Mitigation of Marine Dinoflagellates Using Hydrogen Peroxide (H2O2) Increases Toxicity towards Epithelial Gill Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Culture Conditions
2.1.1. Marine Dinoflagellates
2.1.2. Epithelial RTgill-W1 Cell Line
2.2. Culture Chronic and Acute Effect of H2O2 on Toxic Dinoflagellates
2.2.1. Photosynthetic Efficiency (PE) Measurement
2.2.2. Dinoflagellate Cell Viability (DCV) Test
2.3. RTgill-W1 Bioassay to Test Environmental Cytotoxic Effects
2.3.1. Marine Temporal Effect of H2O2 on the RTgill-W1 Cell Line
2.3.2. Combined Effects of H2O2 and Toxic and Non-Toxic Dinoflagellates on Gill Cells
2.4. Gill Cell Viability Endpoint
2.5. Statistical Analysis
3. Results
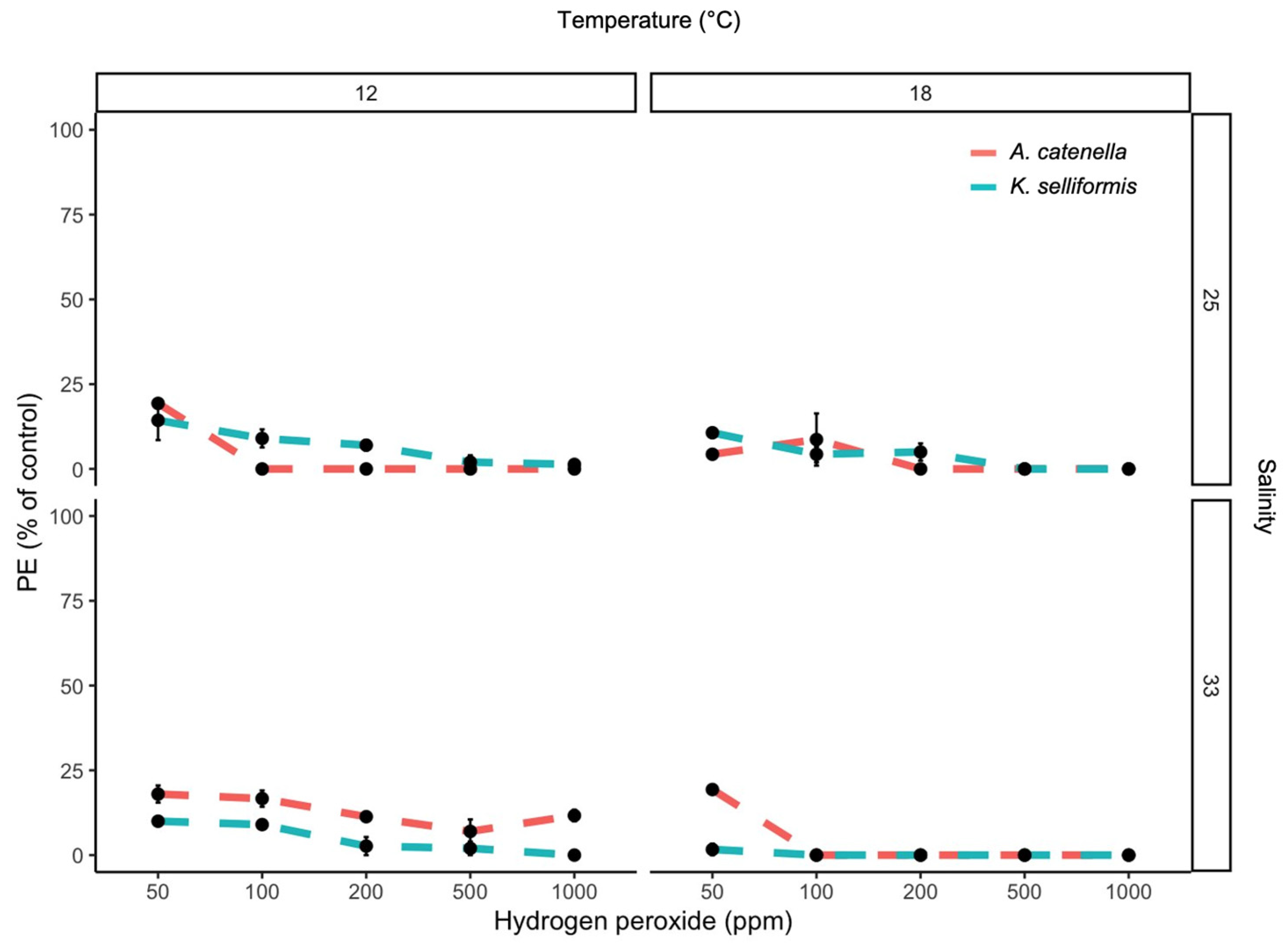
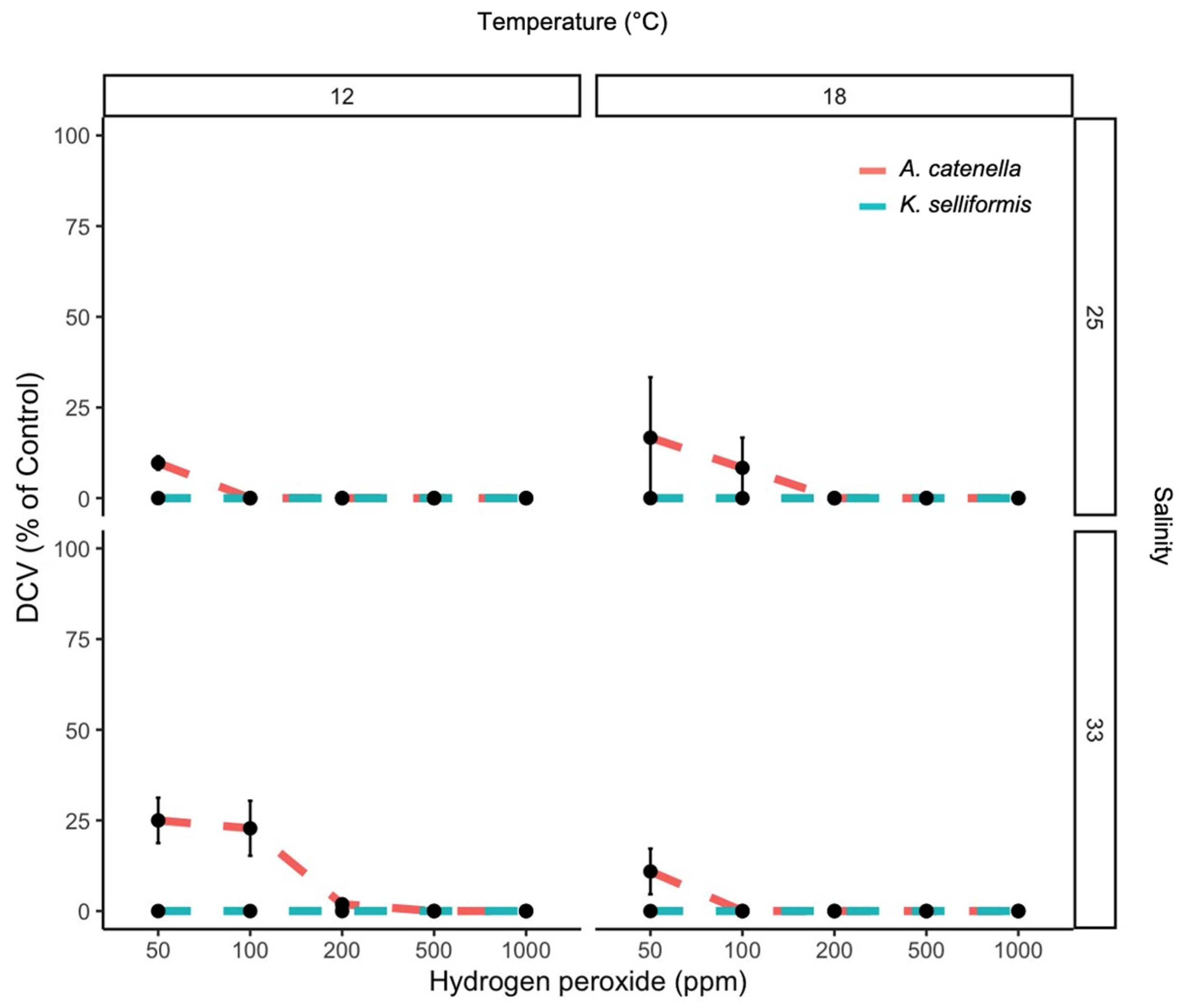
3.1. Effect of H2O2 Concentration, Species, Temperature and Salinity on PE, DCV and GCV
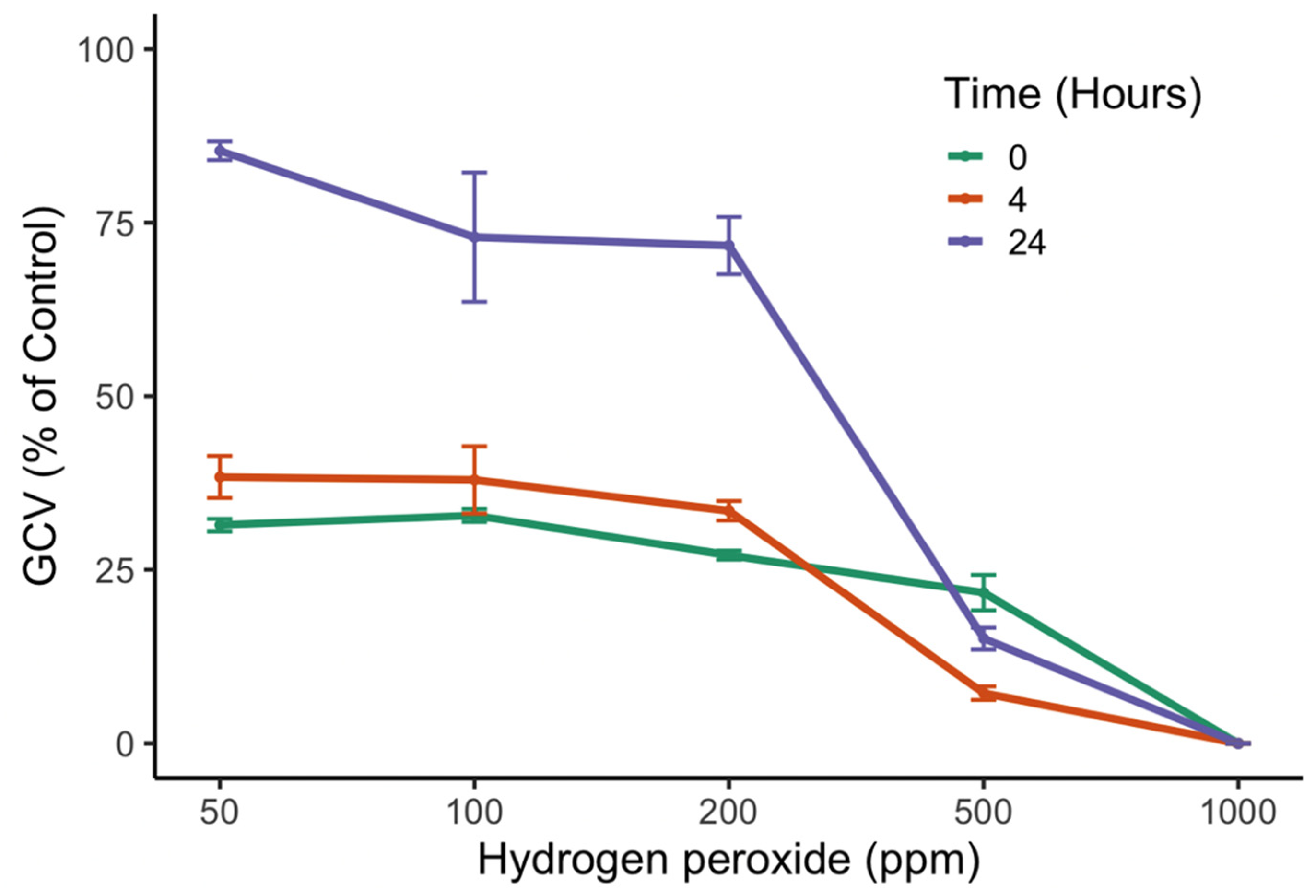
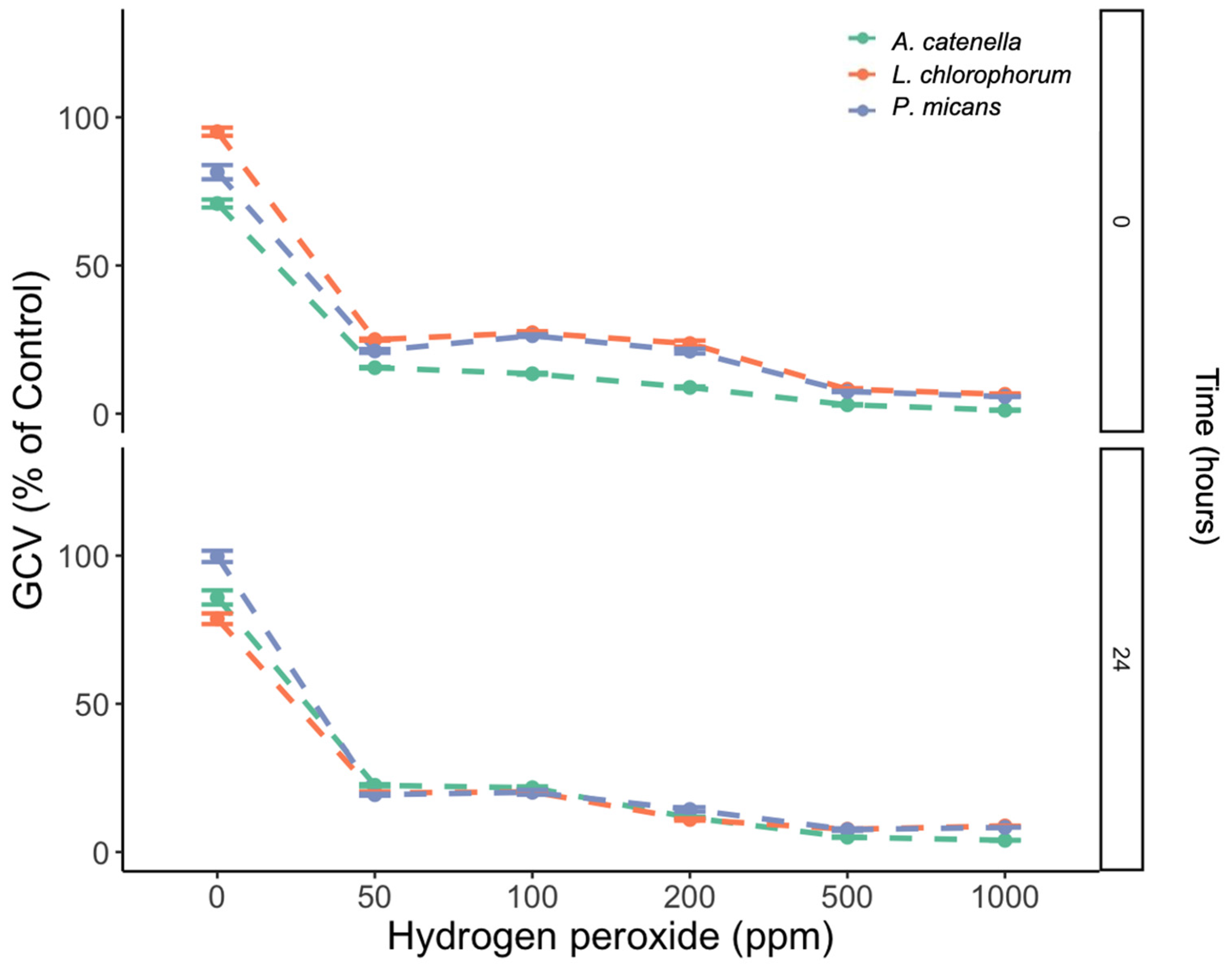
3.2. Effects of H2O2 Concentration and Time of Exposure on GCV
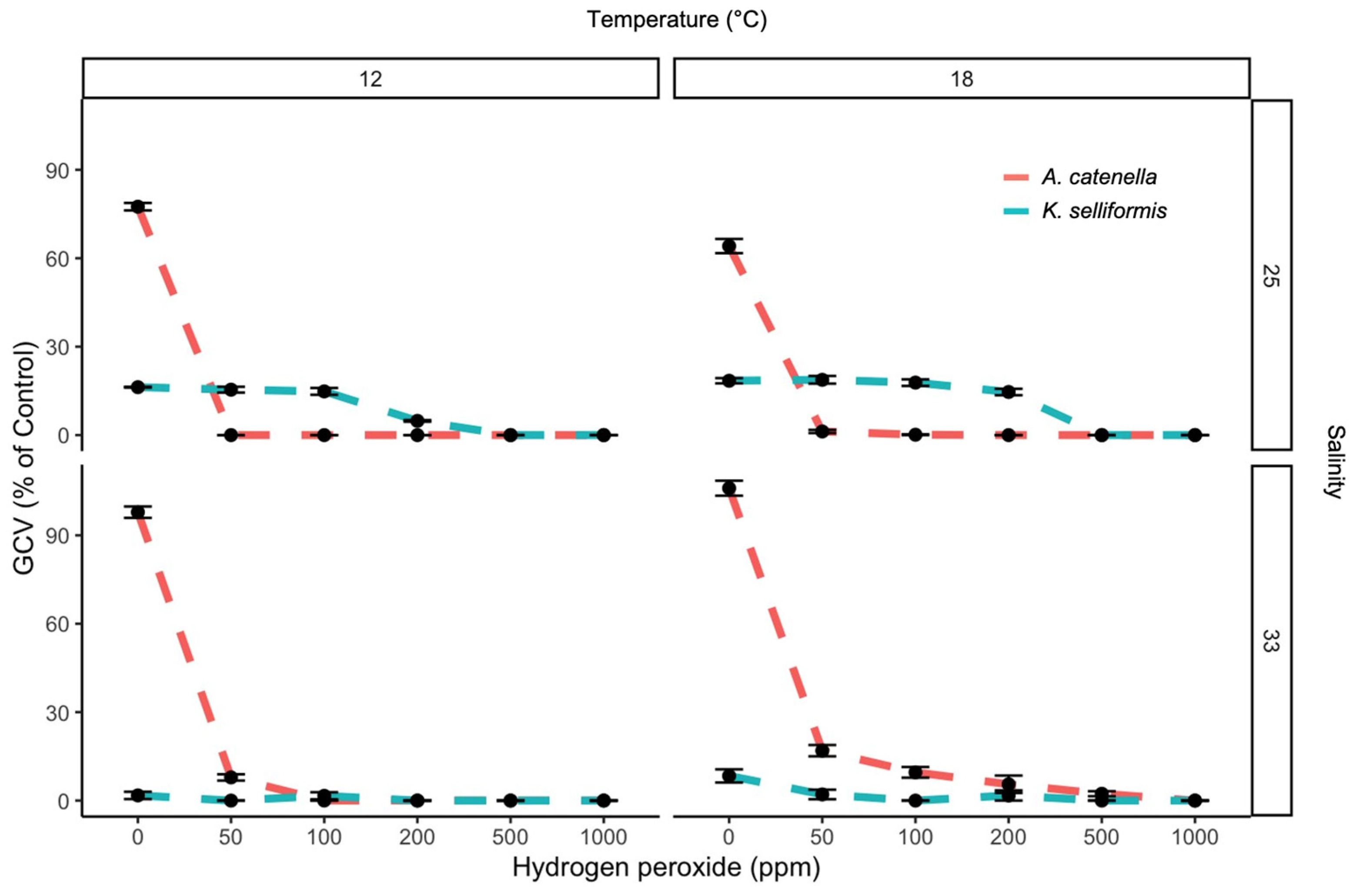
3.3. Synergistic Effect of H2O2 and Toxic and Non-Toxic Dinoflagellates on GCV
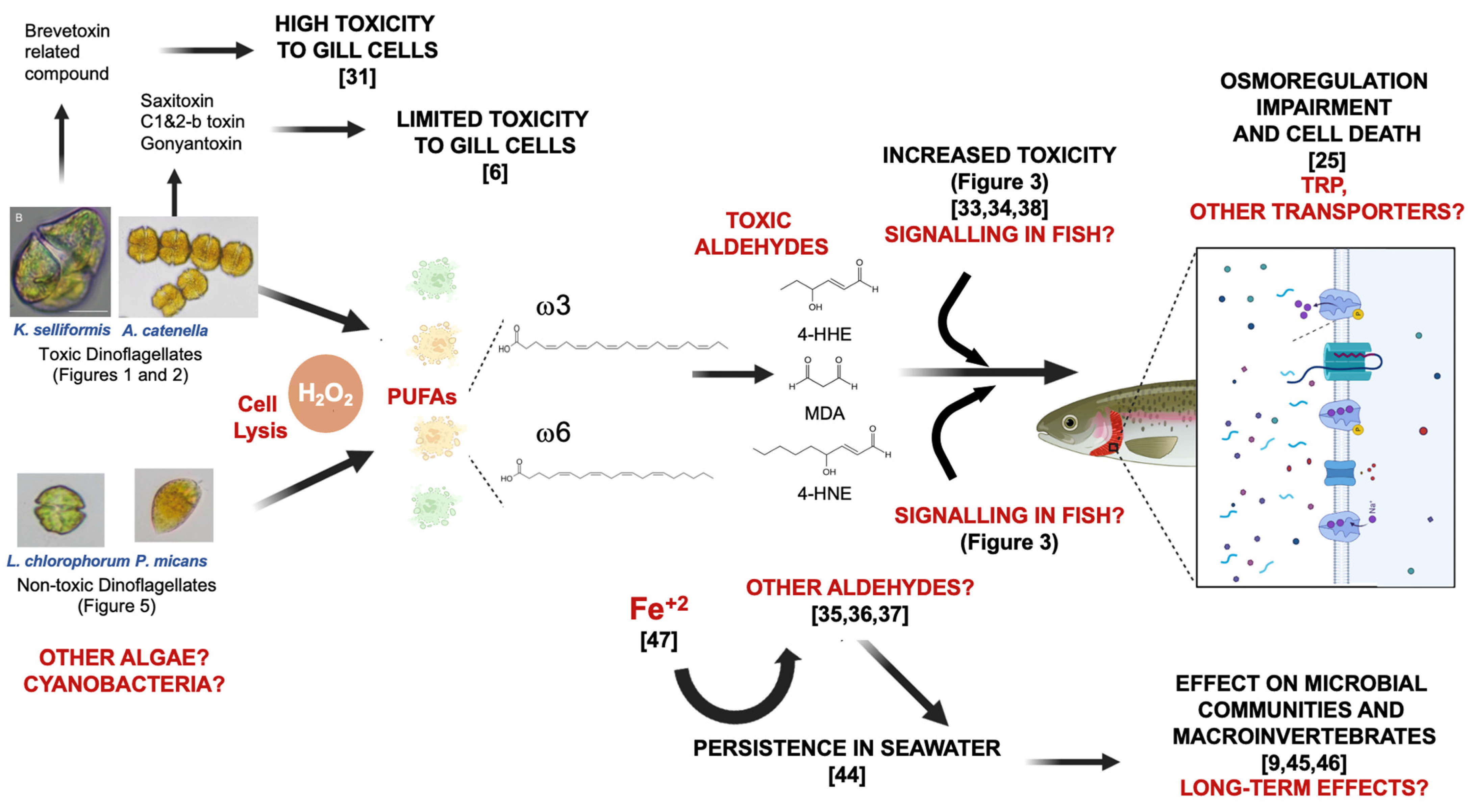
4. Discussion
4.1. Mitigation of Dinoflagellates Using H2O2
4.2. Toxicity in Dinoflagellate Bloom Mitigation Using H2O2
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mardones, J.I.; Paredes-Mella, J.; Flores-Leñero, A.; Yarimizu, K.; Godoy, M.; Artal, O.; Corredor-Acosta, A.; Marcus, L.; Cascales, E.; Espinoza, J.P.; et al. Extreme harmful algal blooms, climate change, and potential risk of eutrophication in Patagonian fjords: Insights from an exceptional Heterosigma akashiwo fish-killing event. Prog. Oceanogr. 2023, 210, 102921. [Google Scholar] [CrossRef]
