Recurrent Subcutaneous Phaeohyphomycosis Due to Medicopsis romeroi: A Case Report in a Dermatomyositis Patient and Review of the Literature
Abstract
1. Introduction
Case Presentation
2. Materials and Methods
3. Results
3.1. Epidemiological Characteristics of M. romeroi Infections
3.2. Clinical Characteristics of M. romeroi Infections
3.3. Underlying Diseases
3.4. Treatment and Outcomes
3.5. Treatment and Outcomes of M. romeroi PHM
3.6. Antifungal Medications in PHM Cases
3.7. Treatment and Outcomes of M. romeroi Eumycetoma
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lieberman, J.A.; Fiorito, J.; Ichikawa, D.; Fang, F.C.; Rakita, R.M.; Bourassa, L. Long-Term Carriage of Medicopsis romeroi, an Agent of Black-Grain Mycetoma, Presenting as Phaeohyphomycosis in a Renal Transplant Patient. Mycopathologia 2019, 184, 671–676. [Google Scholar] [CrossRef] [PubMed]
- Guégan, S.; Garcia-Hermoso, D.; Sitbon, K.; Ahmed, S.; Moguelet, P.; Dromer, F.; Lortholary, O.; Ait-Ammar, N.; Dunand, J.; Levy, B.; et al. Ten-Year Experience of Cutaneous and/or Subcutaneous Infections Due to Coelomycetes in France. Open Forum Infect. Dis. 2016, 3, ofw106. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Hermoso, D.; Valenzuela-Lopez, N.; Rivero-Menendez, O.; Alastruey-Izquierdo, A.; Guarro, J.; Cano-Lira, J.F.; Stchigel, A.M. Diversity of Coelomycetous fungi in human infections: A 10-y experience of two European reference centres. Fungal Biol. 2019, 123, 341–349. [Google Scholar] [CrossRef] [PubMed]
- André, M.; Brumpt, V.; Destombes, P.; Segretain, G. Fungal mycetoma with black grains due to Pyrenochaeta romeroi in Cambodia. Bull. Soc. Pathol. Exot. Fil. 1968, 61, 108–112. [Google Scholar]
- Baylet, R.; Camain, R.; Chabal, J.; Izarn, R. Recent contribution to the study of mycetoma in Senegal. Neotestudina rosatii. Pyrenochaeta romeroi. Aspergillus nidulans. Bull. Soc. Med. Afr. Noire Lang Fr. 1968, 13, 311–313. [Google Scholar] [PubMed]
- David-Chaussé, J.; Texier, L.; Darrasse, H.; Moulinier. Autochthonous myecetoma of the foot due to Pyrenochaeta romeroi. Bull. Soc. Fr. Dermatol. Syphiligr. 1968, 75, 452–453. [Google Scholar]
- Borelli, D. Opportunistic fungi as producers of gray colonies and mycetomata. Dermatologica 1979, 159, 168–174. [Google Scholar] [CrossRef]
- Thammayya, A.; Sanyal, M.; Basu, N. Pyrenochaeta romeroi causing mycetoma pedis in India. J. Indian Med. Assoc. 1979, 73, 47–49. [Google Scholar]
- Zaniboni, M.C.; Borrelli, B.L.; Soares, M.M.; Cintra, M.L.; Nogueira, J.C.P.; Neto Maduromycosis of Black Grains Caused by Pyrenochaeta romeroi. Report of One Case. Am. J. Dermatopathol. 1996, 18, 436. [Google Scholar] [CrossRef]
- Mohanty, J.C.; Mohanty, S.K.; Sahoo, A.; Ghosh, S.K.; Pattnaik, K.L. Eumycetoma caused by Pyrenochaeta romeroi—A case report. Indian J. Dermatol. 2000, 45, 76. [Google Scholar]
- Destombes, P.; Mariat, F.; Rosati, L.; Segretain, G. Mycetoma in Somalia—Results of a survey done from 1959 to 1964. Acta Trop. 1977, 34, 355–373. [Google Scholar] [PubMed]
- Venugopal, P.V.; Venugopal, T.V. Treatment of eumycetoma with ketoconazole. Australas. J. Dermatol. 1993, 34, 27–29. [Google Scholar] [CrossRef] [PubMed]
