A Randomized Large-Scale Cross-Sectional Serological Survey of Hepatitis E Virus Infection in Belgian Pig Farms
Abstract
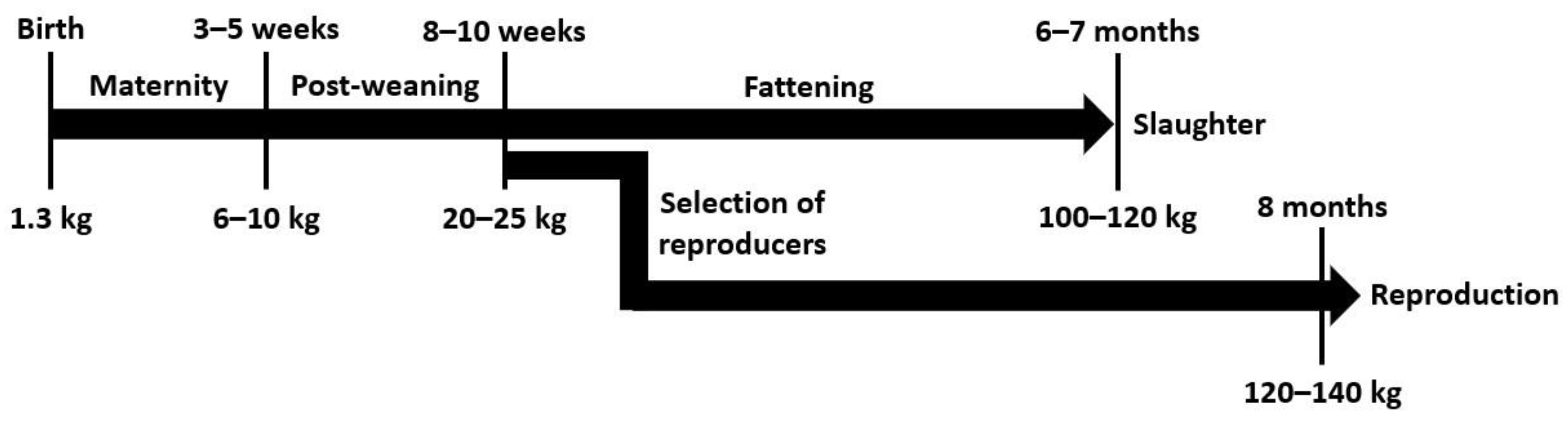
1. Introduction
2. Materials and Methods
2.1. Sampling Protocol and Study Design
2.2. Detection of Anti-HEV Antibodies
2.3. RNA Extraction
2.4. Nested RT-PCR
2.5. Sequencing and Phylogenetic Analysis of HEV Sequences
2.6. Statistical Analysis
3. Results
3.1. HEV Widely Circulates amongst the Belgian Pig Population
3.2. Herd Size and Type Influence the HEV-Serological Status of a Pig Herd
3.3. HEV Genotype 3 RNA Is Present in the Sera of Young Pigs in Belgium
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

| Region | Categories within Province Variable | N | Univariate Model | |
|---|---|---|---|---|
| OR (95% CI) | p-Value | |||
| Flanders | West Flanders | 130 | 1.10 (0.37–3.21) | 0.87 |
| East Flanders | 40 | 0.73 (0.21–2.46) | 0.61 | |
| Antwerp | 28 | - | - | |
| Limburg | 21 | 0.82 (0.19–3.53) | 0.79 | |
| Flemish Brabant | 11 | 0.87 (0.14–5.40) | 0.88 | |
| Wallonia | Hainaut | 14 | 0.35 (0.08–1.52) | 0.16 * |
| Liège | 7 | 1.09 (0.10–11.45) | 0.82 | |
| Namur | 7 | 1.3 (0.13–13.37) | 0.82 | |
| Walloon Brabant | 5 | 0.33 (0.04–3.53) | 0.28 | |
| Luxemburg | 3 | 0.43 (0.03–5.78) | 0.53 | |
References
- Smith, D.B.; Simmonds, P.; Jameel, S.; Emerson, S.U.; Harrison, T.J.; Meng, X.J.; Okamoto, H.; van der Poel, W.H.M.; Purdy, M.A. Consensus Proposals for Classification of the Family Hepeviridae. J. Gen. Virol. 2014, 95 Pt 10, 2223–2232. [Google Scholar] [CrossRef]
- World Health Organization. Hepatitis E. Available online: https://www.who.int/news-room/fact-sheets/detail/hepatitis-e (accessed on 14 September 2022).
