Bacterial Profiles of Brain in Downer Cattle with Unknown Etiology
Abstract
1. Introduction
2. Materials and Methods
2.1. Bovine Brain Sample Collection and Diagnosis Based on the Necropsy, Pathological, and Clinical Examination
2.2. DNA Extraction from Brain Tissues
2.3. Sequencing Library Preparation and Amplicon Sequencing
2.4. Pre-Processing of Sequence Data and Microbiota Analysis
2.5. Statistical Analysis
3. Results
3.1. Diagnosis Result of Downer and Normal Cattle
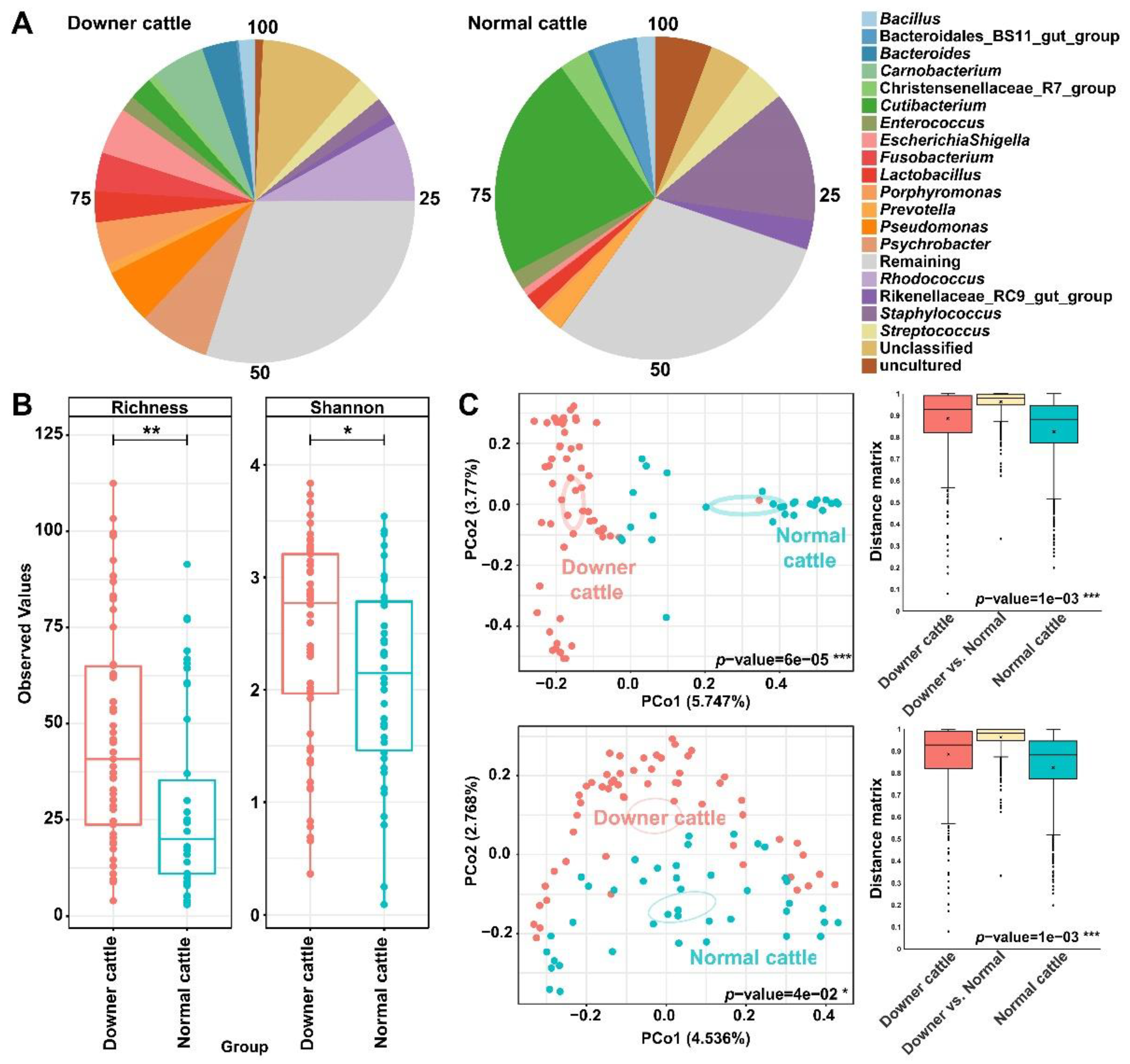
3.2. Differences in Bacterial Structure and Diversity between the Downer and Normal Cattle
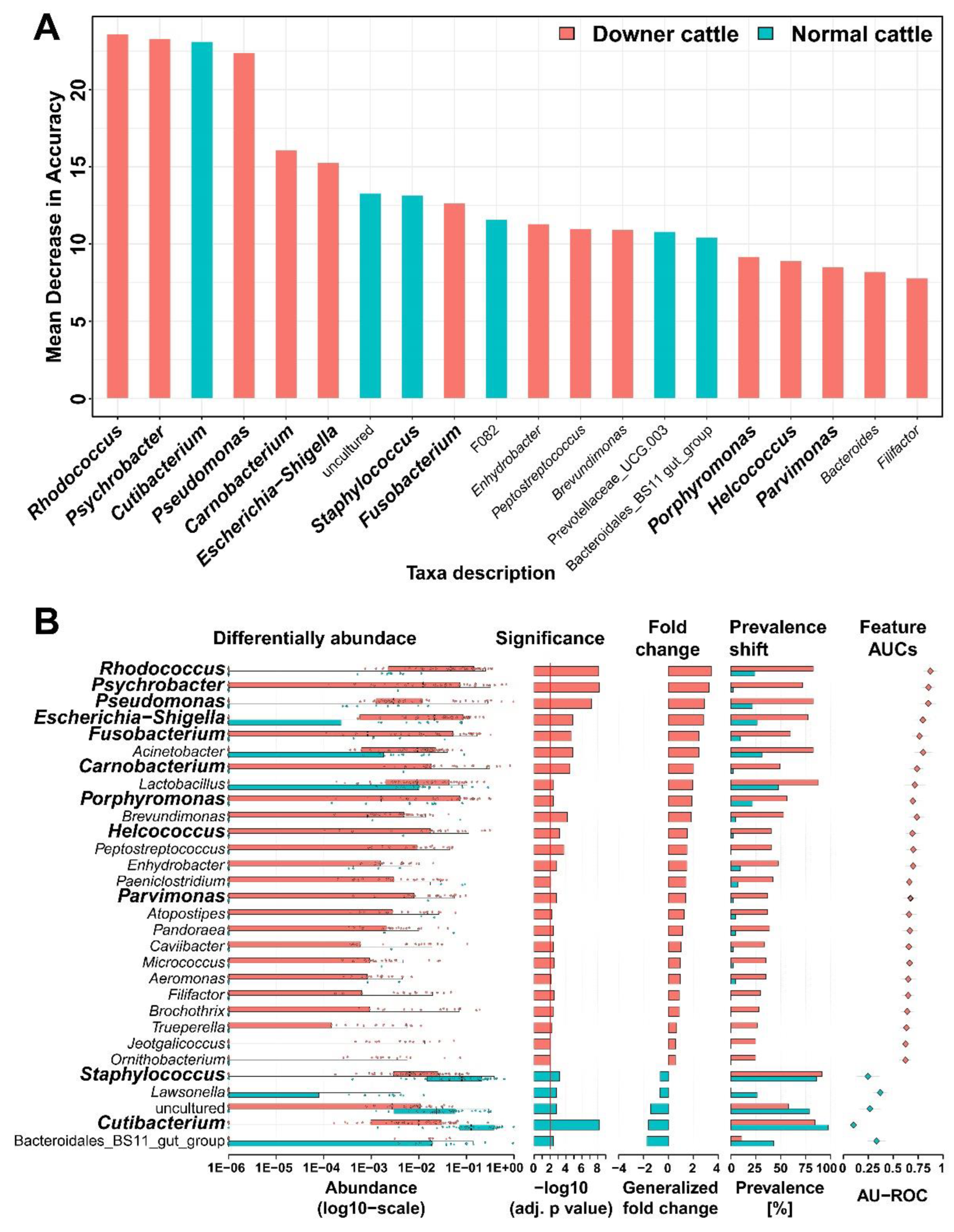
3.3. Representative Genera in the Brain Microbiota of the Downer and Normal Cattle
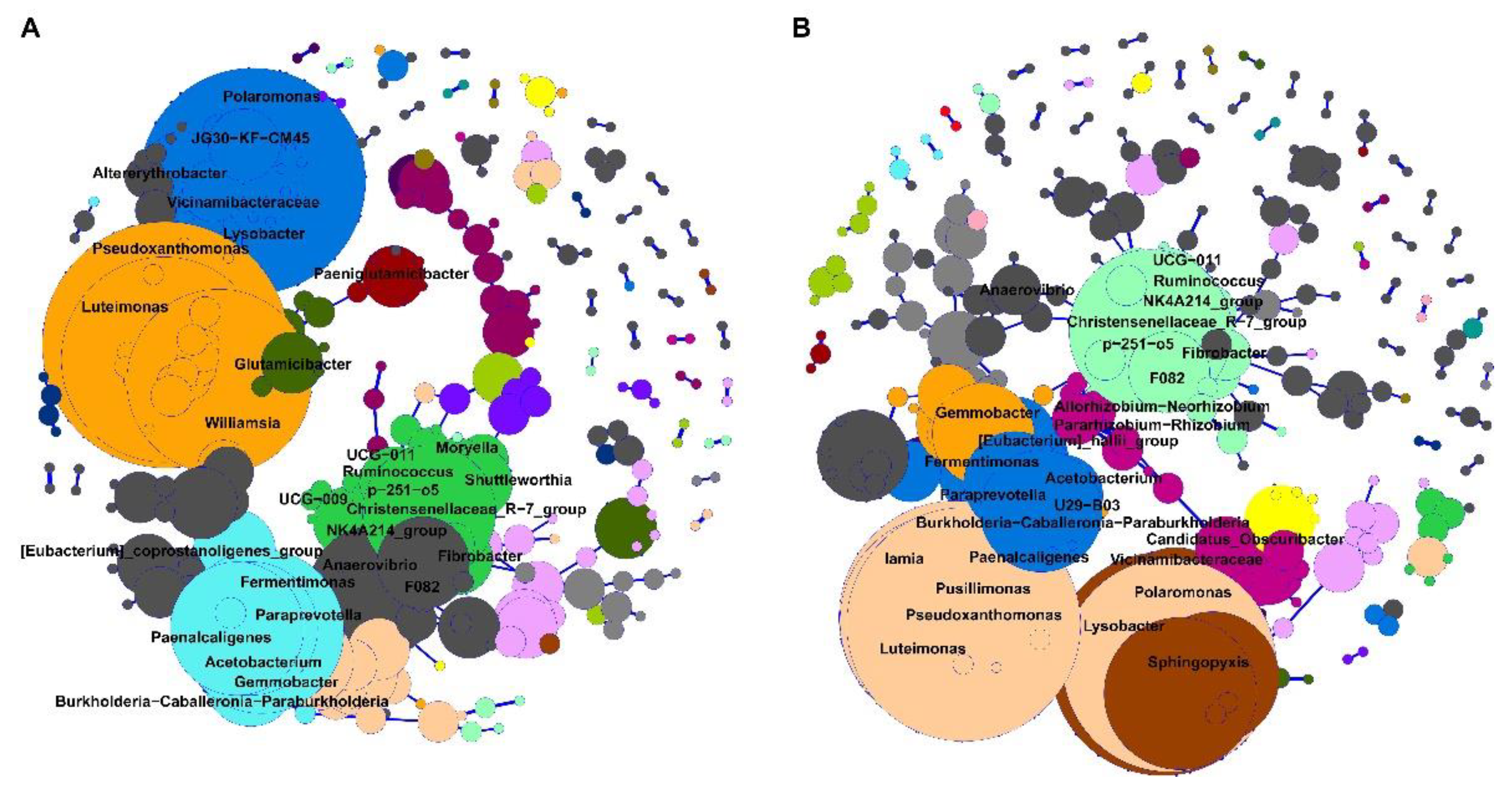
3.4. Bacterial Network from Brain Microbiota between Downer and Normal Cattle
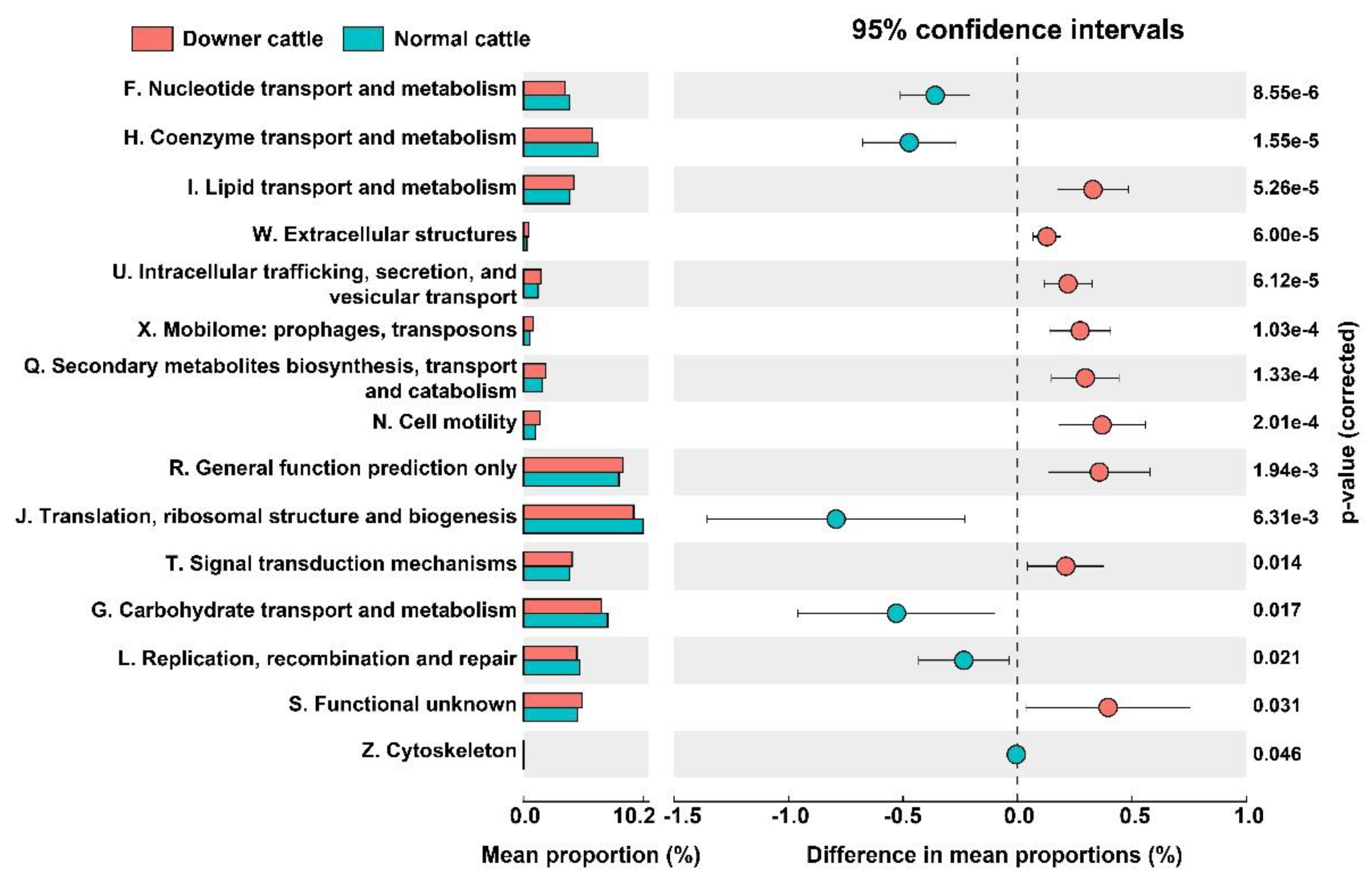
3.5. Predicted Functional genes in the Brain Microbiota between Downer and Normal Cattle
