Abstract
Background: Klebsiella pneumoniae is an important multidrug-resistant (MDR) pathogen, causing both community- and healthcare-associated infections. The resistance is due to the continuous accumulation of multiple antibiotic-resistance-genes (ARGs) through spontaneous genomic mutations and the acquisition of conjugative plasmids. This study presents antibiotics resistance genes, plasmids replicons, and virulence genes of K. pneumoniae isolates from clinical specimens in a tertiary hospital, Mwanza, Tanzania. Methods: Whole genome sequencing (WGS) of 34 K. pneumoniae was performed, using an Illumina NextSeq 500, followed by in silco analysis. Results: A total of 34 extended-spectrum beta-lactamase-producing K. pneumoniae, isolated from blood samples from neonatal units were whole-genome sequenced. Of these, 28 (82.4%) had an identified sequence type (ST), with ST14 (39.3%, n = 11) being frequently identified. Moreover, 18 (52.9%) of the bacteria harbored at least one plasmid, from which a total of 25 plasmid replicons were identified with a predominance of IncFIB(K) 48.0% (n = 12). Out of 34 sequenced K. pneumoniae, 32 (94.1%) were harboring acquired antibiotic/biocides-resistance-genes (ARGs) with a predominance of blaCTX-M-15 (90.6%), followed by oqxB (87.5%), oqxA (84.4%), blaTEM-1B (84.4%) and sul2 (84.4%). Interestingly, we observed the ColRNAI plasmid-replicon (n = 1) and qacE gene (n = 4) for the first time in this setting. Conclusion: Global high-risk clones of K. pneumoniae isolates carry multiple ARGs in multiple plasmid-replicons. Findings from this study warrant genomic-based surveillance to monitor high-risk global clones, epidemic plasmids and ARGs in low- and middle-income countries.
1. Introduction
Klebsiella pneumoniae is an opportunistic nosocomial pathogen that is responsible for a number of infectious diseases, namely urinary tract infections (UTIs), skin and soft tissue infections (SSTIs), both community- and health-care-associated pneumonia, and blood-stream infections (BSIs) [1,2,3]. Infections by K. pneumoniae are reported to be associated with increased health-care costs from treatment failure and prolonged hospitalization, which in turn results in a high risk of mortality [4]. Recently, K. pneumoniae has been reported as the commonest member of Enterobacterales, with high rates of resistance towards beta-lactams, aminoglycosides, quinolones, and folate-pathway antagonists antibiotics [5]. K. pneumoniae is an important multidrug-resistant (MDR) pathogen, which continuously accumulates for itself multiple antibiotic-resistance-genes (ARGs) through spontaneous genomic mutations and acquisitions of transferable genetic elements, particularly conjugative plasmids [4]. Therefore, K. pneumoniae has been identified as the major source and shuttle for antibiotic resistance genes [4].
A report from the Infectious Diseases Society of America listed ESBL-producing Klebsiella pneumoniae as one of the six pathogens for which new therapies are urgently needed [6]. Furthermore, the WHO has identified MDR K. pneumoniae as a critical-priority pathogen. New antibiotics are urgently needed, because MDR K. pneumoniae isolates pose a threat in hospitals among patients requiring devices such as ventilators and blood catheters, and have also been found to cause community infections. Limited treatment-options are available in low- and middle-income countries, and these drugs are too expensive to be afforded by the majority of the population [7,8]. Due to the increasing importance of multi-resistant MDR K. pneumoniae in the community and hospital settings, genomic data to track the clones, acquired ARGs, and virulence genes, are of critical importance.
A number of sequence types (STs) of K. pneumoniae including ST11, ST14, ST15, ST26, ST37, ST45, ST101, ST147, ST149, ST231, ST258, ST627, and ST977 have been reported, some of which are geographically localized, while others are globally epidemic [9]. K. pneumoniae ST11, ST13, ST14, ST15, ST37, ST45 and ST147 are well disseminated globally [10,11,12,13]. On the other hand, replicons belonging to conjugative FIB(K), FII(K), IncHI, IncR, IncQ and Col plasmids are the commonest reservoirs of acquired ARGs among K. pneumoniae [9,11,14]. Genes, namely aac(3)-IIa and aac(6′)-Ib-cr for aminoglycosides and quinolones resistance; aph(3′′)-Ib, aph(6)-Id, and aph(3′)-Ia for aminoglycosides resistance; blaOXA-48, blaNMD, blaCTX-M-15, blaOXA-1 and blaTEM-1B for β-lactams resistance; dfrA14, and dfrA7 for trimethoprim resistance; sul1 and sul2 for sulfamethoxazole resistance; qnrB1 for quinolones resistance; tet(A) for tetracycline resistance; and qacE for biocides resistance are among the multiple genes harbored in K. pneumoniae [5,13]. Moreover, a group of virulence traits: adhesion encoded by fimH-1 and mrkD genes; iron-sequestering proteins (siderophores) encoded by fyuA, iroBCDN, irp1/2, and iucABCD genes; complement resistance encoded by traT gene; heavy-metal resistance, e.g., tellurium-ion-resistance protein encoded by terC gene; plasmid-encoded enterotoxin encoded by senB gene; outer-membrane proteins encoded by OmpK35/36 genes; and glutamate decarboxylase protein encoded by the gad gene, have been reported in K. pneumoniae [1,15,16,17,18].
The above information justifies the fact that K. pneumoniae is the main pathogen that is threatening the effectiveness of the treatment of infectious diseases during this era of global emergence and increasing antibiotic-resistance. Therefore, genomic-based sequencing studies are mandatory in monitoring the epidemic plasmids and resistance genes in K. pneumoniae, and here, we present data of acquired ARGs, plasmid-replicons types and virulence genes, related to Klebsiella pneumoniae clinical isolates, from a tertiary hospital in Tanzania.