- Alves-de-Souza, C.; Mardones, J.I.; Yñiguez, A.T.; Le Bihan, V.; Guillotreau, P.; Gatti, C.M.I.; Richlen, M.L.; Larsen, J.; Berdalet, E. Harmful Algae. In Blue Economy: An Ocean Perspective; Urban, E.R., Jr., Ittekkot, V., Eds.; Springer: Singapore, 2022; Volume 10, pp. 287–317. [Google Scholar] [CrossRef]
- Müller, M.N.; Mardones, J.I.; Dorantes-Aranda, J.J. Editorial: Harmful algal blooms (HABs) in Latin America. Front. Mar. Sci. 2020, 7, 34. [Google Scholar] [CrossRef]
- Diaz, J.M.; Plummer, S. Production of extracellular reactive oxygen species by phytoplankton: Past and future directions. J. Plankton Res. 2018, 40, 655–666. [Google Scholar] [CrossRef] [PubMed]
- Mccord, J.M.; Fridovich, I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J. Biol. Chem. 1969, 244, 6049–6055. [Google Scholar] [CrossRef] [PubMed]
- Diaz, J.M.; Plummer, S.; Tomas, C.; Alves-De-Souza, C. Production of extracellular superoxide and hydrogen peroxide by five marine species of harmful bloom-forming algae. J. Plankton Res. 2018, 40, 667–677. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Nakashima, T.; Matsuyama, Y.; Niwano, Y.; Yamaguchi, K.; Oda, T. Presence of the distinct systems responsible for superoxide anion and hydrogen peroxide generation in red tide phytoplankton Chattonella marina and Chattonella ovata. J. Plankton Res. 2007, 29, 241–247. [Google Scholar] [CrossRef]
- Tang, Y.; Gobler, C. Allelopathic effects of Cochlodinium polykrikoides isolates and blooms from the estuaries of Long Island, New York, on co-occurring phytoplankton. Mar. Ecol. Prog. Ser. 2010, 406, 19–31. [Google Scholar] [CrossRef]
