The Molecular Identification and Antifungal Susceptibility of Clinical Isolates of Aspergillus Section Flavi from Three French Hospitals
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Patients and Isolates
2.2. Molecular Identification
2.3. Antifungal Susceptibility Testing
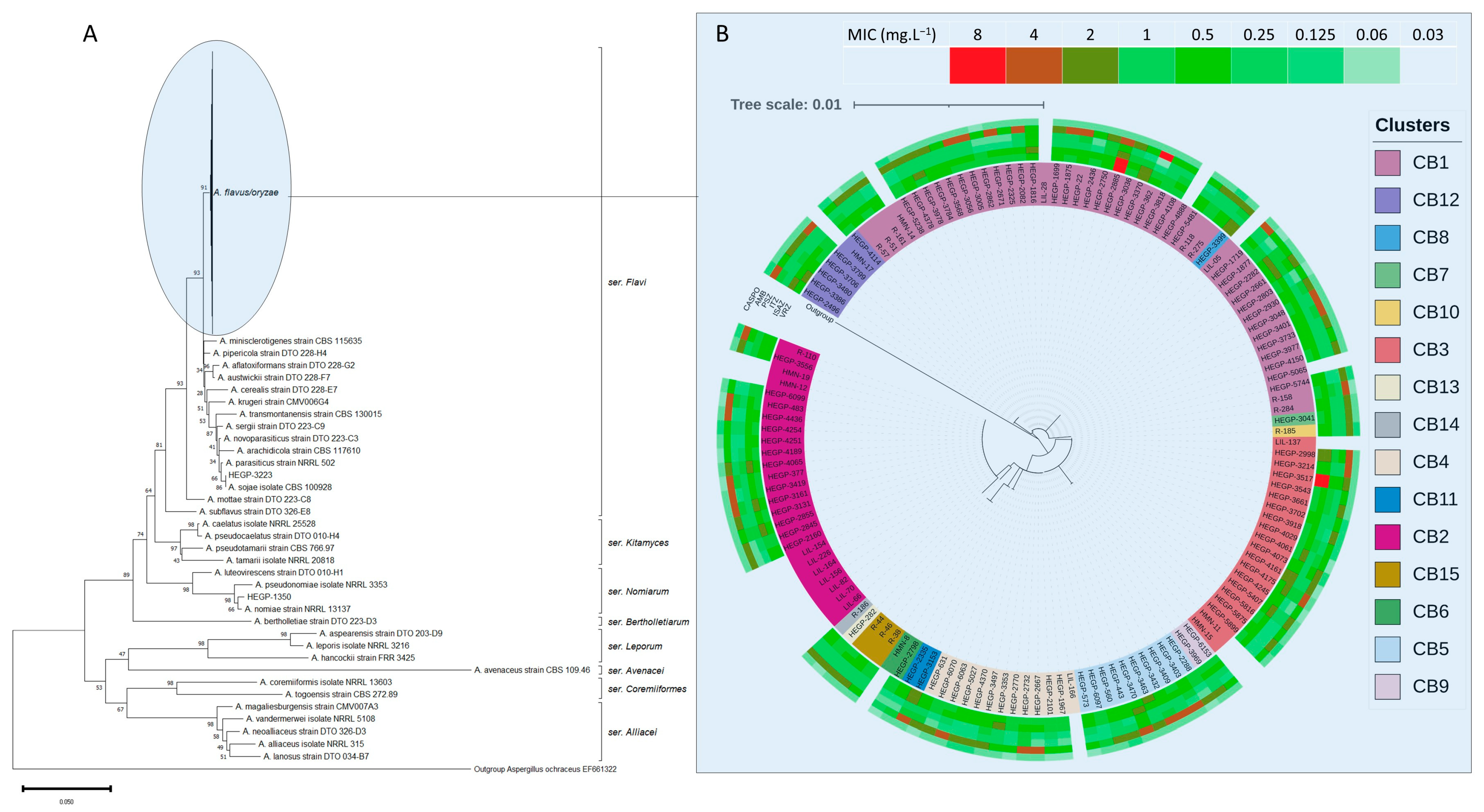
3. Results
3.1. Molecular Identification and Cryptic Species
3.2. Antifungal Susceptibility Testing and Azole MICs Distribution
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hedayati, M.T.; Pasqualotto, A.C.; Warn, P.A.; Bowyer, P.; Denning, D.W. Aspergillus flavus: Human Pathogen, Allergen and Mycotoxin Producer. Microbiology 2007, 153, 1677–1692. [Google Scholar] [CrossRef] [PubMed]
- Rudramurthy, S.M.; Paul, R.A.; Chakrabarti, A.; Mouton, J.W.; Meis, J.F. Invasive Aspergillosis by Aspergillus flavus: Epidemiology, Diagnosis, Antifungal Resistance, and Management. J. Fungi 2019, 5, 55. [Google Scholar] [CrossRef] [PubMed]
- Bernardeschi, C.; Foulet, F.; Ingen-Housz-Oro, S.; Ortonne, N.; Sitbon, K.; Quereux, G.; Lortholary, O.; Chosidow, O.; Bretagne, S. Cutaneous Invasive Aspergillosis: Retrospective Multicenter Study of the French Invasive-Aspergillosis Registry and Literature Review. Medicine 2015, 94, e1018. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, A.; Gupta, V.; Biswas, G.; Kumar, B.; Sakhuja, V.K. Primary Cutaneous Aspergillosis: Our Experience in 10 Years. J. Infect. 1998, 37, 24–27. [Google Scholar] [CrossRef] [PubMed]
- Adoubryn, K.D.; N’Gattia, V.K.; Kouadio-Yapo, G.C.; Nigué, L.; Zika, D.K.; Ouhon, J. Epidemiology of otomycoses at the University Hospital of Yopougon (Abidjan-Ivory Coast). J. Mycol. Med. 2014, 24, e9–e15. [Google Scholar] [CrossRef]
- Al-Hatmi, A.M.S.; Castro, M.A.; de Hoog, G.S.; Badali, H.; Alvarado, V.F.; Verweij, P.E.; Meis, J.F.; Zago, V.V. Epidemiology of Aspergillus Species Causing Keratitis in Mexico. Mycoses 2019, 62, 144–151. [Google Scholar] [CrossRef]
- Manikandan, P.; Varga, J.; Kocsubé, S.; Anita, R.; Revathi, R.; Németh, T.M.; Narendran, V.; Vágvölgyi, C.; Panneer Selvam, K.; Shobana, C.S.; et al. Epidemiology of Aspergillus Keratitis at a Tertiary Care Eye Hospital in South India and Antifungal Susceptibilities of the Causative Agents. Mycoses 2013, 56, 26–33. [Google Scholar] [CrossRef]
- Cuadros, J.; Gros-Otero, J.; Gallego-Angui, P.; Scheu, A.K.; Montes-Mollon, A.; Pérez-Rico, C.; Moreno, J.P.; Gomez-Herruz, P.; Soliveri, J.; Teus, M. Aspergillus tamarii Keratitis in a Contact Lens Wearer. Med. Mycol. Case Rep. 2017, 11, 21–24. [Google Scholar] [CrossRef]
- Kredics, L.; Varga, J.; Kocsubé, S.; Doczi, I.; Samson, R.A.; Rajaraman, R.; Narendran, V.; Bhaskar, M.; Vagvolgyi, C.; Manikandan, P. Case of Keratitis Caused by Aspergillus tamarii. J. Clin. Microbiol. 2007, 45, 3464–3467. [Google Scholar] [CrossRef]
- Homa, M.; Manikandan, P.; Szekeres, A.; Kiss, N.; Kocsubé, S.; Kredics, L.; Papp, T. Characterization of Aspergillus tamarii Strains from Human Keratomycoses: Molecular Identification, Antifungal Susceptibility Patterns and Cyclopiazonic Acid Producing Abilities. Front. Microbiol. 2019, 10, 2249. [Google Scholar] [CrossRef]
- Baranyi, N.; Kocsubé, S.; Szekeres, A.; Raghavan, A.; Narendran, V.; Vágvölgyi, C.; Panneer Selvam, K.; Babu Singh, Y.R.; Kredics, L.; Varga, J.; et al. Keratitis Caused by Aspergillus pseudotamarii. Med. Mycol. Case Rep. 2013, 2, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Pichon, M.; Joly, V.; Argy, N.; Houze, S.; Bretagne, S.; Alanio, A.; Wassef, M.; Verillaud, B.; Yazdanpanah, Y. Aspergillus flavus Malignant External Otitis in a Diabetic Patient: Case Report and Literature Review. Infection 2020, 48, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Morgand, M.; Rammaert, B.; Poirée, S.; Bougnoux, M.-E.; Tran, H.; Kania, R.; Chrétien, F.; Jouvion, G.; Lortholary, O. Chronic Invasive Aspergillus Sinusitis and Otitis with Meningeal Extension Successfully Treated with Voriconazole. Antimicrob. Agents Chemother. 2015, 59, 7857–7861. [Google Scholar] [CrossRef] [PubMed]
