Susceptibility and Virulence of Enterobacteriaceae Isolated from Urinary Tract Infections in Benin
Abstract
1. Introduction
2. Materials and Methods
2.1. Urine Sample Collection
2.2. Isolation and Identification of Enterobacteriaceae Strains
2.3. Antibiotic Susceptibility of Isolates
2.4. ESBL Production Detection Tests
2.5. Detection of the Bacterial Ability to Form Biofilm
2.6. Molecular Identification of Virulence Factors
2.7. Data Analysis
3. Results
3.1. Sociodemographic Characteristics of Patients
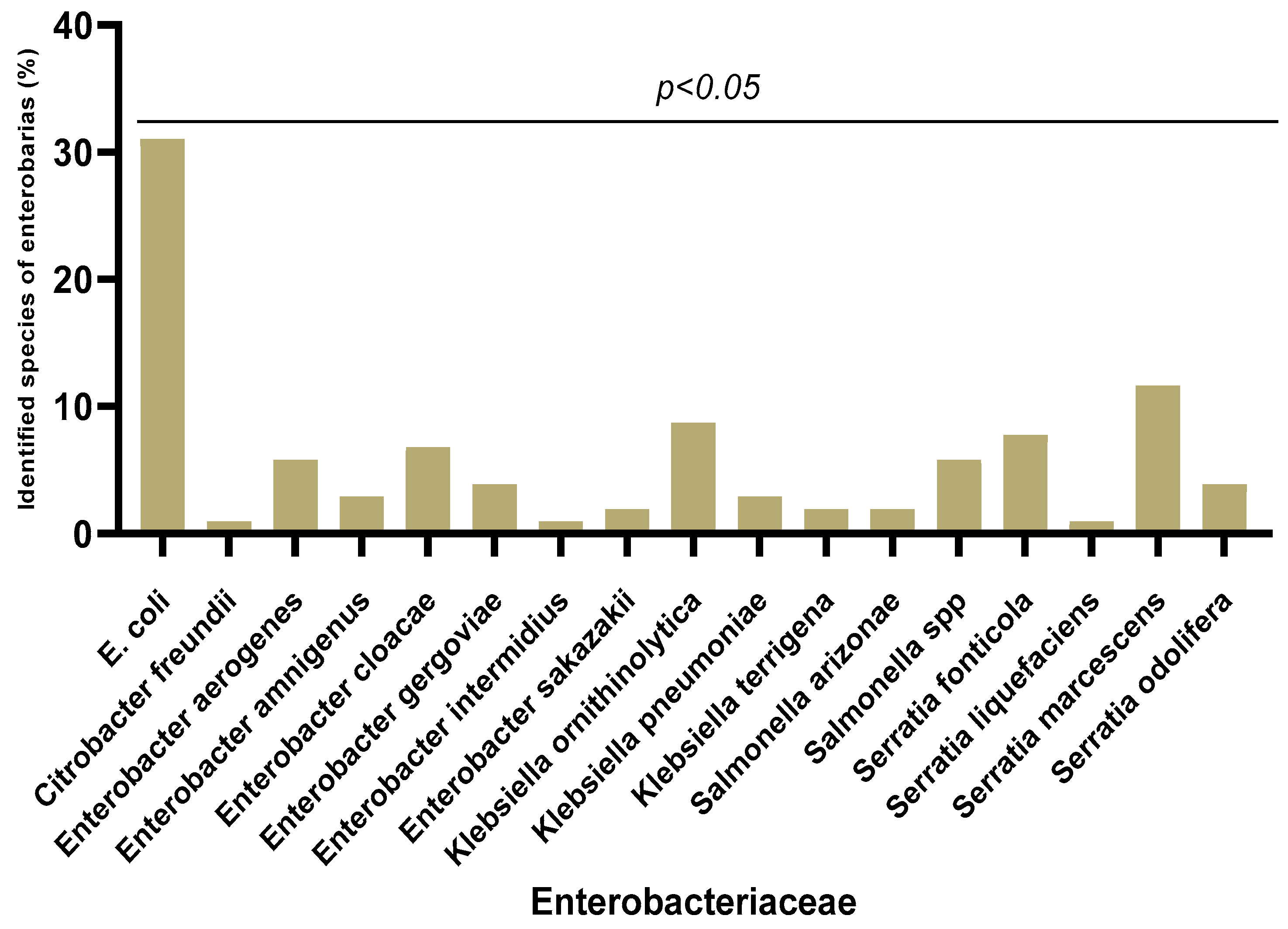
3.2. Enterobacteriaceae Strains’ Isolation Frequency
3.3. Antibiotic Susceptibility of Enterobacteriaceae Strains
3.4. ESBL Production by Isolated Enterobacteriaceae Strains
3.5. Biofilm Formation Capability
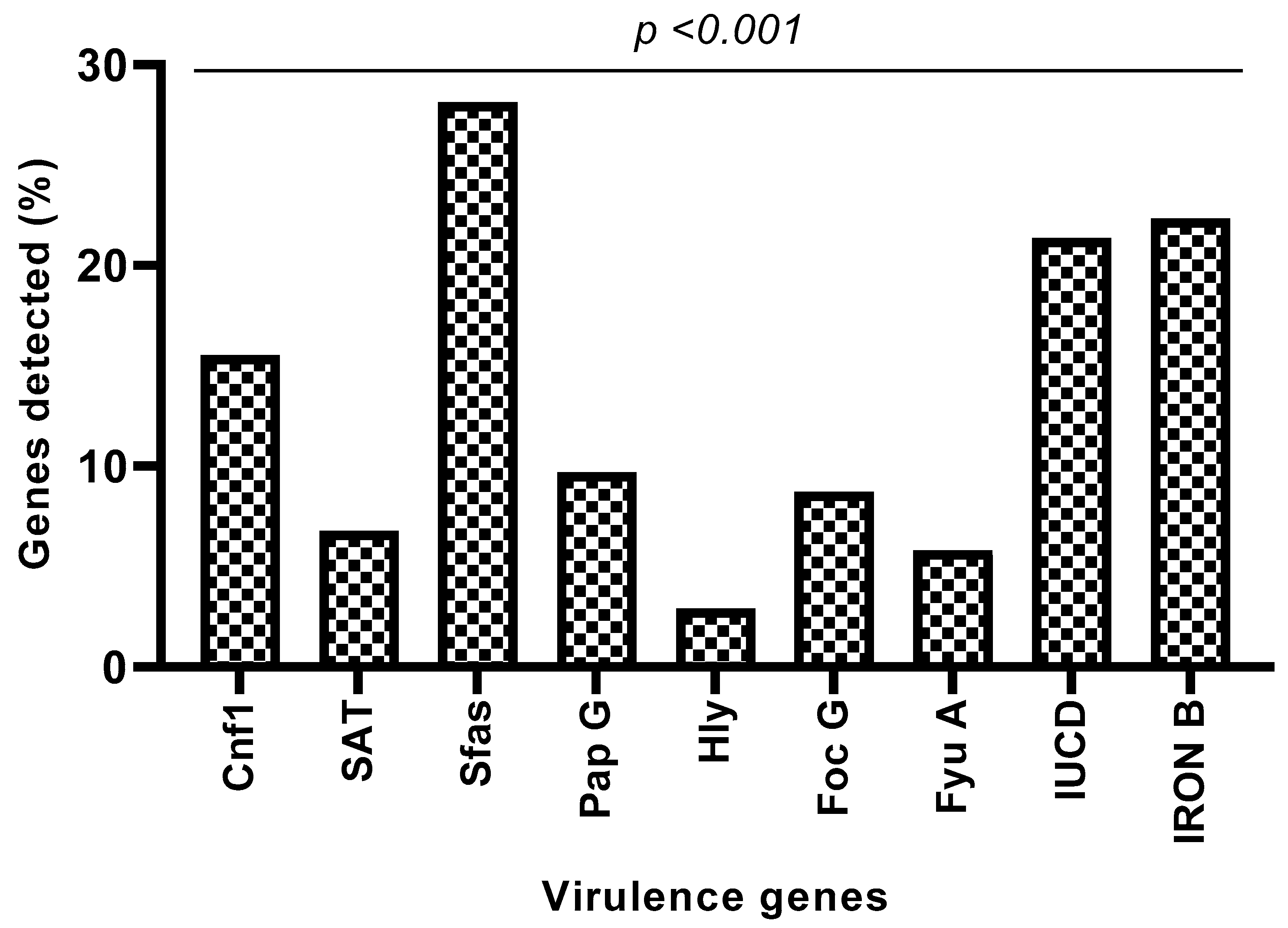
3.6. Detection of Virulence Genes
3.7. Relationship between Virulence Genes and Antibiotic Resistance
3.8. The Relationship between Biofilm Production and Virulence Genes
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Griebling, T.L. Urologic diseases in American project: Trends in resource use for urinary tract infections in women. J Urol. 2005, 173, 1281–1287. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.W.; Chlebicki, M.P. Urinary tract infections in adults. Singap. Med. J. 2016, 57, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Agarwa, J.; Srivastava, S.; Singh, M. Pathogenomics of uropathogenic Escherichia coli. Indian J. Med. Microbiol. 2012, 30, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Launay, E.; Bingen, E.; Cohen, R. Stratégies thérapeutiques dans les infections urinaires du nourrisson et de l’enfant. Arch. Pediatr. 2012, 19, S109–S116. [Google Scholar] [CrossRef] [PubMed]
- Toner, L.; Papa, N.; Aliyu, S.H.; Dev, H.; Lawrentschuk, N.; Al-Hayek, S. Extended spectrum beta-lactamase-producing Enterobacteriaceae in hospital urinary tract infections: Incidence and antibiotic susceptibility profile over 9 years. World J. Urol. 2016, 34, 1031–1037. [Google Scholar] [CrossRef] [PubMed]
- Spencer, J.D.; Schwaderer, A.L.; Becknell, B.; Watson, J.; Hains, D.S. The innate immune response during urinary tract infection and pyelonephritis. Pediatr. Nephrol. 2013, 29, 1139–1149. [Google Scholar] [CrossRef]