- Lee, G.H.; Tan, B.H.; Chi-Yuan Teo, E.; Lim, S.G.; Dan, Y.Y.; Wee, A.; Kim Aw, P.P.; Zhu, Y.; Hibberd, M.L.; Tan, C.K.; et al. Chronic Infection with Camelid Hepatitis E Virus in a Liver Transplant Recipient Who Regularly Consumes Camel Meat and Milk. Gastroenterology 2016, 150, 355–357. [Google Scholar] [CrossRef]
- Thiry, D.; Mauroy, A.; Pavio, N.; Purdy, M.A.; Rose, N.; Thiry, E.; de Oliveira-Filho, E.F. Hepatitis E Virus and Related Viruses in Animals. Transbound. Emerg. Dis. 2017, 64, 37–52. [Google Scholar] [CrossRef]
- Khuroo, M.S.; Khuroo, M.S.; Khuroo, N.S. Transmission of Hepatitis E Virus in Developing Countries. Viruses 2016, 8, 253. [Google Scholar] [CrossRef]
- Singh, M.P.; Majumdar, M.; Goyal, K.; Lakshmi, P.V.M.; Bhatia, D.; Ratho, R.K. Investigation of Suspected Viral Hepatitis Outbreaks in North West India. Diagn. Microbiol. Infect. Dis. 2016, 84, 309–314. [Google Scholar] [CrossRef]
- Belei, O.; Ancusa, O.; Mara, A.; Olariu, L.; Amaricai, E.; Folescu, R.; Zamfir, C.L.; Gurgus, D.; Motoc, A.G.; Stânga, L.C.; et al. Current Paradigm of Hepatitis E Virus among Pediatric and Adult Patients. Front. Pediatr. 2021, 9, 721918. [Google Scholar] [CrossRef]
- Kamar, N.; Legrand-Abravanel, F.; Izopet, J.; Rostaing, L. Hepatitis E Virus: What Transplant Physicians Should Know. Am. J. Transplant. 2012, 12, 2281–2287. [Google Scholar] [CrossRef]
- Sue, P.K.; Pisanic, N.; Heaney, C.D.; Forman, M.; Valsamakis, A.; Jackson, A.M.; Ticehurst, J.R.; Montgomery, R.A.; Schwarz, K.B.; Nelson, K.E.; et al. Hepatitis E Virus Infection Among Solid Organ Transplant Recipients at a North American Transplant Center. Open Forum Infect. Dis. 2016, 3, ofw006. [Google Scholar] [CrossRef]
- Kamar, N.; Dalton, H.R.; Abravanel, F.; Izopet, J. Hepatitis E Virus Infection. Clin. Microbiol. Rev. 2014, 27, 116–138. [Google Scholar] [CrossRef]
- Ho, E.; Schenk, J.; Hutse, V.; Suin, V.; Litzroth, A.; Blaizot, S.; Herzog, S.A.; Verburgh, V.; Jacques, M.; Rahman, A.; et al. Stable HEV IgG Seroprevalence in Belgium between 2006 and 2014. J. Viral Hepat. 2020, 27, 1253–1260. [Google Scholar] [CrossRef]
- Meng, X.-J.; Halbur, P.G.; Shapiro, M.S.; Govindarajan, S.; Bruna, J.D.; Mushahwar, I.K.; Purcell, R.H.; Emerson, S.U. Genetic and Experimental Evidence for Cross-Species Infection by Swine Hepatitis E Virus. J. Virol. 1998, 72, 9714–9721. [Google Scholar] [CrossRef]
- Halbur, P.G.; Kasorndorkbua, C.; Gilbert, C.; Guenette, D.; Potters, M.B.; Purcell, R.H.; Emerson, S.U.; Toth, T.E.; Meng, X.J. Comparative Pathogenesis of Infection of Pigs with Hepatitis E Viruses Recovered from a Pig and a Human. J. Clin. Microbiol. 2001, 39, 918–923. [Google Scholar] [CrossRef]
- Li, P.; Liu, J.; Li, Y.; Su, J.; Ma, Z.; Bramer, W.M.; Cao, W.; de Man, R.A.; Peppelenbosch, M.P.; Pan, Q. The Global Epidemiology of Hepatitis E Virus Infection: A Systematic Review and Meta-Analysis. Liver Int. 2020, 40, 1516–1528. [Google Scholar] [CrossRef]
- Leblanc, D.; Ward, P.; Gagné, M.J.; Poitras, E.; Müller, P.; Trottier, Y.L.; Simard, C.; Houde, A. Presence of Hepatitis E Virus in a Naturally Infected Swine Herd from Nursery to Slaughter. Int. J. Food Microbiol. 2007, 117, 160–166. [Google Scholar] [CrossRef]