- Marchionni, E.; Parize, P.; Lefevre, A.; Vironneau, P.; Bougnoux, M.E.; Poiree, S.; Coignard-Biehler, H.; DeWolf, S.E.; Amazzough, K.; Barchiesi, F.; et al. Aspergillus spp. Invasive External Otitis: Favourable Outcome with a Medical Approach. Clin. Microbiol. Infect. 2016, 22, 434–437. [Google Scholar] [CrossRef] [PubMed]
- Moniot, M.; Montava, M.; Ranque, S.; Scemama, U.; Cassagne, C.; Arthur, V. Malignant Aspergillus flavus Otitis Externa with Jugular Thrombosis. Emerg. Infect. Dis. 2019, 25, 830–832. [Google Scholar] [CrossRef]
- Noroy, L.; Pujo, K.; Leghzali-Moise, H.; Saison, J. Aspergillus Necrotizing Otitis Externa with Temporomandibulozygomatic Involvement. Eur. Ann. Otorhinolaryngol. Head. Neck Dis. 2020, 137, 127–129. [Google Scholar] [CrossRef]
- Clerc, N.L.; Verillaud, B.; Duet, M.; Guichard, J.-P.; Herman, P.; Kania, R. Skull Base Osteomyelitis: Incidence of Resistance, Morbidity, and Treatment Strategy. Laryngoscope 2014, 124, 2013–2016. [Google Scholar] [CrossRef]
- Gabrielli, E.; Fothergill, A.W.; Brescini, L.; Sutton, D.A.; Marchionni, E.; Orsetti, E.; Staffolani, S.; Castelli, P.; Gesuita, R.; Barchiesi, F. Osteomyelitis Caused by Aspergillus Species: A Review of 310 Reported Cases. Clin. Microbiol. Infect. 2014, 20, 559–565. [Google Scholar] [CrossRef]
- Langlois, M.-E.; Lorillou, M.; Ferry, T.; Chidiac, C.; Valour, F. Cystic Lung Lesions Revealing a Pneumocystis jirovecii and Aspergillus flavus Co-Infection in an HIV-Infected Patient. Int. J. Infect. Dis. 2015, 37, 143–144. [Google Scholar] [CrossRef]
- Caillet, A.; Bellanger, A.-P.; Navellou, J.C.; Daguindau, E.; Rocchi, S.; Scherer, E.; Berceanu, A.; Millon, L. Refractory Invasive Pulmonary Aspergillosis Due to Aspergillus flavus Detected with the Combination of Two in-House Aspergillus qPCR. J. Mycol. Med. 2023, 33, 101350. [Google Scholar] [CrossRef]
- Florent, M.; Katsahian, S.; Vekhoff, A.; Levy, V.; Rio, B.; Marie, J.-P.; Bouvet, A.; Cornet, M. Prospective Evaluation of a Polymerase Chain Reaction–ELISA Targeted to Aspergillus fumigatus and Aspergillus flavus for the Early Diagnosis of Invasive Aspergillosis in Patients with Hematological Malignancies. J. Infect. Dis. 2006, 193, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Lortholary, O.; Gangneux, J.-P.; Sitbon, K.; Lebeau, B.; de Monbrison, F.; Le Strat, Y.; Coignard, B.; Dromer, F.; Bretagne, S. French Mycosis Study Group Epidemiological Trends in Invasive Aspergillosis in France: The SAIF Network (2005–2007). Clin. Microbiol. Infect. 2011, 17, 1882–1889. [Google Scholar] [CrossRef] [PubMed]
- Comacle, P.; Belaz, S.; Jegoux, F.; Ruaux, C.; Le Gall, F.; Gangneux, J.-P.; Robert-Gangneux, F. Contribution of Molecular Tools for the Diagnosis and Epidemiology of Fungal Chronic Rhinosinusitis. Med. Mycol. 2016, 54, 794–800. [Google Scholar] [CrossRef] [PubMed]
- Brun, S.; Fekkar, A.; Busse, A.; Seilhean, D.; Lecsö, M.; Adler, D.; Prodanovic, H.; Mazier, D.; Datry, A. Aspergillus flavus Brain Abscesses Associated with Hepatic Amebiasis in a Non-Neutropenic Man in Senegal. Am. J. Trop. Med. Hyg. 2009, 81, 583–586. [Google Scholar] [CrossRef] [PubMed]
- Zaidan, M.; Ottaviani, S.; Polivka, M.; Bouldouyre, M.-A.; Orcel, P.; Richette, P. Aspergillus-Related Myositis: A Case Report and Review of the Literature. Semin. Arthritis Rheum. 2011, 41, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Li, D.M.; Xiu, D.R.; Li, R.Y.; Samson, R.A.; de Hoog, G.S.; Wang, D.L. Aspergillus flavus Myositis in a Patient after Liver Transplantation. Clin. Transplant. 2008, 22, 508–511. [Google Scholar] [CrossRef]
- Gamaletsou, M.N.; Rammaert, B.; Bueno, M.A.; Sipsas, N.V.; Moriyama, B.; Kontoyiannis, D.P.; Roilides, E.; Zeller, V.; Taj-Aldeen, S.J.; Henry, M.; et al. Aspergillus Arthritis: Analysis of Clinical Manifestations, Diagnosis, and Treatment of 31 Reported Cases. Med. Mycol. 2017, 55, 246–254. [Google Scholar] [CrossRef][Green Version]