- Mardones, J.I.; Dorantes-Aranda, J.J.; Nichols, P.D.; Hallegraeff, G.M. Fish gill damage by the dinoflagellate Alexandrium catenella from Chilean fjords: Synergistic action of ROS and PUFA. Harmful Algae 2015, 49, 40–49. [Google Scholar] [CrossRef]
- Cooper, W.J.; Zepp, R.G. Hydrogen peroxide decay in waters with suspended soils: Evidence for biologically mediated processes. Can. J. Fish. Aquat. Sci. 1990, 47, 888–893. [Google Scholar] [CrossRef]
- Matthijs, H.C.; Visser, P.M.; Reeze, B.; Meeuse, J.; Slot, P.C.; Wijn, G.; Talens, R.; Huisman, J. Selective suppression of harmful cyanobacteria in an entire lake with hydrogen peroxide. Water Res. 2012, 46, 1460–1472. [Google Scholar] [CrossRef]
- Burson, A.; Matthhijs, H.C.P.; de Bruijne, W.; Talens, R.; Hoogenboom, R.; Gerssen, A.; Visser, P.M.; Stomp, M.; Steur, K.; van Scheppingen, Y.; et al. Termination of a toxic Alexandrium bloom with hydrogen peroxide. Harmful Algae 2014, 31, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Barrington, D.J.; Ghadouani, A. Application of hydrogen peroxide for the removal of toxic cyanobacteria and other phytoplankton from wastewater. Environ. Sci. Technol. 2008, 42, 8916–8921. [Google Scholar] [CrossRef] [PubMed]
- Samuilov, V.; Timofeev, K.; Sinitsyn, S.; Bezryadnov, D. H2O2-induced inhibition of photosynthetic O2 evolution by Anabaena variabilis cells. Biochemistry 2004, 69, 926–933. [Google Scholar] [PubMed]
- Barrington, D.J.; Ghadouani, A.; Ivey, G.N. Environmental Factors and the Application of Hydrogen Peroxide for the Removal of Toxic Cyanobacteria from Waste Stabilization Ponds. J. Environ. Eng. 2011, 137, 952–960. [Google Scholar] [CrossRef]
- Barroin, G.; Feuillade, M. Hydrogen peroxide as a potential algicide for Oscillatoria rubescens D.C. Water Res. 1986, 20, 619–623. [Google Scholar] [CrossRef]