- Casas, M.; Pujols, J.; Rosell, R.; de Deus, N.; Peralta, B.; Pina, S.; Casal, J.; Martín, M. Retrospective Serological Study on Hepatitis E Infection in Pigs from 1985 to 1997 in Spain. Vet. Microbiol. 2009, 135, 248–252. [Google Scholar] [CrossRef]
- Rose, N.; Lunazzi, A.; Dorenlor, V.; Merbah, T.; Eono, F.; Eloit, M.; Madec, F.; Pavio, N. High Prevalence of Hepatitis E Virus in French Domestic Pigs. Comp. Immunol. Microbiol. Infect. Dis. 2011, 34, 419–427. [Google Scholar] [CrossRef]
- Martinelli, N.; Luppi, A.; Cordioli, P.; Lombardi, G.; Lavazza, A. Prevalence of Hepatitis E Virus Antibodies in Pigs in Northern Italy. Infect. Ecol. Epidemiol. 2011, 1, 7331. [Google Scholar] [CrossRef]
- Lange, H.; Overbo, J.; Borgen, K.; Dudman, S.; Hoddevik, G.; Urdahl, A.M.; Vold, L.; Sjurseth, S.K. Hepatitis E in Norway: Seroprevalence in Humans and Swine. Epidemiol. Infect. 2017, 145, 181–186. [Google Scholar] [CrossRef]
- Pavio, N.; Merbah, T.; Thébault, A. Frequent Hepatitis E Virus Contamination in Food Containing Raw Pork Liver, France. Emerg. Infect. Dis. 2014, 20, 1925–1927. [Google Scholar] [CrossRef]
- Salaün, Y.; Hémonic, A.; Marcon, M.; Courboulay, V.; Quiniou, N.; Bidanel, J.; Duflot, B. Mémento de l’Éleveur de Porc, 7th ed.; Institut Technique du Porc: Paris, France, 2013. [Google Scholar]
- Collège des Producteurs. Filagri—Le Secteur Porcs. Available online: https://filagri.be/porcs/le-secteur-porcs/ (accessed on 16 September 2022).
- Wavreille, J.; Rondia, P.; Decruyenaere, V.; Jamar, D.; Stilmant, D.; Gengler, N.; Mayeres, P.; Beckers, Y.; Sindic, M.; Bartiaux-Thill, N. La Diversification En Productions Animales. In 10ème Carrefour des Productions Animales: L’élevage: Hier, Aujourd’hui, Demain Quelles Attentes Pour Quels Enjeux? CRA-W et FUSAGx: Gembloux, Belgique, 2005; pp. 53–57. [Google Scholar]
- Thiry, D.; Mauroy, A.; Saegerman, C.; Licoppe, A.; Fett, T.; Thomas, I.; Brochier, B.; Thiry, E.; Linden, A. Belgian Wildlife as Potential Zoonotic Reservoir of Hepatitis E Virus. Transbound. Emerg. Dis. 2017, 64, 764–773. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. Int. J. Surg. 2014, 12, 1495–1499. [Google Scholar] [CrossRef] [PubMed]
- Thiry, D.; Mauroy, A.; Saegerman, C.; Thomas, I.; Wautier, M.; Miry, C.; Czaplicki, G.; Berkvens, D.; Praet, N.; van der Poel, W.; et al. Estimation of Hepatitis E Virus (HEV) Pig Seroprevalence Using ELISA and Western Blot and Comparison between Human and Pig HEV Sequences in Belgium. Vet. Microbiol. 2014, 172, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Angus, C. Survey Toolbox for Livestock Diseases: A Practical Manual and Software Package for Active Surveillance of Livestock Diseases in Developing Countries; Australian Centre for International Agricultural Research, Ed.; Australian Government Publishing Service: Canberra, Australia, 1999. [Google Scholar]
- Arrêté Ministériel Portant Exécution de l’Arrêté Royal du 12 Octobre 2010 Relatif à la Lutte Contre la Maladie d’Aujeszky. Moniteur Belge. 23 July 2013. Available online: http://www.ejustice.just.fgov.be/mopdf/2013/07/30_1.pdf#Page19 (accessed on 22 November 2022).
