Strain-Level Characterization of Legionella Environmental Isolates via MALDI-TOF-MS
Abstract
1. Introduction
2. Materials and Methods
2.1. Environmental Sampling
2.2. Legionella Culturing
2.3. DNA Extraction and Molecular Analysis
2.4. MALDI-TOF-MS Sample Preparation
2.5. MALDI-TOF-MS Data Acquisition and Analysis
2.6. MALDI-TOF-MS Optimization
2.7. MALDI-TOF-MS Cluster Analysis
2.8. 16S rRNA Analysis of Isolates
2.9. Statistical Analysis
3. Results and Discussion
3.1. MALDI-TOF-MS Optimization
3.2. MALDI-TOF-MS Cluster Analysis
3.3. 16S rRNA Analysis of Isolates
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brunkard, J.M.; Ailes, E.; Roberts, V.A.; Hill, V.; Hillborn, E.D.; Craun, G.F.; Rajasingham, A.; Kahler, A.; Garrison, L.; Hicks, L.; et al. Surveillance for Waterborne Disease Outbreaks Associated with Drinking Water—United States, 2007–2008. CDC MMWR Surveill. Summ. 2011, 60, 38–68. [Google Scholar] [PubMed]
- Hicks, L.A.; Garrison, L.E.; Nelson, G.E.; Hampton, L.M. Legionellosis—United States, 2000–2009. Centers for Disease Control and Prevention Morbidity and Mortality. Wkly. Rep. 2011, 60, 1083–1086. [Google Scholar]
- Blanco, S.B.; Sanz, C.; Gutiérrez, M.P.; Simarro, M.; López, I.; Escribano, I.; Eiros, J.M.; Zarzosa, P.; Orduña, A.; López, J.; et al. A new MALDI-TOF approach for the quick sequence type identification of Legionella pneumophila. J. Microbiol. Methods 2021, 188, 106292. [Google Scholar] [CrossRef]
- Gaia, V.; Casati, S.; Tonolla, M. Rapid identification of Legionella spp. by MALDI-TOF MS based protein mass fingerprinting. Syst. Appl. Microbiol. 2011, 34, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Jarraud, S.; Descours, G.; Ginevra, C.; Lina, G.; Etienne, J. Identification of legionella in clinical samples. Methods Mol. Biol. 2013, 954, 27–56. [Google Scholar]
- Kliem, M.; Sauer, S. The essence of mass spectrometry based microbial diagnostics. Curr. Opin. Microbiol. 2012, 15, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Clark, C.G.; Kruczkiewicz, P.; Guan, C.; McCorrister, S.J.; Chong, P.; Wylie, J.; van Caeseele, P.; Tabor, H.A.; Snarr, P.; Gilmour, M.W.; et al. Evaluation of MALDI-TOF mass spectrometry methods for determination of Escherichia coli pathotypes. J. Microbiol. Methods 2013, 94, 180–191. [Google Scholar] [CrossRef]