- Drábková, M.; Matthijs, H.C.P.; Admiraal, W.; Maršálek, B. Selective effects of H2O2 on cyanobacterial photosynthesis. Photosynthetica 2007, 45, 363–369. [Google Scholar] [CrossRef]
- Mattheiss, J.; Sellner, K.G.; Ferrier, D. Lake Anita Louise peroxide treatment summary—December 2016; CCWS Contribution #17-01, 2017; Center for Coastal and Watershed Studies (CCWS) Hood College: Frederick, MD, USA, 2017; 9p. [Google Scholar]
- Barrington, D.; Reichwaldt, E.; Ghadouani, A. The use of hydrogen peroxide to remove cyanobacteria and microcystins from waste stabilization ponds and hypereutrophic systems. Ecol. Eng. 2013, 50, 86–94. [Google Scholar] [CrossRef]
- NOWPAP CEARAC. Booklet on Countermeasures against Harmful Algal Blooms (HABs) in the NOWPAP Region; NOWPAP: Toyama City, Japan, 2007; 58p. [Google Scholar]
- Bolch, C.J.; Hallegraeff, G.M. Chemical and physical treatment options to kill toxic dinoflagellate cysts in ship´ ballast waters. J. Mar. Environ. Eng. 1993, 1, 23–29. [Google Scholar]
- Dorantes-Aranda, J.J.; Seger, A.; Mardones, J.I.; Nichols, P.D.; Hallegraeff, G.M. Progress in understanding algal bloom-mediated fish kills: The role of superoxide radicals, phycotoxins and fatty acids. PLoS ONE 2015, 10, e0133549. [Google Scholar] [CrossRef]
- Mardones, J.I.; Fuenzalida, G.; Zenteno, K.; Alves-de-Souza, C.; Astuya, A.; Dorantes-Aranda, J.J. Salinity-growth response and ichthyotoxic potency of the Chilean Pseudochattonella verruculosa. Front. Mar. Sci. 2019, 6, 24. [Google Scholar] [CrossRef]
- Mardones, J.I.; Paredes, J.; Godoy, M.; Suarez, R.; Norambuena, L.; Vargas, V.; Fuenzalida, G.; Pinilla, E.; Artal, O.; Rojas, X.; et al. Disentangling the Environmental Processes Responsible for the World’s Largest Farmed Fish-Killing Harmful Algal Bloom: Chile, 2016. Sci. Total Environ. 2021, 766, 144383. [Google Scholar] [CrossRef]
