Role of Bacillus subtilis Spore Core Water Content and pH in the Accumulation and Utilization of Spores’ Large 3-Phosphoglyceric Acid Depot, and the Crucial Role of This Depot in Generating ATP Early during Spore Germination
Abstract
1. Introduction
2. Materials and Methods
2.1. B. subtilis Strains Used, and Spore Preparation and Purification
2.2. Extraction and Quantitation of Low mol wt Compounds in Spores
2.3. Spore Core pH Determination
2.4. Analysis of ATP and Spore Viability during CaDPA Germination
3. Results
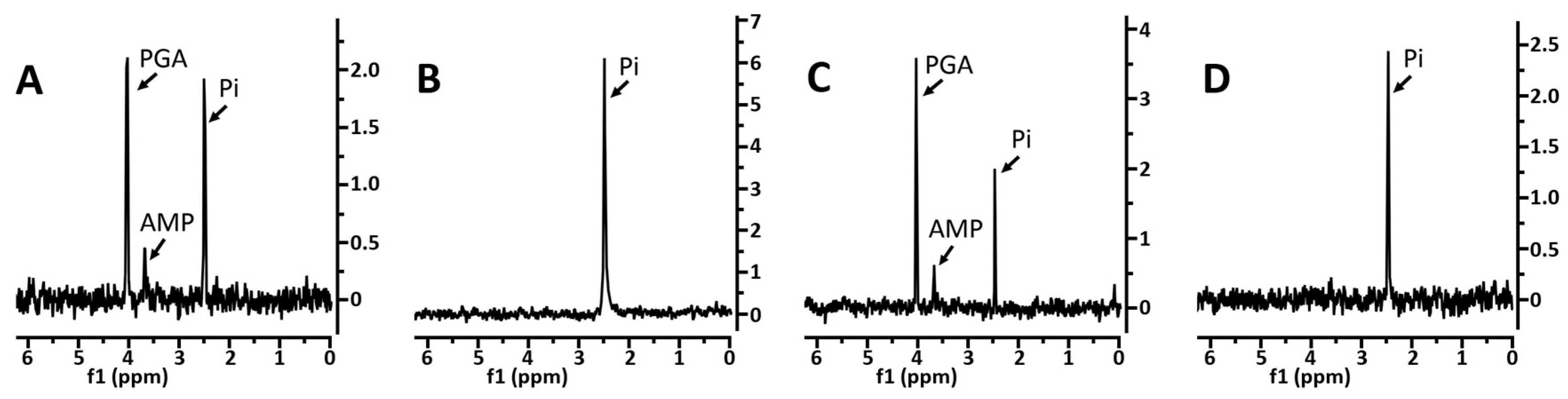
3.1. Levels of 3PGA and Other Phosphorylated Small Molecules in Various Spores
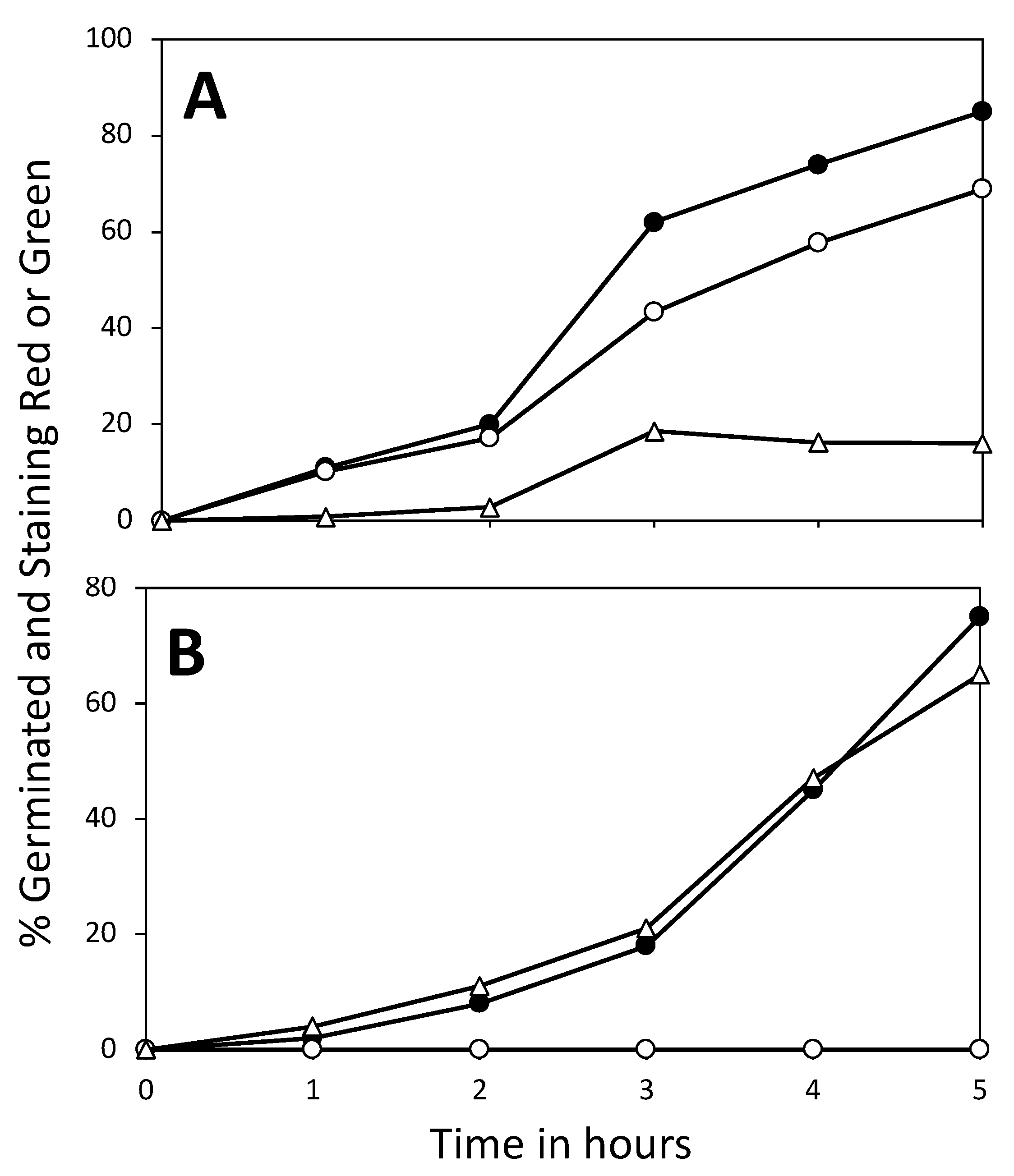
3.2. Effects of Core pH
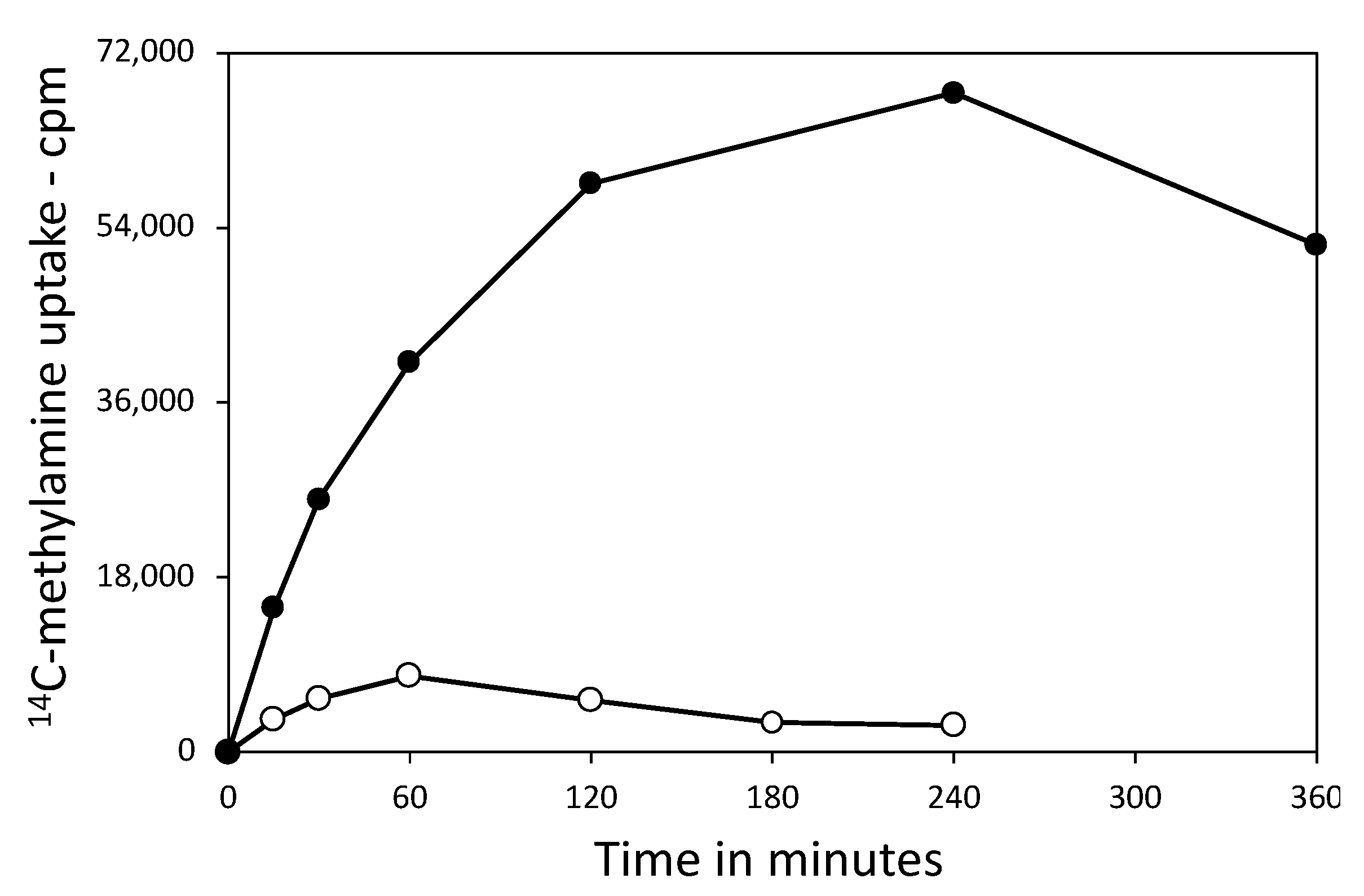
3.3. Effect of 3PGA on ATP Accumulation and the Viability of Germinated Spores
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kennedy, M.J.; Reader, S.J.; Swierczynski, L.M. Preservation records of micro-organisms: Evidence of the tenacity of life. Microbiology 1994, 140, 2513–2529. [Google Scholar] [CrossRef] [PubMed]
- Setlow, P.; Johnson, E.A. Spores and their significance. In Food Microbiology, Fundamentals and Frontiers, 5th ed.; Doyle, M.P., Buchanan, R., Eds.; ASM Press: Washington, DC, USA, 2019; pp. 23–64. [Google Scholar]
- Setlow, P.; Wang, S.; Li, Y.-Q. Germination of spores of the orders Bacillales and Clostridiales. Annu. Rev. Microbiol. 2017, 71, 459–477. [Google Scholar] [CrossRef] [PubMed]
- Gerhardt, P.; Marquis, R.E. Spore thermoresistance mechanisms. In Mechanisms of Prokaryotic Development; Smith, I., Slepecky, R., Setlow, P., Eds.; American Society for Microbiology: Washington, DC, USA, 1989; pp. 17–63. [Google Scholar]
- Paidhungat, M.; Setlow, B.; Driks, A.; Setlow, P. Characterization of spores of Bacillus subtilis which lack dipicolinic acid. J. Bacteriol. 2000, 182, 5505–5512. [Google Scholar] [CrossRef] [PubMed]
- Laue, M.; Han, H.-M.; Dittmann, C.; Setlow, P. Intracellular membranes of bacterial endospores are reservoirs for spore core membrane expansion during spore germination. Sci. Rep. 2018, 8, 11388. [Google Scholar] [CrossRef]