- Flores-Mireles, A.L.; Walker, J.N.; Caparon, M.; Hultgren, S.J. Urinary tract infections: Epidemiology, mechanisms of infection and treatment options. Nat. Rev. Microbiol. 2015, 13, 269–284. [Google Scholar] [CrossRef]
- Ny, S.; Edquist, P.; Dumpis, U.; Gröndahl-Yli-Hannuksela, K.; Hermes, J. Antimicrobial resistance of Escherichia coli isolates from outpatient urinary tract infections in women in six European countries including Russia. J. Glob. Antimicrob. Resist. 2018, 17, 25–34. [Google Scholar] [CrossRef]
- Bader, M.S.; Loeb, M.; Brooks, A.A. An update on the management of urinary tract infections in the era of antimicrobial resistance. Postgrad. Med. 2016, 129, 242–258. [Google Scholar] [CrossRef]
- Ali, I.; Rafaque, Z.; Ahmed, S.; Malik, S.; Dasti, J.I. Prevalence of multi-drug resistant uropathogenic Escherichia coli in Potohar region of Pakistan. Asian Pac. J. Trop. Biomed. 2016, 6, 60–66. [Google Scholar] [CrossRef]
- Neupane, S.; Pant, N.D.; Khatiwada, S.; Chaudhary, R.; Banjara, M.R. Correlation between biofilm formation and resistance toward different commonly used antibiotics along with extended spectrum beta lactamase production in uropathogenic Escherichia coli isolated from the patients suspected of urinary tract infections visit. Antimicrob. Resist. Infect. Control. 2016, 5, 5. [Google Scholar] [CrossRef]
- Hammami, S.; Saidani, M.; Ferjeni, S.; Aissa, I.; Slim, A.; Boutiba-Ben Boubaker, I. Characterization of extended spectrum beta-lactamase-producing Escherichia coli in community-acquired urinary tract infections in Tunisia. Microb. Drug Resist. 2013, 19, 231–236. [Google Scholar] [CrossRef]
- Barguigua, A.; El Otmani, F.; Talmi, M.; Zerouali, K.; Timinouni, M. Prevalence and types of extended spectrum beta-lactamases among urinary Escherichia coli isolates in Moroccan community. Microb. Pathog. 2013, 61–62, 16–22. [Google Scholar] [CrossRef]
- Paterson, D.L.; Bonomo, R.A. Extended-spectrum beta-lactamases: A clinical update. Clin. Microbiol. Rev. 2005, 18, 657–686. [Google Scholar] [CrossRef]
- Naboka, Y.L.; Mavzyutov, A.R.; Kogan, M.I.; Gudima, I.A.; Dzhalagoniya, K.T.; Ivanov, S.N.; Naber, K.G. The gene profile of Enterobacteriaceae virulence factors in relation to bacteriuria levels between the acute episodes of recurrent uncomplicated lower urinary tract infection. Expert Rev. Anti-Infect. Ther. 2021, 19, 1061–1066. [Google Scholar] [CrossRef]