- Flores-Leñero, A.; Torres-Vargas, V.; Paredes-Mella, J.; Norambuena, L.; Fuenzalida, G.; Lee-Chang, K.; Mardones, J.I. Heterosigma akashiwo in Patagonian fjords: Genetics, growth, pigment signature and role of PUFA and ROS in ichthyotoxicity. Toxins 2022, 14, 577. [Google Scholar] [CrossRef] [PubMed]
- Trainer, V.L.; Moore, S.K.; Hallegraeff, G.; Kudela, R.M.; Clement, A.; Mardones, J.I.; Cochlan, W.P. Pelagic harmful algal blooms and climate change: Lessons from nature´s experiments with extremes. Harmful Algae 2020, 91, 101591. [Google Scholar] [CrossRef] [PubMed]
- Mardones, J.I. Screening of Chilean fish-killing microalgae using a gill cell-based assay. Lat. Am. J. Aquat. Res. 2020, 48, 329–335. [Google Scholar] [CrossRef]
- Mardones, J.I.; Shabala, L.; Shabala, S.; Dorantes-Aranda, J.J.; Seger, A.; Hallegraeff, G.M. Fish gill damage by harmful microalgae newly explored by microelectrode ion flux estimation techniques. Harmful Algae 2018, 80, 55–63. [Google Scholar] [CrossRef]
- Van Kooten, O.; Snel, J.F.H. The use of chlorophyll fluorescence nomenclature in plant stress physiology. Photosynth. Res. 1990, 25, 147–150. [Google Scholar] [CrossRef]
- Onji, M.; Sawabe, T.; Ezura, Y. An evaluation of viable staining dyes suitable for marine phytoplankton. Bull. Fac. Fish. Hokkaido Univ. 2000, 51, 153–157. [Google Scholar]
- Bates, D.; Machler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar]
- Bruno, D.W.; Raynard, R.S. Studies on the use of hydrogen peroxide as a method for the control of sea lice on Atlantic salmon. Aquac. Int. 1994, 2, 10–18. [Google Scholar] [CrossRef]