- Cerar, D.; Malallah, Y.M.; Howard, S.J.; Bowyer, P.; Denning, D.W. Isolation, identification and susceptibility of Pyrenochaeta romeroi in a case of eumycetoma of the foot in the UK. Int. J. Antimicrob. Agents 2009, 34, 613–614. [Google Scholar] [CrossRef] [PubMed]
- Dubey, N.; Capoor, M.R.; Hasan, A.S.; Gupta, A.; Ramesh, V.; Sharma, S.; Singh, A.; Rudramurthy, S.M.; Chakrabarti, A. Epidemiological profile and spectrum of neglected tropical disease eumycetoma from Delhi, North India. Epidemiol. Infect. 2019, 147, e294. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Armstrong-James, D.; Teo, I.A.; Shrivastava, S.; Petrou, M.A.; Taube, D.; Dorling, A.; Shaunak, S. Exogenous Interferon-γ Immunotherapy for Invasive Fungal Infections in Kidney Transplant Patients. Am. J. Transplant. 2010, 10, 1796–1803. [Google Scholar] [CrossRef]
- Galezowski, A.; Delyon, J.; Le Cleach, L.; Guégan, S.; Ducroux, E.; Alanio, A.; Lastennet, D.; Moguelet, P.; Dadban, A.; Leccia, M.T.; et al. Deep cutaneous fungal infections in solid-organ transplant recipients. J. Am. Acad. Dermatol. 2020, 83, 455–462. [Google Scholar] [CrossRef]
- Waldman, A.; Ezaldein, H.; Tomayko, M.; Galan, A. Nodular plaque in a cardiac transplant patient. J. Am. Acad. Dermatol. 2015, 72, e111–e112. [Google Scholar] [CrossRef]
- Los-Arcos, I.; Royuela, M.; Martín-Gómez, M.T.; Alastruey-Izquierdo, A.; Sellarès, J.; Perelló, M.; Castells, L.; Dopazo, C.; Gavaldà, J.; Len, O. Phaeohyphomycosis caused by Medicopsis romeroi in solid organ transplant recipients: Report of two cases and comprehensive review of the literature. Transpl. Infect. Dis. 2019, 21, e13072. [Google Scholar] [CrossRef]
- Thiyagarajan, U.M.; Bagul, A.; Nicholson, M.L. A nodulo-cystic eumycetoma caused by Pyrenochaeta romeroi in a renal transplant recipient: A case report. J. Med. Case Rep. 2011, 5, 460. [Google Scholar] [CrossRef]
- Sum, S.; Bru, V.; Cammuzet, G.; Ortéga, F.; Kessler, R.; Pinget, M.; Kessler, L. P160 Infection du pied à eumycétome chez un patient diabétique greffé du poumon: À propos d’une observation. Diabetes Metab. 2009, 35, A66. [Google Scholar] [CrossRef]
- Babu, K.; Murthy, P.R.; Prakash, P.Y.; Kattige, J.; Rangaswamy, S.; Murthy, V.R.; Murthy, K.R. Chronic endophthalmitis due to Pyrenocheta romeroi in an immunocompetent host—A case report from southern india. Retin. Cases Brief Rep. 2014, 8, 197–199. [Google Scholar] [CrossRef] [PubMed]
- Garofalo, F.; Wilcock, J.; Lahey, T. Posttraumatic Endophthalmitis Caused by Medicopsis romeroi: Case Report and Analysis of a Comprehensive Case Series. Infect. Dis. Clin. Pract. 2022, 30, e1138. [Google Scholar] [CrossRef]
- Parta, M.; Kelly, C.; Kwatemaa, N.; Theobald, N.; Hilligoss, D.; Qin, J.; Kuhns, D.B.; Zerbe, C.; Holland, S.M.; Malech, H.; et al. Allogeneic Reduced-Intensity Hematopoietic Stem Cell Transplantation for Chronic Granulomatous Disease: A Single-Center Prospective Trial. J. Clin. Immunol. 2017, 37, 548–558. [Google Scholar] [CrossRef] [PubMed]
