Occurrence of Rickettsia spp., Hantaviridae, Bartonella spp. and Leptospira spp. in European Moles (Talpa europaea) from the Netherlands
Abstract
1. Introduction
2. Material and Methods
2.1. Sample Collection
2.2. Nucleic Acid Extraction, qPCR and Sequencing
2.3. TBEV Serology
2.4. Phylogenetic Analysis
3. Results
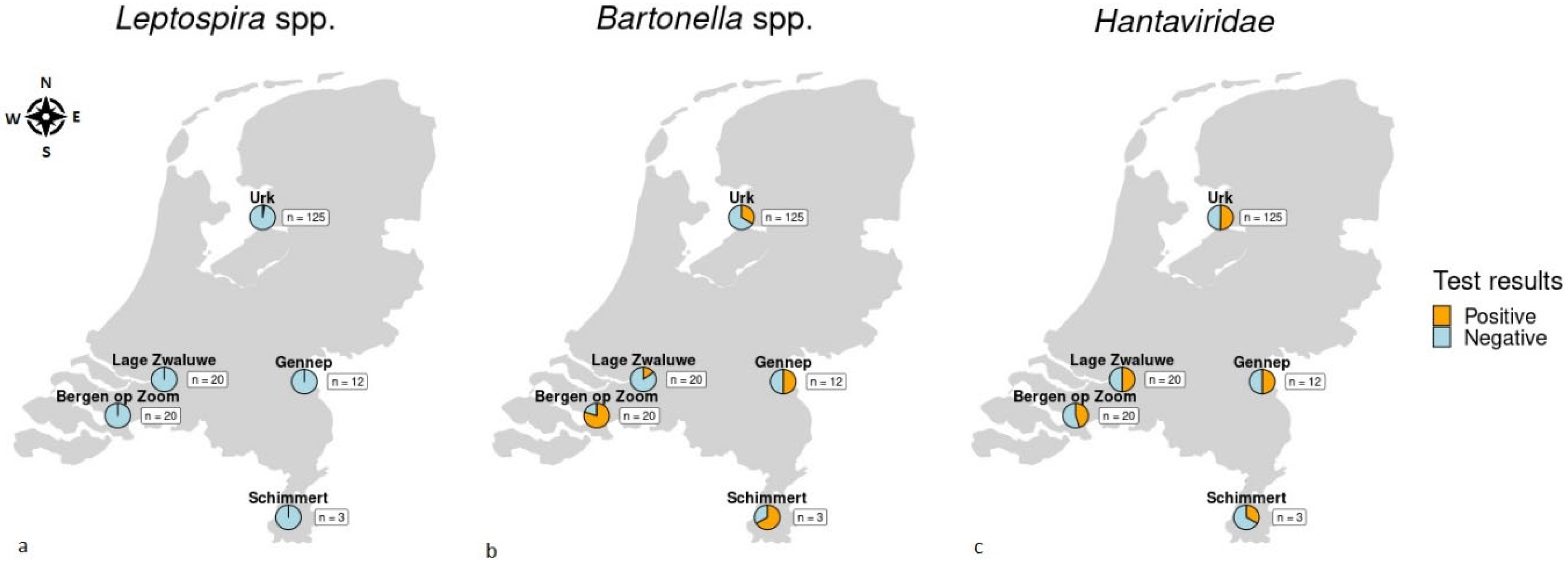
3.1. Apparent Pathogen Prevalence
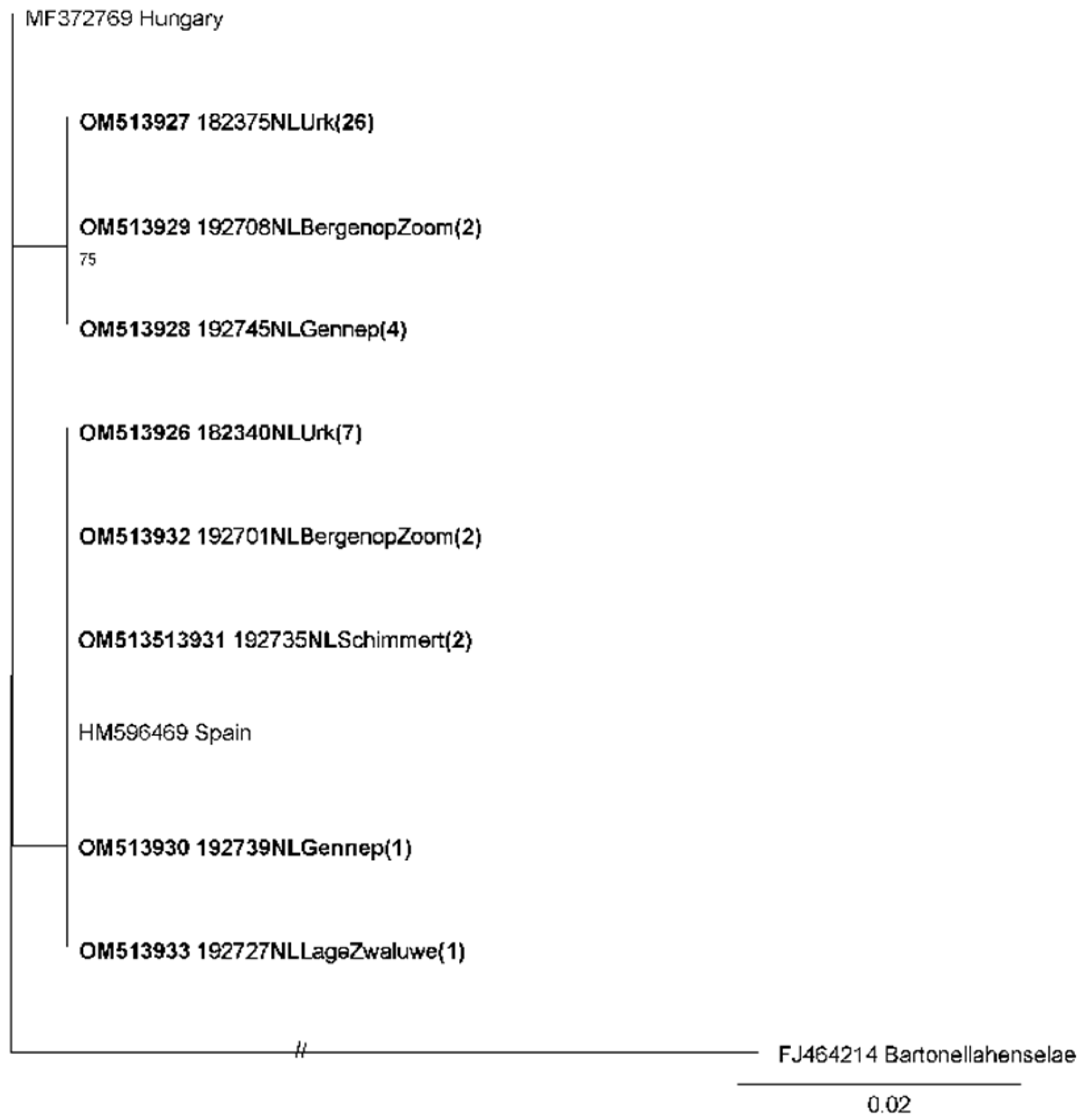
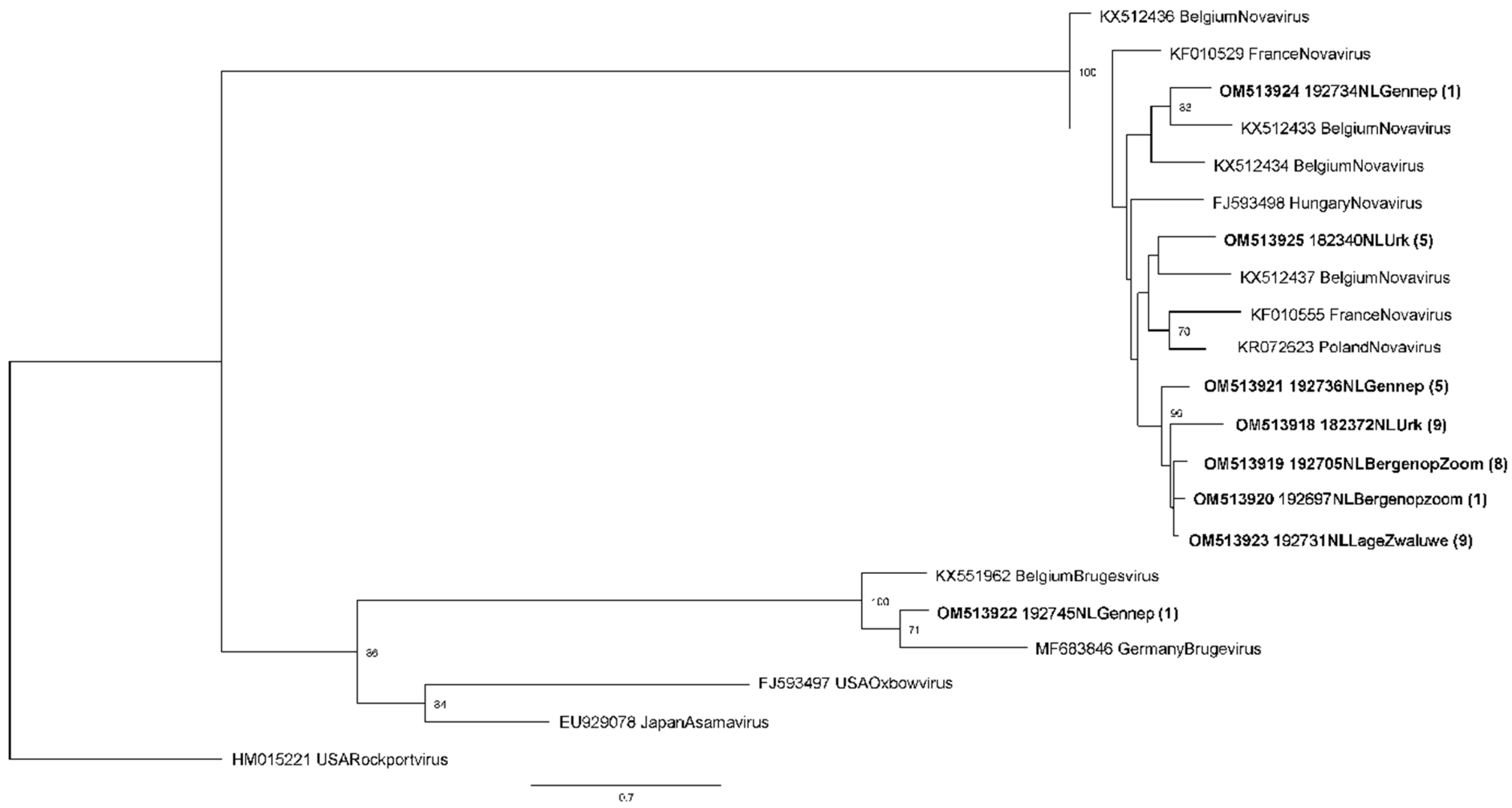
3.2. Phylogenetic Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amori, G.; Hutterer, R.; Mitsainas, G.; Yigit, N.; Krystufek, B.; Palomo, L. Talpa europaea. The IUCN Red List of Threatened Species 2017. Available online: https://www.iucnredlist.org/species/41481/22320754 (accessed on 17 May 2022).