2. Materials and Methods
2.1. Laboratory Procedures
A total of 34 Klebsiella pneumoniae isolated between 2012 and 2015 with resistance towards third-generation cephalosporins were retrieved and whole-genome sequenced. The bacteria were isolated from blood samples of neonates who were admitted to neonatal units (neonatal ICU and premature unit) between 2012 and 2015, presenting with signs and symptoms of bloodstream infections. Conventional bacteriological culture, in-house biochemical-identification testing, and the disk-diffusion method using the Kirby–Bauer technique [19] were used for isolation, identification and antibiotics-susceptibility testing of K. pneumoniae, respectively. The Clinical and Laboratory Standards Institute (CLSI) guidelines were used for the interpretations of the zone of inhibition [20]. The isolates were archived at −80 °C in the Microbiology Research Laboratory at the Catholic University of Health and Allied Sciences (CUHAS).
For DNA extraction prior to sequencing, K. pneumoniae isolates were recovered by plating on plates of Columbia Blood Agar (Becton Dickinson GmbH, D-69126 Heidelberg, Germany) and then incubated aerobically at 35 ± 2 °C, for 20 h.
DNA extraction and purification was carried out using QIAmp® DNA Mini kit (QIAGEN, Hilden, Germany). Briefly, a loopful of 1 µL was used to transfer bacterial colonies into a 1.5 mL safe-lock microcentrifuge tube containing 500 µL nuclease-free ultra-pure molecular water, followed by vortex-mixing to make homogenous suspensions. The suspensions were centrifuged at 13,000 rpm for 10 min, and then the supernatants were discarded. Cell pellets were re-suspended in 180 µL of ATL buffer. Further procedures of DNA extractions and purifications were conducted as instructed by the manufacturer. NanoDrop was used to determine the quantity and quality of the extracted and purified DNA samples.
2.2. Whole Genome Sequencing and In-Silico Analysis
Whole genome sequencing (WGS) was performed on an Illumina NextSeq 500/550 instrument (Illumina, San Diego, CA, USA,) using an Illumina Nextera XT library with 2 × 150 bp paired-end reads. Sequences data (mean length: 5084 kb) were quality checked before being assembled for multi-locus sequence typing (MLST) and phylogenetic-tree construction using Neighbor Joining tree (NJ-tree) algorithm(Center for Demographic and Population Genetics, University of Texas Health Science Center, Houston 77225)., and analyzed for virulence genes (employing VFDB platform) on SeqSphere+ v.8.4.0 (Ridom GmbH, Münster, Germany) pipelines [21]. Genes coding for virulence factors with an identity of ≥95% and an alignment of 100% with the reference sequence were considered present in our sequenced isolates. The detection and typing of plasmid replicons [22] and acquired antibiotics resistance genes (ARGs) were performed on Center for Genomic Epidemiology database (https://www.genomicepidemiology.org/services/; accessed on 9 September 2022).
To confirm the carriage of ARGs in plasmids replicons, the plasmid contigs were extracted from the selected WGS of K. pneumoniae representing each cluster, using Platon software(Justus-Liebig-Universität Gießen, Giessen, Germany) [23]. Plasmid-born contigs were then examined for carriage of ARGs using ResFinder 4.1((https://cge.food.dtu.dk/services/ResFinder/; accessed on 24 November 2022) server on Center for Genomic Epidemiology [24,25,26].
2.3. Systematic Review
For the comparison of changes and/or persistence of sequence types (STs), acquired ARGs, virulence factors (VRFs) and plasmid replicons, we systematically reviewed our own studies between 2011 and 2022 in Mwanza, Tanzania. We included studies that established sequence types (STs), acquired ARGs, virulence factors (VRFs), and plasmid replicons among Enterobacterales from humans, animals and the environment both colonizing and causing invasive infections.
3. Results
3.1. General Overview of Genome-Sequenced Klebsiella pneumoniae
A total of 34 Klebsiella pneumoniae isolated from blood samples from neonatal units were whole-genome sequenced. All isolates were extended spectrum beta-lactamase-producing isolates. These isolates were multidrug resistant (MDR), showing resistance to third- and fourth-generation cephalosporin, gentamicin, tetracycline and trimethoprim-sulfamethoxazole. Regarding ciprofloxacin, only 3 (8.8%) isolates were sensitive to this antibiotic, and all isolates were sensitive to meropenem.
Twenty-eight (82.4%) had an identified sequence-type (ST) with ST14 (39.3%, n = 11) being frequently identified. About 64.3% (n = 18) of the identified STs are known as high-risk (HiR) clones; ST14 (n = 11), ST37 (n = 6) and ST340 (n = 1). Moreover, 18 (52.9%) of the bacteria harbored at least one plasmid from which a total of 25 plasmid replicons were identified, with a predominance of IncFIB(K) 48.0% (n = 12). Out of 34 sequenced K. pneumoniae, 32 (94.1%) were harboring genes encoding for antibiotics and/or biocides resistance. Moreover, all sequenced K. pneumoniae had acquired genes for virulence factors (Table 1).

Table 1.
General overview of genome-sequenced Klebsiella pneumoniae.
3.2. Types and Distributions of Resistance Genes in Sequenced-Klebsiella pneumoniae
The majority of K. pneumoniae were harboring blaCTX-M-15 (90.6%; n = 29), followed by oqxB (87.5%; n = 28), oqxA (84.4%; n = 27), blaTEM-1B (84.4%, n = 27) and sul2 (84.4%, n = 27), out of 32 isolates found to harbor genes encoding for antibiotics and/or biocides resistance (Table 2).

Table 2.
Types and distributions of acquired antibiotic resistance genes (ARGs) in sequenced Klebsiella pneumoniae.