- Sarowska, J.; Futoma-Koloch, B.; Jama-Kmiecik, A.; Frej-Madrzak, M.; Ksiazczyk, M.; Bugla-PLoskonska, G. Virulence factors, prevalence and potential transmission of extraintestinal pathogenic Escherichia coli isolated from different sources: Recent reports. Gut Pathog. 2019, 11, 10. [Google Scholar] [CrossRef]
- Katongole, P.; Nalubega, F.; Florence, N.C.; Asiimwe, B.; Andia, I. Biofilm formation, antimicrobial susceptibility and virulence genes of Uropathogenic Escherichia coli isolated from clinical isolates in Uganda. BMC Infect. Dis. 2020, 20, 453. [Google Scholar] [CrossRef]
- Yazdanpour, Z.; Tadjrobehkar, O.; Shahkhah, M. Significant association between genes encoding virulence factors with antibiotic resistance and phylogenetic groups in community acquired uropathogenic Escherichia coli isolates. MC Microbiol. 2020, 20, 241. [Google Scholar] [CrossRef]
- Khairy, R.M.; Mohamed, E.S.; Abdel Ghany, H.M.; Abdelrahim, S.S. Phylogenic classification and virulence genes profiles of uropathogenic E. coli and diarrhegenic E. coli strains isolated from community acquired infections. PLoS ONE 2019, 14, e0222441. [Google Scholar] [CrossRef]
- Donelli, G.; Vuotto, C. Biofilm-based infections in long-term care facilities. Future Microbiol. 2014, 9, 175–188. [Google Scholar] [CrossRef]
- Karam, M.R.A.; Habibi, M.; Bouzari, S. Relationships between virulence factors and antimicrobial resistance among Escherichia coli isolated from urinary tract infections and commensal isolates in Tehran, Iran. Osong Public Health Res. Perspect. 2018, 9, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, D. La méthode statistique en médecine: Les enquêtes éthiologiques. Rev. Stat. Appliquée 1960, 8, 5–27. [Google Scholar]
- Riegel, P.; Archambaud, M.; Clavé, D.; Vergnaud, M. Bactérie de Culture et D’identification Difficiles; Biomérieux: Nancy l’Etoile, France, 2006; pp. 93–112. [Google Scholar]
- CASFM “Comité de L’antibiogramme de la Société Française de Microbi Logie: Recommandations. 2021. Available online: https://www.sfmmicrobiologie.org/2021/05/06/casfm-eucast-2021-v2/ (accessed on 25 September 2022).