- Krijger, I.M.; Ahmed, A.A.A.; Goris, M.G.A.; Cornelissen, J.B.W.J.; Groot Koerkamp, P.W.G.; Meerburg, B.G. Wild rodents and insectivores as carriers of pathogenic Leptospira and Toxoplasma gondii in The Netherlands. Vet. Med. Sci. 2020, 6, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Szekeres, S.; Docters van Leeuwen, A.; Tóth, E.; Majoros, G.; Sprong, H.; Földvári, G. Road-killed mammals provide insight into tick-borne bacterial pathogen communities within urban habitats. Transbound. Emerg. Dis. 2019, 66, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Špitalská, E.; Minichová, L.; Kocianová, E.; Škultéty, Ľ.; Mahríková, L.; Hamšíková, Z.; Slovák, M.; Kazimírová, M. Diversity and prevalence of Bartonella species in small mammals from Slovakia, Central Europe. Parasitol. Res. 2017, 116, 3087–3095. [Google Scholar] [CrossRef] [PubMed]
- Gil, H.; García-Esteban, C.; Barandika, J.F.; Peig, J.; Toledo, A.; Escudero, R.; Jado, I.; Rodríguez-Vargas, M.; García-Amil, C.; Lobo, B.; et al. Variability of Bartonella genotypes among small mammals in Spain. Appl. Environ. Microbiol. 2010, 76, 8062–8070. [Google Scholar] [CrossRef] [PubMed]
- Silaghi, C.; Woll, D.; Hamel, D.; Pfister, K.; Mahling, M.; Pfeffer, M. Babesia spp. and Anaplasma phagocytophilum in questing ticks, ticks parasitizing rodents and the parasitized rodents—Analyzing the host-pathogen-vector interface in a metropolitan area. Parasites Vectors 2012, 5, 191. [Google Scholar] [CrossRef]
- Krijger, I.M.; Cornelissen, J.B.; Wisselink, H.J.; Meerburg, B.G. Prevalence of Toxoplasma gondii in common moles (Talpa europaea). Acta Vet. Scand. 2014, 56, 48. [Google Scholar] [CrossRef]
- Barandika, J.F.; Hurtado, A.; García-Esteban, C.; Gil, H.; Escudero, R.; Barral, M.; Jado, I.; Juste, R.A.; Anda, P.; García-Pérez, A.L. Tick-borne zoonotic bacteria in wild and domestic small mammals in northern Spain. Appl. Environ. Microbiol. 2007, 73, 6166–6171. [Google Scholar] [CrossRef]
- Obiegala, A.; Woll, D.; Karnath, C.; Silaghi, C.; Schex, S.; Eßbauer, S.; Pfeffer, M. Prevalence and genotype allocation of pathogenic Leptospira species in small mammals from various habitat types in Germany. PLoS Negl. Trop. Dis. 2016, 10, e0004501. [Google Scholar] [CrossRef]
- Bártová, E.; Kučerová, H.L.; Žákovská, A.; Budíková, M.; Nejezchlebová, H. Coxiella burnetii and Francisella tularensis in wild small mammals from the Czech Republic. Ticks Tick Borne Dis. 2020, 11, 101350. [Google Scholar] [CrossRef]
- Kabwe, E.; Davidyuk, Y.; Shamsutdinov, A.; Garanina, E.; Martynova, E.; Kitaeva, K.; Malisheni, M.; Isaeva, G.; Savitskaya, T.; Urbanowicz, R.A.; et al. Orthohantaviruses, Emerging Zoonotic Pathogens. Pathogens 2020, 9, 775. [Google Scholar] [CrossRef]
- Schlegel, M.; Jacob, J.; Krüger, D.H.; Rang, A.; Ulrich, R.G. Chapter 10—Hantavirus emergence in rodents, insectivores and bats: What comes next? In The Role of Animals in Emerging Viral Diseases; Johnson, N., Ed.; Academic Press: Boston, MA, USA, 2014; pp. 235–292. [Google Scholar] [CrossRef]