- Cannon, R.M.; Roe, R.T. Livestock Disease Surveys. A Field Manual for Veterinarians; Australian Government Publishing Service: Canberra, Australia, 1982. [Google Scholar]
- De Deus, N.; Casas, M.; Peralta, B.; Nofrarías, M.; Pina, S.; Martín, M.; Segalés, J. Hepatitis E Virus Infection Dynamics and Organic Distribution in Naturally Infected Pigs in a Farrow-to-Finish Farm. Vet. Microbiol. 2008, 132, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.F.; Haqshenas, G.; Guenette, D.K.; Halbur, P.G.; Schommer, S.K.; Pierson, F.W.; Toth, T.E.; Meng, X.J. Detection by Reverse Transcription-PCR and Genetic Characterization of Field Isolates of Swine Hepatitis E Virus from Pigs in Different Geographic Regions of the United States. J. Clin. Microbiol. 2002, 40, 1326–1332. [Google Scholar] [CrossRef]
- Cooper, K.; Huang, F.F.; Batista, L.; Rayo, C.D.; Bezanilla, J.C.; Toth, T.E.; Meng, X.J. Identification of Genotype 3 Hepatitis E Virus (HEV) in Serum and Fecal Samples from Pigs in Thailand and Mexico, Where Genotype 1 and 2 HEV Strains Are Prevalent in the Respective Human Populations. J. Clin. Microbiol. 2005, 43, 1684–1688. [Google Scholar] [CrossRef]
- Baylis, S.A.; Hanschmann, K.M.; Blümel, J.; Nübling, C.M. Standardization of Hepatitis E Virus (HEV) Nucleic Acid Amplification Technique-Based Assays: An Initial Study to Evaluate a Panel of HEV Strains and Investigate Laboratory Performance. J. Clin. Microbiol. 2011, 49, 1234–1239. [Google Scholar] [CrossRef]
- Shukla, P.; Nguyen, H.T.; Torian, U.; Engle, R.E.; Faulk, K.; Dalton, H.R.; Bendall, R.P.; Keane, F.E.; Purcell, R.H.; Emerson, S.U. Cross-Species Infections of Cultured Cells by Hepatitis E Virus and Discovery of an Infectious Virus-Host Recombinant. Proc. Natl. Acad. Sci. USA 2011, 108, 2438–2443. [Google Scholar] [CrossRef]
- Graff, J.; Torian, U.; Nguyen, H.; Emerson, S.U. A Bicistronic Subgenomic mRNA Encodes Both the ORF2 and ORF3 Proteins of Hepatitis E Virus. J. Virol. 2006, 80, 5919–5926. [Google Scholar] [CrossRef]
- Smith, D.B.; Simmonds, P.; Izopet, J.; Oliveira-Filho, E.F.; Ulrich, R.G.; Johne, R.; Koenig, M.; Jameel, S.; Harrison, T.J.; Meng, X.J.; et al. Proposed Reference Sequences for Hepatitis E Virus Subtypes. J. Gen. Virol. 2016, 97 Pt 3, 537–542. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Li, C.; Hagedorn, C.H. Phylogenetic Analysis of Global Hepatitis E Virus Sequences: Genetic Diversity, Subtypes and Zoonosis. Rev. Med. Virol. 2006, 16, 5–36. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Nei, M. Estimation of the Number of Nucleotide Substitutions in the Control Region of Mitochondrial DNA in Humans and Chimpanzees. Mol. Biol. Evol. 1993, 10, 512–526. [Google Scholar] [CrossRef] [PubMed]
- Faes, C.; Aerts, M.; Litière, S.; Méroc, E.; van der Stede, Y.; Mintiens, K. Estimating Herd Prevalence on the Basis of Aggregate Testing of Animals. J. R. Stat. Soc. Ser. A Stat. Soc. 2011, 174, 155–174. [Google Scholar] [CrossRef]