- Warr, S.R.C.; Reed, R.H.; Stewart, W.D.P. Carbohydrate accumulation in osmotically stressed cyanobacteria (blue-green algae): Interactions of temperature and salinity. New Phytol. 1985, 100, 285–292. [Google Scholar] [CrossRef]
- Mardones, J.I.; Norambuena, L.; Paredes, J.; Fuenzalida, G.; Dorantes-Aranda, J.J.; Chang, K.J.L.; Guzmán, L.; Krock, B.; Hallegraeff, G. Unraveling the Karenia selliformis complex with the description of a non-gymnodimine producing Patagonian phylotype. Harmful Algae 2020, 98, 101892. [Google Scholar] [CrossRef]
- Tort, M.J.; Fletcher, C.; Wooster, G.A.; Bowser, P.R. Stability of Hydrogen Peroxide in Aquaria as a Fish Disease Treatment. J. Appl. Aquat. 2004, 14, 37–45. [Google Scholar] [CrossRef]
- Riahi, Y.; Cohen, G.; Shamni, O.; Sasson, S. Signaling and cytotoxic functions of 4-hydroxyalkenals. Am. J. Physiol. Metab. 2010, 299, E879–E886 . [Google Scholar] [CrossRef] [PubMed]
- Gaschler, M.M.; Stockwell, B.R. Lipid peroxidation in cell death. Biochem. Biophys. Res. Commun. 2017, 482, 419–425. [Google Scholar] [CrossRef]
- Catalá, A. An overview of lipid peroxidation with emphasis in outer segments of photoreceptors and the chemiluminescence assay. Int. J. Biochem. Cell Biol. 2006, 38, 1482–1495. [Google Scholar] [CrossRef] [PubMed]
- Kawai, Y.; Takeda, S.; Terao, J. Lipidomic Analysis for Lipid Peroxidation-Derived Aldehydes Using Gas Chromatography-Mass Spectrometry. Chem. Res. Toxicol. 2006, 20, 99–107. [Google Scholar] [CrossRef]
- Esterbauer, H.; Schaur, R.J.; Zollner, H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic. Biol. Med. 1991, 11, 81–128. [Google Scholar] [CrossRef]
- Hellenthal, K.E.M.; Brabenec, L.; Gross, E.R.; Wagner, N.-M. TRP Channels as Sensors of Aldehyde and Oxidative Stress. Biomolecules 2021, 11, 1401. [Google Scholar] [CrossRef]