- Laenen, L.; Vergote, V.; Kafetzopoulou, L.E.; Wawina, T.B.; Vassou, D.; Cook, J.A.; Hugot, J.P.; Deboutte, W.; Kang, H.J.; Witkowski, P.T.; et al. A novel hantavirus of the European mole, Bruges Virus, is involved in frequent Nova virus coinfections. Genome Biol. Evol. 2018, 10, 45–55. [Google Scholar] [CrossRef]
- Kang, H.J.; Bennett, S.N.; Sumibcay, L.; Arai, S.; Hope, A.G.; Mocz, G.; Song, J.W.; Cook, J.A.; Yanagihara, R. Evolutionary insights from a genetically divergent hantavirus harbored by the European common mole (Talpa europaea). PLoS ONE 2009, 4, e6149. [Google Scholar] [CrossRef]
- Gu, S.H.; Dormion, J.; Hugot, J.P.; Yanagihara, R. High prevalence of Nova hantavirus infection in the European mole (Talpa europaea) in France. Epidemiol. Infect. 2014, 142, 1167–1171. [Google Scholar] [CrossRef]
- Gu, S.H.; Hejduk, J.; Markowski, J.; Kang, H.J.; Markowski, M.; Połatyńska, M.; Sikorska, B.; Liberski, P.P.; Yanagihara, R. Co-circulation of soricid- and talpid-borne hantaviruses in Poland. Infect. Genet. Evol. 2014, 28, 296–303. [Google Scholar] [CrossRef]
- Laenen, L.; Dellicour, S.; Vergote, V.; Nauwelaers, I.; De Coster, S.; Verbeeck, I.; Vanmechelen, B.; Lemey, P.; Maes, P. Spatio-temporal analysis of Nova virus, a divergent hantavirus circulating in the European mole in Belgium. Mol. Ecol. 2016, 25, 5994–6008. [Google Scholar] [CrossRef]
- Verner-Carlsson, J.; Lõhmus, M.; Sundström, K.; Strand, T.M.; Verkerk, M.; Reusken, C.; Yoshimatsu, K.; Arikawa, J.; van de Goot, F.; Lundkvist, Å. First evidence of Seoul hantavirus in the wild rat population in the Netherlands. Infect. Ecol. Epidemiol. 2015, 5, 27215. [Google Scholar] [CrossRef][Green Version]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation: Vienna, Austria, 2021. [Google Scholar]
- Swanink, C.; Reimerink, J.; Gisolf, J.; de Vries, A.; Claassen, M.; Martens, L.; Waegemaekers, T.; Rozendaal, H.; Valkenburgh, S.; Hoornweg, T.; et al. Autochthonous human case of Seoul virus infection, the Netherlands. Emerg. Infect. Dis. 2018, 24, 2158–2163. [Google Scholar] [CrossRef]
- Heylen, D.; Tijsse, E.; Fonville, M.; Matthysen, E.; Sprong, H. Transmission dynamics of Borrelia burgdorferi s.l. in a bird tick community. Environ. Microbiol. 2013, 15, 663–673. [Google Scholar] [CrossRef]
- Hovius, J.W.; de Wever, B.; Sohne, M.; Brouwer, M.C.; Coumou, J.; Wagemakers, A.; Oei, A.; Knol, H.; Narasimhan, S.; Hodiamont, C.J.; et al. A case of meningoencephalitis by the relapsing fever spirochaete Borrelia miyamotoi in Europe. Lancet 2013, 382, 658. [Google Scholar] [CrossRef]
- Jahfari, S.; Fonville, M.; Hengeveld, P.; Reusken, C.; Scholte, E.J.; Takken, W.; Heyman, P.; Medlock, J.M.; Heylen, D.; Kleve, J.; et al. Prevalence of Neoehrlichia mikurensis in ticks and rodents from North-west Europe. Parasites Vectors 2012, 5, 74. [Google Scholar] [CrossRef]