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alzaidi, B.M.; Saed, O.A.S.; Hameed, K.A. Study of Some Biochemical Changes Associated with Downer Cow Syndrome in Local Cattle. Ann. Romanian Soc. Cell Biol. 2022, 26, 2886–2891. [Google Scholar]
- Lee, J.C.; Lee, C.Y.; Lee, J.G. A Study on Recumbency in Cattle. J. Korean Vet. Med. Assoc. 2002, 38, 1123–1133. [Google Scholar]
- Rulff, R.; Schrödl, W.; Basiouni, S.; Neuhaus, J.; Krüger, M. Is downer cow syndrome related to chronic botulism? Pol. J. Vet. Sci. 2015, 18, 759–765. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Animal and Plant Quarantine Agency. Report of disease diagnosis results for mammal in 2020. Unpublished data, South Korea. 2020; 6–7. [Google Scholar]
- Halfvarson, J.; Brislawn, C.J.; Lamendella, R.; Vázquez-Baeza, Y.; Walters, W.A.; Bramer, L.M.; D’amato, M.; Bonfiglio, F.; McDonald, D.; Gonzalez, A.; et al. Dynamics of the human gut microbiome in inflammatory bowel disease. Nat. Microbiol. 2017, 2, 17004. [Google Scholar] [CrossRef]
- Vogt, N.M.; Kerby, R.L.; Dill-McFarland, K.A.; Harding, S.J.; Merluzzi, A.P.; Johnson, S.C.; Carlsson, C.M.; Asthana, S.; Zetterberg, H.; Blennow, K.; et al. Gut microbiome alterations in Alzheimer’s disease. Sci. Rep. 2017, 7, 13537. [Google Scholar] [CrossRef]