- Cortet, B.; Richard, R.; Deprez, X.; Lucet, L.; Flipo, R.M.; Le Loët, X.; Duquesnoy, B.; Delcambre, B. Aspergillus Spondylodiscitis: Successful Conservative Treatment in 9 Cases. J. Rheumatol. 1994, 21, 1287–1291. [Google Scholar]
- Nicolle, A.; de la Blanchardière, A.; Bonhomme, J.; Hamon, M.; Leclercq, R.; Hitier, M. Aspergillus Vertebral Osteomyelitis in Immunocompetent Subjects: Case Report and Review of the Literature. Infection 2013, 41, 833–840. [Google Scholar] [CrossRef]
- Dupouey, J.; Faucher, B.; Normand, A.-C.; Hadrich, I.; Ranque, S.; Dumon, H.; Casalta, J.-P.; Collart, F.; Piarroux, R. Late Post-Operative Aspergillus flavus Endocarditis: Demonstration of a Six Years Incubation Period Using Microsatellite Typing. Med. Mycol. Case Rep. 2012, 1, 29–31. [Google Scholar] [CrossRef]
- Demaria, R.G.; Dürrleman, N.; Rispail, P.; Margueritte, G.; Macia, J.C.; Aymard, T.; Frapier, J.M.; Albat, B.; Chaptal, P.A. Aspergillus flavus Mitral Valve Endocarditis after Lung Abscess. J. Heart Valve Dis. 2000, 9, 786–790. [Google Scholar] [PubMed]
- Stern, J.-B.; Wyplosz, B.; Validire, P.; Angoulvant, A.; Fregeville, A.; Caliandro, R.; Gossot, D. Bulky Mediastinal Aspergillosis Mimicking Cancer in an Immunocompetent Patient. Ann. Thorac. Surg. 2014, 98, 1472–1475. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.A.; Abbas, M.A.; Jouvion, G.; Al-Hatmi, A.M.S.; de Hoog, G.S.; Kolecka, A.; Mahgoub, E.S. Seventeen Years of Subcutaneous Infection by Aspergillus flavus; Eumycetoma Confirmed by Immunohistochemistry. Mycoses 2015, 58, 728–734. [Google Scholar] [CrossRef] [PubMed]
- Serrano, E.; Percodani, J.; Uro-Coste, E.; Yardeni, E.; Abbal, M.; Linas, M.D.; Recco, P.; Delisle, M.B. Value of Investigation in the Diagnosis of Allergic Fungal Rhinosinusitis: Results of a Prospective Study. J. Laryngol. Otol. 2001, 115, 184–189. [Google Scholar] [CrossRef]
- Saussereau, J.; Reboux, G.; Sfeir, G.; Dalphin, J.-C. Two cases of hot tub lung disease: Environmental investigations. Rev. Mal. Respir. 2019, 36, 204–208. [Google Scholar] [CrossRef]
- Varga, J.; Frisvad, J.C.; Samson, R.A. Two New Aflatoxin Producing Species, and an Overview of Aspergillus Section Flavi. Stud. Mycol. 2011, 69, 57–80. [Google Scholar] [CrossRef] [PubMed]
- Khairallah, S.H.; Byrne, K.A.; Tabbara, K.F. Fungal Keratitis in Saudi Arabia. Doc. Ophthalmol. 1992, 79, 269–276. [Google Scholar] [CrossRef]
- Kameswaran, M.; al-Wadei, A.; Khurana, P.; Okafor, B.C. Rhinocerebral Aspergillosis. J. Laryngol. Otol. 1992, 106, 981–985. [Google Scholar] [CrossRef]
- Mahgoub, E.S.; El Hassan, A.M. Pulmonary Aspergillosis Caused by Aspergillus flavus. Thorax 1972, 27, 33–37. [Google Scholar] [CrossRef]
- Frisvad, J.C.; Hubka, V.; Ezekiel, C.N.; Hong, S.-B.; Nováková, A.; Chen, A.J.; Arzanlou, M.; Larsen, T.O.; Sklenář, F.; Mahakarnchanakul, W.; et al. Taxonomy of Aspergillus Section Flavi and Their Production of Aflatoxins, Ochratoxins and Other Mycotoxins. Stud. Mycol. 2019, 93, 1–63. [Google Scholar] [CrossRef]
- Houbraken, J.; Kocsubé, S.; Visagie, C.M.; Yilmaz, N.; Wang, X.-C.; Meijer, M.; Kraak, B.; Hubka, V.; Bensch, K.; Samson, R.A.; et al. Classification of Aspergillus, Penicillium, Talaromyces and Related Genera (Eurotiales): An Overview of Families, Genera, Subgenera, Sections, Series and Species. Stud. Mycol. 2020, 95, 5–169. [Google Scholar] [CrossRef] [PubMed]
- Kjærbølling, I.; Vesth, T.; Frisvad, J.C.; Nybo, J.L.; Theobald, S.; Kildgaard, S.; Petersen, T.I.; Kuo, A.; Sato, A.; Lyhne, E.K.; et al. A Comparative Genomics Study of 23 Aspergillus Species from Section Flavi. Nat. Commun. 2020, 11, 1106. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, S.; Manavathu, E.K.; Chandrasekar, P.H. Aspergillus flavus: An Emerging Non-fumigatus Aspergillus Species of Significance. Mycoses 2009, 52, 206–222. [Google Scholar] [CrossRef] [PubMed]