- Guh, Y.J.; Lin, C.H.; Hwang, P.P. Osmorregulation in zebrafish: Ion transport mechanisms and functional regulation. EXCLI J. 2015, 14, 627–659. [Google Scholar]
- Nisembaum, L.G.; Loentgen, G.; L´Honoré, T.; Martin, P.; Paulin, C.-H.; Fuentes, M.; Escoubeyrou, K.; Delgado, M.J.; Besseau, L.; Falcón, J. Transient receptor potential-vanilloid (TRPV1-TRPV4) channels in the Atlantic salmon, Salmo salar. A focus on the pineal gland and melatonin production. Front. Physiol. 2022, 12, 784416. [Google Scholar] [CrossRef]
- Kumari, P.; Can´ani, A.; Didi-Cohen, S.; Tzin, V.; Khozin-Goldberg, I. Nitrogen deprivation-induced production of volatile organic compounds in the arachidonic-acid-accumulating microalga Lobosphaera incisa underspin their role as ROS scavengers and chemical messengers. Front. Mar. Sci. 2020, 7, 410. [Google Scholar] [CrossRef]
- Goulitquer, S.; Ritter, A.; Thomas, F.; Ferec, C.; Salaün, J.-P.; Potin, P. Release of volatile aldehydes by the brown algal kelp Laminaria digitata in response to both biotic and abiotic stress. ChemBioChem 2009, 10, 977–982. [Google Scholar] [CrossRef] [PubMed]
- Koteska, D.; Garcia, S.S.; Wagner-Döbler, I.; Schulz, S. Identification of Volatiles of the Dinoflagellate Prorocentrum cordatum. Mar. Drugs 2022, 20, 371. [Google Scholar] [CrossRef] [PubMed]
- Bartual, A.; Ortega, M.J. Temperature differentially affects the persistence of polyunsaturated aldehydes in seawater. Environ. Chem. 2013, 10, 403–408. [Google Scholar] [CrossRef]
- Lusty, M.W.; Gobler, C.J. The efficacy of hydrogen peroxide in mitigating cyanobacterial blooms and altering microbial communities across four lakes in NY, USA. Toxins 2020, 12, 428. [Google Scholar] [CrossRef]