- Allouch, P.Y.; Labia, R.; Pina, P.; Morin, E. Observatoires hospitaliers de la sensibilité de E. coli et de Klebsiella à l’association amoxicilline-acide clavulanique en 1994. Med. Mal. Infect. 1995, 25, 934–939. [Google Scholar] [CrossRef]
- Christensen, G.D.; Simpson, W.A.; Bisno, A.L.; Beachy, E.H. Adherence of biofilm producing strains of Staphylococci epidermidis to smooth surfaces. Infect. Imm. 1982, 37, 318–326. [Google Scholar] [CrossRef]
- Stepanović, S.; Vuković, D.; Dakić, I.; Savić, B.; Švabić-Vlahović, M. A modified microtiter-plate test for quantification of staphylococcal biofilm formation. J. Microbiol. Methods 2000, 40, 175–179. [Google Scholar] [CrossRef]
- Rasmussen, R.S.; Morrissey, M.T. DNA-based methods for the identification of commercial fish and seafood species. Compr. Rev. Food Sci. Food Saf. 2008, 7, 280–295. [Google Scholar] [CrossRef]
- Maris, S. Caractérisation de Souches d’Escherichia coli Pathogènes Urinaires Provenant de Guadeloupe: Portrait de la Diversité des Facteurs de Virulence Présentes. Ph.D. Dissertation, Université du Québec, Institut National de la Recherche Scientifique, Quebec City, QC, Canada, 2016. Available online: https://espace.inrs.ca/id/eprint/4854/1/Maris-S-M-Aout2016.pdf (accessed on 25 December 2021).
- Römling, U.; Balsalobre, C. Biofilm infections, their resilience to therapy and innovative treatment strategies. J. Intern. Med. 2012, 272, 541–561. [Google Scholar] [CrossRef]
- Tarchouna, M.; Ferjani, A.; Ben-Selma, W.; Boukadida, J. Distribution of uropathogenic virulence genes in Escherichia coli isolated from patients with urinary tract infection. Int. J. Infect. Dis. 2013, 17, e450–e453. [Google Scholar] [CrossRef]
- Kalambry, A.C. Profil de résistance aux bêtalactamines des entérobactéries isolées des prélèvements urinaires à l’Hôpital du Mali. Rev. Afr. Médecine Interne 2019, 14, 1–10. [Google Scholar]
- Thaden, J.T.; Pogue, J.M.; Kaye, K.S. Role of newer and re-emerging older agents in the treatment of infections caused by carbapenem-resistant Enterobacteriaceae. Virulence 2016, 8, 403–416. [Google Scholar] [CrossRef]
- Maraki, S.; Mantadakis, E.; Michailidis, L.; Samonis, G. Changing antibiotic susceptibilities of community-acquired uropathogens in Greece, 2005–2010. J. Microbiol. Immunol. Infect. 2013, 46, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Soubra, L.; Kabbani, S.; Anwar, M.F.; Dbouk, R. Spectrum and patterns of antimicrobial resistance of uropathogens isolated from a sample of hospitalised Lebanese patients with urinary tract infections. J. Glob. Antimicrob. Resist. 2014, 2, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Farfour, E.; Dortet, L.; Guillard, T.; Chatelain, N.; Poisson, A.; Mizrahi, A.; Fournier, D.; Bonnin, R.A.; Degand, N.; Morand, P.; et al. Antimicrobial Resistance in Enterobacterales Recovered from Urinary Tract Infections in France. Pathogens 2022, 11, 356. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.; Stephen, B.M.; Joseph, N.; Asiphas, O.; Musa, K.; Taseera, K. Prevalence and bacteriology of culture-positive urinary tract infection among pregnant women with suspected urinary tract infection at Mbarara regional referral hospital, South-Western Uganda. BMC Pregnancy Childbirth 2021, 21, 159. [Google Scholar] [CrossRef] [PubMed]
- Bush, L.M.; Vazquez-Pertejo, M.T. Infections par Klebsiella, Enterobacter, et Serratia. Manuels Professional Mal Infect. 2022. Available online: https://www.msdmanuals.com/fr/professional/maladies-infectieuses/bacilles-gram-n%C3%A9gatifs/introduction-aux-bacilles-gram-n%C3%A9gatifs (accessed on 3 December 2022).
- Yehouenou, C.L.; Kpangon, A.A.; Affolabi, D.; Rodriguez-Villalobos, H.; Van Bambeke, F.; Dalleur, O.; Simon, A. Antimicrobial resistance in hospitalized surgical patients: A silently emerging public health concern in Benin. Ann. Clin. Microbiol. Antimicrob. 2020, 19, 54. [Google Scholar] [CrossRef]
- Sabor, H. Phénotypes de résistance des entérobactéries isolées au CHUNU de fann de Dakar de 2014 à 2016. Mémoire DES de biologie clinique, Université Cheikh Anta Diop de Dakar, Faculté de Médecine de Pharmacie et d’Odontologie, Dakar, Senegal. 2017. Available online: http://196.1.97.20/greenstone/collect/mmoires/import/memm_2017_0201.pdf (accessed on 25 September 2022).