- Courtney, J.W.; Kostelnik, L.M.; Zeidner, N.S.; Massung, R.F. Multiplex real-time PCR for detection of Anaplasma phagocytophilum and Borrelia burgdorferi. J. Clin. Microbiol. 2004, 42, 3164–3168. [Google Scholar] [CrossRef] [PubMed]
- Azagi, T.; Jaarsma, R.I.; Docters van Leeuwen, A.; Fonville, M.; Maas, M.; Franssen, F.F.J.; Kik, M.; Rijks, J.M.; Montizaan, M.G.; Groenevelt, M.; et al. Circulation of Babesia Species and Their Exposure to Humans through Ixodes Ricinus. Pathogens 2021, 10, 386. [Google Scholar] [CrossRef] [PubMed]
- Øines, Ø.; Radzijevskaja, J.; Paulauskas, A.; Rosef, O. Prevalence and diversity of Babesia spp. in questing Ixodes ricinus ticks from Norway. Parasites Vectors 2012, 5, 156. [Google Scholar] [CrossRef]
- Stenos, J.; Graves, S.R.; Unsworth, N.B. A highly sensitive and specific real-time PCR assay for the detection of spotted fever and typhus group Rickettsiae. Am. J. Trop. Med. Hyg. 2005, 73, 1083–1085. [Google Scholar] [CrossRef] [PubMed]
- Diaz, M.H.; Bai, Y.; Malania, L.; Winchell, J.M.; Kosoy, M.Y. Development of a novel genus-specific real-time PCR assay for detection and differentiation of Bartonella species and genotypes. J. Clin. Microbiol. 2012, 50, 1645–1649. [Google Scholar] [CrossRef]
- Versage, J.L.; Severin, D.D.; Chu, M.C.; Petersen, J.M. Development of a multitarget real-time TaqMan PCR assay for enhanced detection of Francisella tularensis in complex specimens. J. Clin. Microbiol. 2003, 41, 5492–5499. [Google Scholar] [CrossRef]
- Krawczyk, A.I.; van Duijvendijk, G.L.A.; Swart, A.; Heylen, D.; Jaarsma, R.I.; Jacobs, F.H.H.; Fonville, M.; Sprong, H.; Takken, W. Effect of rodent density on tick and tick-borne pathogen populations: Consequences for infectious disease risk. Parasites Vectors 2020, 13, 34. [Google Scholar] [CrossRef]
- Ahmed, A.; Engelberts, M.F.; Boer, K.R.; Ahmed, N.; Hartskeerl, R.A. Development and validation of a real-time PCR for detection of pathogenic leptospira species in clinical materials. PLoS ONE 2009, 4, e7093. [Google Scholar] [CrossRef]
- Klempa, B.; Fichet-Calvet, E.; Lecompte, E.; Auste, B.; Aniskin, V.; Meisel, H.; Denys, C.; Koivogui, L.; Meulen, J.; Krüger, D.H. Hantavirus in African Wood Mouse, Guinea. Emerg. Infect. Dis. J. 2006, 12, 838. [Google Scholar] [CrossRef]
- Norman, A.F.; Regnery, R.; Jameson, P.; Greene, C.; Krause, D.C. Differentiation of Bartonella-like isolates at the species level by PCR-restriction fragment length polymorphism in the citrate synthase gene. J. Clin. Microbiol. 1995, 33, 1797–1803. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
- Minh, B.Q.; Nguyen, M.A.; von Haeseler, A. Ultrafast approximation for phylogenetic bootstrap. Mol. Biol. Evol. 2013, 30, 1188–1195. [Google Scholar] [CrossRef]
- Rambaut, A. FigTree: Tree Figure Drawing Tool, 1.4.4. 2018. Available online: http://tree.bio.ed.ac.uk/software/figtree/ (accessed on 5 October 2022).