- Li, H.; Wu, J.; Sheng, Y.; Lu, Q.; Liu, B.; Chen, Y.; Sun, Y.; Zhou, E.M.; Zhao, Q. Prevalence of Hepatitis E Virus (HEV) Infection in Various Pig Farms from Shaanxi Province, China: First Detection of HEV RNA in Pig Semen. Transbound. Emerg. Dis. 2019, 66, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Risalde, M.A.; Frias, M.; Caballero-Gómez, J.; Lopez-Lopez, P.; Fast, C.; Jiménez-Ruiz, S.; Agulló-Ros, I.; Eiden, M.; Jiménez-Martín, D.; García-Bocanegra, I.; et al. Presence of Hepatitis E Virus in Testis of Naturally Infected Wild Boars. Transbound. Emerg. Dis. 2022, in press. [Google Scholar] [CrossRef]
- Zhang, W.; Shen, Q.; Mou, J.; Gong, G.; Yang, Z.; Cui, L.; Zhu, J.; Ju, G.; Hua, X. Hepatitis E Virus Infection among Domestic Animals in Eastern China. Zoonoses Public Health 2008, 55, 291–298. [Google Scholar] [CrossRef]
- Seminati, C.; Mateu, E.; Peralta, B.; de Deus, N.; Martin, M. Distribution of Hepatitis E Virus Infection and Its Prevalence in Pigs on Commercial Farms in Spain. Vet. J. 2008, 175, 130–132. [Google Scholar] [CrossRef]
- Vitral, C.L.; Pinto, M.A.; Lewis-Ximenez, L.L.; Khudyakov, Y.E.; dos Santos, D.R.; Gaspar, A.M.C. Serological Evidence of Hepatitis E Virus Infection in Different Animal Species from the Southeast of Brazil. Mem. Inst. Oswaldo Cruz 2005, 100, 117–122. [Google Scholar] [CrossRef]
- Kasorndorkbua, C.; Thacker, B.J.; Halbur, P.G.; Guenette, D.K.; Buitenwerf, R.M.; Royer, R.L.; Meng, X.J. Experimental Infection of Pregnant Gilts with Swine Hepatitis E Virus. Can. J. Vet. Res. 2003, 67, 303–306. [Google Scholar]
- Andraud, M.; Casas, M.; Pavio, N.; Rose, N. Early-Life Hepatitis E Infection in Pigs: The Importance of Maternally-Derived Antibodies. PLoS ONE 2014, 9, e105527. [Google Scholar] [CrossRef]
- Andraud, M.; Dumarest, M.; Cariolet, R.; Aylaj, B.; Barnaud, E.; Eono, F.; Pavio, N.; Rose, N. Direct Contact and Environmental Contaminations Are Responsible for HEV Transmission in Pigs. Vet. Res. 2013, 44, 102. [Google Scholar] [CrossRef] [PubMed]
- Walachowski, S.; Dorenlor, V.; Lefevre, J.; Lunazzi, A.; Eono, F.; Merbah, T.; Eveno, E.; Pavio, N.; Rose, N. Risk Factors Associated with the Presence of Hepatitis E Virus in Livers and Seroprevalence in Slaughter-Age Pigs: A Retrospective Study of 90 Swine Farms in France. Epidemiol. Infect. 2014, 142, 1934–1944. [Google Scholar] [CrossRef] [PubMed]
- Hinjoy, S.; Nelson, K.E.; Gibbons, R.V.; Jarman, R.G.; Chinnawirotpisan, P.; Fernandez, S.; Tablerk, P.; Labrique, A.B.; Patchanee, P. A Cross-Sectional Study of Hepatitis E Virus Infection in Pigs in Different-Sized Farms in Northern Thailand. Foodborne Pathog. Dis. 2013, 10, 698–704. [Google Scholar] [CrossRef] [PubMed]
- Jinshan; Jirintai; Manglai, D.; Takahashi, M.; Nagashima, S.; Okamoto, H. Molecular and Serological Survey of Hepatitis E Virus Infection among Domestic Pigs in Inner Mongolia, China. Arch. Virol. 2010, 155, 1217–1226. [Google Scholar] [CrossRef]
- Li, W.; She, R.; Wei, H.; Zhao, J.; Wang, Y.; Sun, Q.; Zhang, Y.; Wang, D.; Li, R. Prevalence of Hepatitis E Virus in Swine under Different Breeding Environment and Abattoir in Beijing, China. Vet. Microbiol. 2009, 133, 75–83. [Google Scholar] [CrossRef]