- Djahida, S.; Imane, S.; Mourad, D. Résistance aux antibiotiques des entérobactéries au niveau du CHU de Sidi Bel Abbes (Algerie). MHA 2011, 23, 37–41. [Google Scholar]
- Huemer, M.; Mairpady Shambat, S.; Brugger, S.D.; Zinkernagel, A.S. Antibiotic resistance and persistence—Implications for human health and treatment perspectives. EMBO Rep. 2020, 21, e51034. [Google Scholar] [CrossRef]
- Haque, M.; Sartelli, M.; McKimm, J.; Bakar, M.A. Health care-associated infections–an overview. Infect Drug Resist. 2018, 11, 2321. [Google Scholar] [CrossRef]
- Shafiq, M.; Zeng, M.; Permana, B.; Bilal, H.; Huang, J.; Yao, F.; Algammal, A.M.; Li, X.; Yuan, Y.; Jiao, X. Coexistence of blaNDM−5 and tet(X4) in international high-risk Escherichia coli clone ST648 of human origin in China. Front. Microbiol. 2022, 13, 1031688. [Google Scholar] [CrossRef]
- Bilal, H.; Zhang, G.; Rehman, T.; Han, J.; Khan, S.; Shafiq, M.; Yang, X.; Yan, Z.; Yang, X. First Report of blaNDM-1 Bearing IncX3 Plasmid in Clinically Isolated ST11 Klebsiella pneumoniae from Pakistan. Microorganisms 2021, 9, 951. [Google Scholar] [CrossRef]
- Namuwenge, J.M.; Mwesige, O.G.; Mukanga, N. Over-the-counter suboptimal dispensing of antibiotics in Uganda. J. Multidiscip. Health 2013, 6, 303–310. [Google Scholar] [CrossRef]
- Alonso, C.A.; Zarazaga, M.; Ben Sallem, R.; Jouini, A.; Ben Slama, K.; Torres, C. Antibiotic resistance in Escherichia coli in husbandry animals: The African perspective. Lett. Appl. Microbiol. 2017, 64, 318–334. [Google Scholar] [CrossRef]
- Hashemi, S.H.; Esna-Ashari, F.; Tavakoli, S.; Mamani, M. The prevalence of antibiotic resistance of Enterobacteriaceae strains isolated in community-and hospital-acquired infections in teaching hospitals of Hamadan, west of Iran. J. Res. Health Sci. 2013, 13, 75–80. [Google Scholar]
- Hameed, M.F.; Chen, Y.; Wang, Y.; Shafiq, M.; Bilal, H.; Liu, L.; Ma, J.; Gu, P.; Ge, H. Epidemiological Characterization of Colistin and Carbapenem Resistant Enterobacteriaceae in a Tertiary: A Hospital from Anhui Province. Infect. Drug Resist. 2021, 14, 1325–1333. [Google Scholar] [CrossRef]
- Akel, Z. Profil épidémiologique des Entérobactéries Productrices de Carbapénémases Isolées au CHU Ibn Sina-Rabat. Doctoral Dissertation, Mohammed V University of Rabat Faculty of Medicine and Pharmacy, Rabat, Morocco, 2014. Available online: http://ao.um5.ac.ma/xmlui/bitstream/handle/123456789/14692/P075%202014.pdf?sequence=2&isAllowed=y (accessed on 23 July 2022).
- Affolabi, D.; Sogbo, F.; Haag, U.; Orekan, J.; Anagonou, S. Bacteriological profile of Enterobacteriaceae producing broad-spectrum beta-lactamases at the Cotonou National Center Hubert Koutoukou Maga university Hospital, Benin. Ann. Clin. Microbiol. Antimicrob. 2016, 10. Available online: https://anafrimed.net/download/3319/?tmstv=1672855058 (accessed on 3 January 2023).
- European Centre for Disease Prevention and Control. Surveillance Atlas of Infectious Diseases. 2019. Available online: http://atlas.ecdc.europa.eu/public/index.aspx (accessed on 27 October 2022).