3.3. Descriptions of Acquired Virulence Genes among Sequenced K. pneumoniae
We grouped the virulence factors (VFs) into five groups, namely group A (attachment/adhesion factors), group B (plasma/protein-resistant factors), group C (iron-sequestering factors), group D (capsule factors) and group E (lipopolysaccharide O-antigen). All of the sequenced isolates were harboring fimABCDEFGHIK and mrkABCDFHIJ genes, encoding for adhesion; acrAB genes encoding for plasma/protein resistance; and entABCDEF and fepABCDG genes encoding for iron acquisition (100%; n = 34 each). Further, the majority of isolates were harboring fes (91.2%; n = 31), iroE (85.3%; n = 29), and ybdA (85.3%; n = 29) genes, encoding for iron acquisition; rcsAB and wzi genes (97.1%; n = 33 each) encoding for capsule-formation; and wzm (64.7%; n = 22), wbbMNO (64.7%; n = 22), and wzt (61.8%; n = 21) genes, encoding for lipopolysaccharide O-antigen (Figure 1).
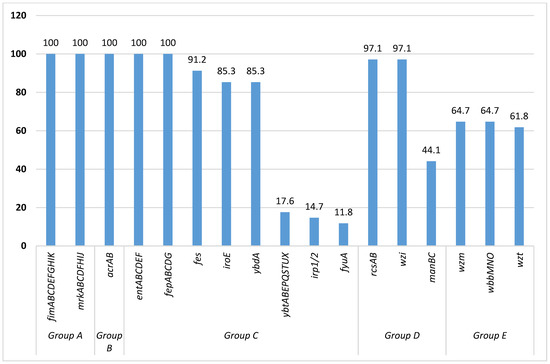
Figure 1.
Percentages of virulence genes found in sequenced Klebsiella pneumoniae.
3.4. Clonal Distributions of Sequenced K. pneumoniae
The phylogenetic tree constructed by the NJ tree algorithm showed six MLST clonal-clusters. Those MLST clonal-clusters include: cluster 1, made with K. pneumoniae ST14 (n = 10) and unknown ST (n = 1); cluster 2, made with K. pneumoniae ST37 (n = 3); cluster 3, made with K. pneumoniae ST37 (n = 2); cluster 4, made with K. pneumoniae ST322 (n = 2); cluster 5, made with K. pneumoniae ST896 (n = 2); and cluster 6, made with K. pneumoniae ST1562 (n = 2). Unidentified STs are denoted by question marks (Figure 2).
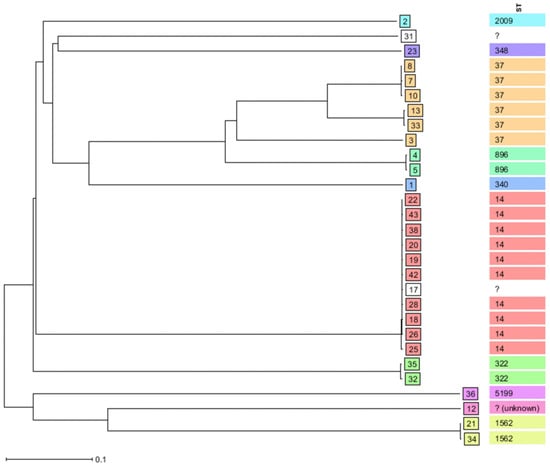
Figure 2.
Clonal distribution by Neighbor-Joining tree (NJ tree) algorithm of sequenced K. pneumoniae isolated from neonates’ blood samples between 2012 and 2015 by SeqSphere+ v.8.4.0 database.
3.5. Sequence Types (STs), Antibiotic Resistance Genes (ARGs), Virulence Genes (VRGs) and Plasmid Replicons Circulating between 2011 and 2022 in Mwanza among Enterobacterales
In the nine previous studies reviewed, the majority involved humans (n = 6 studies), working with blood samples (n = 4 studies). These studies focused on E. coli (n = 8 studies) and K. pneumoniae complex (n = 7 studies). In characterizing the isolates, conventional PCR and sequencing was used in five studies, while WGS was used in four studies.
K. pneumoniae from Blood (n = 61), pus/wound swabs (n = 19) and urine (n = 12), isolated between 2009 and 2010 using PCR and sequencing showed the predominance of ST14. Other STs detected were ST101, ST48, ST348 and ST147. These isolates were found to carry predominantly blaCTX-M-15 in conjugative plasmids of IncFII and IncFIA. Other resistance genes detected were blaSHV-11, blaTEM-10 and blaTEM-170. Using WGS, the three K. pneumoniae from fish isolated in 2015 were typed as ST37 (n = 2) and ST280. These isolates were found to carry blaCTX-M-15 and other resistance genes such as strA/B, qnrS1, aac(3)-IIa and aac(6′)-Ib-cr in multiple plasmids of replicon types IncFII, IncFIB(K), IncHI1B and IncR. Furthermore, the 38 K. pneumoniae from neonates (15 blood and 23 swabs), isolated in 2016, were predominantly typed as ST45 (seven from blood and eleven from swabs), using WGS. Other high-risk clones detected were STs ST348 (n = 3), ST101 and ST14. Multiple acquired ARGs such as blaCTX-M-15, blaSHV-1, blaTEM-1B, blaOXA-1, oqxA, oqxB, qnrB2, aac(6′)Ib-cr, qnrS1, aadA2 sul2 and dfrA1 were found in plasmid with replicon types InFIB, IncFII, IncFR, IncHI1B, IncR and IncHI1A. The virulence genes involved in iron-acquisition, such as irp1, irp2, fyuA, etc., were detected in the majority of K. pneumoniae isolates. The blaCTX-M gene was found to be predominant in K. pneumoniae from humans (blood, rectal swabs, urine, stool) and the environment in those isolated between 2018 and 2021.
