Gut Microbiota Manipulation in Irritable Bowel Syndrome
Abstract
1. Introduction
2. Dysbiosis and IBS
3. Diet
4. Prebiotics, Probiotics and Antibiotics
5. Fecal Microbiota Transplantation
| Authors, Years | Diagnostic Criteria, Study Duration | Sample Size, IBS Subtypes | Allocation | Donors | Bowel Cleansing | FMT Route and Location (Upper/Lower GI Tract), Frequency | Dosage of FMT Group | Dosage of Control Group | Microbial Analysis | Findings |
|---|---|---|---|---|---|---|---|---|---|---|
| Aroniadis et al., 2019 [93] | Rome III, 3 months | n = 48: 100% IBS-D | 1:1 | Four donors, not mixed | No | Oral capsule ( upper), multiple lasted 3 days | 25 frozen capsules (0.38 g FMT) per day | 25 placebo capsules per day | 16S rRNA | Bacterial composition of FMT recipients shifted closer to that of the donors. |
| El-salhy et al., 2019 [91] | Rome IV, 3 months | n = 165: 37.8% IBS-C; 38.4% IBS-D; 23.8% IBS-M | 1:1:1 | One donor, not mixed | No | Gastroscopy (upper), single FMT | Frozen 30 g FMT and 60 g FMT | Frozen 30 g autologous feces | 16S rRNA | Higher abundance of Eubacterium biforme, Lactobacillus spp. and Alistipes spp., lower abundance of Bacteroides spp. Inverse correlation between IBS symptoms and the concentrations of Lactobacillus spp. and Alistipes spp. Negative correlation between the Fatigue Assessment Scale score and the concentration of Alistipes spp. |
| Halkjær et al., 2018 [94] | Rome III, 6 months | n = 52: 33.3% IBS-C; 29.4% IBS-D; 37.3% IBS-M | 1:1 | Four donors, mixed FMT | Yes | Oral capsule (upper), multiple administrations lasted 12 days | 25 frozen capsules (50 g FMT) | 25 placebo capsules per day | 16S rRNA | Fecal donors had higher biodiversity than IBS patients. Microbiota of FMT recipients are more similar to the donors’ microbiota than to that of the placebo recipient. Microbiota of placebo recipient did not become more similar to the donors’ microbiota than patients with IBS before randomization. Bacteroides genus and Ruminococcaceae family correlate positively with IBS symptoms score. Blautia genus and Clostridiales correlate negatively with IBS symptoms score. |
| Holster et al., 2019 [90] | Rome III, 6 months | n = 17: 25% IBS-C; 56.3% IBS-D; 18.8% IBS-M | 1:1 | Two donors, not mixed | Yes | Colonoscopy (lower), single FMT | Frozen 30 g FMT | Frozen 30 g autologous feces | Human Intestinal Tract Chip (fecal and mucosa) | The abundance of butyrate-producing bacteria in patients’ fecal samples was not lower than the donors at baseline. Microbial composition of patients had changed to resemble that of the donor after FMT. No effect on microbial diversity was observed after FMT in fecal or mucosal microbiota. |
| Holvoet et al., 2020 [89] | Rome III, 3 months | n = 62: 100% IBS-D/IBS-M. | 2:1 | Two donors; not mixed | No | Naso-jejunal tube (upper), single FMT | Donor fresh feces | Autologous feces | 16S rRNA | Donors’ fecal samples had higher diversity than the patients. Responders to FMT had a higher microbial diversity at baseline compared to non-responders. There was a significant difference in overall bacterial composition between responder and non-responders before treatment. Bacterial composition of FMT recipients shifted closer to that of the donors. |
| Johnsen et al., 2018 [92] | Rome III, 12 months | n = 90: 53% IBS-D; 47% IBS-M | 2:1 | Two donors, mixed | Yes | Colonoscopy (lower), single FMT | Frozen or fresh 50–80 g FMT | Frozen or fresh 50– 80 g autologous feces | Not reported | Not reported |