- Setlow, P.; Kornberg, A. Biochemical studies of bacterial sporulation and germination. XXII. Energy metabolism in early stages of germination of Bacillus megaterium spores. J. Biol. Chem. 1970, 245, 3637–3644. [Google Scholar] [CrossRef]
- Setlow, P. Mechanisms which contribute to the long-term survival of spores of Bacillus species. J. Appl. Bacteriol. 1994, 76, 49S–60S. [Google Scholar] [CrossRef]
- Swerdlow, B.M.; Setlow, B.; Setlow, P. Levels of H+ and monovalent cations in dormant and germinating spores of Bacillus megaterium. J. Bacteriol. 1981, 148, 20–29. [Google Scholar] [CrossRef]
- Magill, N.G.; Cowan, A.E.; Koppel, D.E.; Setlow, P. The internal pH of the forespore compartment of Bacillus megaterium decreases by about 1 pH unit during sporulation. J. Bacteriol. 1994, 176, 2252–2258. [Google Scholar] [CrossRef]
- van Beilin, J.W.; Brul, S. Compartment-specific pH monitoring in Bacillus subtilis using fluorescent sensor proteins: A tool to analyze the antibacterial effect of weak organic acids. Front. Microbiol. 2013, 4, 157. [Google Scholar] [CrossRef]
- Setlow, B.; Melly, E.; Setlow, P. Properties of spores of Bacillus subtilis blocked at an intermediate stage of spore germination. J. Bacteriol. 2001, 183, 4894–4899. [Google Scholar] [CrossRef]
- Cowan, A.E.; Koppel, D.E.; Setlow, B.; Setlow, P. A soluble protein is immobile in dormant spores of Bacillus subtilis but is mobile in germinated spores: Implications for spore dormancy. Proc. Natl. Acad. Sci. USA 2003, 100, 4209–4214. [Google Scholar] [CrossRef] [PubMed]
- Scott, I.R.; Ellar, D.J. Metabolism and triggering of germination of Bacillus megaterium. Concentrations of amino acids, organic acids, adenine nucleotides and nicotinamide nucleotides during germination. Biochem. J. 1978, 174, 627–634. [Google Scholar] [CrossRef]