- Fischer, S.; Mayer-Scholl, A.; Imholt, C.; Spierling, N.G.; Heuser, E.; Schmidt, S.; Reil, D.; Rosenfeld, U.M.; Jacob, J.; Nöckler, K.; et al. Leptospira genomospecies and sequence type prevalence in small mammal populations in Germany. Vector Borne Zoonotic Dis. 2018, 18, 188–199. [Google Scholar] [CrossRef]
- Millán, J.; Cevidanes, A.; Chirife, A.D.; Candela, M.G.; León-Vizcaíno, L. Risk factors of Leptospira infection in Mediterranean periurban micromammals. Zoonoses Public Health 2018, 65, e79–e85. [Google Scholar] [CrossRef]
- Desai, S.; van Treeck, U.; Lierz, M.; Espelage, W.; Zota, L.; Czerwinski, M.; Sadkowska-Todys, M.; Avdicová, M.; Reetz, J.; Luge, E.; et al. Resurgence of Field Fever in a Temperate Country: An Epidemic of Leptospirosis among Seasonal Strawberry Harvesters in Germany in 2007. Clin. Infect. Dis. 2009, 48, 691–697. [Google Scholar] [CrossRef]
- Samrot, A.V.; Sean, T.C.; Bhavya, K.S.; Sahithya, C.S.; Chan-drasekaran, S.; Palanisamy, R.; Robinson, E.R.; Subbiah, S.K.; Mok, P.L. Leptospiral Infection, Pathogenesis and Its Diagnosis—A Review. Pathogens 2021, 10, 145. [Google Scholar] [CrossRef]
- Rood, E.J.; Goris, M.G.; Pijnacker, R.; Bakker, M.I.; Hartskeerl, R.A. Environmental risk of leptospirosis infections in the Netherlands: Spatial modelling of environmental risk factors of leptospirosis in the Netherlands. PLoS ONE 2017, 12, e0186987. [Google Scholar] [CrossRef]
- Cheslock, M.A.; Embers, M.E. Human Bartonellosis: An Underappreciated Public Health Problem? Trop. Med. Infect. Dis. 2019, 4, 69. [Google Scholar] [CrossRef]
- von Loewenich, F.D.; Seckert, C.; Dauber, E.; Kik, M.J.L.; de Vries, A.; Sprong, H.; Buschmann, K.; Aardema, M.L.; Brandstetter, M. Prosthetic Valve Endocarditis with Bartonella washoensis in a Human European Patient and Its Detection in Red Squirrels (Sciurus vulgaris). J. Clin. Microbiol. 2019, 58, e01404-19. [Google Scholar] [CrossRef]
- Downey, R.D.; Russo, S.M.; Hauger, S.B.; Murphey, D.K.; Marx, G.; Huynh, T.; Denison, A.M.; Quirt, R.; Bailey, A.; Fernandez, M. Identification of an Emergent Pathogen, Bartonella vinsonii, Using Next-Generation Sequencing in a Patient With Culture-Negative Endocarditis. J. Pediatr. Infect. Dis. Soc. 2021, 10, 213–216. [Google Scholar] [CrossRef] [PubMed]
- Okaro, U.; Addisu, A.; Casanas, B.; Anderson, B. Bartonella Species, an Emerging Cause of Blood-Culture-Negative Endocarditis. Clin. Microbiol. Rev. 2017, 30, 709–746. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.H.; Kumar, M.; Sikorska, B.; Hejduk, J.; Markowski, J.; Markowski, M.; Liberski, P.P.; Yanagihara, R. Isolation and partial characterization of a highly divergent lineage of hantavirus from the European mole (Talpa europaea). Sci. Rep. 2016, 6, 21119. [Google Scholar] [CrossRef] [PubMed]
- Heinemann, P.; Tia, M.; Alabi, A.; Anon, J.C.; Auste, B.; Essbauer, S.; Gnionsahe, A.; Kigninlman, H.; Klempa, B.; Kraef, C.; et al. Human infections by non-rodent-associated hantaviruses in Africa. J. Infect. Dis. 2016, 214, 1507–1511. [Google Scholar] [CrossRef]