Regarding the STs, ARG, plasmid replicons and virulence genes from other Enterobacterales in the study setting, a similar picture was observed as that for K. pneumoniae. Using WGS, E. coli from humans, the environment and animals, global clones such as ST131, ST38, ST617, ST648 and ST10 were observed circulating in humans, animals and the environment. E.coli strains harbored multiple acquired ARGs similar to those observed in K. pneumoniae. These genes were located in multiple plasmids of replicon types IncI2, Col156, IncFIA, IncFIB, IncFII, IncQ1, and IncY.
Changes in the predominance of K. pneumoniae STs were observed between 2010 and 2016. However, there was a persistence of acquired ARGs and conjugative plasmid circulating among Enterobacterales and other species such as Acinetobacter spp. and Pseudomonas spp. (Supplementary Material Table S1) [10,11,27,28,29,30,31].
3.6. Plasmids Harboring Genes Encoding for Antibiotics Resistance
We observed that extracted plasmid-contigs of the sequenced K. pneumoniae were harboring ARGs, conferring resistance to beta-lactams, aminoglycosides, sulfamethoxazole, trimethoprim, tetracyclines, and quinolones, and the biocides, ammonium quaternary compounds (Table 3).

Table 3.
Plasmids harboring genes encoding for antibiotics resistance.
4. Discussion
The Klebsiella pneumoniae isolated between 2012 and 2015 from blood samples confirmed the existence of global high-risk (HiR)-clones ST14, ST37 and ST340, carrying multidrug-resistance genes in multiple plasmid-replicons in Tanzania, causing invasive infections among neonates. Furthermore, the predominance of the HiR clone ST14, causing invasive infections in neonates was observed in 2009. However, in 2016 other HiR clones, ST 45, ST17, ST20 and ST48 were found to be more predominant in the same units, causing invasive infections and colonizing neonates [11].
The insight from our own studies and the findings from the present study show the persistence of K. pneumoniae ST14 from 2009 in the neonatal units, as observed by Mshana et al 2011 [10] through 2015. Changes in the predominance of K. pneumoniae ST in the neonatal units were observed and documented by Marando et al in 2018, who observed ST45 and ST35 to be the predominant STs in 2016 [11]. Furthermore, we observed the persistence of acquired ARGs circulating among HiR clones of K. pneumoniae and E. coli, especially blaCTX-M-15 and other quinolones and aminoglycosides genes. These acquired ARGs were found in similar conjugative-plasmid replicon types, pointing to the horizontal transfer of these genes among Enterobacterales. The persistence of a clone in the hospital setting can be due to poor infection-prevention practices in the presence of carriers of these clones. These HiR K. pneumoniae clones are present beyond the hospital settings, as observed by Moremi et al. in 2015, who found that the HiR clone ST37 was found to colonize the gut of tilapia fish in the same region [27].
As observed in this study and by Marando et al [11] and another study in Germany, HiR K. pneumoniae clones ST14, ST15, ST17, ST20, ST37, ST48, ST147 and ST307 are multidrug resistant (MDR) and are predominantly colonizing and causing sepsis in both hospital and community settings [28]. These clones of the K. pneumoniae complex have a global distribution and a potential association with outbreaks of health-care-associated infections [28,29].
K. pneumoniae ST14 and ST37 have been found to carry high levels of colistin-resistance and carbapenem-resistance genes such as blaNDM and blaOXA-48 [30,31]; however, these genes were not observed in the current study or in previous studies that used WGS from the same setting [10,11,27]. This fact therefore underscores the need for the continuous genomic surveillance of MDR pathogens in the community and in hospital settings, for the early detection of the changes in clones and the acquisition of other acquired ARGs.
Eighteen out of 34 sequenced K. pneumoniae in the current study were harboring mobile genetic elements, notably plasmids of various replicon types including ColRNAI, which was observed in the clinical isolates of K. pneumoniae at our setting for the first time. Similar common replicon-types were observed among Enterobacterales in the study setting, with a predominance of conjugative IncFII and IncFIB plasmids (Supplementary Material Table S1). The ColRNAI was detected in one K. pneumoniae ST340. K. pneumoniae ST340 with ColRNAI plasmid was also harboring other plasmids, including IncFII(K), IncFIB(K) and IncR, of which altogether were carrying acquired ARGs, namely aac(6′)-Ib-cr, aadA2, aph(3′)-Ia, blaCTX-M-15, blaOXA-1, OqxA, OqxB, qacE, sul1 and tet(A). A recent study in Japan reported that K. pneumoniae ST258, harboring ColRNAI plasmid [32] reserves multiple ARGs, as observed in the current study.
We observed some isolates harboring more than one different plasmid-replicon, with one isolate carrying four plasmids, namely ColRNAI, IncR, IncFII(K), and IncFIB(K). As previously described [11,33] and observed in our previous studies (Supplementary Material Table S1), the commonest replicon types are IncY and IncF i.e., IncFIA, IncFIB and IncFII. The IncFIB and IncFII(K) replicons are commonly reported in clinical isolates of K. pneumoniae and E.coli, and are highly associated with the dissemination of acquired ARGs [34]. On the other hand, we identified two K. pneumoniae with IncHI2/2A plasmid-replicons, despite the fact that IncHI2/2A replicons have been identified as predominant reservoirs of acquired ARGs in multidrug-resistant Salmonella spp. [32,35]. Our findings are also supported by a recent study which observed IncHI1B as the commonest plasmid among K. pneumoniae isolated from blood samples [36]. Rare plasmids of replicon types IncQ1 and IncR were also identified in the current study and in other previous studies in the same setting (Supplementary Material Table S1). IncQ1 and IncR plasmids have been found to carry multiple resistance-genes, such as blaNDM-1, blaKPC-2, blaDHA-1, blaVIM-1, qnrS1, or armA [37,38].