- Santos, A.A.; Guedes, D.O.; Barros, M.U.; Oliveira, S.; Pacheco, A.B.; Azevedo, S.M.; Magalhães, V.F.; Pestana, C.J.; Edwards, C.; Lawton, L.A.; et al. Effect of hydrogen peroxide on natural phytoplankton and bacterioplankton in a drinking water reservoir: Mesocosm-scale study. Water Res. 2021, 197, 117069. [Google Scholar] [CrossRef]
- Repetto, M.G.; Ferrarotti, N.F.; Boveris, A. The involvement of transition metal ions on iron-dependent lipid peroxidation. Arch. Toxicol. 2009, 84, 255–262. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mardones, J.I.; Flores-Leñero, A.; Pinto-Torres, M.; Paredes-Mella, J.; Fuentes-Alburquenque, S. Mitigation of Marine Dinoflagellates Using Hydrogen Peroxide (H2O2) Increases Toxicity towards Epithelial Gill Cells. Microorganisms 2023, 11, 83. https://doi.org/10.3390/microorganisms11010083
Mardones JI, Flores-Leñero A, Pinto-Torres M, Paredes-Mella J, Fuentes-Alburquenque S. Mitigation of Marine Dinoflagellates Using Hydrogen Peroxide (H2O2) Increases Toxicity towards Epithelial Gill Cells. Microorganisms. 2023; 11(1):83. https://doi.org/10.3390/microorganisms11010083
Chicago/Turabian StyleMardones, Jorge I., Ana Flores-Leñero, Marco Pinto-Torres, Javier Paredes-Mella, and Sebastián Fuentes-Alburquenque. 2023. "Mitigation of Marine Dinoflagellates Using Hydrogen Peroxide (H2O2) Increases Toxicity towards Epithelial Gill Cells" Microorganisms 11, no. 1: 83. https://doi.org/10.3390/microorganisms11010083
APA StyleMardones, J. I., Flores-Leñero, A., Pinto-Torres, M., Paredes-Mella, J., & Fuentes-Alburquenque, S. (2023). Mitigation of Marine Dinoflagellates Using Hydrogen Peroxide (H2O2) Increases Toxicity towards Epithelial Gill Cells. Microorganisms, 11(1), 83. https://doi.org/10.3390/microorganisms11010083