- Ocampo, M.; Kanitakis, J.; Bienvenu, A.-L.; Chauvet, C.; Euvrard, S. Phaeohyphomycosis caused by Pyrenochaeta romeroi mimicking a plantar wart in a kidney transplant recipient. Transpl. Infect. Dis. 2012, 14, E173–E174. [Google Scholar] [CrossRef]
- Sharma, S.; Capoor, M.R.; Singh, M.; Kiran, D.; Mandal, A.K. Subcutaneous Phaeohyphomycosis Caused by Pyrenochaeta romeroi in a Rheumatoid Arthritis Patient: A Case Report with Review of the Literature. Mycopathologia 2016, 181, 735–743. [Google Scholar] [CrossRef]
- Kulkarni, M.; Jamale, T.; Hase, N.; Ubale, M.; Keskar, V.; Jagadish, P.K. Subcutaneous Phaeohyphomycosis Caused By Pyrenochaeta romeroi in a Kidney Transplant Recipient: A Case Report. Exp. Clin. Transplant. 2017, 15, 226–227. [Google Scholar] [CrossRef][Green Version]
- Detroyer, D.; Deraedt, K.; Schöffski, P.; Hauben, E.; Lagrou, K.; Naesens, M.; Delforge, M.-L.; Kuypers, D. Resolution of diffuse skin and systemic Kaposi's sarcoma in a renal transplant recipient after introduction of everolimus: A case report. Transpl. Infect. Dis. 2015, 17, 303–307. [Google Scholar] [CrossRef]
- Badali, H.; Chander, J.; Gulati, N.; Attri, A.; Chopra, R.; Najafzadeh, M.J.; Chhabra, S.; Meis, J.F.G.M.; de Hoog, G.S. Subcutaneous phaeohyphomycotic cyst caused by Pyrenochaeta romeroi. Med Mycol. 2010, 48, 763–768. [Google Scholar] [CrossRef]
- Bains, A.; Singh, S.; Dutt, N.; Asfahan, S.; Vedant, D.; Nalwa, A. Medicopsis romeroi infection presenting as disseminated nodules and sinuses in a patient with chronic rheumatoid arthritis. J. Eur. Acad. Dermatol. Venereol. 2020, 35, e70–e72. [Google Scholar] [CrossRef]
- Girard, C.; Dereure, O.; Rispail, P.; Durand, L.; Guilhou, J.-J. Subcutaneous Phaeohyphomycosis due to Pyrenochaeta romeroi in a Patient with Leprosy. Acta Derm. Venereol. 2004, 84, 154–155. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.; Ahmad, S.; Kapila, K.; Ramaswamy, N.V.; Alath, P.; Joseph, L.; Chandy, R. Pyrenochaeta romeroi: A causative agent of phaeohyphomycotic cyst. J. Med. Microbiol. 2011, 60, 842–846. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, Y.-W.; Chia, J.-H.; Lu, C.-F.; Chung, W.-H. Molecular diagnosis and therapeutic experience of subcutaneous Pyrenochaeta romeroi infection: A case report and review of the literature. Int. J. Dermatol. 2013, 52, 1237–1240. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.; Tan, A.; Tan, B. Subcutaneous abscess due to Pyrenochaeta romeroi in a renal transplant recipient. Singap. Med. J. 2014, 55, e64–e66. [Google Scholar] [CrossRef]
- Yadav, S.; Agarwal, R.; Singh, S.; Goel, S. Pyrenochaeta romeroi causing subcutaneous phaeohyphomycotic cyst in a diabetic female. Med. Mycol. Case Rep. 2015, 8, 47–49. [Google Scholar] [CrossRef]
- Abdolrasouli, A.; Gonzalo, X.; Jatan, A.; McArthur, G.J.; Francis, N.; Azadian, B.S.; Borman, A.M.; Johnson, E.M. Subcutaneous Phaeohyphomycosis Cyst Associated with Medicopsis romeroi in an Immunocompromised Host. Mycopathologia 2016, 181, 717–721. [Google Scholar] [CrossRef] [PubMed]