- Prados-Bo, A.; Casino, G. Microbiome research in general and business newspapers: How many microbiome articles are published and which study designs make the news the most? PLoS ONE 2021, 16, e0249835. [Google Scholar] [CrossRef]
- Wüthrich, D.; Boujon, C.L.; Truchet, L.; Selimovic-Hamza, S.; Oevermann, A.; Bouzalas, I.G.; Bruggmann, R.; Seuberlich, T. Exploring the virome of cattle with non-suppurative encephalitis of unknown etiology by metagenomics. Virology 2016, 493, 22–30. [Google Scholar] [CrossRef]
- Vidal, S.; Kegler, K.; Posthaus, H.; Perreten, V.; Rodriguez-Campos, S. Amplicon sequencing of bacterial microbiota in abortion material from cattle. Vet. Res. 2017, 48, 64. [Google Scholar] [CrossRef]
- Daneman, R.; Prat, A. The blood–brain barrier. Cold Spring Harb. Perspect. Biol. 2015, 7, a020412. [Google Scholar] [CrossRef]
- Obermeier, B.; Daneman, R.; Ransohoff, R.M. Development, maintenance, and disruption of the blood-brain barrier. Nat. Med. 2013, 19, 1584–1596. [Google Scholar] [CrossRef] [PubMed]
