The Effect of Anaerobically Cultivated Human Intestinal Microbiota Compared to Fecal Microbiota Transplantation on Gut Microbiota Profile and Symptoms of Irritable Bowel Syndrome, a Double-Blind Placebo-Controlled Study
Abstract
:1. Introduction
2. Material and Methods
2.1. Eligible Patients
2.2. Randomization of Patients
2.3. Donors
2.4. Screening
2.5. Transplantation Procedure
2.6. ACHIM Transplantation Protocol
2.7. Donor-Fecal Microbiota Transplantation Protocol
2.8. Placebo Protocol
2.9. Stool Sample Collections
2.10. Microbial DNA Analysis
2.11. Study Questionnaires
3. Statistical Analyses
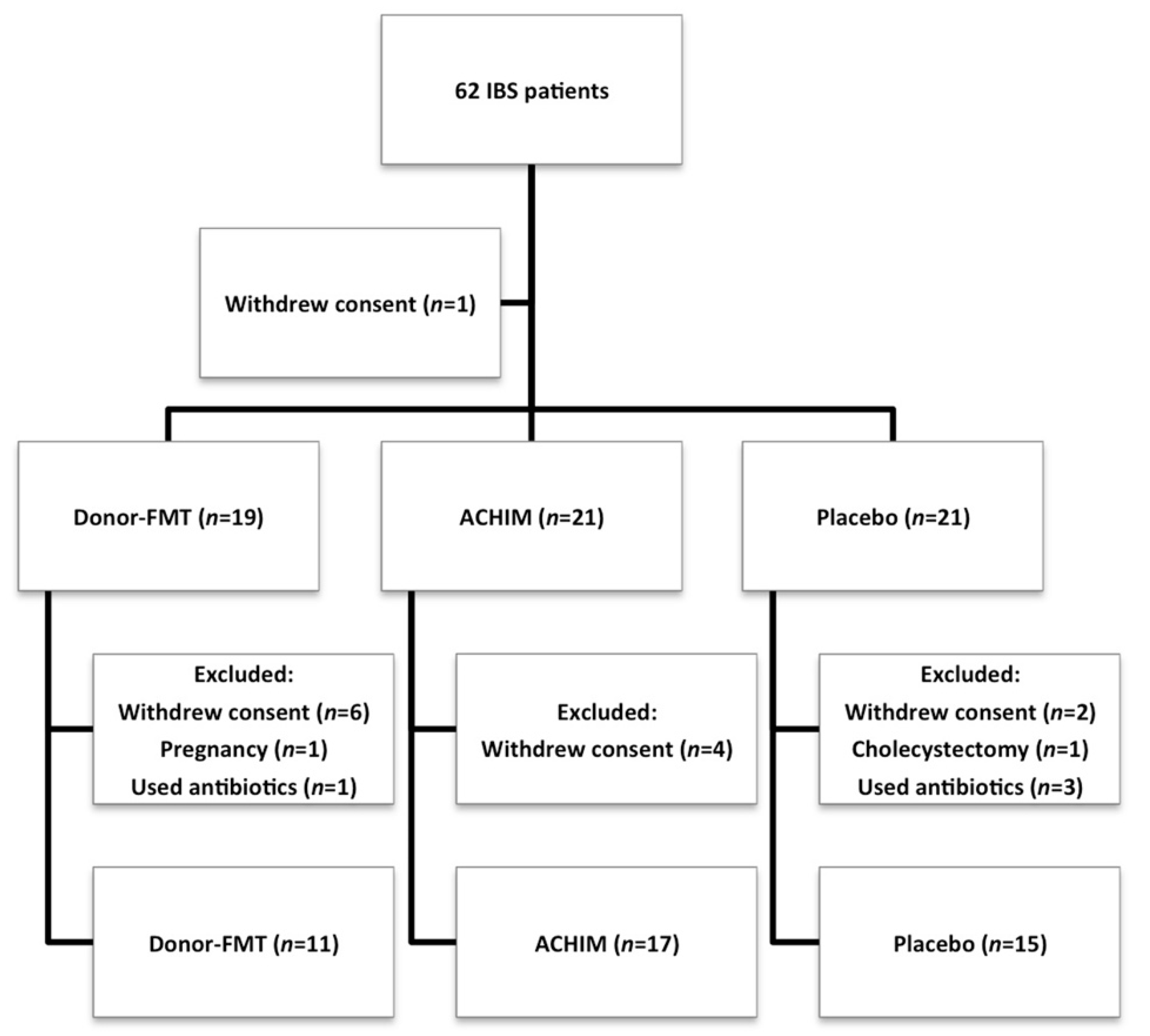
4. Results
Participants
5. Gut Microbiota
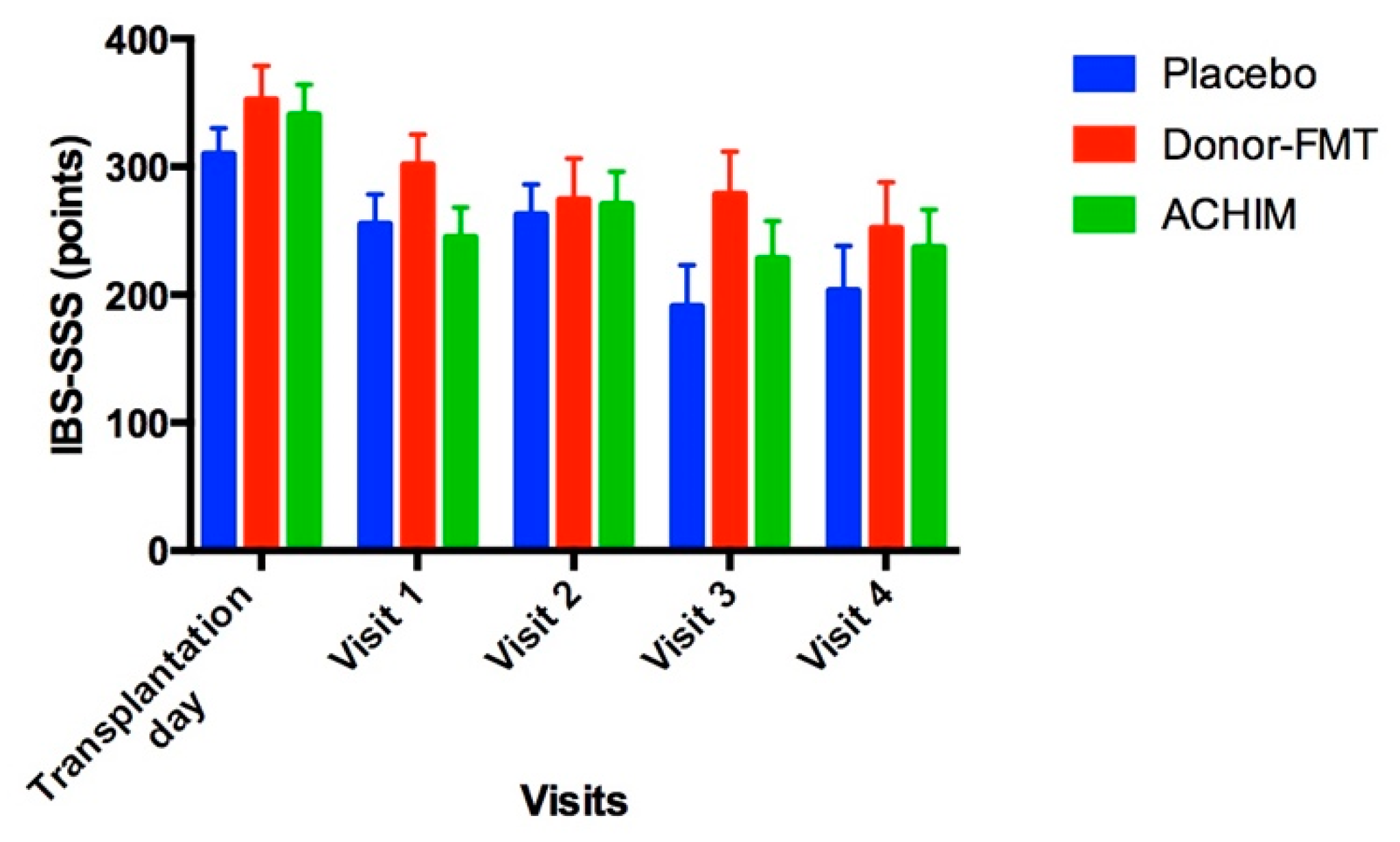
6. Study Questionnaires
7. Post-Transplantation Complications
8. Discussion
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oka, P.; Parr, H.; Barberio, B.; Black, C.J.; Savarino, E.V.; Ford, A.C. Global prevalence of irritable bowel syndrome according to Rome III or IV criteria: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2020, 5, 908–917. [Google Scholar] [CrossRef]
- Talley, N.J.; Gabriel, S.E.; Harmsen, W.; Zinsmeister, A.R.; Evans, R.W. Medical costs in community subjects with irritable bowel syndrome. Gastroenterology 1995, 109, 1736–1741. [Google Scholar] [CrossRef]
- Sandler, R.S.; Everhart, J.E.; Donowitz, M.; Adams, E.; Cronin, K.; Goodman, C.; Gemmen, E.; Shah, S.; Avdic, A.; Rubin, R. The burden of selected digestive diseases in the United States. Gastroenterology 2002, 122, 1500–1511. [Google Scholar] [CrossRef] [PubMed]
- Longstreth, G.F.; Thompson, W.G.; Chey, W.D.; Houghton, L.A.; Mearin, F.; Spiller, R.C. Functional Bowel Disorders. Gastroenterology 2006, 130, 1480–1491. [Google Scholar] [CrossRef] [PubMed]
- El-Salhy, M.; Gundersen, D.; Hatlebakk, J.G.; Hausken, T. Irritable Bowel Syndrome; Nova Science Publisher: New York, NY, USA, 2012. [Google Scholar]
- El-Salhy, M.; Hatlebakk, J.G.; Gilja, O.H.; Hausken, T. Irritable bowel syndrome: Recent developments in diagnosis, pathophysiology, and treatment. Expert Rev. Gastroenterol. Hepatol. 2014, 8, 435–443. [Google Scholar] [CrossRef]
- Codling, C.; O’Mahony, L.; Shanahan, F.; Quigley, E.M.M.; Marchesi, J.R. A Molecular Analysis of Fecal and Mucosal Bacterial Communities in Irritable Bowel Syndrome. Am. J. Dig. Dis. 2009, 55, 392–397. [Google Scholar] [CrossRef]