- Dinh, A.; Levy, B.; Bouchand, F.; Davido, B.; Duran, C.; Cristi, M.; Felter, A.; Salomon, J.; Ammar, N.A. Subcutaneous Phaeohyphomycosis Due to Pyrenochaeta romeroi Mimicking a Synovial Cyst. Front. Microbiol. 2016, 7, 1405. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Singh, P.K.; Pandey, K.; Mittal, G.; Ramesh, V.; Deb, M. Subcutaneous Cyst due to Medicopsis romeroi in a Diabetic Lepromatous Leprosy Patient: An Interesting Case Report and Review from India. J. Clin. Diagn. Res. 2017, 11, 1–3. [Google Scholar] [CrossRef]
- Chanyachailert, P.; Leeyaphan, C.; Bunyaratavej, S.; Chongtrakool, P. Subcutaneous phaeohyphomycosis from Medicopsis romeroi in a diabetic patient. Med. Mycol. Case Rep. 2019, 26, 69–72. [Google Scholar] [CrossRef]
- Prasad, S.; Khurana, U.; Karuna, T.; Brahmachari, S.; Sinha, J.K.; Tandon, A.; Kapoor, N. Fine needle aspiration of nodular cystic swelling showing a rare melanized fungus Medicopsis romeroi: A case report. Diagn. Cytopathol. 2019, 48, 401–404. [Google Scholar] [CrossRef]
- Jeddi, F.; Paugam, C.; Hartuis, S.; Denis-Musquer, M.; Sabou, M.; Lavergne, R.-A.; Muguet, L.; Le Pape, P. Medicopsis romeroi nodular subcutaneous infection in a kidney transplant recipient. Int. J. Infect. Dis. 2020, 95, 262–264. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Sun, P.; Lee, C.; Su, C.; Tseng, H. Deep cutaneous fungal infection by Pleosporales: An exceptional pathogen in tropical Taiwan. J. Dermatol. 2020, 48, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; O'Driscoll, D.; Bhandary, N.; Mohteshamzadeh, M.; Pay, A.; Johnson, E.; de la Roche, H.M.; Goolamali, S. Phaeohyphomycotic cyst due to Medicopsis romeroi in an immunosuppressed patient. Br. J. Dermatol. 2017, 177, 142. [Google Scholar]
- Deshkar, S.; Patil, N.; Lad, A.; Amberkar, S.; Sharan, S. Nodular Subcutaneous Phaeohyphomycosis due to Medicopsis romeroi in an Immunocompetent Patient. J. Clin. Diagn. Res. 2021, 15, 1–3. [Google Scholar] [CrossRef]
- Gopal, L.; Balajee, G.; Mouleeswaran, K.; Kindo, A.J.; Swaminathan, S.; Srividhya, G.; Seetharaman, S.; Kumar, A.; Periasamy, M.; Symss, N.P. Phaeohyphomycosis and role of internal transcribed spacer. J. Acad. Clin. Microbiol. 2021, 23, 51. [Google Scholar] [CrossRef]
- Ahmed, S.; van de Sande, W.; Stevens, D.; Fahal, A.; van Diepeningen, A.; Menken, S.; de Hoog, G. Revision of agents of black-grain eumycetoma in the order Pleosporales. Persoonia-Mol. Phylogeny Evol. Fungi 2014, 33, 141–154. [Google Scholar] [CrossRef]
- Revankar, S.G. Dematiaceous Fungi. Semin. Respir. Crit. Care Med. 2004, 25, 183–189. [Google Scholar] [CrossRef]
- Ahmed, S.A.; Ende, B.H.G.G.V.D.; Fahal, A.H.; van de Sande, W.W.J.; de Hoog, G.S. Rapid Identification of Black Grain Eumycetoma Causative Agents Using Rolling Circle Amplification. PLoS Neglected. Trop. Dis. 2014, 8, e3368. [Google Scholar] [CrossRef]
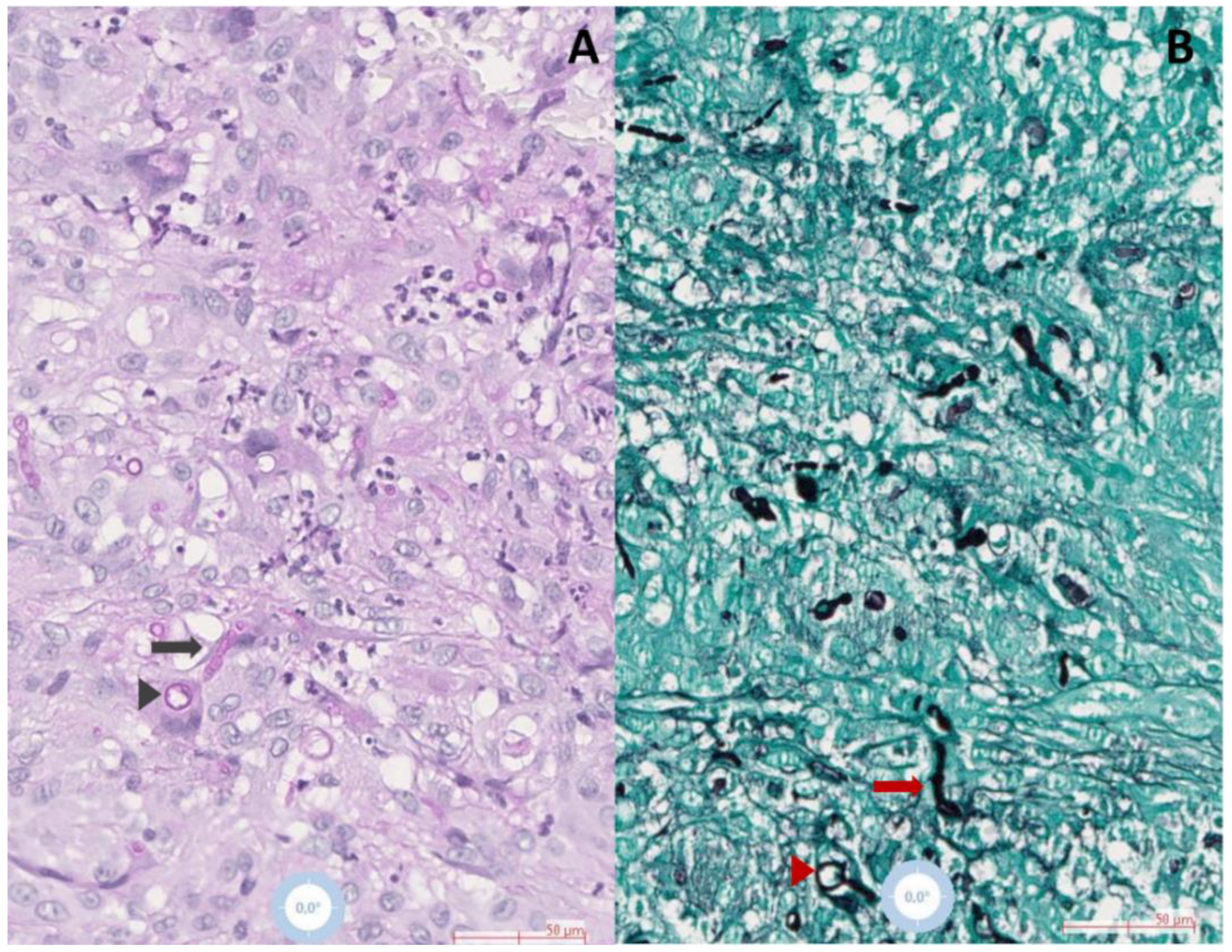
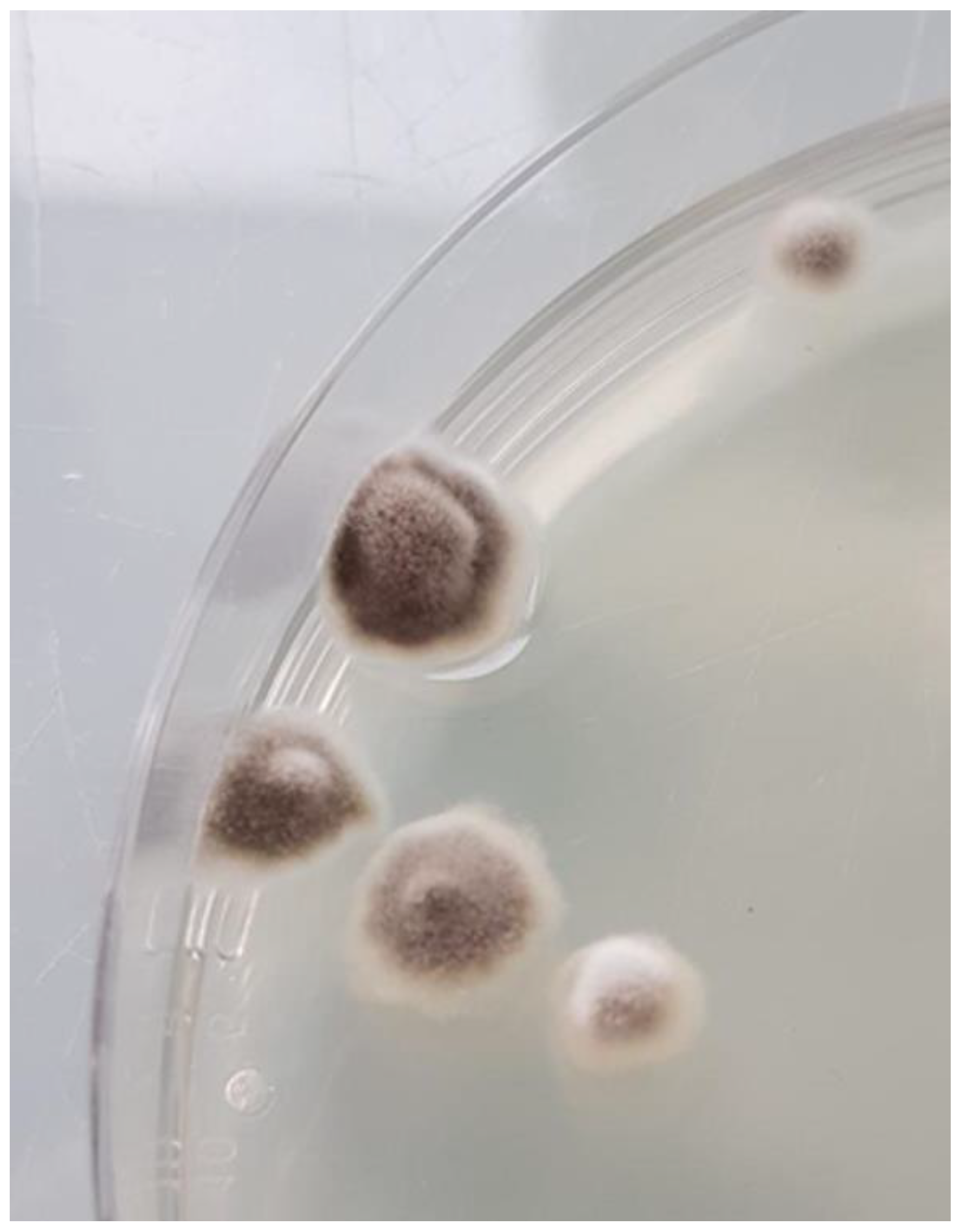

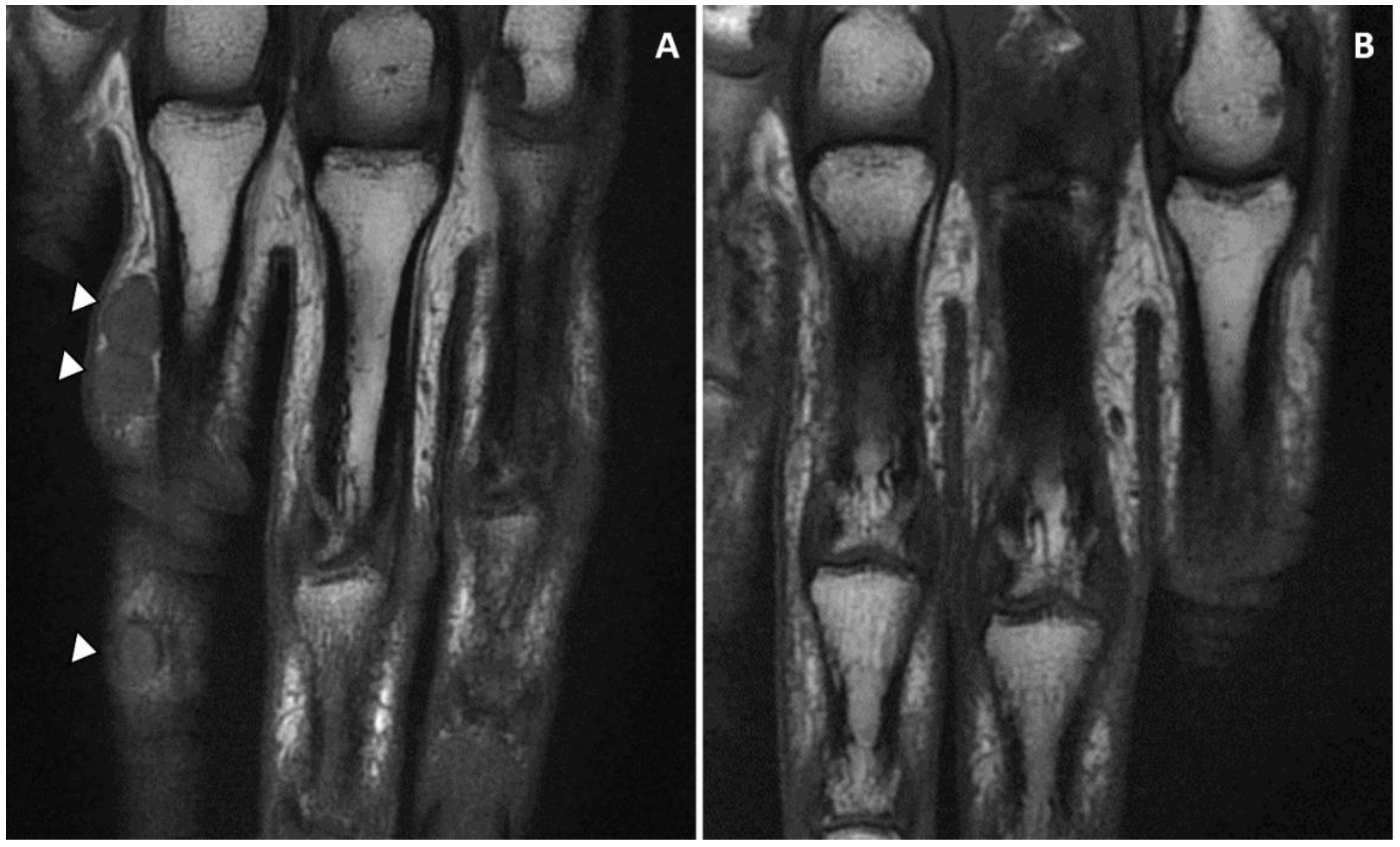
| Demographics | Clinical Characteristics | Diagnosis | Treatment | Outcome | Reference | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (Years) | Sex | Country of Occurrence | Country of Origin | Site | Pain | Bone Involvement | Pre-Treatment Period (Months) | Histology & Culture | Molecular Identification | |||
