CrossRef] [PubMed]
- Luan, X.Y.; Chen, J.X.; Zhang, X.H.; Jia, J.T.; Sun, F.R.; Li, Y. Comparison of different primers for rapid detection of Vibrio parahaemolyticus using the polymerase chain reaction. Lett. Appl. Microbiol. 2007, 44, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Almuhaideb, E.; Chintapenta, L.K.; Abbott, A.; Parveen, S.; Ozbay, G. Assessment of Vibrio parahaemolyticus levels in oysters (Crassostrea virginica) and seawater in Delaware Bay in relation to environmental conditions and the prevalence of molecular markers to identify pathogenic Vibrio parahaemolyticus strains. PLoS ONE 2020, 15, 0242229. [Google Scholar] [CrossRef] [PubMed]
- Guin, S.; Saravanan, M.; Chowdhury, G.; Pazhani, G.P.; Ramamurthy, T.; Das, S.C. Pathogenic Vibrio parahaemolyticus in diarrhoeal patients, fish and aquatic environments and their potential for inter-source transmission. Heliyon 2019, 5, e01743. [Google Scholar] [CrossRef]


| Municipality | Sampling Site | Coordinates |
|---|---|---|
| Buffalo City | Tertiary hospital wastewater effluent (THWE) | 32°55′37″ S 27°44′42″ E |
| Amathole District | Secondary hospital wastewater effluent (SHWE) | 32°77′53″ S 26°84′64″ E |
| Limbede community wastewater (LCWE) | 32°77′53″ S 26°84′64″ E |
| Target Organism | Gene Target | Oligonucleotide Sequence (5′-3′) | Length (bp) | PCR Cycling Conditions | References |
|---|---|---|---|---|---|
| Vibrio genus | 16S rRNA | FP:CGG TGAAATGCGTAGAGAT RP:TACTAGCGATTCCGAGTTC | 663 | Firstly, denaturation at 93 °C for 15 min accompanied by 35 cycles of denaturation at 92 °C for 40 s, annealing at 57 °C for 1 min, elongation at 72 °C for 1.5 min and lastly, elongation at 72 °C for 7 min | [31] |
| V. cholerae | OmpW | FP:CACCAAGAAGGTGACTTTATTGTG RP:GGTTTGTCGAATTAGCTTCACC | 304 | Firstly, denaturation at 93 °C for 15 min accompanied by 35 cycles of denaturation: 92 °C for 40 s, annealing: 57 °C for 1 min, elongation 72 °C for 1.5 min, and lastly, elongation at 72 °C for 7 min | [32] |
| V. parahaemolyticus | flaE | FP:GCAGCTGATCAAAACGTTGAGT RP:ATTATCGATCGTGCCACTCAC | 897 | Firstly, denaturation at 94 °C for 5 min, accompanied by 30 cycles of 94 °C for 40 s, 64 °C for 40 s, 72 °C for 90 s, and lastly, elongation at 72 °C for 7 min | [33] |
| V. vulnificus | Hsp0 | FP: GTCTTAAAGCGGTTGCTGC RP: CGCTTCAAGTGCTGGTAGAAG | 410 | Firstly, denaturation at 94 °C for 5 min, accompanied by 35 cycles of 94 °C for 30 s, 55 °C for 30 s, 72 °C for 30 s, and lastly, elongation at 72 °C for 10 min | [33] |
| V. fluvialis | toxR | FP:GACCAG GGCTTTGAGGTGGAC RP:GGATACGGCACTTGAGTAAGACTC | 217 | Firstly, denaturation at 94 °C for 5 min, accompanied by 30 cycles of 94 °C for 40 s, 65 °C for 40 s, 72 °C for 1 min, and lastly elongation at 72 °C for 7 min | [34] |
| Species | Gene | Oligonucleotide Sequence (5′-3′) | Amplicon Size (bp) | Annealing Temp (°C) | References |
|---|---|---|---|---|---|
| V. cholerae | tcpA | F:GAAGAAGTTTRTAAAAGAAGAACA R:GAAAGGACCTTCTTTCACGTTG | 451 | 55 | [35] |
| toxR | F:ATGTTCGGATTAGGACAC R:TACTCACACACTTTGATGGC | 779 | 60 | [36] | |
| ompU | F:ACGCTGACGGAATCAACCAAAG R:GCGGAAGTTTGGCTTGAAGTAG | 869 | 62 | [37] | |
| zot | F:TCGCTTAACGATGGCGCGTTTT R:AACCCCGTTTCACTTCTACCCA | 947 | 62 | [37] | |
| ctx | F:CTCAGACGGGATTTGTTAGGCACG R:TCTATCTCTGTAGCCCCTATTACG | 301 | 55 | [38] | |
| VPI | F:GCAATTTAGGGGCGCGACGT R:CCGCTCTTTCTTGATCTGGTAG | 680 | 52 | [39] | |
| hylA | F:GAGCCGGCATTCATC TGAAT R:CTCAGCGGGCTAATACGGTTTA | 481 | 60 | [40] | |
| V. vulnificus | vcgA | F:AGCTGCCGATAGCGATCT R:CGCTTAGGATGATCGGTG | 278 | 56 | [41] |
| vcgB | F:CTCAATTGACAATGATCT R:CGCTTAGGATGATCGGTG | 278 | 56 | [41] | |
| V. fluvialis | vfh | F:GCGCGTCAGTGGTGGTGAAG R:TCGGTCGAACCGCTCTCGCTT | 800 | 61 | [42] |
| hupO | F:ATTACGCACAACGAGTCGAAC R:ATTGAGATGGTAAACAGCGCC | 600 | 56 | [42] | |
| vfpA | F:TACAACGTCAAGTTAAAGGC R:GTAGGCGCTGTAGCCTTTCA | 1790 | 55 | [42] | |
| V. parahaemolyticus | Stn | F:GGTGCAACATAATAAACAGTCAACAA R:TAGTGGTATGCGTTGCCAGC | 375 | 53 | [42] |
| Tdh | F-GTAAAGGTCTCTGACTTT TGGAC R-TGGAATAGAACCTTCATCTTCACC | 269 | 58 | [43] | |
| Tlh | F:AAAGCGGATTATGCAGAAGCACTG R:GCTACTTTCTAGCATTTTCTCTGC | 450 | 58 | [44] | |
| Trh | F:TTGGCTTCGATATTTTCAGTATCT R:CATAACAAACATATGCCCATTTCCG | 500 | 58 | [43] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mavhungu, M.; Digban, T.O.; Nwodo, U.U. Incidence and Virulence Factor Profiling of Vibrio Species: A Study on Hospital and Community Wastewater Effluents. Microorganisms 2023, 11, 2449. https://doi.org/10.3390/microorganisms11102449
Mavhungu M, Digban TO, Nwodo UU. Incidence and Virulence Factor Profiling of Vibrio Species: A Study on Hospital and Community Wastewater Effluents. Microorganisms. 2023; 11(10):2449. https://doi.org/10.3390/microorganisms11102449
Chicago/Turabian StyleMavhungu, Mashudu, Tennison O. Digban, and Uchechukwu U. Nwodo. 2023. "Incidence and Virulence Factor Profiling of Vibrio Species: A Study on Hospital and Community Wastewater Effluents" Microorganisms 11, no. 10: 2449. https://doi.org/10.3390/microorganisms11102449
APA StyleMavhungu, M., Digban, T. O., & Nwodo, U. U. (2023). Incidence and Virulence Factor Profiling of Vibrio Species: A Study on Hospital and Community Wastewater Effluents. Microorganisms, 11(10), 2449. https://doi.org/10.3390/microorganisms11102449