Similarly to previous studies from the same setting [10,11], blaCTX-M-15 and blaTEM-1B conferring resistance towards β-lactams, sul2 conferring resistance to sulfamethoxazole; aph(3”)-Ib, and aph(6)-Id conferring resistance to aminoglycosides were the commonest acquired ARG genes detected in the sequenced K. pneumoniae. However, this is the first study to report blaLEN10 and qacE genes conferring resistance towards β-lactam antibiotics and quaternary ammonium compound, respectively, in K. pneumoniae, in this setting. Our findings support observations by Damiano and colleagues, who reported phenotypic resistance of Enterobacterales towards antiseptics/biocides, in this setting [39]. The blaLEN10 has been previously reported among the environmental isolates of Klebsiella spp. in Nigeria [40] and the USA [41]. The presence of qacE genes was also reported previously in K. pneumoniae from one study in Iran [42] and another study in the UK [43]. The blaLEN10 gene was identified among two K. pneumoniae of the ST1562 type; however, the only IncR plasmid which was present in these isolates did not carry this gene. The blaLEN has been identified as class A chromosomal Beta-Lactamase gene in Klebsiella pneumoniae previously [44]. On the other hand, the four qacE genes were identified among three K. pneumoniae of STs 340, 5199 and 14 and one K. pneumoniae with an unknown ST.
The acquired virulence-genes in the current study were categorized into five groups, namely adhesins (group A), plasma/protein-resistance factors (group B), iron-acquisition/sequestering system (group C), capsule-formation factors (group D), and lipopolysaccharide O-antigen (group E) [45,46,47]. However, the majority of our sequenced isolates were harboring fimABCDEFGHIK and mrkABCDFHIJ genes, encoding for adhesion/attachment, an essential first step in developing infectious disease; entABCDEF, fepABCDG, fes, iroE, and ybdA genes, encoding for iron acquisition from host tissues, which ensures the availability of essential micronutrients for bacterial survival as well as the destruction of host tissues; acrAB genes that encode for plasma/protein resistance to facilitate evading the host immune-response; rcsAB and wzi genes, encoding for capsule formation and facilitating the evading of the host immune-response through anti-phagocytosis; and wzm, wzt, and wbbMNO genes, responsible for hypervirulence [45,46,47]. Other virulence-encoding genes such as irp1/2 and fyuA were reported in the same setting by Marando and colleagues; these virulence genes are responsible for iron acquisition from the environment [11], and might indicate that these K. pneumoniae might be from the community in origin [48]. Moreover, acrAB, manBC, wzi, wzt, and wzm genes were reported for the first time among clinical isolates of K. pneumoniae in this setting.
The neighbor-joining tree (NJ tree) algorithm grouped sequenced K. pneumoniae into six MLST clonal-clusters. The large MLST clonal-cluster is made with 11 K. pneumoniae, of which 10 belong to ST14 and 1 is of unidentified ST. Other MLST clonal-clusters are made of three K. pneumoniae (n = 1; ST37); and two K. pneumoniae (n = 2 each; ST37, ST322, ST896 and ST1562). These findings indicate the possible cross-transmission of K. pneumoniae, particularly ST14, ST37, ST322, ST896 and ST1562, causing neonatal sepsis in neonatal units in this setting. Similar findings were reported previously, from the same setting [49]. Therefore, the stringent implementation of IPC measures is mandatory for minimizing the spread of HiR clones, i.e., K. pneumoniae ST14 and ST37, causing neonatal sepsis in this setting.
5. Conclusions
K. pneumoniae ST14 harboring hypervirulent genes (wzm, wzt, and wbbMNO) and multiple acquired-ARGs is an important and successful disseminated sequence-type among clinical isolates, causing bloodstream infections in our setting between 2009 and 2015, with changes in 2016, whereby the ST45 was predominant. The FIB(K) and FII(K) plasmid replicons carrying blaCTX-M-15, sul2 and aph(3”)-Ib and aph(6)-Id ARGs were predominantly identified in the high-risk global clones of K. pneumoniae from this setting. The less-common plasmid-replicon ColRNAI, and qacE and blaLEN10 genes were also reported for the first time among clinical isolates of K. pneumoniae in this setting. The majority of isolates are harboring genes encoding for the iron-sequestering system that can indicate the community spread of MDR pathogens. The predominance and the persistence of the blaCTX-M-15 gene and other acquired-ARGs among Enterobacterales in our setting might be due to circulating conjugative-plasmids and the spread of HiR-clones of K. pneumoniae in the hospital setting, due to poor IPC-practices. These findings warrant sustained improved IPC-practices and genomic-based sequencing surveillance to monitor K. pneumoniae HiR clones, epidemic plasmids, and virulence and acquired antibiotic-resistance genes among MDR pathogens causing invasive infections in developing countries.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microorganisms10122396/s1, Supplementary Table S1: Sequence types (STs), antibiotic resistance genes (ARGs), virulence factors (VRFs) and plasmid replicons circulating between 2011 and 2022 in Mwanza.
Author Contributions
Conceptualization; methodology; formal analysis; writing—original draft preparation; writing—review and editing, V.S. and S.E.M.; supervision, S.E.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding. The APC was funded by the Catholic University of Health and Allied Sciences.
Institutional Review Board Statement
The permission was obtained from an Institutional research ethics review board known as the joint Catholic University of Health and Allied Sciences and Bugando Medical Centre Research Ethics and Review Committee (CREC) by a letter with reference number BU/36/DRI/001/Vol.1.
Informed Consent Statement
Not applicable because the study used archived clinical isolates and permission to use the isolates was obtained from the joint Catholic University of Health and Allied Sciences and Bugando Medical Centre Research Ethics and Review Committee (CREC) by a letter with reference number BU/36/DRI/001/Vol.1.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The sequence data have been deposited in the database with accession numbers ERR829918-ERR829962 (https://www.ebi.ac.uk/ena/browser/view/ERR829918-62, accessed on 22 November 2022).