- Muriuki, C.W.; Ogonda, L.A.; Kyanya, C.; Matano, D.; Masakhwe, C.; Odoyo, E. Phenotypic and genotypic characteristics of uropathogenic Escherichia coli isolates from Kenya. Microb. Drug Resist. 2022, 28, 31–38. [Google Scholar] [CrossRef]
- Vazouras, K.; Velali, K.; Tassiou, I.; Anastasiou-Katsiardani, A.; Athanasopoulou, K.; Barbouni, A. Antibiotic treatment and antimicrobial resistance in children with urinary tract infections. J. Glob. Antimicrob. Resist. 2019, 20, 4–10. [Google Scholar] [CrossRef]
- Shakya, P.; Shrestha, D.; Maharjan, E.; Sharma, V.K.; Paudyal, R. ESBL production among E. coli and Klebsiella spp. causing urinary tract infection: A hospital-based study. Open Microbiol. J. 2017, 11, 23–30. [Google Scholar] [CrossRef]
- Sudheendra, K.R.; Basavaraj, P.V. Analysis of antibiotic sensitivity profile of biofilm-forming uropathogenic Escherichia coli. J. Nat. Sci. Biol. Med. 2018, 9, 175. [Google Scholar] [CrossRef]
- Nandakumar, V.; Chittaranjan, S.; Kurian, V.M.; Doble, M. Characteristics of bacterial biofilm associated with implant material in clinical practice. Polym. J. 2013, 45, 137. [Google Scholar] [CrossRef]
- Davies, J.; Davies, D. Origins and evolution of antibiotic resistance. Microbiol. Mol. Biol. Rev. 2010, 74, 417–433. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.D.; Hultgren, S.J. Urinary tract infections: Microbial pathogenesis, host-pathogen interactions and new treatment strategies. Nat. Rev. Microbiol. 2020, 18, 211–226. [Google Scholar] [CrossRef] [PubMed]
- Biesecker, S.G.; Nicastro, L.K.; Wilson, R.P.; Tukel, C. The Functional Amyloid Curli Protects Escherichia coli against Complement-Mediated Bactericidal Activity. Biomolecules 2018, 8, 5. [Google Scholar] [CrossRef] [PubMed]
- Hollenbeck, E.C.; Antonoplis, A.; Chai, C.; Thongsomboon, W.; Fuller, G.G.; Cegelski, L. Phosphoethanolamine cellulose enhances curli-mediated adhesion of uropathogenic Escherichia coli to bladder epithelial cells. Proc. Natl. Acad. Sci. USA 2018, 115, 10106–10111. [Google Scholar] [CrossRef] [PubMed]
- Usein, C.R.; Damian, M.; Tatu-Chitoiu, D.; Capusa, C.; Fagaras, R.; Tudorache, D. Prevalence of virulence genes in Escherichia coli strains isolated from Romanian adult urinary tract infection cases. J. Cell. Mol. Med. 2001, 5, 303–310. [Google Scholar] [CrossRef]
- Munkhdelger, Y.; Gunregjav, N.; Dorjpurev, A.; Juniichiro, N.; Sarantuya, J. Detection of virulence genes, phylogenetic group and antibiotic resistance of uropathogenic Escherichia coli in Mongolia. J. Infect. Dev. Ctries. 2017, 11, 51–57. [Google Scholar] [CrossRef]
- Alabsi, M.S.; Ghazal, A.; Sabry, S.A.; Alasaly, M.M. Association of some virulence genes with antibiotic resistance among uropathogenic Escherichia coli isolated from urinary tract infection patients in Alexandria, Egypt: A hospital-based study. J. Glob. Antimicrob. Resist. 2014, 2, 83–86. [Google Scholar] [CrossRef]
- Lasaro, M.A.; Salinger, N.; Zhang, J.; Wang, Y.; Zhong, Z.; Goulian, M.; Zhu, J. F1C fimbriae play an important role in biofilm formation and intestinal colonization by the Escherichia coli commensal strain Nissle 1917. Appl. Environ. Microbiol. 2009, 75, 246–251. [Google Scholar] [CrossRef]
- Paniagua-Contreras, G.L.; Monroy-Pérez, E.; Rodrı’guez-Moctezuma, J.R.; Domı’nguez-Trejo, P.; Vaca-Paniagua, F.; Vaca, S. Virulence factors, antibiotic resistance phenotypes and O-serogroups of Escherichia coli strains isolated from community-acquired urinary tract infection patients in Mexico. J. Microbiol. Immunol. Infect. 2017, 50, 478–485. [Google Scholar] [CrossRef]
- Yun, K.W.; Kim, H.Y.; Park, H.K.; Kim, W.; Lim, I.S. Virulence factors of uropathogenic Escherichia coli of urinary tract infections and asymptomatic bacteriuria in children. J. Microbiol. Immunol. Infect. 2014, 47, 455–461. [Google Scholar] [CrossRef]
- Lawlor, M.S.; O’connor, C.; Miller, V.L. Yersiniabactin is a virulence factor for Klebsiella pneumoniae during pulmonary infection. Infect. Immun. 2007, 75, 1463–1472. [Google Scholar] [CrossRef]