- Mencacci, A.; Monari, C.; Leli, C.; Merlini, L.; De Carolis, E.; Vella, A.; Cacioni, M.; Buzi, S.; Nardelli, E.; Bistoni, F.; et al. Typing of nosocomial outbreaks of Acinetobacter baumannii by use of matrix-assisted laser desorption ionization-time of flight mass spectrometry. J. Clin. Microbiol. 2013, 51, 603–606. [Google Scholar] [CrossRef]
- Siegrist, T.J.; Anderson, P.D.; Huen, W.H.; Kleinheinz, G.T.; McDermott, C.M.; Sandrin, T.R. Discrimination and characterization of environmental strains of Escherichia coli by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF-MS). J. Microbiol. Methods 2007, 68, 554–562. [Google Scholar] [CrossRef]
- Giebel, R.A.; Fredenber, W.; Sandrin, T.R. Characterization of environmental isolates of Enterococcus spp. by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Water Res. 2008, 42, 931–940. [Google Scholar] [CrossRef]
- Doan, N.T.; Van Hoorde, K.; Cnockaert, M.; De Brandt, E.; Aerts, M.; Le Thanh, B.; Vandamme, P. Validation of MALDI-TOF MS for rapid classification and identification of lactic acid bacteria, with a focus on isolates from traditional fermented foods in Northern Vietnam. Lett. Appl. Microbiol. 2012, 55, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Sandrin, T.R.; Goldstein, J.E.; Schumaker, S. MALDI TOF MS profiling of bacteria at the strain level: A review. Mass Spectrom. Rev. 2012, 32, 188–217. [Google Scholar] [CrossRef] [PubMed]
- Moliner, C.; Gnevra, C.; Jarraud, S.; Flaudrops, C.; Bedotto, M.; Couderc, C.; Etienne, J.; Fournier, P.E. Rapid identification of Legionella species by mass spectrometry. J. Med. Microbiol. 2010, 59, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Svarrer, C.W.; Uldum, S.A. The occurrence of Legionella species other than Legionella pneumophila in clinical and environmental samples in Denmark identified by mip gene sequencing and matrix-assisted laser desorption ionization time-of-flight mass spectrometry. Clin. Microbiol. Infect. 2011, 18, 1004–1009. [Google Scholar] [CrossRef]