| 63 | F | Somalia | - | NA | NA | NA | 18 | Yes | No | Surgery | NA | [11] * |
| 39 | M | Cambodia | - | Foot | Yes | Yes | 21 | Yes | No | Excision | Recurrence | [4] |
| NA | M | Senegal | - | Leg | NA | NA | NA | Yes | No | NA | NA | [5] |
| NA | M | Sole | ||||||||||
| NA | M | Hand | ||||||||||
| 53 | M | France | - | Foot | Yes | Yes | 24 | Yes | No | Amputation | NA | [6] |
| NA | NA | Venezuela | - | NA | NA | NA | NA | Yes | No | NA | NA | [7] |
| 37 | M | India | India | Foot, ankle | No | NS | 216 | Yes | No | NA | NA | [8] |
| 42 | M | Foot | No | Yes | 60 | Yes | No | |||||
| 30 | F | NA | NA | Foot, leg | NA | Yes | 180 | Yes | No | KTC (400 mg/d) for 8 months | Great improvement without further recorded follow-up | [12] ** |
| 42 | M | Brazil | - | Sole | NA | No | 36 | Yes | No | KTC | Failure | [9] |
| ITC | Slight improvement | |||||||||||
| 36 | M | India | - | Foot | NA | NA | NA | Yes | No | NA | NA | [10] |
| 56 | M | UK | Pakistan | Foot | Yes | Yes | 204 | Yes | Yes | ITC 200 mg b.i.d + 5FC 1000 mg t.i.d for 9 months | Failure | [13] |
| VRC 200 mg b.i.d for 7 weeks, then 150 mg b.i.d for 4 years | Minimal improvement | |||||||||||
| POS 400 mg t.i.d for 17 months | Decrease in pain and swelling, then relapse | |||||||||||
| 24 | M | India | - | NA | NA | NA | NA | Yes | Yes | AMB and surgery | Recurrence | [14] *** |
| Demographics | Clinical Characteristics | Treatment | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (Years) /Sex | Country of Occurrence | Country of Origin * | Last Travel (Years) ** | Site | Clinical Presentation | IS Duration (mo) ‡ | Comorbidities | 1st Line | Outcome | 2nd Line | Outcome | 3rd Line | Outcome | Follow-Up (mo) | Reference |
| 45/M | France | Senegal | 6 | Leg | Subcutaneous swelling, sinuses | 20 | IM steroids, multibacillary leprosy | Surgery ITC (4 mo) | Cured | - | - | - | - | 12 | [31] |
| 54/M | France | Ghana | NA | Hallux | Subcutaneous swelling | 36 | SOT, diabetes | VRC (6 mo) | Failure | VRC + CAS | Failure | Surgery | NA | NA | [21] |
| 45/F | India | NA | NA | Forearm | Subcutaneous swelling/Verrucous plaque | 0 | None | Surgery | Cured | - | - | - | - | 12 | [29] |
| 43/M | UK | NA | NA | Arms Legs | Nodules | 12 | SOT | LAMB | Stability | ITC (0.5 mth) | Stability | INF-γ (1.5 mth) IST withdrawal | Cured | 20 | [16] |
| 47/F | Kuwait | India | 3 | Finger | Subcutaneous swelling | 34 | ALL Chemotherapy | Surgery | Cured | - | - | - | - | 2 | [32] |
| 57/M | UK | Bangladesh | 8 | Knee | Subcutaneous swelling, sinuses | 8 | SOT | Surgery VRC (2 mths) | Cured | - | - | - | - | 6 | [20] |
| 66/M | France | Central Africa | 10 | Heel | Verrucous nodule | 14 | SOT | Surgery IST reduction | Cured | - | - | - | - | 9 | [25] |
| 78/M | Taiwan | NA | NA | Forearm | Papulopustular lesions | 72 | OS | Surgery ITC (6 mths) | Failure | LAMB (0.75 mth) | Cured | - | - | 6 | [33] |
| 55/M | Singapore | NA | NA | Thigh | Nodule | 12 | SOT | Surgery | Failure | ITC (36 mths) | Stability | - | - | 36 | [34] |