- Scott, I.R.; Ellar, D.J. Metabolism and triggering of germination of Bacillus megaterium. Use of L-[3H]alanine and tritiated water to detect metabolism. Biochem. J. 1978, 174, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Korza, G.; Maciejewski, M.; Setlow, P. Analysis of metabolism in dormant spores of Bacillus species by 31P-NMR of low molecular weight compounds. J. Bacteriol. 2015, 197, 991–1001. [Google Scholar] [CrossRef] [PubMed]
- Setlow, P. I will survive: DNA protection in bacterial spores. Trends Microbiol. 2007, 15, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Setlow, P.; Primus, G. Protein metabolism during germination of Bacillus megaterium spores. I. Protein synthesis and amino acid metabolism. J. Biol. Chem. 1975, 250, 623–630. [Google Scholar] [CrossRef]
- Tovar-Rojo, F.; Cabrera-Martinez, R.M.; Setlow, B.; Setlow, P. Studies on the osmoresistance of spores of Bacillus subtilis. J. Appl. Microbiol. 2003, 184, 584–587. [Google Scholar] [CrossRef]
- Magill, N.G.; E Cowan, A.; Leyva-Vazquez, M.A.; Brown, M.; E Koppel, D.; Setlow, P. Analysis of the relationship between the decrease in pH and accumulation of 3-phosphoglyceric acid in developing forespores of Bacillus species. J. Bacteriol. 1996, 178, 2204–2210. [Google Scholar] [CrossRef]
- Kuhn, N.J.; Setlow, B.; Setlow, P.; Cammack, R.; Williams, R. Cooperative manganese (II) activation of 3-phosphoglycerate mutase of Bacillus megaterium: A biological pH-sensing mechanism in bacterial spore formation and germination. Arch. Biochem. Biophys. 1995, 319, 35–42. [Google Scholar] [CrossRef]
- Mokashi, S.; Kanaan, J.; Craft, D.L.; Byrd, B.; Zenick, B.; Laue, M.; Korza, G.; Mok, W.W.; Setlow, P. Killing of bacterial spores by dodecylamine and its effects on spore inner membrane properties. J. Appl. Microbiol. 2020, 129, 1511–1522. [Google Scholar] [CrossRef]
- Setlow, B.; Setlow, P. Small, acid-soluble proteins bound to DNA protect Bacillus subtilis spores from killing by dry heat. Appl. Environ. Microbiol. 1995, 61, 2787–2790. [Google Scholar] [CrossRef] [PubMed]