- Al-Wathiqi, F.; Ahmad, S.; Khan, Z. Molecular Identification and Antifungal Susceptibility Profile of Aspergillus flavus Isolates Recovered from Clinical Specimens in Kuwait. BMC Infect. Dis. 2013, 13, 126. [Google Scholar] [CrossRef]
- Dehghan, P.; Bui, T.; Campbell, L.T.; Lai, Y.-W.; Tran-Dinh, N.; Zaini, F.; Carter, D.A. Multilocus Variable-Number Tandem-Repeat Analysis of Clinical Isolates of Aspergillus flavus from Iran Reveals the First Cases of Aspergillus minisclerotigenes Associated with Human Infection. BMC Infect. Dis. 2014, 14, 358. [Google Scholar] [CrossRef][Green Version]
- Fernandez-Pittol, M.; Alejo-Cancho, I.; Rubio-García, E.; Cardozo, C.; Puerta-Alcalde, P.; Moreno-García, E.; Garcia-Pouton, N.; Garrido, M.; Villanueva, M.; Alastruey-Izquierdo, A.; et al. Aspergillosis by Cryptic Aspergillus Species: A Case Series and Review of the Literature. Rev. Iberoam. Micol. 2022, 39, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Dannaoui, E.; Persat, F.; Monier, M.F.; Borel, E.; Piens, M.A.; Picot, S. In-Vitro Susceptibility of Aspergillus spp. Isolates to Amphotericin B and Itraconazole. J. Antimicrob. Chemother. 1999, 44, 553–555. [Google Scholar] [CrossRef]
- Meis, J.F.; Chowdhary, A.; Rhodes, J.L.; Fisher, M.C.; Verweij, P.E. Clinical Implications of Globally Emerging Azole Resistance in Aspergillus fumigatus. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2016, 371, 460. [Google Scholar] [CrossRef]
- Imbert, S.; Normand, A.C.; Ranque, S.; Costa, J.M.; Guitard, J.; Accoceberry, I.; Bonnal, C.; Fekkar, A.; Bourgeois, N.; Houzé, S.; et al. Species Identification and in Vitro Antifungal Susceptibility of Aspergillus terreus Species Complex Clinical Isolates from a French Multicenter Study. Antimicrob. Agents Chemother. 2018, 62, e02315-17. [Google Scholar] [CrossRef]
- Simon, L.; Déméautis, T.; Dupont, D.; Kramer, R.; Garnier, H.; Durieu, I.; Sénéchal, A.; Reix, P.; Couraud, S.; Devouassoux, G.; et al. Azole Resistance in Aspergillus fumigatus Isolates from Respiratory Specimens in Lyon University Hospitals, France: Prevalence and Mechanisms Involved. Int. J. Antimicrob. Agents 2021, 58, 106447. [Google Scholar] [CrossRef]
- Monpierre, L.; Desbois-Nogard, N.; Valsecchi, I.; Bajal, M.; Angebault, C.; Miossec, C.; Botterel, F.; Dannaoui, É. Azole Resistance in Clinical and Environmental Aspergillus Isolates from the French West Indies (Martinique). J. Fungi 2021, 7, 355. [Google Scholar] [CrossRef] [PubMed]
- Hamprecht, A.; Morio, F.; Bader, O.; Le Pape, P.; Steinmann, J.; Dannaoui, E. Azole Resistance in Aspergillus fumigatus in Patients with Cystic Fibrosis: A Matter of Concern? Mycopathologia 2018, 183, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Sun, Y.; Chen, W.; Liu, W.; Wan, Z.; Bu, D.; Li, R. The T788G Mutation in the cyp51C Gene Confers Voriconazole Resistance in Aspergillus flavus Causing Aspergillosis. Antimicrob. Agents Chemother. 2012, 56, 2598–2603. [Google Scholar] [CrossRef] [PubMed]
- Paul, R.A.; Rudramurthy, S.M.; Meis, J.F.; Mouton, J.W.; Chakrabarti, A. A Novel Y319H Substitution in CYP51C Associated with Azole Resistance in Aspergillus flavus. Antimicrob. Agents Chemother. 2015, 59, 6615–6619. [Google Scholar] [CrossRef] [PubMed]
- Paul, R.A.; Rudramurthy, S.M. Magnitude of Voriconazole Resistance in Clinical and Environmental Isolates of Aspergillus flavus and Investigation into the Role of Multidrug Efflux. Antimicrob. Agents Chemother. 2018, 62, e01022-18. [Google Scholar] [CrossRef]
- Choi, M.J.; Won, E.J.; Joo, M.Y.; Park, Y.-J.; Kim, S.H.; Shin, M.G.; Shin, J.H. Emergence of Voriconazole-Resistant Aspergillus flavus Isolates in Korean Hospitals: Microsatellite Typing and Resistance Mechanism Analysis. Antimicrob. Agents Chemother. 2019, 63, e01610–e01618. [Google Scholar] [CrossRef]
- Glass, N.L.; Donaldson, G.C. Development of Primer Sets Designed for Use with the PCR to Amplify Conserved Genes from Filamentous Ascomycetes. Appl. Environ. Microbiol. 1995, 61, 1323–1330. [Google Scholar] [CrossRef]