- di Bartolo, I.; Martelli, F.; Inglese, N.; Pourshaban, M.; Caprioli, A.; Ostanello, F.; Ruggeri, F.M. Widespread Diffusion of Genotype 3 Hepatitis E Virus among Farming Swine in Northern Italy. Vet. Microbiol. 2008, 132, 47–55. [Google Scholar] [CrossRef]
- Meester, M.; Bouwknegt, M.; der Honing, R.H.; Vernooij, H.; Houben, M.; van Oort, S.; van der Poel, W.H.M.; Stegeman, A.; Tobias, T. Repeated Cross-Sectional Sampling of Pigs at Slaughter Indicates Varying Age of Hepatitis E Virus Infection within and between Pig Farms. Vet. Res. 2022, 53, 50. [Google Scholar] [CrossRef]
- Rutjes, S.A.; Bouwknegt, M.; van der Giessen, J.W.; de Roda Husman, A.M.; Reusken, C.B.E.M. Seroprevalence of Hepatitis e Virus in Pigs from Different Farming Systems in the Netherlands. J. Food Prot. 2014, 77, 640–642. [Google Scholar] [CrossRef]
- Bouwknegt, M.; Rutjes, S.A.; Reusken, C.B.E.M.; Stockhofe-Zurwieden, N.; Frankena, K.; de Jong, M.C.M.; de Roda Husman, A.M.; van der Poel, W.H.M. The Course of Hepatitis E Virus Infection in Pigs after Contact-Infection and Intravenous Inoculation. BMC Vet. Res. 2009, 5, 7. [Google Scholar] [CrossRef]
- Bouwknegt, M.; Teunis, P.F.M.; Frankena, K.; de Jong, M.C.M.; de Roda Husman, A.M. Estimation of the Likelihood of Fecal-Oral HEV Transmission Among Pigs. Risk Anal. 2011, 31, 940–950. [Google Scholar] [CrossRef]
- Suin, V.; Klamer, S.E.; Hutse, V.; Wautier, M.; Jacques, M.; Abady, M.; Lamoral, S.; Verburgh, V.; Thomas, I.; Brochier, B.; et al. Epidemiology and Genotype 3 Subtype Dynamics of Hepatitis e Virus in Belgium, 2010 to 2017. Eurosurveillance 2019, 24, 1800141. [Google Scholar] [CrossRef] [PubMed]
- Subissi, L.; Peeters, M.; Lamoral, S.; Klamer, S.; Suin, V.; van Gucht, S. Subtype-Specific Differences in the Risk of Hospitalisation among Patients Infected with Hepatitis E Virus Genotype 3 in Belgium, 2010–2018. Epidemiol. Infect. 2019, 147, E224. [Google Scholar] [CrossRef] [PubMed]
- Thiry, D.; Rose, N.; Mauroy, A.; Paboeuf, F.; Dams, L.; Roels, S.; Pavio, N.; Thiry, E. Susceptibility of Pigs to Zoonotic Hepatitis E Virus Genotype 3 Isolated from a Wild Boar. Transbound. Emerg. Dis. 2017, 64, 1589–1597. [Google Scholar] [CrossRef] [PubMed]
- Ricci, A.; Allende, A.; Bolton, D.; Chemaly, M.; Davies, R.; Fernandez Escamez, P.S.; Herman, L.; Koutsoumanis, K.; Lindqvist, R.; Nørrung, B.; et al. Public Health Risks Associated with Hepatitis E Virus (HEV) as a Food-borne Pathogen. EFSA J. 2017, 15, 4886. [Google Scholar] [CrossRef]




| Number of Herds by Herd Type (%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Slaughter | Closed | Mixed | Other | Total | |||||||
| Region | Province | Population | Sample | Population | Sample | Population | Sample | Population | Sample | Population | Sample |
| Flanders | West Flanders | 1348 (29.97) | 71 (26.69) | 224 (4.98) | 18 (6.77) | 674 (14.98) | 29 (10.90) | 145 (3.22) | 12 (4.51) | 2391 (53.16) | 130 (48.87) |