- Tovar-Rojo, F.; Chander, M.; Setlow, B.; Setlow, P. The products of the spoVA operon are involved in dipicolinic acid uptake into developing spores of Bacillus subtilis. J. Bacteriol. 2002, 184, 584–587. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, W.L.; Setlow, P. Sporulation, germination and outgrowth. In Molecular Biological Methods for Bacillus; Harwood, C.R., Cutting, S.M., Eds.; John Wiley and Sons: Chichester, UK, 1990; pp. 391–450. [Google Scholar]
- Setlow, P. Observations on research with spores of Bacillales and Clostridiales species. J. Appl. Microbiol. 2019, 126, 348–358. [Google Scholar] [CrossRef] [PubMed]
- Korza, G.; Abini-Agbomson, S.; Setlow, B.; Shen, A.; Setlow, P. Levels of malate and other low molecular weight metabolites in spores of Bacillus species and Clostridium difficile. PLoS ONE 2017, 12, e0182656. [Google Scholar] [CrossRef] [PubMed]
- Setlow, B.; Korza, G.; Blatt, K.; Fey, J.; Setlow, P. Mechanism of Bacillus subtilis spore inactivation by and resistance to supercritical CO2 plus peracetic acid. J. Appl. Microbiol. 2015, 120, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Cortezzo, D.; Koziol-Dube, K.; Setlow, B.; Setlow, P. Treatment with oxidizing agents damages the inner membrane of spores of Bacillus subtilis and sensitizes spores to subsequent stress. J. Appl. Microbiol. 2004, 97, 838–852. [Google Scholar] [CrossRef]
- Setlow, B.; Shay, L.; Vary, J.C.; Setlow, P. Production of large amounts of acetate during germination of Bacillus megaterium spores in the absence of exogenous carbon sources. J. Bacteriol. 1977, 132, 744–746. [Google Scholar] [CrossRef]
- Singh, R.P.; Setlow, P. Purification and properties of phosphoglycerate mutase from spores and cells of Bacillus megaterium. J. Bacteriol. 1979, 137, 1024–1027. [Google Scholar] [CrossRef]
- Leyva-Vazquez, M.A.; Setlow, P. Cloning and nucleotide sequences of the genes encoding triose phosphate isomerase, phosphoglycerate mutase, and enolase from Bacillus subtilis. J. Bacteriol. 1994, 176, 3903–3910. [Google Scholar] [CrossRef]
- Singh, R.P.; Setlow, P. Regulation of phosphoglycerate phosphomutase in developing forespores and dormant and germinated spores of Bacillus megaterium by the level of free manganous ions. J. Bacteriol. 1979, 139, 889–898. [Google Scholar] [CrossRef]