- Hong, S.-B.; Go, S.-J.; Shin, H.-D.; Frisvad, J.C.; Samson, R.A. Polyphasic Taxonomy of Aspergillus fumigatus and Related Species. Mycologia 2005, 97, 1316–1329. [Google Scholar] [CrossRef]
- Choukri, F.; Botterel, F.; Sitterlé, E.; Bassinet, L.; Foulet, F.; Guillot, J.; Costa, J.M.; Fauchet, N.; Dannaoui, E. Prospective Evaluation of Azole Resistance in Aspergillus fumigatus Clinical Isolates in France. Med. Mycol. 2015, 53, 593–596. [Google Scholar] [CrossRef][Green Version]
- The European Committee on Antimicrobial Susceptibility Testing. Overview of Antifungal ECOFFs and Clinical Breakpoints for Yeasts, Moulds and Dermatophytes Using the EUCAST E.Def 7.3, E.Def 9.4 and E.Def 11.0 Procedures. Version 3. 2022. Available online: http://www.eucast.org (accessed on 1 September 2023).
- Saitou, N.; Nei, M. The Neighbor-Joining Method: A New Method for Reconstructing Phylogenetic Trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [CrossRef]
- Felsenstein, J. Confidence Limits on Phylogenies: An Approach Using the Bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Nei, M.; Kumar, S. Prospects for Inferring Very Large Phylogenies by Using the Neighbor-Joining Method. Proc. Natl. Acad. Sci. USA 2004, 101, 11030–11035. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, T.R. Identification and Toxigenic Potential of the Industrially Important Fungi, Aspergillus oryzae and Aspergillus sojae. J. Food Prot. 2007, 70, 2916–2934. [Google Scholar] [CrossRef]
- Tam, E.W.T.; Chen, J.H.K.; Lau, E.C.L.; Ngan, A.H.Y.; Fung, K.S.C.; Lee, K.-C.; Lam, C.-W.; Yuen, K.-Y.; Lau, S.K.P.; Woo, P.C.Y. Misidentification of Aspergillus nomius and Aspergillus tamarii as Aspergillus flavus: Characterization by Internal Transcribed Spacer, β-Tubulin, and Calmodulin Gene Sequencing, Metabolic Fingerprinting, and Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry. J. Clin. Microbiol. 2014, 52, 1153–1160. [Google Scholar] [CrossRef]
- Caira, M.; Posteraro, B.; Sanguinetti, M.; de Carolis, E.; Leone, G.; Pagano, L. First Case of Breakthrough Pneumonia Due to Aspergillus nomius in a Patient with Acute Myeloid Leukemia. Med. Mycol. J. 2012, 50, 746–750. [Google Scholar] [CrossRef]
- Perez, L.; Messina, F.; Negroni, R.; Arechavala, A.; Bustamante, J.; Oleastro, M.; Migaud, M.; Casanova, J.-L.; Puel, A.; Santiso, G. Inherited CARD9 Deficiency in a Patient with Both Exophiala spinifera and Aspergillus nomius Severe Infections. J. Clin. Immunol. 2020, 40, 359–366. [Google Scholar] [CrossRef]
- Zotti, M.; Machetti, M.; Persi, A.; Barabino, G.; Parodi, A. Onychomycosis: First Case Due to Aspergillus nomius. Acta Derm. Venereol. 2011, 91, 591–592. [Google Scholar] [CrossRef]
- Manikandan, P.; Varga, J.; Kocsubé, S.; Samson, R.A.; Anita, R.; Revathi, R.; Dóczi, I.; Németh, T.M.; Narendran, V.; Vágvölgyi, C.; et al. Mycotic Keratitis Due to Aspergillus nomius. J. Clin. Microbiol. 2009, 47, 3382–3385. [Google Scholar] [CrossRef]
- Zhou, Y.B.; Li, D.M.; Houbraken, J.; Sun, T.T.; de Hoog, G.S. Fatal Rhinofacial Mycosis Due to Aspergillus nomiae: Case Report and Review of Published Literature. Front. Microbiol. 2020, 11, 595375. [Google Scholar] [CrossRef]
- Salah, H.; Lackner, M.; Houbraken, J.; Theelen, B.; Lass-Flörl, C.; Boekhout, T.; Almaslamani, M.; Taj-Aldeen, S.J. The Emergence of Rare Clinical Aspergillus Species in Qatar: Molecular Characterization and Antifungal Susceptibility Profiles. Front. Microbiol. 2019, 10, 1677. [Google Scholar] [CrossRef] [PubMed]
- Alshehri, B.; Palanisamy, M. Evaluation of Molecular Identification of Aspergillus Species Causing Fungal Keratitis. Saudi J. Biol. Sci. 2020, 27, 751–756. [Google Scholar] [CrossRef] [PubMed]