Acknowledgments
We thank the core-sequencing and informatics team of the Welcome Trust Sanger Institute. We also thank Linda Falgenhauer of the Institute of Medical Microbiology, Justus Liebig University Giessen, for the assistance in the extraction of plasmid contigs.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ballén, V.; Gabasa, Y.; Ratia, C.; Ortega, R.; Tejero, M.; Soto, S. Antibiotic resistance and virulence profiles of Klebsiella pneumoniae strains isolated from different clinical sources. Front. Cell. Infect. Microbiol. 2021, 11, 738223. [Google Scholar] [CrossRef] [PubMed]
- Silago, V.; Kovacs, D.; Msanga, D.R.; Seni, J.; Matthews, L.; Oravcová, K.; Zadoks, R.N.; Lupindu, A.M.; Hoza, A.S.; Mshana, S.E. Bacteremia in critical care units at Bugando Medical Centre, Mwanza, Tanzania: The role of colonization and contaminated cots and mothers’ hands in cross-transmission of multidrug resistant Gram-negative bacteria. Antimicrob. Resist. Infect. Control 2020, 9, 58. [Google Scholar] [CrossRef] [PubMed]
- Kishimbo, P.; Sogone, N.M.; Kalokola, F.; Mshana, S.E. Prevalence of gram negative bacteria causing community acquired pneumonia among adults in Mwanza City, Tanzania. Pneumonia 2020, 12, 7. [Google Scholar] [CrossRef] [PubMed]
- Navon-Venezia, S.; Kondratyeva, K.; Carattoli, A. Klebsiella pneumoniae: A major worldwide source and shuttle for antibiotic resistance. FEMS Microbiol. Rev. 2017, 41, 252–275. [Google Scholar] [CrossRef]
- Edward, E.A.; Mohamed, N.M.; Zakaria, A.S. Whole Genome Characterization of the High-Risk Clone ST383 Klebsiella pneumoniae with a Simultaneous Carriage of blaCTX-M-14 on IncL/M Plasmid and blaCTX-M-15 on Convergent IncHI1B/IncFIB Plasmid from Egypt. Microorganisms 2022, 10, 1097. [Google Scholar] [CrossRef]
- Boucher, H.W.; Talbot, G.H.; Bradley, J.S.; Edwards, J.E.; Gilbert, D.; Rice, L.B.; Scheld, M.; Spellberg, B.; Bartlett, J. Bad bugs, no drugs: No ESKAPE! An update from the Infectious Diseases Society of America. Clin. Infect. Dis. 2009, 48, 1–12. [Google Scholar] [CrossRef]
- Dadgostar, P. Antimicrobial resistance: Implications and costs. Infect. Drug Resist. 2019, 12, 3903. [Google Scholar] [CrossRef]
- Asokan, G.V.; Vanitha, A. WHO global priority pathogens list on antibiotic resistance: An urgent need for action to integrate One Health data. Perspect. Public Health 2018, 138, 87–88. [Google Scholar]
- Enany, S.; Zakeer, S.; Diab, A.A.; Bakry, U.; Sayed, A.A. Whole genome sequencing of Klebsiella pneumoniae clinical isolates sequence type 627 isolated from Egyptian patients. PLoS ONE 2022, 17, e0265884. [Google Scholar] [CrossRef]
- Mshana, S.E.; Hain, T.; Domann, E.; Lyamuya, E.F.; Chakraborty, T.; Imirzalioglu, C. Predominance of Klebsiella pneumoniae ST14 carrying CTX-M-15 causing neonatal sepsis in Tanzania. BMC Infect. Dis. 2013, 13, 466. [Google Scholar] [CrossRef]
- Marando, R.; Seni, J.; Mirambo, M.M.; Falgenhauer, L.; Moremi, N.; Mushi, M.F.; Kayange, N.; Manyama, F.; Imirzalioglu, C.; Chakraborty, T. Predictors of the extended-spectrum-beta lactamases producing Enterobacteriaceae neonatal sepsis at a tertiary hospital, Tanzania. Int. J. Med. Microbiol. 2018, 308, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Giske, C.G.; Fröding, I.; Hasan, C.M.; Turlej-Rogacka, A.; Toleman, M.; Livermore, D.; Woodford, N.; Walsh, T.R. Diverse sequence types of Klebsiella pneumoniae contribute to the dissemination of bla NDM-1 in India, Sweden, and the United Kingdom. Antimicrob. Agents Chemother. 2012, 56, 2735–2738. [Google Scholar] [CrossRef] [PubMed]
- Spadar, A.; Phelan, J.; Elias, R.; Modesto, A.; Caneiras, C.; Marques, C.; Lito, L.; Pinto, M.; Cavaco-Silva, P.; Ferreira, H. Genomic epidemiological analysis of Klebsiella pneumoniae from Portuguese hospitals reveals insights into circulating antimicrobial resistance. Sci. Rep. 2022, 12, 13791. [Google Scholar] [CrossRef] [PubMed]
- Ragupathi, N.K.D.; Bakthavatchalam, Y.D.; Mathur, P.; Pragasam, A.K.; Walia, K.; Ohri, V.; Veeraraghavan, B. Plasmid profiles among some ESKAPE pathogens in a tertiary care centre in south India. Indian J. Med. Res. 2019, 149, 222. [Google Scholar] [PubMed]
- Holt, K.E.; Wertheim, H.; Zadoks, R.N.; Baker, S.; Whitehouse, C.A.; Dance, D.; Jenney, A.; Connor, T.R.; Hsu, L.Y.; Severin, J. Genomic analysis of diversity, population structure, virulence, and antimicrobial resistance in Klebsiella pneumoniae, an urgent threat to public health. Proc. Natl. Acad. Sci. USA 2015, 112, E3574–E3581. [Google Scholar] [CrossRef] [PubMed]