- Pennenac, X.; Dufour, A.; Haras, D.; Réhel, K. A quick and easy method to identify bacteria by matrix-assisted laser desorption/ionisation time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 2010, 24, 384–392. [Google Scholar] [CrossRef]
- Fujinami, Y.; Kikkawa, H.S.; Kurosaki, Y.; Sakurada, K.; Yoshino, M.; Yasuda, J. Rapid discrimination of Legionella by matrix-assisted laser desorption ionization time-of-flight mass spectrometry. Microbiol. Res. 2011, 166, 77–86. [Google Scholar] [CrossRef]
- Kyritsi, M.A.; Kristo, I.; Hadjichristodoulou, C. Serotyping and detection of pathogenicity loci of environmental isolates of Legionella pneumophila using MALDI-TOF MS. Int. J. Hyg. Environ. Health 2020, 224, 113441. [Google Scholar] [CrossRef]
- Ruelle, V.; El Moualij, B.; Zorzi, W.; Ledent, P.; Pauw, E.D. Rapid identification of environmental bacterial strains by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 2004, 18, 2013–2019. [Google Scholar] [CrossRef]
- Schwake, D.O.; Alum, A.; Abbaszadegan, M. Automobile windshield washer fluid: A potential source of transmission for Legionella. Sci. Total Environ. 2015, 526, 271–277. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services Public Health Service Centers for Disease Control and Prevention. Procedures for the Recovery of Legionella from the Environment; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2005.
- Wullings, B.A.; Bakker, G.; van der Kooij, D. Concentration and diversity of uncultured Legionella spp. in two unchlorinated drinking water supplies with different concentrations of natural organic matter. Appl. Environ. Microbiol. 2011, 77, 634–641. [Google Scholar] [CrossRef]
- Pereira, L.; Dias, N.; Santos, C.; Lima, N. The use of MALDI-TOF ICMS as an alternative tool for Trichophyton rubrum identification and typing. Enferm. Infecc. Microbiol. Clín. 2014, 31, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Sievers, F.; Wilm, A.; Dineen, D.G.; Gibson, T.J.; Karplus, K.; Li, W.; Lopez, R.; McWilliam, H.; Remmert, M.; Söding, J.; et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 2011, 7, 539. [Google Scholar] [CrossRef] [PubMed]