- Chander, M.; Setlow, B.; Setlow, P. The enzymatic activity of phosphoglyceratre mutase from Gram-positive endospore-forming bacteria requires Mn2+ and is pH sensitive. Can. J. Microbiol. 1998, 44, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Jedrzejas, M.J.; Chander, M.; Setlow, P.; Krishnasamy, G. Structure and mechanism of action of a novel phosphoglycerate mutase from Bacillus stearothermophilus. EMBO J. 2000, 19, 1419–1431. [Google Scholar] [CrossRef] [PubMed]
- Jedrzejas, M.J.; Setlow, P. Comparison of the binuclear metalloenzymes diphosphoglycerate-independent phosphoglycerate mutase and alkaline phosphatase: Their mechanism of catalysis via a phosphoserine intermediate. Chem. Rev. 2001, 101, 607–618. [Google Scholar] [CrossRef]
- Fothergill-Gilmore, L.A.; Watson, H.C. The phosphoglycerate mutases. Adv. Enzymol. Relat. Areas Mol. Biol. 1989, 62, 227–313. [Google Scholar]
- Jedrzejas, M.J. Structure, function, and evolution of phosphoglycerate mutases: Comparison with fructose-2,6-bis phosphatase, acid phosphatase, and alkaline phosphatase. Prog. Biophys. Mol. Biol. 2000, 73, 263–287. [Google Scholar] [CrossRef] [PubMed]
- Fraser, H.I.; Kvaratskhelia, M.; White, M.F. The two analogous phosphoglycerate mutases of Escherichia coli. FEBS Lett. 1999, 455, 344–348. [Google Scholar] [CrossRef] [PubMed]
- Galperin, M.Y.; Koonin, E.V.; Bairoch, A. A superfamily of metalloenzymes unifies phosphopentomutase and cofactor-independent phosphoglycerate mutase with alkaline phosphatases and sulfatases. Protein Sci. 1998, 7, 1829–1835. [Google Scholar] [CrossRef]
- Pearson, C.L.; Loshon, C.A.; Pedersen, L.; Setlow, B.; Setlow, P. Analysis of the function of a putative 2,3-diphosphoglyceric acid-dependent phosphoglycerate mutase from Bacillus subtilis. J. Bacteriol. 2000, 182, 4121–4123. [Google Scholar] [CrossRef]
- Li, N.; Liu, X. Phosphoglycerate mutase 1: Its glycolytic and non-glycolytic roles in tumor malignant behaviors and potential therapetic significance. OncoTargets Ther. 2020, 13, 1787–1795. [Google Scholar] [CrossRef]