- Qi, R.; Sun, X.F.; Qin, X.R.; Wang, L.J.; Zhao, M.; Jiang, F.; Wang, L.; Lei, X.Y.; Liu, J.W.; Yu, X.J. Suggestive serological evidence of infection with shrew-borne Imjin virus (Hantaviridae) in humans. Viruses 2019, 11, 1128. [Google Scholar] [CrossRef]
- Vlaanderen, F.; Cuperus, T.; Keur, I.; De Rosa, M.; Rozendaal, H.; Friesema, I.; Rietveld, A.; van der Poel, W.; Franz, E.; Maassen, K. Staat van Zoönosen 2020. In State of Zoonoses 2020; Rijksinstituut voor Volksgezondheid en Milieu (RIVM): Bilthoven, The Netherlands, 2021. [Google Scholar] [CrossRef]
- Kozuch, O.; Grulich, I.; Nosek, J. Serological survey and isolation of tick-borne encephalitis virus from the blood of the mole (Talpa europaea) in a natural focus. Acta Virol. 1966, 10, 557–560. [Google Scholar]
- Esser, H.J.; Lim, S.M.; de Vries, A.; Sprong, H.; Dekker, D.J.; Pascoe, E.L.; Bakker, J.W.; Suin, V.; Franz, E.; Martina, B.E. Continued Circulation of Tick-Borne Encephalitis Virus Variants and Detection of Novel Transmission Foci, the Netherlands. Emerg. Infect. Dis. 2022, 28, 2416–2424. [Google Scholar] [CrossRef]
| No. Positive Moles per Capture Location (Prevalence (%), 95% Confidence Interval) | ||||||
|---|---|---|---|---|---|---|
| Pathogen * | Urk (n = 125) | Lage Zwaluwe (n = 20) | Bergen Op Zoom (n = 20) | Gennep (n = 12) | Schimmert (n = 3) | Total (n = 180) |
| Leptospira spp. | 3 (2.4%, 0.5–6.9) | 0 (0%, 0–16.8) | 0 (0%, 0–16.8) | 0 (0%, 0–26.5) | 0 (0%, 0–70.8) | 3 (1.7%, 0.4–4.8) |
| Bartonella spp. | 42 (33.6%, 25.4–42.6) | 3 (15%, 3.2–37.9) | 16 (80%, 56.3–94.3) | 6 (50%, 21.1–78.9) | 2 (66.7%, 9.4–99.2) | 69 (38.3%, 31.2–45.9) |
| Hantaviridae | 63 (50.4%, 41.3–59.5) | 10 (50%, 27.2–72.8) | 9 (45%, 23.1–68.5) | 6 (50%, 21.1–78.9) | 1 (33.3%, 0.8–90.6) | 89 (49.4%, 41.9–56.0) |
| Spotted fever group Rickettsia | 0 (0%, 0–2.9) | 1 (5%, 0.1–24.9) | 0 (0%, 0–16.8) | 0 (0%, 0–26.5) | 0 (0%, 0–70.8) | 1 (0.6%, 0.01–3.1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cuperus, T.; de Vries, A.; Jaarsma, R.I.; Sprong, H.; Maas, M. Occurrence of Rickettsia spp., Hantaviridae, Bartonella spp. and Leptospira spp. in European Moles (Talpa europaea) from the Netherlands. Microorganisms 2023, 11, 41. https://doi.org/10.3390/microorganisms11010041
Cuperus T, de Vries A, Jaarsma RI, Sprong H, Maas M. Occurrence of Rickettsia spp., Hantaviridae, Bartonella spp. and Leptospira spp. in European Moles (Talpa europaea) from the Netherlands. Microorganisms. 2023; 11(1):41. https://doi.org/10.3390/microorganisms11010041
Chicago/Turabian StyleCuperus, Tryntsje, Ankje de Vries, Ryanne I. Jaarsma, Hein Sprong, and Miriam Maas. 2023. "Occurrence of Rickettsia spp., Hantaviridae, Bartonella spp. and Leptospira spp. in European Moles (Talpa europaea) from the Netherlands" Microorganisms 11, no. 1: 41. https://doi.org/10.3390/microorganisms11010041
APA StyleCuperus, T., de Vries, A., Jaarsma, R. I., Sprong, H., & Maas, M. (2023). Occurrence of Rickettsia spp., Hantaviridae, Bartonella spp. and Leptospira spp. in European Moles (Talpa europaea) from the Netherlands. Microorganisms, 11(1), 41. https://doi.org/10.3390/microorganisms11010041