- Kimura, H.; Mitsuto, I.; Taguchi, R.; Anzawa, K.; Mochizuki, T. Primary Cutaneous Aspergillosis Caused by Aspergillus tamarii in a Premature Infant with Extremely Low Birthweight: A Case Report with Short Review. J. Dermatol. 2018, 45, 622–625. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Madhav Yenigalla, B.; Kumar Naidu, S.; Pidakala, P. Primary Cutaneous Aspergillosis Due to Aspergillus tamarii in an Immunocompetent Host. Br. Med. J. Case Rep. 2013, 2013, bcr2013010128. [Google Scholar] [CrossRef] [PubMed]
- Aries, P.; Hoffmann, C.; Schaal, J.-V.; Leclerc, T.; Donat, N.; Cirodde, A.; Masson, Y.; Renner, J.; Soler, C. Aspergillus tamarii: An Uncommon Burn Wound Infection. J. Clin. Pathol. 2018, 71, 379–380. [Google Scholar] [CrossRef] [PubMed]
- Gómez, O.M.; Freyle, C.G.; Torres, S.; Rúa, Á.L.; Tamayo, D.P.; McEwen, J.G.; Borges, C.L.; Hernández, O. Draft Genome Sequences of Clinical and Environmental Isolates of Aspergillus tamarii from Colombia. Microbiol. Resour. Announc. 2020, 9, e01514–e01519. [Google Scholar] [CrossRef]
- Kristensen, L.; Stenderup, J.; Otkjaer, A. Onychomycosis Due to Aspergillus tamarii in a 3-Year-Old Boy. Acta Derm. Venereol. 2005, 85, 261–262. [Google Scholar] [CrossRef]
- Ghosh, A.; Kaur, H.; Gupta, A.; Singh, S.; Rudramurthy, S.M.; Gupta, S.; Chakrabarti, A. Emerging Dematiaceous and Hyaline Fungi Causing Keratitis in a Tertiary Care Centre from North India. Cornea 2020, 39, 868–876. [Google Scholar] [CrossRef]
- Paludetti, G.; Rosignoli, M.; Ferri, E.; Cesari, M.R.; Morace, G.; Fantoni, M.; Galli, J. Invasive nasosinusal aspergillosis in an immunocompetent patient. Acta Otorhinolaryngol. Ital. 1992, 12, 581–591. [Google Scholar]
- Kamble, R.; Joshi, J.; Pendurkar, S. Paranasal Sinus Aspergillosis: A Case Report and Review of Literature. Int. J. Curr. Microbiol. App. Sci. 2015, 4, 314–322. [Google Scholar]
- Ozhak-Baysan, B.; Alastruey-Izquierdo, A.; Saba, R.; Ogunc, D.; Ongut, G.; Timuragaoglu, A.; Arslan, G.; Cuenca-Estrella, M.; Rodriguez-Tudela, J.L. Aspergillus alliaceus and Aspergillus flavus Co-Infection in an Acute Myeloid Leukemia Patient. Med. Mycol. 2010, 48, 995–999. [Google Scholar] [CrossRef] [PubMed]
- Balajee, S.A.; Lindsley, M.D.; Iqbal, N.; Ito, J.; Pappas, P.G.; Brandt, M.E. Nonsporulating Clinical Isolate Identified as Petromyces alliaceus (Anamorph Aspergillus alliaceus) by Morphological and Sequence-Based Methods. J. Clin. Microbiol. 2007, 45, 2701–2703. [Google Scholar] [CrossRef] [PubMed]
- Bayman, P.; Baker, J.L.; Doster, M.A.; Michailides, T.J.; Mahoney, N.E. Ochratoxin Production by the Aspergillus ochraceus Group and Aspergillus alliaceus. Appl. Environ. Microbiol. 2002, 68, 2326–2329. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Chen, M.; Zhu, J.; Gerrits van den Ende, B.; Chen, A.J.; Al-Hatmi, A.M.S.; Li, L.; Zhang, Q.; Xu, J.; Liao, W.; et al. Aspergillus Species in Lower Respiratory Tract of Hospitalized Patients from Shanghai, China: Species Diversity and Emerging Azole Resistance. Infect. Drug Resist. 2020, 13, 4663–4672. [Google Scholar] [CrossRef]
- Le Pape, P.; Ximenes, R.M.; Ariza, B.; Iriarte, J.; Alvarado, J.; Robert, E.; Sierra, C.; Montañez, A.; Álvarez-Moreno, C. First Case of Aspergillus caelatus Airway Colonization in a Chronic Obstructive Pulmonary Disease Patient. Int. J. Infect. Dis. 2019, 81, 85–90. [Google Scholar] [CrossRef]
- Karimizadeh Esfahani, M.; Eslampoor, A.; Dolatabadi, S.; Najafzadeh, M.J.; Houbraken, J. First Case of Fungal Keratitis Due to Aspergillus minisclerotigenes in Iran. Curr. Med. Mycol. 2019, 5, 45–48. [Google Scholar] [CrossRef]
- Gonçalves, S.S.; Stchigel, A.M.; Cano, J.F.; Godoy-Martinez, P.C.; Colombo, A.L.; Guarro, J. Aspergillus novoparasiticus: A New Clinical Species of the Section Flavi. Med. Mycol. 2012, 50, 152–160. [Google Scholar] [CrossRef]
- Arias, R.S.; Orner, V.A.; Martinez-Castillo, J.; Sobolev, V.S. Aspergillus Section Flavi, Need for a Robust Taxonomy. Microbiol. Resour. Announc. 2021, 10, e0078421. [Google Scholar] [CrossRef]