- Pascale, M.R.; Mazzotta, M.; Salaris, S.; Girolamini, L.; Grottola, A.; Simone, M.L.; Cordovana, M.; Bisognin, F.; Monte, P.D.; Sabattini, M.A.B.; et al. Evaluation of MALDI-TOF Mass Spectrometry in Diagnostic and Environmental Surveillance of Legionella Species: A comparison with Culture and Mip-gene Sequence Technique. Front. Microbiol. 2020, 11, 589369. [Google Scholar] [CrossRef] [PubMed]
- Schumaker, S.; Borror, C.M.; Sandrin, T.R. Automating data acquisition affects mass spectrum quality and reproducibility during bacterial profiling using an intact cell sample preparation method with matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 2012, 15, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Sedo, O.; Voráč, A.; Zdráhal, Z. Optimization of mass spectral features in MALDI-TOF MS profiling of Acinetobacter species. Syst. Appl. Microbiol. 2011, 31, 30–34. [Google Scholar] [CrossRef]
- Buse, H.Y.; Donohue, M.J.; Ashbolt, N.J. Hartmannella vermiformis inhibition of Legionella pneumophila Cultivability. Microb. Ecol. 2013, 66, 715–726. [Google Scholar] [CrossRef]
- Kern, C.C.; Usbek, J.C.; Vogel, R.F.; Behr, J. Optimization of Matrix-Assisted-Laser-Desorption-Ionization-Time-Of-Flight Mass Spectrometry for the identification of bacterial contaminants in beverages. J. Microbiol. Methods 2013, 93, 185–191. [Google Scholar] [CrossRef]
- Drevinek, M.; Dresler, J.; Klimentova, J.; Pisa, L.; Hubalek, M. Evaluation of sample preparation methods for MALDI-TOF MS identification of highly dangerous bacteria. Lett. Appl. Microbiol. 2012, 55, 40–46. [Google Scholar] [CrossRef]
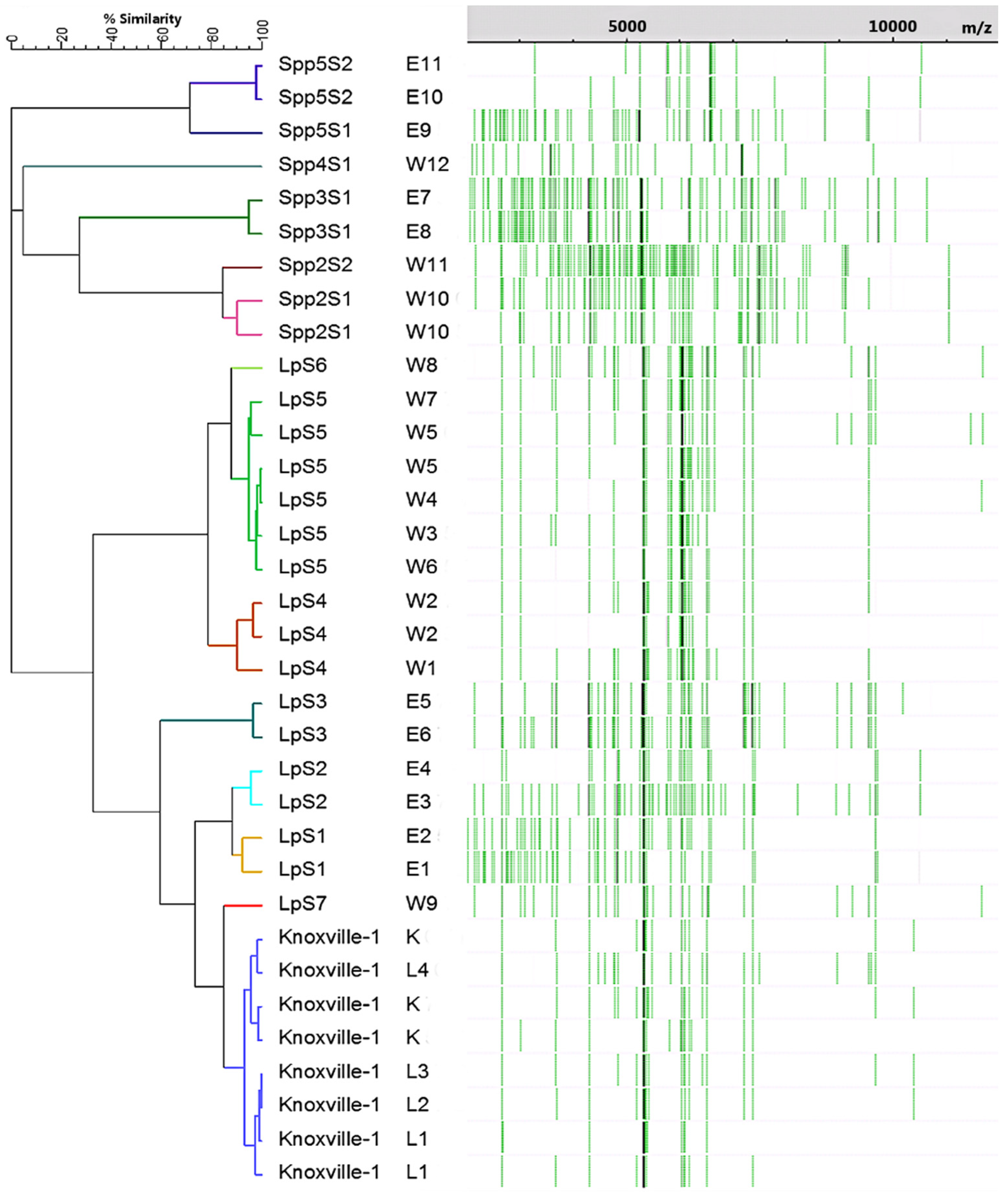
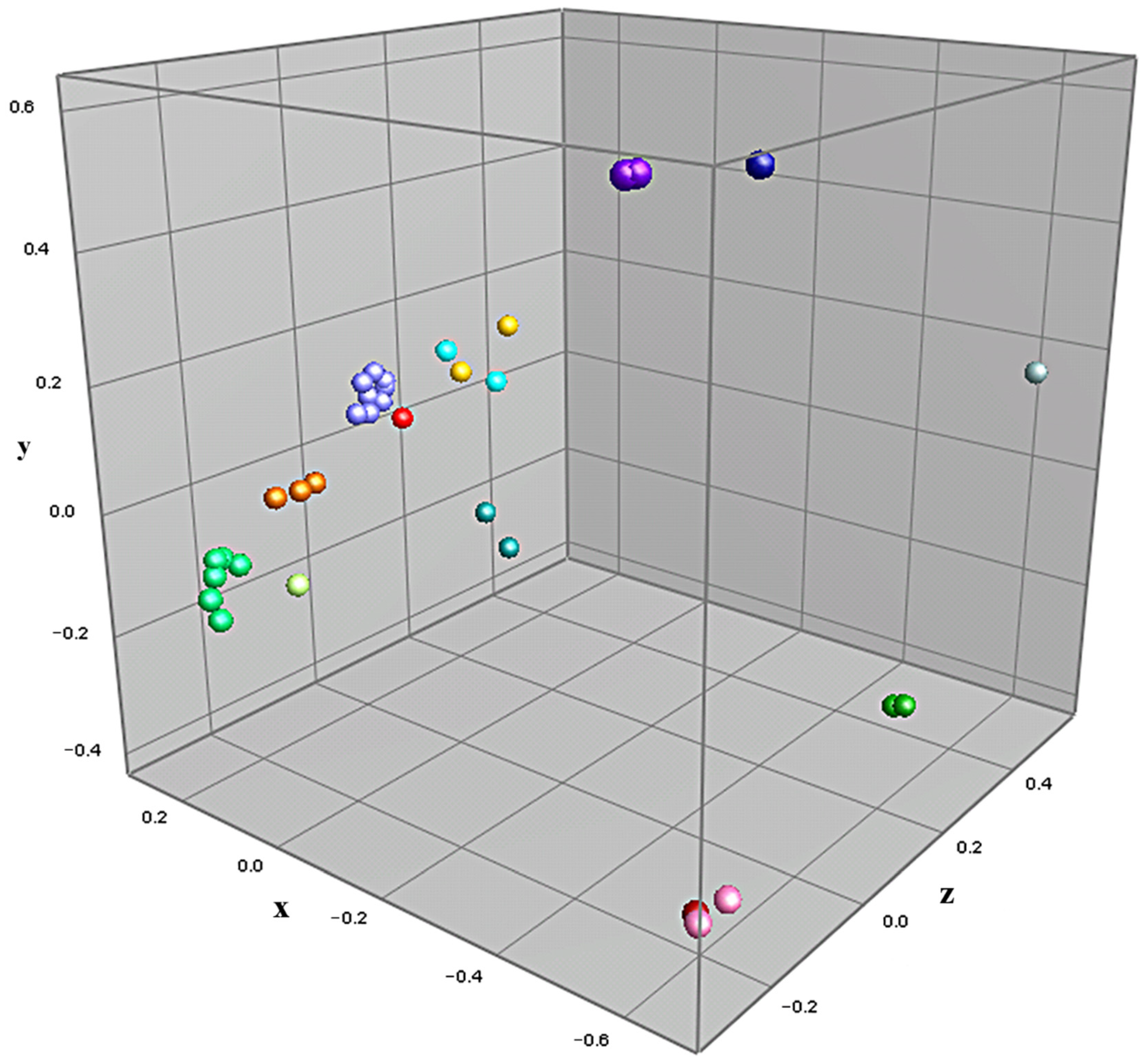

| Isolate | K | L1–L4 | E1–E4 | E5, E6 | E7–E11 | W1, W5 | W2–W4, W6–W9 | W10, W11 | W12 |
|---|---|---|---|---|---|---|---|---|---|
| Water Source | Stock Culture | Laboratory Experiment | East System | East System | East System | West System | West System | West System | West System |