- Tana, C.; Umesaki, Y.; Imaoka, A.; Handa, T.; Kanazawa, M.; Fukudo, S. Altered profiles of intestinal microbiota and organic acids may be the origin of symptoms in irritable bowel syndrome. Neurogastroenterol. Motil. 2009, 22, 512-e115. [Google Scholar] [CrossRef]
- Whorwell, P.; Altringer, L.; Morel, J.; Bond, Y.; Charbonneau, D.; O’Mahony, L.; Kiely, B.; Shanahan, F.; Quigley, E.M.M. Efficacy of an Encapsulated Probiotic Bifidobacterium infantis 35624 in Women with Irritable Bowel Syndrome. Am. J. Gastroenterol. 2006, 101, 1581–1590. [Google Scholar] [CrossRef]
- Sisson, G.; Ayis, S.; Sherwood, R.A.; Bjarnason, I. Randomised clinical trial: A liquid multi-strain probiotic vs. placebo in the irritable bowel syndrome—A 12 week double-blind study. Aliment. Pharmacol. Ther. 2014, 40, 51–62. [Google Scholar] [CrossRef]
- Karlsson, F.; Fåk, F.; Nookaew, I.; Tremaroli, V.; Fagerberg, B.; Petranovic, D.; Bäckhed, F.; Nielsen, J. Symptomatic atherosclerosis is associated with an altered gut metagenome. Nat. Commun. 2012, 3, 1245. [Google Scholar] [CrossRef] [Green Version]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Human Gut Microbes Associated with Obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef]
- Mazzawi, T.; Hausken, T.; Hov, J.R.; Valeur, J.; Sangnes, D.A.; El-Salhy, M.; Gilja, O.H.; Hatlebakk, J.G.; Lied, G.A. Clinical response to fecal microbiota transplantation in patients with diarrhea-predominant irritable bowel syndrome is associated with normalization of fecal microbiota composition and short-chain fatty acid levels. Scand. J. Gastroenterol. 2019, 54, 690–699. [Google Scholar] [CrossRef] [PubMed]
- Mazzawi, T.; Lied, G.A.; Sangnes, D.A.; El-Salhy, M.; Hov, J.R.; Gilja, O.H.; Hatlebakk, J.G.; Hausken, T. The kinetics of gut microbial community composition in patients with irritable bowel syndrome following fecal microbiota transplantation. PLoS ONE 2018, 13, e0194904. [Google Scholar] [CrossRef] [PubMed]
- Crow, J.R.; Davis, S.L.; Chaykosky, D.M.; Smith, T.T.; Smith, J.M. Probiotics and Fecal Microbiota Transplant for Primary and Secondary Prevention of Clostridium difficile Infection. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2015, 35, 1016–1025. [Google Scholar] [CrossRef] [PubMed]