| East Flanders | 383 (8.51) | 19 (7.14) | 89 (1.98) | 8 (3.01) | 193 (4.29) | 10 (3.76) | 39 (0.87) | 3 (1.13) | 704 (15.65) | 40 (15.04) | |
| Antwerp | 282 (6.27) | 13 (4.89) | 75 (1.67) | 5 (1.88) | 149 (3.31) | 7 (2.63) | 29 (0.64) | 3 (1.13) | 535 (11.89) | 28 (10.53) | |
| Limburg | 163 (3.62) | 8 (3.01) | 48 (1.07) | 5 (1.88) | 102 (2.27) | 8 (3.01) | 23 (0.51) | 0 (0.00) | 336 (7.47) | 21 (7.89) | |
| Flemish Brabant | 56 (1.24) | 5 (1.88) | 24 (0.53) | 2 (0.75) | 35 (0.78) | 3 (1.13) | 3 (0.07) | 1 (0.38) | 118 (2.62) | 11 (4.14) | |
| Wallonia | Hainaut | 89 (1.98) | 8 (3.01) | 21 (0.47) | 2 (0.75) | 18 (0.40) | 1 (0.38) | 4 (0.09) | 3 (1.13) | 132 (2.93) | 14 (5.26) |
| Liège | 84 (1.87) | 1 (0.38) | 8 (0.18) | 2 (0.75) | 23 (0.51) | 2 (0.75) | 4 (0.09) | 2 (0.75) | 119 (2.65) | 7 (2.63) | |
| Namur | 60 (1.33) | 3 (1.13) | 10 (0.22) | 2 (0.75) | 10 (0.22) | 2 (0.75) | 3 (0.07) | 0 (0.00) | 83 (1.85) | 7 (2.63) | |
| Walloon Brabant | 14 (0.31) | 2 (0.75) | 4 (0.09) | 2 (0.75) | 8 (0.18) | 1 (0.38) | 0 (0.00) | 0 (0.00) | 26 (0.58) | 5 (1.88) | |
| Luxembourg | 22 (0.49) | 1 (0.38) | 7 (0.16) | 1 (0.38) | 25 (0.56) | 1 (0.38) | 0 (0.00) | 0 (0.00) | 54 (1.20) | 3 (1.13) | |
| Total | 2501 (56.60) | 131 (49.25) | 510 (11.34) | 47 (17.67) | 1237 (27.50) | 64 (24.06) | 250 (5.56) | 24 (9.02) | 4498 (100.00) | 266 (100.00) | |
| Primer Sequence (5′ → 3′) | Amplicon Length & HEV/MNV RNA Position | Final Concentration (nM) |
|---|---|---|
| Hepatitis E virus | ||
| AATTATGCYCAGTAYCGRGTTG (F, external RT-PCR) | 731 (5899–6629) | 800 |
| CCCTTRTCYTGCTGMGCATTCTC (R, external RT-PCR) | 800 | |
| GTWATGCTYTGCATWCATGGCT (F, internal PCR) | 348 (6184–6531) | 800 |
| AGCCGACGAAATCAATTCTGTC (R, internal PCR) | 800 | |
| Murine norovirus CW1 | ||
| CGCTATGGATGCMAAGGA (F) | 84 (389–472) | 200 |
| CCGATGTAGACAGAGTAATGGTA (R) | 200 |
| Number of Pigs by Weight (Prevalence (%) (95% CI)) | |||||||
|---|---|---|---|---|---|---|---|
| <40 kg | 40–59 kg | 60–79 kg | ≥80 kg | Sows | Boars | p-Value | |
| Seropositive | 183 (44.85 (39.96–49.82)) | 222 (28.43 (25.28–31.73)) | 201 (44.36 (36.94–45.88)) | 111 (44.40 (38.14–50.79)) | 496 (80.26 (76.90–83.33)) | 0 (0.00 (0.00–15.33)) | <0.0001 |
| Total | 408 | 781 | 486 | 250 | 618 | 18 | |
| Region | Province | Number of Seropositive Herds (Prevalence (%) (95% CI)) | Total Number of Herds |
|---|---|---|---|
| Flanders | West Flanders | 109 (83.85 (77.69–90.00)) | 130 |
| East Flanders | 31 (77.50 (64.93–90.07)) | 40 | |
| Antwerp | 23 (82.14 (68.33–95.95)) | 28 | |
| Limburg | 16 (76.19 (58.55–93.83)) | 21 | |
| Flemish Brabant | 9 (81.82 (60.11–100.00)) | 11 | |
| Wallonia | Hainaut | 9 (64.29 (40.55–88.02)) | 14 |
| Liège | 6 (85.71 (60.57–100.00)) | 7 | |
| Namur | 6 (85.71 (60.91–100.00) | 7 | |
| Walloon Brabant | 3 (60.00 (21.41–98.59)) | 5 | |
| Luxembourg | 2 (66.67 (14.83–100.00)) | 3 | |
| Total | 214 (80.45 (75.83–85.07)) | 266 |
| Number of Herds by Herd Size (Prevalence (%) (95% CI)) | ||||||
|---|---|---|---|---|---|---|
| 101–200 | 200–500 | 501–1000 | 1001–2000 | >2000 | p-Value | |