- Bernstein, H.D. Type V Secretion in Gram-Negative Bacteria. EcoSal Plus 2019, 8. [Google Scholar] [CrossRef]
- Kot, B.; Wicha, J.; Gruzewska, A.; Piechota, M.; Wolska, K.; Obrebska, M. Virulence factors, biofilm-forming ability, and antimicrobial resistance of urinary Escherichia coli strains isolated from hospitalized patients. Turk. J. Med. Sci. 2016, 46, 1908–1914. [Google Scholar] [CrossRef]
- Tabasi, M.; Karam, M.R.A.; Habibi, M.; Mostafavi, E.; Bouzari, S. Genotypic characterization of virulence factors in Escherichia coli isolated from patients with acute cystitis, pyelonephritis and asymptomatic Bacteriuria. J. Clin. Diagn. Res. 2016, 10, DC01–DC07. [Google Scholar] [CrossRef]
- Stephenson, S.A.M.; Brown, P.D. Distribution of virulence determinants among antimicrobial-resistant and antimicrobial-susceptible Escherichia coli implicated in urinary tract infections. Indian J. Med. Microbiol. 2016, 34, 448–456. [Google Scholar] [CrossRef]
- Zamani, H.; Salehzadeh, A. Biofilm formation in uropathogenic Escherichia coli: Association with adhesion factor genes. Turk. J. Med Sci. 2018, 48, 162–167. [Google Scholar] [CrossRef]





| Screened Gene | Primer | Primer Sequences (5′------->3′) | Expected Sizes (bp) |
|---|---|---|---|
| cnf1 | Cnf1 | 5′-aagatggagtttcctatgcaggag-3′ | 498 |
| Cnf2 | 5′-cattcagagtcctgccctcattatt-3′ | ||
| sat | SAT F | 5′-ggtattgatatctccggtgaac-3′ | 779 |
| SAT R | 5′-atagccgcctgacatcagtaat-3′ | ||
| papG II/III | pF f | 5′-ctgtaattacggaagtgatttctg-3′ | 1070 |
| pG r | 5′-actatccggctccggataaaccat-3 | ||
| iucD | iucD f | 5′-aaaactgacatcggatggc-3′ | 253 |
| iucD r | 5′-gtatttgtggcaacgcagaa-3′ | ||
| fyuA | FyuA f’ | 5′-tgattaaccccgcgacgggaa-3′ | 880 |
| FyuA r’ | 5′-cgcagtaggcacgatgttgta-3 | ||
| focG | FocG f | 5′-cagcacaggcagtggatacga-3′ | 360 |
| FocG r | 5′-gaatgtcgcctgcccattgct-3′ | ||
| sfaS | SfaS f | 5′-gtggatacgacgattactgtg-3′ | 240 |
| SfaS r | 5′-ccgccagcattccctgtattc-3′ | ||
| iroN | IRON1 | 5′-tattcgtggtatggggccgga-3′ | 547 |
| IRON2 | 5′-gcccgcatagatattcccctg-3′ | ||
| hlyA | Hly f | 5′-aacaasgataagcactgttctggct-3′ | 1177 |
| Hly r | 5′-accatataagcggtcattcccrtca-3′ |
| Parameter | Variable | Percentage (%) |
|---|---|---|
| Sex | M | 35.92 |
| F | 64.08 | |
| Age | 10–20 years | 11.65 |
| 21–30 years | 22.23 | |
| 31–40 years | 19.42 | |
| 41–50 years | 6.8 | |
| 51–60 years | 19.42 | |
| 61–70 years | 16.5 | |
| 71–80 years | 3.88 |
| Species | Antibiotics | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CN | CIP | AMC | CXM | E | AMP | IPM | CRO | LEV | SSS | DO | CFM | |
| Escherichia coli | 61.29% | 70.96% | 96.77% | 80.64% | 100% | 100% | 22.58% | 100% | 58.06% | 90.32% | 58.06% | 100% |
| Citrobacter freundii | 0% | 100% | 0% | 100% | 100% | 100% | 0% | 100% | 100% | 100% | 0% | 100% |
| Enterobacter aerogenes | 66.66% | 66.66% | 100% | 100% | 100% | 100% | 33.33% | 100% | 66.66% | 100% | 66.66% | 83.33% |
| Enterobacter amnigenus | 66.66% | 33.33% | 66.66% | 100% | 100% | 100% | 66.66% | 100% | 33.33% | 100% | 33.33% | 100% |
| Enterobacter cloacae | 85.71% | 71.42% | 85.71% | 85.71% | 100% | 100% | 42.85% | 85.71% | 57.14% | 100% | 100% | 100% |
| Enterobacter gergoviae | 75% | 25% | 50% | 75% | 100% | 100% | 25% | 100% | 75% | 100% | 75% | 100% |
| Enterobacter intermidius | 0% | 0% | 100% | 100% | 100% | 100% | 0% | 100% | 0% | 0% | 0% | 100% |
| Enterobacter sakazakii | 100% | 100% | 100% | 100% | 100% | 50% | 50% | 100% | 100% | 100% | 100% | 100% |
| Klebsiella ornithinolytica | 66.66% | 88.88% | 88.88% | 88.88% | 100% | 100% | 33.33% | 88.88% | 66.66% | 88.88% | 77.77% | 100% |
| Klebsiella pneumoniae | 50% | 100% | 100% | 50% | 100% | 50% | 50% | 100% | 50% | 0% | 50% | 100% |
| Klebsiella ssp | 100% | 100% | 100% | 100% | 100% | 100% | 0% | 100% | 100% | 100% | 100% | 100% |
| Klebsiella terrigena | 100% | 100% | 100% | 100% | 100% | 100% | 0% | 100% | 50% | 100% | 100% | 100% |