- Brandt, L.J. American Journal of Gastroenterology Lecture: Intestinal Microbiota and the Role of Fecal Microbiota Transplant (FMT) in Treatment of C. difficile Infection. Am. J. Gastroenterol. 2013, 108, 177–185. [Google Scholar] [CrossRef]
- Brandt, L.J.; Aroniadis, O.C.; Mellow, M.; Kanatzar, A.; Kelly, C.; Park, T.; Stollman, N.; Rohlke, F.; Surawicz, C. Long-Term Follow-Up of Colonoscopic Fecal Microbiota Transplant for Recurrent Clostridium difficile Infection. Am. J. Gastroenterol. 2012, 107, 1079–1087. [Google Scholar] [CrossRef]
- Kunde, S.; Pham, A.; Bonczyk, S.; Crumb, T.; Duba, M.; Conrad, H.; Cloney, D.; Kugathasan, S. Safety, Tolerability, and Clinical Response After Fecal Transplantation in Children and Young Adults With Ulcerative Colitis. J. Pediatr. Gastroenterol. Nutr. 2013, 56, 597–601. [Google Scholar] [CrossRef]
- Cammarota, G.; Ianiro, G.; Tilg, H.; Rajilić-Stojanović, M.; Kump, P.; Satokari, R.; Sokol, H.; Arkkila, P.; Pintus, C.; Hart, A.; et al. European consensus conference on faecal microbiota transplantation in clinical practice. Gut 2017, 66, 569–580. [Google Scholar] [CrossRef]
- Johnsen, P.H.; Hilpüsch, F.; Cavanagh, J.P.; Leikanger, I.S.; Kolstad, C.; Valle, P.C.; Goll, R. Faecal microbiota transplantation versus placebo for moderate-to-severe irritable bowel syndrome: A double-blind, randomised, placebo-controlled, parallel-group, single-centre trial. Lancet Gastroenterol. Hepatol. 2018, 3, 17–24. [Google Scholar] [CrossRef]
- El-Salhy, M.; Hatlebakk, J.G.; Gilja, O.H.; Kristoffersen, A.B.; Hausken, T. Efficacy of faecal microbiota transplantation for patients with irritable bowel syndrome in a randomised, double-blind, placebo-controlled study. Gut 2019, 69, 859–867. [Google Scholar] [CrossRef] [Green Version]
- Benno, P.; Norin, E.; Midtvedt, T.; Hellström, P. Therapeutic potential of an anaerobic cultured human intestinal microbiota, ACHIM, for treatment of IBS. Best Pr. Res. Clin. Gastroenterol. 2019, 40-41, 101607. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, A.; Berstad, A.; Lund-Tonnesen, S.; Midtvedt, T.; Norin, E. The effect of faecal enema on five microflora-associated characteristics in patients with antibiotic-associated diarrhoea. Scand. J. Gastroenterol. 1999, 34, 580–586. [Google Scholar] [PubMed]