- Al-Obaidi, M.M.J.; Desa, M.N.M. Mechanisms of blood brain barrier disruption by different types of bacteria, and bacterial–host interactions facilitate the bacterial pathogen invading the brain. Cell. Mol. Neurobiol. 2018, 38, 1349–1368. [Google Scholar] [CrossRef] [PubMed]
- Le Guennec, L.; Coureuil, M.; Nassif, X.; Bourdoulous, S. Strategies used by bacterial pathogens to cross the blood–brain barrier. Cell. Microbiol. 2022, 22, e13132. [Google Scholar] [CrossRef] [PubMed]
- Herold, R.; Schroten, H.; Schwerk, C. Virulence factors of meningitis-causing bacteria: Enabling brain entry across the blood–brain barrier. Int. J. Mol. Sci. 2019, 20, 5393. [Google Scholar] [CrossRef]
- Kim, K.S. Investigating bacterial penetration of the blood–brain barrier for the pathogenesis, prevention, and therapy of bacterial meningitis. ACS Infect. Dis. 2019, 6, 34–42. [Google Scholar] [CrossRef]
- Whitlock, K.B.; Pope, C.E.; Hodor, P.; Hoffman, L.R.; Limbrick, D.L., Jr.; McDonald, P.J.; Hauptman, J.S.; Ojemann, J.G.; Simon, T.D.; Cerebrospinal FLuId MicroBiota in Shunts Study (CLIMB) Group. Characterization of cerebrospinal fluid (CSF) microbiota from patients with CSF shunt infection and reinfection using high throughput sequencing of 16S ribosomal RNAgenes. PLoS ONE 2021, 16, e0244643. [Google Scholar] [CrossRef]
- Abutarbush, S.M. Veterinary medicine—A textbook of the diseases of cattle, horses, sheep, pigs and goats. Can. Vet. J. 2010, 51, 541. [Google Scholar]
- King, J.M.; Roth, J.L.; Dodd, D.C.; Newsom, M.E. The Necropsy Book: A Guide for Veterinary Students, Residents, Clinicians, Pathologists, and Biological Researchers; The Internet-First University Press: Ithaca, NY, USA, 2014; pp. 14–16. [Google Scholar]
- Salter, S.J.; Cox, M.J.; Turek, E.M.; Calus, S.T.; Cookson, W.O.; Moffatt, M.F.; Turner, P.; Parkhill, J.; Loman, N.J.; Walker, A.W. Reagent and laboratory contamination can critically impact sequence-based microbiome analyses. BMC Biol. 2014, 12, 87. [Google Scholar] [CrossRef]
- FastQC: A Quality Control Tool for High Throughput Sequence Data. Available online: http://www.bioinformatics.babraham.ac.uk/projects/fastqc (accessed on 29 August 2022).