| 25/F | India | NA | NA | Eye | Red painful eye | 0 | None | FLU (4 wks) | Failure | Topical VRC + Oral KTC (0.5 mth) | Failure | Surgery Intravitreal VRC Intravitreal AMB | Cured | 6 | [22] |
| 50/F | India | NA | NA | Foot | Subcutaneous swelling | 12 | Diabetes | Surgery ITC (0.5 mth) | Cured | - | - | - | - | 3 | [35] |
| 63/M | USA | NA | NA | Knee | Nodular plaque & sinuses | NA | SOT | Surgery VRC (1 mth) | Failure | Surgery | NA | - | - | NA | [18] |
| 27/F | Belgium | Gambia | NA | Hallux | Wound | 12 | SOT | Topical TRB (9 mths) IST reduction | NA | - | - | - | - | NA | [28] |
| 61/F | India | NA | NS | Index | Subcutaneous swelling | 120 | OS and MTX for RA | Surgery ITC (3 mths) IST reduction | Cured | - | - | - | - | 6 | [26] |
| 88/M | UK | NA | NA | Hand | Nodule | NS | OS for sarcoidosis, LAD, COPD | Surgery | Cured | - | - | - | - | 0.33 | [36] |
| 47/F | France | Benin | NA | Foot | Subcutaneous swelling | 12 | Diabetes | Surgery | Cured | - | - | - | - | 24 | [37] |
| 59/F | France | Sri Lanka | NA | Foot | Subcutaneous swelling | NA | Diabetes, OS for polymyalgia rheumatica | Surgery | Cured | - | - | - | - | 96 | [2] |
| 73/F | France | India | NA | Foot and leg | Subcutaneous swelling | NA | OS for Giant cell arteritis | Surgery VRC (0.75 mth) | Cured | - | - | - | - | 84 | |
| 65/M | France | West Africa | NA | Knee | Abscess | NA | SOT | POS (1 mth) | Cured | - | - | - | - | 72 | |
| 53/M | France | Pakistan | NA | Foot | Abscess | NA | SOT | POS (0.5 mth) | Failure | Surgery LAMB (2 mths) | Cured | - | - | 10 | |
| 43/M | India | NA | NA | Thigh, leg, toe | Nodules, sinuses | 6 | SOT Diabetes | TRB + ITC (2 mths) IST reduction | Failure | Surgery VRC | Stability | - | - | NA | [27] |
| 48/M | India | NA | NA | Foot | Nodule | 3 | Diabetes, lepromatous leprosy | Surgery ITC | NA | - | - | - | - | NA | [38] |
| 68/F | UK | Nepal | NA | Foot, toes | Multiple nodules | NA | SOT Diabetes | Surgery LAMB (1 mth) then VRC (4–12 mths) | Cured | - | - | - | - | NA | [43] |
| 18/M | USA | NA | NA | Liver and lung | Abscess | NA | CGD | NA | Died | - | - | - | - | NA | [24] |
| 65/M | Spain | India | 1 | Foot | Nodule | 1 | SOT, Diabetes | Surgery POS (1 mth) | Cured | - | - | - | - | 48 | [19] |
| 56/F | Spain | Pakistan | 4 | Index | Abscess | 24 | SOT, Diabetes | Surgery VRC (6 mths) | Cured | - | - | - | - | 36 | |
| 80/M | Thailand | NA | NA | Foot | Nodule | 48 | Diabetes | Surgery Glycemic control | Cured | - | - | - | - | 9 | [39] |
| 65/F | USA | Philippines | NA | Foot | Subcutaneous swelling | 72 | SOT | Surgery VRC then POS (3 mths) | Cured | - | - | - | - | 24 | [1] |
| 33/M | France | Bangladesh | NA † | Knee | Monoarthritis | NA | None | Surgery POS | NA | - | - | - | - | NA | [3] |
| NA/M | France | Senegal | NS | Knee | Subcutaneous swelling | NA | Diabetes, CRF | Surgery | NA | - | - | - | - | NA | |
| 27/F | India | NA | NA | Buttock | Nodule | NA | IM steroids for ITP | Surgery | NA | - | - | - | - | NA | [40] |
| 30/M | France | Guinea | 9 | Hallux | Nodule | 15 | SOT | VRC (2 mths) | Failure | Surgery VRC (0.5 mth) | Cured | - | - | 6 | [41] |