- Ianiro, G.; Mullish, B.H.; Kelly, C.R.; Sokol, H.; Kassam, Z.; Ng, S.C.; Fischer, M.; Allegretti, J.R.; Masucci, L.; Zhang, F.; et al. Screening of faecal microbiota transplant donors during the COVID-19 outbreak: Suggestions for urgent updates from an international expert panel. Lancet Gastroenterol. Hepatol. 2020, 5, 430–432. [Google Scholar] [CrossRef]
- Juul, F.E.; Garborg, K.; Bretthauer, M.; Skudal, H.; Øines, M.N.; Wiig, H.; Rose, Ø.; Seip, B.; Lamont, J.T.; Midtvedt, T.; et al. Fecal Microbiota Transplantation for Primary Clostridium difficile Infection. N. Engl. J. Med. 2018, 378, 2535–2536. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann-Vold, A.-M.; Fretheim, H.H.; Sarna, V.K.; Barua, I.; Carstens, M.N.; Distler, O.; Khanna, D.; Volkmann, E.R.; Midtvedt, Ø.; Didriksen, H.; et al. Safety and efficacy of faecal microbiota transplantation by Anaerobic Cultivated Human Intestinal Microbiome (ACHIM) in patients with systemic sclerosis: Study protocol for the randomised controlled phase II ReSScue trial. BMJ Open 2021, 11, e048541. [Google Scholar] [CrossRef]
- World Medical Association Declaration of Helsinki. Ethical principles for medical research involving human subjects. J. Am. Coll. Dent. 2014, 81, 14–18. [Google Scholar]
- Aroniadis, O.C.; Brandt, L.J. Fecal microbiota transplantation. Curr. Opin. Gastroenterol. 2013, 29, 79–84. [Google Scholar] [CrossRef]
- Cammarota, G.; Ianiro, G.; Gasbarrini, A. Fecal Microbiota Transplantation for the Treatment of Clostridium difficile Infection. J. Clin. Gastroenterol. 2014, 48, 693–702. [Google Scholar] [CrossRef]
- Casén, C.; Vebø, H.C.; Sekelja, M.; Hegge, F.T.; Karlsson, M.K.; Ciemniejewska, E.; Dzankovic, S.; Frøyland, C.J.; Nestestog, R.; Engstrand, L.; et al. Deviations in human gut microbiota: A novel diagnostic test for determining dysbiosis in patients with IBS or IBD. Aliment. Pharmacol. Ther. 2015, 42, 71–83. [Google Scholar] [CrossRef]
- Francis, C.Y.; Morris, J.; Whorwell, P. The irritable bowel severity scoring system: A simple method of monitoring irritable bowel syndrome and its progress. Aliment. Pharmacol. Ther. 1997, 11, 395–402. [Google Scholar] [CrossRef]