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2012, 41, 590–596. [Google Scholar] [CrossRef]
- McMurdie, P.J.; Holmes, S. Phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef] [PubMed]
- MicrobiomeSeq: An R Package for Analysis of Microbial Communities in an Environmental Context. Available online: https://github.com/umerijaz/microbiomeSeq (accessed on 29 August 2022).
- Zakrzewski, M.; Proietti, C.; Ellis, J.J.; Hasan, S.; Brion, M.J.; Berger, B.; Krause, L. Calypso: A user-friendly web-server for mining and visualizing microbiome–environment interactions. Bioinformatics 2017, 33, 782–783. [Google Scholar] [CrossRef] [PubMed]
- Wirbel, J.; Zych, K.; Essex, M.; Karcher, N.; Kartal, E.; Salazar, G.; Bork, P.; Sunagawa, S.; Zeller, G. SIAMCAT: User-friendly and versatile machine learning workflows for statistically rigorous microbiome analyses. bioRxiv 2020, 931808. [Google Scholar] [CrossRef]
- Robin, X.; Turck, N.; Hainard, A.; Tiberti, N.; Lisacek, F.; Sanchez, J.C.; Müller, M. pROC: An open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinform. 2011, 12, 77. [Google Scholar] [CrossRef] [PubMed]
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.R.; Yurgel, S.N.; Brown, J.R.; Taylor, C.M.; Huttenhower, C.; Langille, M.G. PICRUSt2 for prediction of metagenome functions. Nat. Biotechnol. 2020, 38, 685–688. [Google Scholar] [CrossRef]
- Parks, D.H.; Tyson, G.W.; Hugenholtz, P.; Beiko, R.G. STAMP: Statistical analysis of taxonomic and functional profiles. Bioinformatics 2014, 30, 3123–3124. [Google Scholar] [CrossRef]
- McFarlane, W.J.; Winder, C.B.; Duffield, T.F.; Kelton, D.F.; Bauman, C.A.; Croyle, S.L.; Renaud, D.L. Factors influencing how Canadian dairy producers respond to a downer cow scenario. J. Dairy Sci. 2022, 105, 684–694. [Google Scholar] [CrossRef]
- Lee, Y.H.; Kim, M.J.; Tark, D.S.; Sohn, H.J.; Yun, E.I.; Cho, I.S.; Choi, Y.P.; Kim, C.L.; Lee, J.H.; Kweon, C.H. Bovine spongiform encephalopathy surveillance in the Republic of Korea. Rev. Sci. Tech. 2012, 31, 861–870. [Google Scholar] [CrossRef]
- Coureuil, M.; Lécuyer, H.; Bourdoulous, S.; Nassif, X. A journey into the brain: Insight into how bacterial pathogens cross blood–brain barriers. Nat. Rev. Microbiol. 2017, 15, 149–159. [Google Scholar] [CrossRef]
- María, O.A.J.; Miguel, S.C.J.; Fabiola, G.A.; Elizabeth, G.D.; Araceli, R.C.; Patricia, A.P.; Claudia, W.A.; Maribel, G.V.; Gloria, L.Á.; Jeanette, G.C.A.; et al. Fatal Psychrobacter sp. infection in a pediatric patient with meningitis identified by metagenomic next-generation sequencing in cerebrospinal fluid. Arch. Microbiol. 2016, 198, 129–135. [Google Scholar] [CrossRef]
- Li, X.Y.; Ke, B.X.; Chen, C.N.; Xiao, H.L.; Liu, M.Z.; Xiong, Y.C.; Bai, R.; Chen, J.D.; Ke, C.W. First co-infection case of melioidosis and Japanese encephalitis in China. BMC Infect. Dis. 2018, 18, 452. [Google Scholar] [CrossRef] [PubMed]
- Vielmo, A.; Bianchi, R.M.; Argenta, F.F.; Rolim, V.M.; GM, G. Thromboembolic encephalitis secondary to bacterial valvular endocarditis in a red-billed curassow (Crax blumenbachii). Braz. J. Vet. Pathol. 2018, 11, 28–31. [Google Scholar] [CrossRef]
- Alonso, J.d.M.; Pedro Filho, T.H.; Ávila, A.R.; Machado, V.M.; Hataka, A.; Bueno, L.M.; Alves, A.L.G.; Hussni, C.A.; Rodrigues, C.A.; Watanabe, M.J. Surgical repair of an occipital meningocele in a foal. J. Equine Vet. Sci. 2019, 81, 102771. [Google Scholar] [CrossRef] [PubMed]