| 60/M | India | NA | NA | Legs, buttocks | Nodules, sinuses | 12 | OS for RA | AMBD + CAS | Failure | ITC | Failure (death) | - | - | NA | [30] |
| 78/F | Taiwan | NA | NA | Arm | Subcutaneous nodules | 180 | OS for RA | ITC (2 mths) | Failure | - | - | - | - | NA | [42] |
| 40/M | India | NA | NA | Foot | Subcutaneous swelling | 78 | Diabetes | Surgery ITC (1.5 mths) | Cured | - | - | - | - | 3 | [45] |
| 62/M | India | NA | NA | Foot | Subcutaneous swelling | 0 | None | Surgery ITC (0.75 mths) | Cured | - | - | - | - | 6 | [44] |
| 64/M | USA | Laos | NA | Eye | Painful red eye | 0 | None | ITC (4 mths) Topical VRC Intravitreal AMB | Failure | VRC | Failure | Surgery VRC | Cured | 3 | [23] |
| 56/F | France | Mali | 4 | Finger | Subcutaneous nodule | 15 | OS, RTX, MMF for Dermatomyositis Diabetes | Surgery | Relapse | TRB, POS (5 mths) | Stability | Surgery POS (3 mo) | Relapse | 12 | Current case |
| Demographics | All Cases (n = 52) | Eumycetoma (n = 14) | Phaeohyphomycosis (n = 38) |
|---|---|---|---|
| Male gender, n (%) * | 34 (66%) | 11 (85%) | 23 (60%) |
| Age at diagnosis (yr), mean, (range) | 52 (18–88) | 42 (24–63) | 54 (18–88) |
| Geographical region of origin, n (%) ** | |||
| Indian subcontinent, n (%) | 24 (51%) | 5 (42%) | 19 (54%) |
| Africa, n (%) | 13 (27%) | 4 (33%) | 9 (26%) |
| Southeast Asia, n (%) | 7 (15%) | 1 (8%) | 6 (17%) |
| Europe, n (%) | 2 (4%) | 1 (8%) | 1 (3%) |
| South America n (%) | 1 (2%) | 1 (8%) | 0 |
| Patients resident in Europe or USA, n (%) * | 25 (49%) | 2 (15%) | 23 (60%) |
| Tropical/subtropical origin in Europe/USA cases n (%) ‡ | 19 (90%) | 1 (50%) | 18 (95%) |
| Time delay between last travel and onset of disease (yr), mean | 6 | 11 | 5 |
| Agricultural work, n (%) | 12 (23%) | 5 (36%) | 7 (18%) |
| Clinical characteristics | |||
| Single lesion, n (%) ⁋ | 40 (82%) | 11 (100%) | 29 (76%) |
| Multiple lesions, n (%) | 9 (18%) | 0 | 9 (24%) |
| Painful lesion (%) ¥ | 16 (53%) | 3 (60%) | 13 (52%) |
| Growth speed § | |||
| Slow (%) | 10 (77%) | NA | 10 (77%) |
| Fast (%) | 3 (23%) | NA | 3 (23%) |
| Presentation | |||
| Nodules (%) | 33 (63%) | NA | 33 (87%) |
| Verrucous lesions (%) | 2 (4%) | NA | 2 (5%) |
| Draining sinus (%) | - | - | 5 (13%) |
| Body area involved Þ | |||
| Lower limb, n (%) | 37 (75%) | 10 (91%) | 27 (71%) |
| Upper limb, n (%) | 10 (20%) | 1 (9%) | 9 (24%) |
| Underlying disease, n (%) | 32 (61%) | 0 | 32 (84%) |
| Solid-organ transplantation | 15 (29%) | 0 | 15 (39%) |
| Systemic steroids for inflammatory diseases | 9 (17%) | 0 | 9 (24%) |
| Diabetes | 13 (25%) | 0 | 13 (34%) |
| None Disease mean duration before treatment (mth), (range) | 19 (36%) 34 (1–204) | 14 (100%) 94 (18–204) | 5 (13%) 12 (1–48) |
| Outcome after treatment | |||
| First-line treatment outcome, n of cases | 44 | 7 | 37 |
| Surgery alone | 14 | 3 | 11 |
| Complete remission n (%) | 7 (50%) | 0 (0%) | 7 (63%) |
| Antifungal treatment alone | 14 | 3 | 11 |
| Complete remission n (%) Stability/partial improvement | 1 (7%) 2 (14%) | 0 (0%) 1 (33%) | 1 (9%) 1 (9%) |
| Surgery and antifungal treatment | 16 | 1 | 15 |
| Complete remission n (%) | 11 (68%) | 0 (0%) | 11 (73%) |