- Arslan, G.; Lind, R.; Olafsson, S.; Florvaag, E.; Berstad, A. Quality of Life in Patients with Subjective Food Hypersensitivity: Applicability of the 10-Item Short Form of the Nepean Dyspepsia Index. Am. J. Dig. Dis. 2004, 49, 680–687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewis, S.J.; Heaton, K.W. Stool Form Scale as a Useful Guide to Intestinal Transit Time. Scand. J. Gastroenterol. 1997, 32, 920–924. [Google Scholar] [CrossRef] [PubMed]
- Heaton, K.W.; Radvan, J.; Cripps, H.; A Mountford, R.; E Braddon, F.; O Hughes, A. Defecation frequency and timing, and stool form in the general population: A prospective study. Gut 1992, 33, 818–824. [Google Scholar] [CrossRef] [PubMed]
- Pickford, R.W.; Eysenck, H.J.; Notcutt, B. The Structure of Human Personality. Br. J. Sociol. 1954, 5, 183. [Google Scholar] [CrossRef]
- Larsen, A.R.; Engsbro, A.L.; Bytzer, P. Screening instruments for anxiety and depression in patients with irritable bowel syndrome are ambiguous. Dan. Med. J. 2014, 61, A4785. [Google Scholar] [PubMed]
- Sugaya, N.; Nomura, S.; Shimada, H. Relationship Between Cognitive Factors and Anxiety in Individuals with Irritable Bowel Syndrome. Int. J. Behav. Med. 2011, 19, 308–315. [Google Scholar] [CrossRef]
- Thijssen, A.Y.; Jonkers, D.M.; Leue, C.; van der Veek, P.P.; Vidakovic-Vukic, M.; van Rood, Y.R.; Clemens, C.H.; Masclee, A.A. Dysfunctional Cognitions, Anxiety and Depression in Irritable Bowel Syndrome. J. Clin. Gastroenterol. 2010, 44, e236–e241. [Google Scholar] [CrossRef]
- Holvoet, T.; Joossens, M.; Wang, J.; Boelens, J.; Verhasselt, B.; Laukens, D.; van Vlierberghe, H.; Hindryckx, P.; de Vos, M.; de Looze, D.; et al. Assessment of faecal microbial transfer in irritable bowel syndrome with severe bloating. Gut 2016, 66, 980–982. [Google Scholar] [CrossRef]
- Bennet, S.M.; Ohman, L.; Simren, M. Gut Microbiota as Potential Orchestrators of Irritable Bowel Syndrome. Gut Liver 2015, 9, 318–331. [Google Scholar] [CrossRef]
- Duboc, H.; Rainteau, D.; Rajca, S.; Humbert, L.; Farabos, D.; Maubert, M.; Grondin, V.; Jouet, P.; Bouhassira, D.; Seksik, P.; et al. Increase in fecal primary bile acids and dysbiosis in patients with diarrhea-predominant irritable bowel syndrome. Neurogastroenterol. Motil. 2012, 24, 513-e247. [Google Scholar] [CrossRef]
- Kassinen, A.; Krogius-Kurikka, L.; Mäkivuokko, H.; Rinttilä, T.; Paulin, L.; Corander, J.; Malinen, E.; Apajalahti, J.; Palva, A. The Fecal Microbiota of Irritable Bowel Syndrome Patients Differs Significantly From That of Healthy Subjects. Gastroenterology 2007, 133, 24–33. [Google Scholar] [CrossRef] [PubMed]