- Mirzaie, A.; Ranjbar, R. Antibiotic resistance, virulence-associated genes analysis and molecular typing of Klebsiella pneumoniae strains recovered from clinical samples. AMB Express 2021, 11, 122. [Google Scholar] [CrossRef]
- Ranjbar, R.; Kelishadrokhi, A.F.; Chehelgerdi, M. Molecular characterization, serotypes and phenotypic and genotypic evaluation of antibiotic resistance of the Klebsiella pneumoniae strains isolated from different types of hospital-acquired infections. Infect. Drug Resist. 2019, 12, 603. [Google Scholar] [CrossRef]
- Olusoga, O.D.; Majeed, A.A.; Sunday, O.A.; Adegboyega, O.A.; Alexander, W.M. Emergence of Diarrhoeagenic Klebsiella pneumoniae Carrying astA and senB genes in Nigeria. Afr. J. Microbiol. Res. 2022, 16, 264–267. [Google Scholar]
- Hudzicki, J. Kirby-Bauer disk diffusion susceptibility test protocol. Am. Soc. Microbiol. 2009, 15, 55–63. [Google Scholar]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, 32nd ed.; CLSI supplement M100; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2022. [Google Scholar]
- Jünemann, S.; Sedlazeck, F.J.; Prior, K.; Albersmeier, A.; John, U.; Kalinowski, J.; Mellmann, A.; Goesmann, A.; Von Haeseler, A.; Stoye, J. Updating benchtop sequencing performance comparison. Nat. Biotechnol. 2013, 31, 294–296. [Google Scholar] [CrossRef]
- Carattoli, A.; Zankari, E.; García-Fernández, A.; Voldby Larsen, M.; Lund, O.; Villa, L.; Møller Aarestrup, F.; Hasman, H. In silico detection and typing of plasmids using PlasmidFinder and plasmid multilocus sequence typing. Antimicrob. Agents Chemother. 2014, 58, 3895–3903. [Google Scholar] [CrossRef]
- Schwengers, O.; Barth, P.; Falgenhauer, L.; Hain, T.; Chakraborty, T.; Goesmann, A. Platon: Identification and characterization of bacterial plasmid contigs in short-read draft assemblies exploiting protein sequence-based replicon distribution scores. Microb. Genom. 2020, 6, mgen000398. [Google Scholar] [CrossRef]
- Bortolaia, V.; Kaas, R.S.; Ruppe, E.; Roberts, M.C.; Schwarz, S.; Cattoir, V.; Philippon, A.; Allesoe, R.L.; Rebelo, A.R.; Florensa, A.F. ResFinder 4.0 for predictions of phenotypes from genotypes. J. Antimicrob. Chemother. 2020, 75, 3491–3500. [Google Scholar] [CrossRef] [PubMed]
- Zankari, E.; Allesøe, R.; Joensen, K.G.; Cavaco, L.M.; Lund, O.; Aarestrup, F.M. PointFinder: A novel web tool for WGS-based detection of antimicrobial resistance associated with chromosomal point mutations in bacterial pathogens. J. Antimicrob. Chemother. 2017, 72, 2764–2768. [Google Scholar] [CrossRef] [PubMed]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and applications. BMC Bioinform. 2009, 10, 421. [Google Scholar] [CrossRef] [PubMed]
- Moremi, N.; Manda, E.V.; Falgenhauer, L.; Ghosh, H.; Imirzalioglu, C.; Matee, M.; Chakraborty, T.; Mshana, S.E. Predominance of CTX-M-15 among ESBL producers from environment and fish gut from the shores of Lake Victoria in Mwanza, Tanzania. Front. Microbiol. 2016, 7, 1862. [Google Scholar] [CrossRef] [PubMed]
- Xanthopoulou, K.; Imirzalioglu, C.; Walker, S.V.; Behnke, M.; Dinkelacker, A.G.; Eisenbeis, S.; Gastmeier, P.; Gölz, H.; Käding, N.; Kern, W.V. Surveillance and Genomic Analysis of Third-Generation Cephalosporin-Resistant and Carbapenem-Resistant Klebsiella pneumoniae Complex in Germany. Antibiotics 2022, 11, 1286. [Google Scholar] [CrossRef]
- Esteban-Cantos, A.; Aracil, B.; Bautista, V.; Ortega, A.; Lara, N.; Saez, D.; Fernández-Romero, S.; Pérez-Vázquez, M.; Navarro, F.; Grundmann, H. The carbapenemase-producing Klebsiella pneumoniae population is distinct and more clonal than the carbapenem-susceptible population. Antimicrob. Agents Chemother. 2017, 61, e02520-16. [Google Scholar] [CrossRef]
- Moubareck, C.A.; Mouftah, S.F.; Pál, T.; Ghazawi, A.; Halat, D.H.; Nabi, A.; AlSharhan, M.A.; AlDeesi, Z.O.; Peters, C.C.; Celiloglu, H. Clonal emergence of Klebsiella pneumoniae ST14 co-producing OXA-48-type and NDM carbapenemases with high rate of colistin resistance in Dubai, United Arab Emirates. Int. J. Antimicrob. Agents 2018, 52, 90–95. [Google Scholar] [CrossRef]
- Mouftah, S.F.; Pál, T.; Higgins, P.G.; Ghazawi, A.; Idaghdour, Y.; Alqahtani, M.; Omrani, A.S.; Rizvi, T.A.; Sonnevend, Á. Diversity of carbapenem-resistant Klebsiella pneumoniae ST14 and emergence of a subgroup with KL64 capsular locus in the Arabian Peninsula. Eur. J. Clin. Microbiol. Infect. Dis. 2021. online ahead of print. [Google Scholar] [CrossRef]
- Zhao, H.; Chen, W.; Xu, X.; Zhou, X.; Shi, C. Transmissible ST3-IncHI2 plasmids are predominant carriers of diverse complex IS 26-Class 1 integron arrangements in multidrug-resistant Salmonella. Front. Microbiol. 2018, 9, 2492. [Google Scholar] [CrossRef] [PubMed]