- Yang, G.-J.; Tao, F.; Zhong, H.-J.; Yang, C.; Chen, J. Targeting PGAM1 in cancer: An emerging therapeutic opportunity. Eur. J. Med. Chem. 2022, 244, 114798. [Google Scholar] [CrossRef]
- Riley, E.P.; Lopez-Garrido, J.; Sugie, J.; Liu, R.B.; Pogliano, K. Metabolic differentiation and intercellular nurturing underpin bacterial endospore formation. Sci. Adv. 2021, 7, eabd6385. [Google Scholar] [CrossRef] [PubMed]

| Core pH | Spores |
|---|---|
| 6.4 | PS533 |
| 7.4 | PS3406 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korza, G.; Goulet, M.; DeMarco, A.; Wicander, J.; Setlow, P. Role of Bacillus subtilis Spore Core Water Content and pH in the Accumulation and Utilization of Spores’ Large 3-Phosphoglyceric Acid Depot, and the Crucial Role of This Depot in Generating ATP Early during Spore Germination. Microorganisms 2023, 11, 195. https://doi.org/10.3390/microorganisms11010195
Korza G, Goulet M, DeMarco A, Wicander J, Setlow P. Role of Bacillus subtilis Spore Core Water Content and pH in the Accumulation and Utilization of Spores’ Large 3-Phosphoglyceric Acid Depot, and the Crucial Role of This Depot in Generating ATP Early during Spore Germination. Microorganisms. 2023; 11(1):195. https://doi.org/10.3390/microorganisms11010195
Chicago/Turabian StyleKorza, George, Michelle Goulet, Angela DeMarco, James Wicander, and Peter Setlow. 2023. "Role of Bacillus subtilis Spore Core Water Content and pH in the Accumulation and Utilization of Spores’ Large 3-Phosphoglyceric Acid Depot, and the Crucial Role of This Depot in Generating ATP Early during Spore Germination" Microorganisms 11, no. 1: 195. https://doi.org/10.3390/microorganisms11010195
APA StyleKorza, G., Goulet, M., DeMarco, A., Wicander, J., & Setlow, P. (2023). Role of Bacillus subtilis Spore Core Water Content and pH in the Accumulation and Utilization of Spores’ Large 3-Phosphoglyceric Acid Depot, and the Crucial Role of This Depot in Generating ATP Early during Spore Germination. Microorganisms, 11(1), 195. https://doi.org/10.3390/microorganisms11010195