- El-Salhy, M.; Mazzawi, T.; Hausken, T.; Hatlebakk, J.G. Irritable bowel syndrome patients who are not likely to respond to fecal microbiota transplantation. Neurogastroenterol. Motil. 2022, 34, e14353. [Google Scholar] [CrossRef]
- Li, J.; Sung, C.Y.J.; Lee, N.; Ni, Y.; Pihlajamäki, J.; Panagiotou, G.; El-Nezami, H. Probiotics modulated gut microbiota suppresses hepatocellular carcinoma growth in mice. Proc. Natl. Acad. Sci. USA 2016, 113, E1306–E1315. [Google Scholar] [CrossRef]
- Dalton, A.; Mermier, C.; Zuhl, M. Exercise influence on the microbiome–gut–brain axis. Gut Microbes 2019, 10, 555–568. [Google Scholar] [CrossRef] [PubMed]
- Halkjær, S.I.; Christensen, A.H.; Lo, B.Z.S.; Browne, P.; Günther, S.; Hansen, L.H.; Petersen, A.M. Faecal microbiota transplantation alters gut microbiota in patients with irritable bowel syndrome: Results from a randomised, double-blind placebo-controlled study. Gut 2018, 67, 2107–2115. [Google Scholar] [CrossRef]
- Kurokawa, S.; Kishimoto, T.; Mizuno, S.; Masaoka, T.; Naganuma, M.; Liang, K.-C.; Kitazawa, M.; Nakashima, M.; Shindo, C.; Suda, W.; et al. The effect of fecal microbiota transplantation on psychiatric symptoms among patients with irritable bowel syndrome, functional diarrhea and functional constipation: An open-label observational study. J. Affect. Disord. 2018, 235, 506–512. [Google Scholar] [CrossRef] [PubMed]

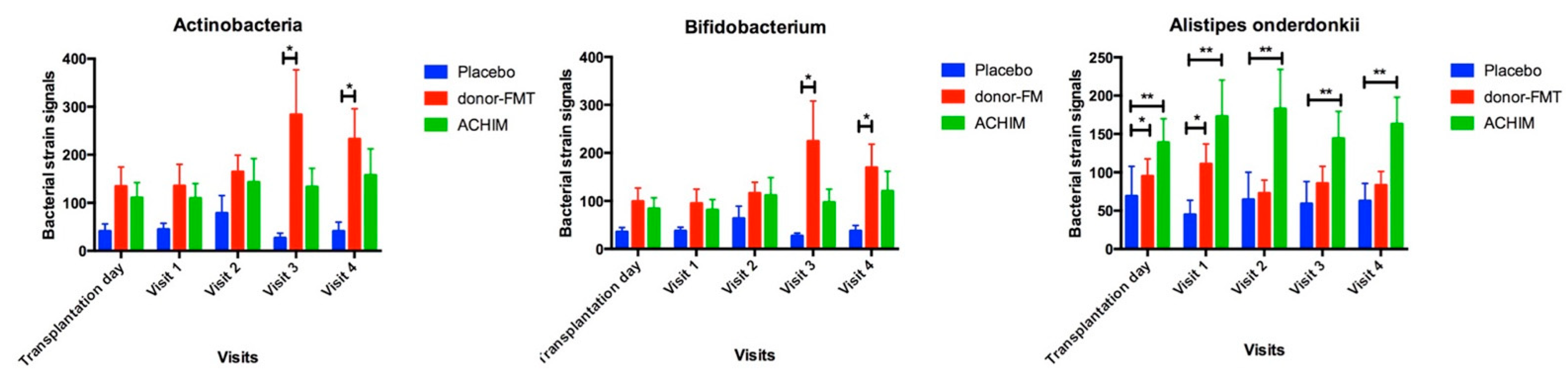
| Bacteria Strain | Healthy Controls | Transplant Type | Transplantation Day | Visit 1 | Visit 2 | Visit 3 | Visit 4 | pa | pb | pc | pd | pe |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Actinobacteria | 220 ± 71 | Donor-FMT | 134.6 ± 40 | 135.4 ± 45 | 164.3 ± 35 | 283.5 ± 93 | 232.9 ± 63 | >0.9 | >0.9 | >0.9 | >0.9 | >0.9 |
| ACHIM | 110.6 ± 32 | 109.4 ± 31 | 143.2 ± 49 | 133.4 ± 39 | 157.4 ± 55 | 0.8 | 0.7 | >0.9 | >0.9 | >0.9 | ||
| Placebo | 41 ± 15 | 45 ± 13 | 79 ± 37 | 27 ± 10 | 41 ± 19 | 0.057 | 0.04 | 0.15 | 0.01 | 0.03 | ||
| Bifidobacteria | 167 ± 62 | Donor-FMT | 99 ± 28 | 95 ± 30 | 116 ± 23 | 225 ± 84 | 170 ± 49 | >0.9 | >0.9 | >0.9 | >0.9 | >0.9 |
| ACHIM | 84 ± 23 | 81 ± 21 | 112 ± 37 | 97 ± 27 | 121 ± 41 | 0.9 | 0.6 | >0.9 | >0.9 | 0.9 | ||