| Seropositive | 3 (60.00 (14.66–94.73)) | 36 (72.00 (57.51–83.77)) | 46 (77.97 (65.27–87.71)) | 65 (77.38 (66.95–85.80)) | 64 (94.12 (85.62–98.37)) | <0.01 |
| Total | 5 | 50 | 59 | 84 | 68 | |
| Number of Herds by Herd Type (Prevalence (%) (95% CI)) | |||||
|---|---|---|---|---|---|
| Slaughter | Closed | Mixed | Other | p-Value | |
| Seropositive | 96 (73.28 (64.85–80.63)) | 45 (95.74 (84.46–99.48)) | 57 (89.06 (78.75–95.49)) | 16 (66.67 (44.68–84.37)) | <0.01 |
| Total | 131 | 47 | 64 | 24 | |
| Number of Herds (Prevalence (%) (95% CI)) | |||
|---|---|---|---|
| Free Range System | No Free Range System | p-Value | |
| Seropositive | 19 (95.00 (75.13–99.87)) | 195 (79.27 (73.66–84.16)) | 0.12 |
| Total | 20 | 246 | |
| Variable | Category | N | Univariate Model | Multivariate Model | ||
|---|---|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | |||
| Type | Closed f-to-f | 47 | - | - | - | - |
| Mixed f-to-f | 64 | 0.36 (0.07–1.83) | 0.219 | 0.33 (0.06–1.69) | 0.184 * | |
| Other | 24 | 0.09 (0.02–0.46) | 0.004 *** | 0.10 (0.02–0.55) | 0.008 *** | |
| Slaughter | 131 | 0.12 (0.03–0.53) | 0.005 *** | 0.14 (0.03–0.60) | 0.008 *** | |
| Size | - | 266 | 3.65 (1.59–8.36) | 0.002 *** | 2.64 (1.10–6.34) | 0.029 ** |
| Free-range system | No | 246 | - | - | Not retained | |
| Yes | 20 | 4.97 (0.65–38.00) | 0.122 * | Not retained | ||
| Within-Herd Animal Prevalence (%) (95% CI) | Herd Prevalence (%) (95% CI) | Within-Herd Correlation (%) (95% CI) | |||
|---|---|---|---|---|---|
| Apparent | True | Apparent | True | ||
| Six sera sampled per herd | 53.07 (48.26–57.64) | 31.24 (31.20–31.27) | 74.92 (69.95–79.27) | 51.94 (49.35–54.60) | 59.85 (49.35–64.63) |
| Twelve sera sampled per herd | 53.12 (48.46–57.73) | 31.24 (31.20–31.27) | 80.37 (75.83–84.71) | 58.33 (55.09–61.88) | 59.93 (54.92–64.55) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wielick, C.; Ludwig-Begall, L.; Faes, C.; Ribbens, S.; Saegerman, C.; Thiry, E. A Randomized Large-Scale Cross-Sectional Serological Survey of Hepatitis E Virus Infection in Belgian Pig Farms. Microorganisms 2023, 11, 129. https://doi.org/10.3390/microorganisms11010129
Wielick C, Ludwig-Begall L, Faes C, Ribbens S, Saegerman C, Thiry E. A Randomized Large-Scale Cross-Sectional Serological Survey of Hepatitis E Virus Infection in Belgian Pig Farms. Microorganisms. 2023; 11(1):129. https://doi.org/10.3390/microorganisms11010129
Chicago/Turabian StyleWielick, Constance, Louisa Ludwig-Begall, Christel Faes, Stefaan Ribbens, Claude Saegerman, and Etienne Thiry. 2023. "A Randomized Large-Scale Cross-Sectional Serological Survey of Hepatitis E Virus Infection in Belgian Pig Farms" Microorganisms 11, no. 1: 129. https://doi.org/10.3390/microorganisms11010129
APA StyleWielick, C., Ludwig-Begall, L., Faes, C., Ribbens, S., Saegerman, C., & Thiry, E. (2023). A Randomized Large-Scale Cross-Sectional Serological Survey of Hepatitis E Virus Infection in Belgian Pig Farms. Microorganisms, 11(1), 129. https://doi.org/10.3390/microorganisms11010129