- Seguel, M.; Moroni, M.; Gomez, M.; Hernández, C.; Paredes, E. Bacterial meningoencephalitis in a free Chimango Caracara (Milvago chimango temucoensis). Braz. J. Vet. Pathol. 2012, 5, 16–19. [Google Scholar]
- Afroze, F.; Ahmed, T.; Sarmin, M.; SMSB Shahid, A.; Shahunja, K.M.; Shahrin, L.; Chisti, M.J. Risk factors and outcome of Shigella encephalopathy in Bangladeshi children. PLoS Negl. Trop. Dis. 2017, 11, e0005561. [Google Scholar] [CrossRef]
- Kalay, G.N.; Dalgic, N.; Bozan, T.; Ulger-Toprak, N.; Bayraktar, B.; Soyletir, G. Polymicrobial anaerobic meningitis caused by Bacteroides fragilis, Bacteroides thetaiotaomicron, Fusobacterium necrophorum and Slackia exigua in a patient with mastoiditis following otitis media. Anaerobe 2019, 56, 95–97. [Google Scholar] [CrossRef]
- Hintze, T.; Steed, M.; Sievers, E.; Bagwell, J.T.; Selfa, N. Primary meningitis due to Fusobacterium nucleatum successfully treated with ceftriaxone in a healthy adult male. IDCases 2019, 18, e00616. [Google Scholar] [CrossRef]
- Luo, L.; Wang, C.; Shen, N.; Zhao, R.; Tao, Y.; Mo, X.; Cao, Q. Polymicrobial anaerobic bacterial meningitis secondary to dermal sinus: A case report. Transl. Pediatr. 2021, 10, 3118. [Google Scholar] [CrossRef]
- Bagdure, S.R.; Fisher, M.A.; Ryan, M.E.; Khasawneh, F.A. Rhodococcus erythropolis encephalitis in patient receiving rituximab. Emerg. Infect. Dis. 2012, 18, 1377. [Google Scholar] [CrossRef]
- Mihaila, D.; Donegan, J.; Barns, S.; LaRocca, D.; Du, Q.; Zheng, D.; Vidal, M.; Neville, C.; Uhlig, R.; Middleton, F.A. The oral microbiome of early stage Parkinson’s disease and its relationship with functional measures of motor and non-motor function. PLoS ONE 2019, 14, e0218252. [Google Scholar] [CrossRef]
- Holman, D.B.; Timsit, E.; Alexander, T.W. The nasopharyngeal microbiota of feedlot cattle. Sci. Rep. 2015, 5, 15557. [Google Scholar] [CrossRef] [PubMed]
- Schaffer, P.A.; Lifland, B.; Sommeran, S.V.; Casper, D.R.; Davis, C.R. Meningoencephalitis associated with Carnobacterium maltaromaticum–like bacteria in stranded juvenile salmon sharks (Lamna ditropis). Vet. Pathol. 2013, 50, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Kutzer, P.; Schulze, C.; Engelhardt, A.; Wieler, L.H.; Nordhoff, M. Helcococcus ovis, an emerging pathogen in bovine valvular endocarditis. J. Clin. Microbiol. 2008, 46, 3291–3295. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, S.; Chan, J.F.; Yuen, K.Y. First report of brain abscess caused by a satelliting phenotypic variant of Helcococcus kunzii. J. Clin. Microbiol. 2014, 52, 370–373. [Google Scholar] [CrossRef]
- Sonneville, R.; Mourvillier, B.; Bouadma, L.; Wolff, M. Management of neurological complications of infective endocarditis in ICU patients. Ann. Intensive Care 2011, 1, 10. [Google Scholar] [CrossRef]
- Heintz, E.; Pettengill, M.A.; Gangat, M.A.; Hardy, D.J.; Bonnez, W.; Sobhanie, M.M. Oral flora meningoencephalitis diagnosis by next-generation DNA sequencing. Access Microbiol. 2019, 1, e000056. [Google Scholar] [CrossRef]
- Mollerup, S.; Friis-Nielsen, J.; Vinner, L.; Hansen, T.A.; Richter, S.R.; Fridholm, H.; Herrera, J.A.R.; Lund, O.; Brunak, S.; Izarzugaza, J.M. Propionibacterium acnes: Disease-causing agent or common contaminant? Detection in diverse patient samples by next-generation sequencing. J. Clin. Microbiol. 2016, 54, 980–987. [Google Scholar] [CrossRef]