- Minja, C.A.; Shirima, G.; Mshana, S.E. Conjugative plasmids disseminating ctx-m-15 among human, animals and the environment in Mwanza Tanzania: A need to intensify one health approach. Antibiotics 2021, 10, 836. [Google Scholar] [CrossRef] [PubMed]
- Bi, D.; Zheng, J.; Li, J.-J.; Sheng, Z.-K.; Zhu, X.; Ou, H.-Y.; Li, Q.; Wei, Q. In silico typing and comparative genomic analysis of IncFIIK plasmids and insights into the evolution of replicons, plasmid backbones, and resistance determinant profiles. Antimicrob. Agents Chemother. 2018, 62, e00764-18. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Fang, T.; Zhou, X.; Zhang, D.; Shi, X.; Shi, C. IncHI2 plasmids are predominant in antibiotic-resistant Salmonella isolates. Front. Microbiol. 2016, 7, 1566. [Google Scholar] [CrossRef] [PubMed]
- Shankar, C.; Sethuvel, D.P.M.; Neeravi, A.R.; Venkatesan, M.; Ragupathi, N.K.D.; Anandan, S.; Veeraraghavan, B. Identification of plasmids by PCR based replicon typing in bacteremic Klebsiella pneumoniae. Microb. Pathog. 2020, 148, 104429. [Google Scholar] [CrossRef]
- Qu, D.; Shen, Y.; Hu, L.; Jiang, X.; Yin, Z.; Gao, B.; Zhao, Y.; Yang, W.; Yang, H.; Han, J. Comparative analysis of KPC-2-encoding chimera plasmids with multi-replicon IncR: IncpA1763-KPC: IncN1 or IncFIIpHN7A8: IncpA1763-KPC: IncN1. Infect. Drug Resist. 2019, 12, 285. [Google Scholar] [CrossRef]
- Fuga, B.; Cerdeira, L.; Moura, Q.; Fontana, H.; Fuentes-Castillo, D.; Carvalho, A.C.; Lincopan, N. Genomic data reveals the emergence of an IncQ1 small plasmid carrying blaKPC-2 in Escherichia coli of the pandemic sequence type 648. J. Glob. Antimicrob. Resist. 2021, 25, 8–13. [Google Scholar] [CrossRef]
- Damiano, P.; Salema, E.J.; Silago, V. The susceptibility of multidrug resistant and biofilm forming Klebsiella pneumoniae and Escherichia coli to antiseptic agents used for preoperative skin preparations at zonal referral hospital in Mwanza, Tanzania. Malawi Med. J. 2021, 33, 59–64. [Google Scholar]
- Jesumirhewe, C.; Springer, B.; Allerberger, F.; Ruppitsch, W. Genetic Characterization of Antibiotic Resistant Enterobacteriaceae Isolates from Bovine Animals and the Environment in Nigeria. Front. Microbiol. 2022, 13, 793541. [Google Scholar] [CrossRef]
- Donner, L.; Staley, Z.R.; Petali, J.; Sangster, J.; Li, X.; Mathews, W.; Snow, D.; Howe, A.; Soupir, M.; Bartelt-Hunt, S. The Human Health Implications of Antibiotic Resistance in Environmental Isolates from Two Nebraska Watersheds. Microbiol. Spectr. 2022, 10, e02082-21. [Google Scholar] [CrossRef]
- Mahmoudvand, H.; Azadpour, M.; Nowroozi, J.; Goudarzi, G. Presence of qacEΔ1 and cepA genes and susceptibility to a hospital biocide in clinical isolates of Klebsiella pneumoniae in Iran. Trop. Biomed. 2015, 32, 109–115. [Google Scholar]
- Abuzaid, A.; Hamouda, A.; Amyes, S. Klebsiella pneumoniae susceptibility to biocides and its association with cepA, qacΔE and qacE efflux pump genes and antibiotic resistance. J. Hosp. Infect. 2012, 81, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Haeggman, S.; Löfdahl, S.; Paauw, A.; Verhoef, J.; Brisse, S. Diversity and evolution of the class A chromosomal beta-lactamase gene in Klebsiella pneumoniae. Antimicrob Agents Chemother. 2004, 48, 2400–2408. [Google Scholar] [CrossRef] [PubMed]
- Casadevall, A.; Pirofski, L.A. Virulence factors and their mechanisms of action: The view from a damage–response framework. J. Water Health 2009, 7, S2–S18. [Google Scholar] [CrossRef]
- Peterson, J.W. Bacterial pathogenesis. In Medical Microbiology, 4th ed.; University of Texas Medical Branch: Galveston, TX, USA, 1996. [Google Scholar]
- Liu, B.; Zheng, D.; Zhou, S.; Chen, L.; Yang, J. VFDB 2022: A general classification scheme for bacterial virulence factors. Nucleic Acids Res. 2022, 50, D912–D917. [Google Scholar] [CrossRef]
- Gorrie, C.L.; Mirčeta, M.; Wick, R.R.; Judd, L.M.; Lam, M.; Gomi, R.; Abbott, I.J.; Thomson, N.R.; Strugnell, R.A.; Pratt, N.F. Genomic dissection of Klebsiella pneumoniae infections in hospital patients reveals insights into an opportunistic pathogen. Nat. Commun. 2022, 13, 3017. [Google Scholar] [CrossRef]
- Silago, V.; Kovacs, D.; Samson, H.; Seni, J.; Matthews, L.; Oravcová, K.; Lupindu, A.M.; Hoza, A.S.; Mshana, S.E. Existence of multiple ESBL genes among phenotypically confirmed ESBL producing Klebsiella pneumoniae and Escherichia coli concurrently isolated from clinical, colonization and contamination samples from neonatal units at Bugando Medical Center, Mwanza, Tanzania. Antibiotics 2021, 10, 476. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).