| Water Type | N/A | Sterile Water | Tap Water | Tap Water | Tap Water | Tap Water | Washer Fluid | Washer Fluid | Tap Water |
| Spp | Lp | Lp | Lp | Lp | Non-Lp | Lp | Lp | Non-Lp | Non-Lp |
| Sample | Base Peak S:N Ratio | Base Peak Resolution | Peak Number | Reproducibility (%) |
|---|---|---|---|---|
| (a) | ||||
| K IC | 364.4 ± 275.2 | 627.0 ± 30.2 | 94.7 ± 156.2 | 80.9 ± 6.5 |
| K PE | 480.9 ± 219.0 | 647.6 ± 35.0 | 23 ± 2.6 | 92.8 ± 4.7 |
| W5 IC | 616.9 ± 657.7 | 650.2 ± 31.1 | 120.0 ± 79.2 | 83.5 ± 9.6 |
| W5 PE | 327.1 ± 188.9 | 655.3 ± 27.5 | 33.3 ± 0.6 | 87.2 ± 8.8 |
| W11 IC | 103.0 ± 44.4 | 742.1 ± 47.7 | 444.3 ± 275.2 | 50.0 ± 23.7 |
| W11 PE | 275.4 ± 24.9 | 782.8 ± 68.8 | 104.0 ± 32.9 | 91.9 ± 1.2 |
| Avg IC | 361.4 ± 256.9 | 673.1 ± 60.9 | 219.7 ± 194.9 | 71.5 ± 18.6 |
| Avg PE | 361.1 ± 106.9 | 695.2 ± 75.9 | 53.4 ± 44.1 | 90.7 ± 2.9 |
| (b) | ||||
| K Broth | 140.3 ± 28.8 | 521.2 ± 9.7 | 765.3 ± 71.9 | 76.6 ± 9.1 |
| W5 Broth | 117.6 ± 46.3 | 546.3 ± 49.7 | 772.7 ± 42.2 | 45.7 ± 14.7 |
| W11 Broth | 40.4 ± N/A | 707.6 ± N/A | 1193 ± N/A | N/A |
| Avg Broth | 99.4 ± 52.4 | 591.7 ± 101.1 | 910.3 ± 244.8 | 61.2 ± 21.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schwake, D.O.; Sandrin, T.; Zhang, L.; Abbaszadegan, M. Strain-Level Characterization of Legionella Environmental Isolates via MALDI-TOF-MS. Microorganisms 2023, 11, 8. https://doi.org/10.3390/microorganisms11010008
Schwake DO, Sandrin T, Zhang L, Abbaszadegan M. Strain-Level Characterization of Legionella Environmental Isolates via MALDI-TOF-MS. Microorganisms. 2023; 11(1):8. https://doi.org/10.3390/microorganisms11010008
Chicago/Turabian StyleSchwake, David Otto, Todd Sandrin, Lin Zhang, and Morteza Abbaszadegan. 2023. "Strain-Level Characterization of Legionella Environmental Isolates via MALDI-TOF-MS" Microorganisms 11, no. 1: 8. https://doi.org/10.3390/microorganisms11010008
APA StyleSchwake, D. O., Sandrin, T., Zhang, L., & Abbaszadegan, M. (2023). Strain-Level Characterization of Legionella Environmental Isolates via MALDI-TOF-MS. Microorganisms, 11(1), 8. https://doi.org/10.3390/microorganisms11010008