| Salmonalla spp | 50% | 66.66% | 100% | 83.33% | 100% | 100% | 50% | 100% | 33.33% | 100% | 83.33% | 100% |
| Salmonella arizonae | 100% | 100% | 100% | 100% | 100% | 100% | 0% | 100% | 50% | 50% | 50% | 100% |
| Serratia fonticola | 37.50% | 62.50% | 75% | 100% | 100% | 75% | 12.50% | 100% | 37.50% | 75% | 75% | 100% |
| Serratia liquefaciens | 100% | 100% | 0 | 100% | 100% | 100% | 0 | 100% | 100% | 100% | 0 | 100% |
| Serratia marcescens | 66.66% | 66.66% | 91.66% | 91.66% | 100% | 100% | 25% | 75% | 91.66% | 75% | 66.66% | 100% |
| Serratia odolifera | 75% | 75% | 75% | 75% | 100% | 100% | 0% | 100% | 75% | 50% | 50% | 100% |
| Species | Frequency of Virulence Genes (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Cnf1 | SAT | Sfas | Pap G | Hly | Foc G | Fyu A | IUCD | IronB | |
| C. freundii | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| E. coli | 19.35 | 6.45 | 25.8 | 9.67 | 0 | 16.12 | 12.9 | 35.48 | 32.25 |
| En. aerogenes | 0 | 0 | 16.66 | 16.66 | 0 | 0 | 16.66 | 16.66 | 33.33 |
| En. amnigenus | 33.33 | 0 | 33.33 | 0 | 0 | 0 | 0 | 33.33 | 0 |
| En. cloacae | 0 | 0 | 28.57 | 0 | 0 | 14.28 | 0 | 14.28 | 0 |
| En. gergoviae | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 25 |
| En. intermidius | 0 | 100 | 100 | 0 | 0 | 100 | 0 | 100 | 100 |
| En. sakazakii | 0 | 0 | 100 | 0 | 0 | 0 | 50 | 0 | 0 |
| K. ornithinolytica | 11.11 | 0 | 33.33 | 11.11 | 11.11 | 0 | 0 | 0 | 11.11 |
| K. pneumoniae | 0 | 0 | 0 | 50 | 50 | 0 | 0 | 0 | 0 |
| K. terrigena | 50 | 0 | 50 | 0 | 0 | 0 | 0 | 0 | 0 |
| S. arizonae | 50 | 0 | 50 | 0 | 0 | 0 | 0 | 50 | 50 |
| Salmonella spp | 0 | 0 | 16.66 | 0 | 16.66 | 0 | 0 | 16.66 | 0 |
| Serratia fonticola | 12.5 | 12.5 | 25 | 0 | 0 | 12.5 | 0 | 12.5 | 50 |
| S. liquefaciens | 0 | 0 | 0 | 100 | 0 | 0 | 0 | 100 | 0 |
| S. marcescens | 33.33 | 16.66 | 41.66 | 25 | 0 | 0 | 0 | 16.66 | 25 |
| S. odolifera | 0 | 0 | 0 | 0 | 0 | 25 | 0 | 0 | 0 |
| Main Components | ||||
|---|---|---|---|---|
| Settings | Dim,1 | Dim,2 | Dim,3 | Dim,4 |
| Own value | 4.74 | 2.18 | 1.13 | 0.66 |
| Percentage of variance | 47.43 | 2.,80 | 11.28 | 6.62 |
| Cumulative percentage of variance | 47.43 | 69.23 | 80.51 | 87.13 |
| Dim,1 | Dim,2 | |
|---|---|---|
| IRON.B | −0.870 | 0.076 |
| SAT | −0.928 | −0.136 |
| Foc.G | −0.911 | −0.100 |
| AMC | −0.292 | 0.828 |
| DO | 0.358 | 0.873 |
| LEV | 0.751 | −0.365 |
| CN | 0.685 | 0.331 |
| CIP | 0.755 | −0.090 |
| SSS | 0.632 | 0.128 |
| Pap.G | 0.285 | −0.657 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Assouma, F.F.; Sina, H.; Adjobimey, T.; Noumavo, A.D.P.; Socohou, A.; Boya, B.; Dossou, A.D.; Akpovo, L.; Konmy, B.B.S.; Mavoungou, J.F.; et al. Susceptibility and Virulence of Enterobacteriaceae Isolated from Urinary Tract Infections in Benin. Microorganisms 2023, 11, 213. https://doi.org/10.3390/microorganisms11010213
Assouma FF, Sina H, Adjobimey T, Noumavo ADP, Socohou A, Boya B, Dossou AD, Akpovo L, Konmy BBS, Mavoungou JF, et al. Susceptibility and Virulence of Enterobacteriaceae Isolated from Urinary Tract Infections in Benin. Microorganisms. 2023; 11(1):213. https://doi.org/10.3390/microorganisms11010213
Chicago/Turabian StyleAssouma, Funkè F., Haziz Sina, Tomabu Adjobimey, Agossou Damien Pacôme Noumavo, Akim Socohou, Bawa Boya, Ange D. Dossou, Lauriane Akpovo, Basile Boni Saka Konmy, Jacques F. Mavoungou, and et al. 2023. "Susceptibility and Virulence of Enterobacteriaceae Isolated from Urinary Tract Infections in Benin" Microorganisms 11, no. 1: 213. https://doi.org/10.3390/microorganisms11010213
APA StyleAssouma, F. F., Sina, H., Adjobimey, T., Noumavo, A. D. P., Socohou, A., Boya, B., Dossou, A. D., Akpovo, L., Konmy, B. B. S., Mavoungou, J. F., Adjanohoun, A., & Baba-Moussa, L. (2023). Susceptibility and Virulence of Enterobacteriaceae Isolated from Urinary Tract Infections in Benin. Microorganisms, 11(1), 213. https://doi.org/10.3390/microorganisms11010213