| Placebo | 35 ± 9 | 37 ± 8 | 63 ± 26 | 27 ± 6 | 37 ± 11 | 0.03 | 0.03 | 0.1 | 0.009 | 0.04 | ||
| Alistipes onderdonkii | 132 ± 33 | Donor-FMT | 95 ± 23 | 111 ± 26 | 73 ± 17 | 85 ± 22 | 83 ± 18 | >0.9 | >0.9 | 0.9 | >0.9 | >0.9 |
| ACHIM | 139 ± 31 | 173 ± 47 | 183 ± 51 | 144 ± 35 | 163 ± 35 | >0.9 | >0.9 | >0.9 | >0.9 | >0.9 | ||
| Placebo | 70 ± 39 | 45 ± 19 | 65 ± 36 | 59 ± 29 | 63 ± 22 | 0.01 | 0.009 | 0.008 | 0.01 | 0.04 |
| Questionnaire | Transplantation Day | Visit 1 | Visit 2 | Visit 3 | Visit 4 | pa | pb | pc | pd |
|---|---|---|---|---|---|---|---|---|---|
| IBS-SSS | 340.3 ± 24 | 244.7 ± 23 | 270.4 ± 26 | 228 ± 29 | 236.7 ± 30 | <0.0001 | 0.001 | 0.003 | 0.0008 |
| Bristol stool form | 5 ± 0.4 | 4.7 ± 0.3 | 4.3 ± 0.4 | 4.3 ± 0.3 | 4.4 ± 0.4 | 0.4 | 0.04 | 0.03 | 0.057 |
| SF-NDI | 33.9 ± 1.5 | 26 ± 2 | 25.7 ± 2 | 24.1 ± 2 | 26.3 ± 2 | 0.001 | 0.0003 | 0.002 | 0.008 |
| HAD total | 15.2 ± 1.6 | 15.13 ± 1.4 | 14.2 ± 1.5 | 15 ± 1.6 | 12.6 ± 1.4 | 0.9 | 0.4 | 0.9 | 0.1 |
| HAD anxiety | 9.2 ± 1.2 | 8.8 ± 0.9 | 7.8 ± 0.9 | 9.2 ± 1.2 | 7.2 ± 0.9 | 0.6 | 0.1 | >0.9 | 0.06 |
| HAD depression | 5.8 ± 0.8 | 6.1 ± 0.8 | 5.9 ± 0.8 | 5.6 ± 0.9 | 5.1 ± 0.8 | 0.6 | 0.8 | 0.9 | 0.5 |
| EPQ-N-12 | 7.3 ± 0.6 | 5.2 ± 0.7 | 6.2 ± 0.8 | 6.1 ± 0.8 | 4.9 ± 0.8 | 0.005 | 0.04 | 0.1 | 0.001 |
| Questionnaire | Transplantation Day | Visit 1 | Visit 2 | Visit 3 | Visit 4 | pa | pb | pc | pd |
|---|---|---|---|---|---|---|---|---|---|
| IBS-SSS | 352.1 ± 27 | 301.4 ± 24 | 274.3 ± 32 | 278.6 ± 33 | 251.9 ± 36 | 0.03 | 0.2 | 0.04 | 0.04 |
| Bristol stool form | 5.3 ± 0.3 | 5.1 ± 0.3 | 4.5 ± 0.5 | 4.8 ± 0.3 | 4.4 ± 0.4 | 0.6 | 0.2 | 0.2 | 0.04 |
| SF-NDI | 32.8 ± 2.3 | 27.8 ± 2.2 | 27.1 ± 2.5 | 27.1 ± 3.2 | 27.2 ± 2.5 | 0.004 | 0.02 | 0.051 | 0.052 |
| HAD total | 12.5 ± 2.3 | 12 ± 2.3 | 11.4 ± 2.3 | 11.89 ± 2.4 | 11.56 ± 2.9 | 0.5 | 0.4 | 0.4 | 0.5 |
| HAD anxiety | 7.3 ± 1.1 | 7.1 ± 1.1 | 6.7 ± 1.3 | 6.9 ± 1.3 | 7.7 ± 1.7 | 0.7 | 0.4 | 0.2 | >0.9 |
| HAD depression | 5.2 ± 1.6 | 4.7 ± 1.6 | 4.7 ± 1.4 | 4.9 ± 1.4 | 3.9 ± 1.3 | 0.2 | 0.5 | 0.5 | 0.3 |
| EPQ-N-12 | 6.8 ± 1.1 | 5.4 ± 1.1 | 4.9 ± 1 | 4.6 ± 1.2 | 5.2 ± 1.2 | 0.02 | 0.05 | 0.02 | 0.1 |
| Questionnaire | Transplantation Day | Visit 1 | Visit 2 | Visit 3 | Visit 4 | pa | pb | pc | pd |
|---|---|---|---|---|---|---|---|---|---|