- Peles, F.; Wagner, M.; Varga, L.; Hein, I.; Rieck, P.; Gutser, K.; Keresztúri, P.; Kardos, G.; Turcsányi, I.; Béri, B.; et al. Characterization of Staphylococcus aureus strains isolated from bovine milk in Hungary. Int. J. Food Microbiol. 2007, 118, 186–193. [Google Scholar] [CrossRef]
- Francisco, C.C.; Chamberlain, C.S.; Waldner, D.N.; Wettemann, R.P.; Spicer, L.J. Propionibacteria fed to dairy cows: Effects on energy balance, plasma metabolites and hormones, and reproduction. J. Dairy Sci. 2002, 85, 1738–1751. [Google Scholar] [CrossRef]
- Ametaj, B.N.; Zebeli, Q.; Iqbal, S. Nutrition, microbiota, and endotoxin-related diseases in dairy cows. Rev. Bras. De Zootec. 2010, 39, 433–444. [Google Scholar] [CrossRef]
- Oikawa, S.; Katoh, N. Decreases in serum apolipoprotein B-100 and AI concentrations in cows with milk fever and downer cows. Can. Vet. J. 2002, 66, 31–34. [Google Scholar]
- Kalaitzakis, E.; Panousis, N.; Roubies, N.; Giadinis, N.; Kaldrymidou, E.; Georgiadis, M.; Karatzias, H. Clinicopathological evaluation of downer dairy cows with fatty liver. Can. Vet. J. 2010, 51, 615. [Google Scholar]
- Williams, J.M.; Tsai, B. Intracellular trafficking of bacterial toxins. Curr. Opin. Cell Biol. 2016, 41, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Sharon, G.; Sampson, T.R.; Geschwind, D.H.; Mazmanian, S.K. The central nervous system and the gut microbiome. Cell 2016, 167, 915–932. [Google Scholar] [CrossRef] [PubMed]
- Spichak, S.; Bastiaanssen, T.F.; Berding, K.; Vlckova, K.; Clarke, G.; Dinan, T.G.; Cryan, J.F. Mining microbes for mental health: Determining the role of microbial metabolic pathways in human brain health and disease. Neurosci. Biobehav. Rev. 2021, 125, 698–761. [Google Scholar] [CrossRef] [PubMed]
| Variable | Downer Cattle | Normal Cattle |
|---|---|---|
| Number of brain tissue sample (Mixed brain, forebrain, midbrain, cerebellum, hindbrain, brainstem) | 57 (10, 11, 11, 9, 10, 6) | 42 (0, 8, 9, 9, 8, 8) |
| Number of bovine type (Korean beef, beef cattle, dairy cattle) | 21 (12, 1, 8) | 9 (9, 0, 0) |
| Average age of bovine sample (months) | 52.2 | 29.1 |
| Sex (male, female) | 9, 48 | 42, 0 |
| Number of brain samples with pathogenic bacteria isolated | 9 | 0 |
| Number of brain samples with virus detected | 5 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, Y.-J.; Kang, G.-U.; Jeong, M.; Singh, V.; Kim, J.; Lee, K.; Choi, E.-J.; Kim, H.-J.; Lee, S.; Lee, S.-Y.; et al. Bacterial Profiles of Brain in Downer Cattle with Unknown Etiology. Microorganisms 2023, 11, 98. https://doi.org/10.3390/microorganisms11010098
Park Y-J, Kang G-U, Jeong M, Singh V, Kim J, Lee K, Choi E-J, Kim H-J, Lee S, Lee S-Y, et al. Bacterial Profiles of Brain in Downer Cattle with Unknown Etiology. Microorganisms. 2023; 11(1):98. https://doi.org/10.3390/microorganisms11010098
Chicago/Turabian StylePark, Yeong-Jun, Gi-Ung Kang, Minsoo Jeong, Vineet Singh, Jongho Kim, Kyunghyun Lee, Eun-Jin Choi, Heui-Jin Kim, Seungjun Lee, Sook-Young Lee, and et al. 2023. "Bacterial Profiles of Brain in Downer Cattle with Unknown Etiology" Microorganisms 11, no. 1: 98. https://doi.org/10.3390/microorganisms11010098
APA StylePark, Y.-J., Kang, G.-U., Jeong, M., Singh, V., Kim, J., Lee, K., Choi, E.-J., Kim, H.-J., Lee, S., Lee, S.-Y., Oem, J.-K., & Shin, J.-H. (2023). Bacterial Profiles of Brain in Downer Cattle with Unknown Etiology. Microorganisms, 11(1), 98. https://doi.org/10.3390/microorganisms11010098