- Abbas, A.; Hussien, T.; Yli-Mattila, T. A Polyphasic Approach to Compare the Genomic Profiles of Aflatoxigenic and Non-Aflatoxigenic Isolates of Aspergillus Section Flavi. Toxins 2020, 12, 56. [Google Scholar] [CrossRef]
- Quéro, L.; Courault, P.; Cellière, B.; Lorber, S.; Jany, J.-L.; Puel, O.; Girard, V.; Vasseur, V.; Nodet, P.; Mounier, J. Application of MALDI-TOF MS to Species Complex Differentiation and Strain Typing of Food Related Fungi: Case Studies with Aspergillus Section Flavi Species and Penicillium roqueforti Isolates. Food Microbiol. 2020, 86, 103311. [Google Scholar] [CrossRef]
- Tuchtan, L.; Piercecchi-Marti, M.-D.; Dumon, H.; Métras, D.; Léonetti, G.; Bartoli, C. Medicolegal Implications of Nosocomial Infections: A Case Report of Aspergillus Contamination during Cardiac Surgery. J. Forensic Sci. 2017, 62, 815–818. [Google Scholar] [CrossRef] [PubMed]
- Sharma, C.; Kumar, R.; Kumar, N.; Masih, A.; Gupta, D.; Chowdhary, A. Investigation of Multiple Resistance Mechanisms in Voriconazole-Resistant Aspergillus flavus Clinical Isolates from a Chest Hospital Surveillance in Delhi, India. Antimicrob. Agents Chemother. 2018, 62, e01928-17. [Google Scholar] [CrossRef] [PubMed]
- Rivero-Menendez, O.; Soto-Debran, J.C.; Medina, N.; Lucio, J.; Mellado, E.; Alastruey-Izquierdo, A. Molecular Identification, Antifungal Susceptibility Testing, and Mechanisms of Azole Resistance in Aspergillus Species Received within a Surveillance Program on Antifungal Resistance in Spain. Antimicrob. Agents Chemother. 2019, 63, e00865-19. [Google Scholar] [CrossRef] [PubMed]
- Lucio, J.; Gonzalez-Jimenez, I.; Rivero-Menendez, O.; Alastruey-Izquierdo, A.; Pelaez, T.; Alcazar-Fuoli, L.; Mellado, E. Point Mutations in the 14-α Sterol Demethylase Cyp51A or Cyp51C Could Contribute to Azole Resistance in Aspergillus flavus. Genes. 2020, 11, 1217. [Google Scholar] [CrossRef]
- Ferraz, T.L.L.; Araújo, E.M.; Calixto, R.F.; Sampaio, M.L.M.; de Mello, L.R.B.; Torres, K.B.; de Aguiar Cordeiro, R.; Le Pape, P.; Gonçalves de Lima-Neto, R. Lethal Destructive Sinusopathy Due to Amphotericin B-Resistant Aspergillus flavus: A Case Report. Rev. Iberoam. Micol. 2022, 39, 21–24. [Google Scholar] [CrossRef]
- Dannaoui, E.; Espinel-Ingroff, A. Antifungal Susceptibly Testing by Concentration Gradient Strip Etest Method for Fungal Isolates: A Review. J. Fungi 2019, 5, 108. [Google Scholar] [CrossRef]
| Antifungal Drugs | Number of Isolates for the Following MIC (mg/L) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0.015 | 0.03 | 0.06 | 0.125 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | |
| VRZ | - | - | - | - | - | 58 | 53 | 7 | - | 2 |
| ISA | - | - | - | - | - | 19 | 81 | 18 | - | 2 |
| ITZ | - | - | - | 29 | 64 | 23 | 3 | 1 | - | - |
| PSZ | - | 1 | 15 | 24 | 54 | 18 | 8 | - | - | |
| AMB | - | - | - | - | 1 | 2 | 34 | 51 | 31 | 1 |
| CAS | - | 20 | 83 | 17 | - | - | - | - | - | - |
| Indice (mg/L) | Antifungal Drug (mg/L) | |||||
|---|---|---|---|---|---|---|
| VRZ | ISA | ITZ | PSZ | AMB | CAS | |
| Min | 0.5 | 0.5 | 0.125 | 0.031 | 0.25 | 0.03 |
| Max | 8 | 8 | 2 | 1 | 8 | 0.125 |
| GMean | 0.77 | 1.03 | 0.25 | 0.22 | 1.91 | 0.06 |
| MIC50 | 1 | 1 | 0.25 | 0.25 | 2 | 0.06 |
| MIC90 | 1 | 2 | 0.5 | 0.5 | 4 | 0.125 |
| Species | MIC (mg/L) | |||||
|---|---|---|---|---|---|---|
| VRZ | ISA | ITZ | PSZ | AMB | CAS | |
| A. parasiticus | 0.5 | 0.5 | 0.25 | 0.125 | 2 | 0.06 |
| A. nomiae | 0.5 | 1 | 0.5 | 0.25 | 4 | 0.03 |
| Species | Localization of Infection | Treatment | Outcome | Reference | |
|---|---|---|---|---|---|
| Local | Systemic | ||||
| A. alliaceus | Lung | None | VRZ | Survived | [46] |
| A. alliaceus | Lung | None | VRZ | Death | [82] |
| A. alliaceus | Lung | None | AMB + CAS, then ITZ | Death | [83] |
| A. caelatus | Lung (colonization) | none | none | NA | [86] |
| A. minisclerotigenes | Sinus | NA | NA | NA | [45] |
| A. minisclerotigenes | Paranasal | NA | NA | NA | [45] |
| A. minisclerotigenes | Eye | AMB + VRZ | FCZ | Corneal transplant | [87] |