| n of treatment events considering all lines received as independent events | 61 | 10 | 51 |
| Surgery alone ŧ | 15 | 3 | 12 |
| Complete remission n (%) | 7 (58%) | 0 (0%) | 7 (78%) |
| Antifungal treatment alone £ | 24 | 6 | 18 |
| Complete remission n (%) | 2 (9%) | 0 (0%) | 2 (12%) |
| Stability/partial improvement | 6 (27%) | 2 (33%) | 4 (25%) |
| Surgery and antifungal treatment © | 22 | 1 | 21 |
| Complete remission n (%) | 16 (80%) | 0 (0%) | 16 (84%) |
| KTC (MIC) | TRB (MIC) | FLC (MIC) | ISC (MIC) | ITC (MIC) | POS (MIC) | VRC (MIC) | CAS (MEC) | ANF (MEC) | MFG (MEC) | AMB (MIC) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| PV. Venugopal et al., 1993 [12] | 0.5 | - | - | - | - | - | - | - | - | - | - |
| Cerar et al., 2009 [13] | - | - | - | - | - | 0.25 MFC 1.0 | >8 MFC > 8 | - | - | - | - |
| Badali et al., 2010 [29] | - | - | >64 | 0.125 | 0.5 | 0.5 | 4.0 | 8.0 | 1.0 | - | 4.0 |
| Khan et al., 2011 [32] | - | - | >256 | - | 3.0 | 0.064 | 0.008 | 6.0 | 0.5 | - | 8.0 |
| Abdolrasouli et al., 2016 [36] | - | 0.125 | >64 | - | 0.5 | 0.25 | 0.5 | 4.0 | - | - | 0.25 |
| Guégan et al., 2016 [2] | - | 0.06 | - | - | 4 | 2 | 0.125 | 1 | - | 4 | 0.25 |
| - | 0.25 | - | - | 8 | 4 | 0.5 | 1 | - | 2 | 0.5 | |
| - | 0.06 | - | - | 4 | 8 | 0.5 | 0.25 | - | 8 | 1 | |
| - | 2.79 | 128 | - | 5.77 | 1.80 | 1.81 | 4.6 | - | 4.6 | 2.78 | |
| Los-Arcos et al., 2019 [19] | - | 0.25 | - | - | 8 | 0.5 | 2.0 | 1.0 | - | - | 0.25 |
| - | >16 | - | - | >8 | 2.0 | 0.5 | >16 | - | - | 8.0 | |
| Lieberman et al., 2019 [1] | - | - | - | - | >16 | 0.5 | 2.0 | - | - | - | - |
| Current case | - | - | - | 0.012 | >32 | 0.047 | 0.016 | >32 | - | - | 4 |
| Median | 0.5 | 0.25 | 96 | 0.0685 | 5.77 | 0.5 | 0.5 | 4.3 | 0.75 | 4.3 | 1.89 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aljundi, M.; Brun, S.; Akhoundi, M.; Didier, M.; Jabbour, R.; Izri, A.; Caux, F.; Bohelay, G. Recurrent Subcutaneous Phaeohyphomycosis Due to Medicopsis romeroi: A Case Report in a Dermatomyositis Patient and Review of the Literature. Microorganisms 2023, 11, 3. https://doi.org/10.3390/microorganisms11010003
Aljundi M, Brun S, Akhoundi M, Didier M, Jabbour R, Izri A, Caux F, Bohelay G. Recurrent Subcutaneous Phaeohyphomycosis Due to Medicopsis romeroi: A Case Report in a Dermatomyositis Patient and Review of the Literature. Microorganisms. 2023; 11(1):3. https://doi.org/10.3390/microorganisms11010003
Chicago/Turabian StyleAljundi, Mohanad, Sophie Brun, Mohammad Akhoundi, Morgane Didier, Roula Jabbour, Arezki Izri, Frédéric Caux, and Gérôme Bohelay. 2023. "Recurrent Subcutaneous Phaeohyphomycosis Due to Medicopsis romeroi: A Case Report in a Dermatomyositis Patient and Review of the Literature" Microorganisms 11, no. 1: 3. https://doi.org/10.3390/microorganisms11010003
APA StyleAljundi, M., Brun, S., Akhoundi, M., Didier, M., Jabbour, R., Izri, A., Caux, F., & Bohelay, G. (2023). Recurrent Subcutaneous Phaeohyphomycosis Due to Medicopsis romeroi: A Case Report in a Dermatomyositis Patient and Review of the Literature. Microorganisms, 11(1), 3. https://doi.org/10.3390/microorganisms11010003