| IBS-SSS | 309.8 ± 20 | 255.1 ± 23 | 262.6 ± 24 | 190.9 ± 32 | 203.1 ± 35 | 0.03 | 0.049 | 0.001 | 0.005 |
| Bristol stool form | 4.9 ± 0.4 | 4 ± 0.4 | 4.313 ± 0.4 | 3.9 ± 0.4 | 4 ± 0.4 | 0.01 | 0.03 | 0.01 | 0.04 |
| SF-NDI | 34.7 ± 1.7 | 29.6 ± 1.8 | 27.8 ± 2 | 23.1 ± 2 | 22.4 ± 2 | 0.02 | 0.002 | 0.005 | 0.007 |
| HAD total | 9.2 ± 1.6 | 8 ± 1.3 | 7.9 ± 1.6 | 6.6 ± 1.1 | 5.9 ± 1.4 | 0.1 | 0.053 | 0.1 | 0.09 |
| HAD anxiety | 6.3 ± 1.1 | 5.3 ± 0.8 | 5.1 ± 0.9 | 4.9 ± 0.8 | 3.9 ± 0.8 | 0.08 | 0.009 | 0.2 | 0.07 |
| HAD depression | 3.3 ± 0.8 | 3 ± 0.8 | 3.1 ± 1 | 2.1 ± 0.6 | 2.2 ± 0.9 | 0.4 | 0.7 | 0.1 | 0.4 |
| EPQ-N-12 | 3.7 ± 0.8 | 3,5 ± 0.8 | 3.5 ± 0.9 | 2.7 ± 9.7 | 1.7 ± 0.5 | 0.6 | 0.5 | 0.055 | 0.006 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazzawi, T.; Hausken, T.; Refsnes, P.F.; Hatlebakk, J.G.; Lied, G.A. The Effect of Anaerobically Cultivated Human Intestinal Microbiota Compared to Fecal Microbiota Transplantation on Gut Microbiota Profile and Symptoms of Irritable Bowel Syndrome, a Double-Blind Placebo-Controlled Study. Microorganisms 2022, 10, 1819. https://doi.org/10.3390/microorganisms10091819
Mazzawi T, Hausken T, Refsnes PF, Hatlebakk JG, Lied GA. The Effect of Anaerobically Cultivated Human Intestinal Microbiota Compared to Fecal Microbiota Transplantation on Gut Microbiota Profile and Symptoms of Irritable Bowel Syndrome, a Double-Blind Placebo-Controlled Study. Microorganisms. 2022; 10(9):1819. https://doi.org/10.3390/microorganisms10091819
Chicago/Turabian StyleMazzawi, Tarek, Trygve Hausken, Per Førde Refsnes, Jan Gunnar Hatlebakk, and Gülen Arslan Lied. 2022. "The Effect of Anaerobically Cultivated Human Intestinal Microbiota Compared to Fecal Microbiota Transplantation on Gut Microbiota Profile and Symptoms of Irritable Bowel Syndrome, a Double-Blind Placebo-Controlled Study" Microorganisms 10, no. 9: 1819. https://doi.org/10.3390/microorganisms10091819
APA StyleMazzawi, T., Hausken, T., Refsnes, P. F., Hatlebakk, J. G., & Lied, G. A. (2022). The Effect of Anaerobically Cultivated Human Intestinal Microbiota Compared to Fecal Microbiota Transplantation on Gut Microbiota Profile and Symptoms of Irritable Bowel Syndrome, a Double-Blind Placebo-Controlled Study. Microorganisms, 10(9), 1819. https://doi.org/10.3390/microorganisms10091819