| A. novoparasiticus | Lung | NA | NA | NA | [88] |
| A. novoparasiticus | Lung | NA | NA | NA | [88] |
| A. nomiae | Lung | none | AMB + ITZ | Death | [66] |
| A. nomiae | Lung | none | ITZ | Survived | [66] |
| A. nomiae | Corneal | NTM, ECZ, ITZ, KTZ | none | Corneal perforation, scleral extension | [70] |
| A. nomiae | Nail | Amorolfine | ITZ | Cured | [69] |
| A. nomiae | Sputum | VRZ | none | Death | [67] |
| A. nomiae | Lung | AMB nebulized | ISA + Anidulafungin | Death | [46] |
| A. nomiae | Skin | none | AMB | Death | [71] |
| A. nomiae | Lung | none | CAS + PSZ + bilobectomy | Cured | [68] |
| A. nomiae | Eye | NA | NA | [73] | |
| A. oryzea var. effusus | Eye | none | none | Tectonic KP, vitrectomy | [6] |
| A. pseudonomiae | Sinus tissue | surgery | Cured | [72] | |
| A. pseudonomiae | Eye | NA | NA | [73] | |
| A.pseudonomiae | Eye | [7] | |||
| A. pseudotamarii | Eye | NTM; ITZ | ITZ | Improved | [11] |
| A. tamarii | Eye | NTM, ECZ (then CLT), ITZ | KTZ | Good response, no follow-up | [10] |
| A. tamarii | Eye | NTM, ECZ (then CLT), ITZ | KTZ | Healed, no follow-up | [10] |
| A. tamarii | Eye | NTM, ECZ, ITZ, AMB | KTZ | Evisceration | [10] |
| A. tamarii | Eye | NTM, ITZ | KTZ | Healed | [10] |
| A. tamarii | Eye | NTM, ECZ, ITZ | KTZ | Healed | [10] |
| A. tamarii | Eye | NTM, ECZ, ITZ | KTZ | No response, no follow-up | [10] |
| A. tamarii | Eye | NTM, ECZ, FCZ | KTZ | Improved, central nebular scar | [9] |
| A. tamarii | Eye | VRZ, AMB, NTM | AMB, VRZ | Improved, extensive corneal scar | [8] |
| A. tamarii | Eye | VRZ | ITZ | Improved, no surgery | [6] |
| A. tamarii | Eye | VRZ after surgery | none | Tectonic KP, vitrectomy | [6] |
| A. tamarii | Eye | VRZ after surgery | none | Tectonic KP, vitrectomy | [6] |
| A. tamarii | Eye | VRZ | none | Tectonic KP, OKP, intraocular lens | [6] |
| A. tamarii | Skin | CLT | AMB iv | Resolving of cutaneous lesions | [74] |
| A. tamarii | Skin | none | ITZ | Complete recovery | [75] |
| A. tamarii | Nasosinusal | ITZ and surgery | Excellent local results at a follow-up of one year | [80] | |
| A. tamarii | Sputum (colonization) | none | none | Survived | [66] |
| A. tamarii | Nail | Urea cream, Terbinafine | Cured | [78] | |
| A. tamarii | Corneal | NA | NA | NA | [7] |
| A. tamarii | Eye | NTM | ITZ | Vascularized corneal opacity | [79] |
| A. tamarii | Tissue (RTA) | AMB | [72] | ||
| A. tamarii | Skin | AMB | VRZ then AMB | Survived | [76] |
| A. tamarii | Left foot biopsy | NA | NA | NA | [77] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Djenontin, E.; Costa, J.-M.; Mousavi, B.; Nguyen, L.D.N.; Guillot, J.; Delhaes, L.; Botterel, F.; Dannaoui, E. The Molecular Identification and Antifungal Susceptibility of Clinical Isolates of Aspergillus Section Flavi from Three French Hospitals. Microorganisms 2023, 11, 2429. https://doi.org/10.3390/microorganisms11102429
Djenontin E, Costa J-M, Mousavi B, Nguyen LDN, Guillot J, Delhaes L, Botterel F, Dannaoui E. The Molecular Identification and Antifungal Susceptibility of Clinical Isolates of Aspergillus Section Flavi from Three French Hospitals. Microorganisms. 2023; 11(10):2429. https://doi.org/10.3390/microorganisms11102429
Chicago/Turabian StyleDjenontin, Elie, Jean-Marc Costa, Bita Mousavi, Lin Do Ngoc Nguyen, Jacques Guillot, Laurence Delhaes, Françoise Botterel, and Eric Dannaoui. 2023. "The Molecular Identification and Antifungal Susceptibility of Clinical Isolates of Aspergillus Section Flavi from Three French Hospitals" Microorganisms 11, no. 10: 2429. https://doi.org/10.3390/microorganisms11102429
APA StyleDjenontin, E., Costa, J.-M., Mousavi, B., Nguyen, L. D. N., Guillot, J., Delhaes, L., Botterel, F., & Dannaoui, E. (2023). The Molecular Identification and Antifungal Susceptibility of Clinical Isolates of Aspergillus Section Flavi from Three French Hospitals. Microorganisms, 11(10), 2429. https://doi.org/10.3390/microorganisms11102429