| Lahtinen et al., 2020 [88] | Rome III, 3 months | n = 55: 51% IBS-D; 14.3% IBS-M; 28.6% IBS unsubtyped; 6.1% other | 1:1 | One donor, not mixed | Yes | Colonoscopy (lower), single FMT | Frozen 30 g FMT | Fresh 30 g autologous feces | 16S rRNA | Changes in gut microbiota profile was observed. |
6. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Schuster, M.M. Defining and diagnosing irritable bowel syndrome. Am. J. Manag. Care 2001, 7 (Suppl. 8), S246–S251. [Google Scholar] [PubMed]
- El-Salhy, M.; Hatlebakk, J.G.; Gilja, O.H.; Hausken, T. Irritable bowel syndrome: Recent developments in diagnosis, pathophysiology, and treatment. Expert Rev. Gastroenterol. Hepatol. 2014, 8, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Böhn, L.; Störsrud, S.; Törnblom, H.; Bengtsson, U.; Simrén, M. Self-Reported Food-Related Gastrointestinal Symptoms in IBS Are Common and Associated With More Severe Symptoms and Reduced Quality of Life. Am. J. Gastroenterol. 2013, 108, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wang, J.; Ye, Z.; Wang, J.; Liao, X.; Liv, M.; Svn, Z. Risk of Colorectal Cancer in Patients With Irritable Bowel Syndrome: A Meta-Analysis of Population-Based Observational Studies. Front. Med. 2022, 9, 819122. [Google Scholar] [CrossRef] [PubMed]
- Hahn, B.A.; Yan, S.; Strassels, S. Impact of Irritable Bowel Syndrome on Quality of Life and Resource Use in the United States and United Kingdom. Digestion 1999, 60, 77–81. [Google Scholar] [CrossRef]
- Patrick, D.L.; Drossman, D.A.; Frederick, I.O.; Dicesare, J.; Puder, K.L. Quality of Life in Persons with Irritable Bowel Syndrome (Development and Validation of a New Measure). Am. J. Dig. Dis. 1998, 43, 400–411. [Google Scholar] [CrossRef]
- Oka, P.; Parr, H.; Barberio, B.; Black, C.J.; Savarino, E.V.; Ford, A.C. Global prevalence of irritable bowel syndrome according to Rome III or IV criteria: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2020, 5, 908–917. [Google Scholar] [CrossRef]
- Lacy, B.E.; Mearin, F.; Chang, L.; Chey, W.D.; Lembo, A.J.; Simren, M.; Spiller, R. Bowel disorders. Gastroenterology 2016, 150, 1393–1407. [Google Scholar] [CrossRef]
- Hungin, A.P.S.; Whorwell, P.J.; Tack, J.; Mearin, F. The prevalence, patterns and impact of irritable bowel syndrome: An international survey of 40 000 subjects. Aliment. Pharmacol. Ther. 2003, 17, 643–650. [Google Scholar] [CrossRef]
- Rayman, R.B. Irritable bowel syndrome: Aeromedical considerations. Aviat. Space Environ. Med. 2011, 82, 1061–1063. [Google Scholar] [CrossRef]
- Hong, S.N.; Rhee, P.L. Unraveling the ties between irritable bowel syndrome and intestinal microbiota. World J. Gastroenterol. 2014, 20, 2470–2481. [Google Scholar] [CrossRef] [PubMed]
- Mazzawi, T.; El-Salhy, M. Changes in duodenal enteroendocrine cells in patients with irritable bowel syndrome following dietary guidance. Exp. Biol. Med. 2017, 242, 1355–1362. [Google Scholar] [CrossRef] [PubMed]
- El-Salhy, M.; Mazzawi, T.; Gundersen, D.; Hatlebakk, J.G.; Hausken, T. Changes in the symptom pattern and the densities of large-intestinal endocrine cells following Campylobacter infection in irritable bowel syndrome: A case report. BMC Res. Notes 2013, 6, 391. [Google Scholar] [CrossRef][Green Version]
- Grover, M.; Camilleri, M.; Smith, K.; Linden, D.R.; Farrugia, G. On the fiftieth anniversary Postinfectious irritable bowel syndrome: Mechanisms related to pathogens. Neurogastroenterol. Motil. 2014, 26, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, S.; Tada, Y.; Fukuba, N.; Oka, A.; Kusunoki, R.; Mishima, Y.; Oshima, N.; Moriyama, I.; Yuki, T.; Kawashima, K.; et al. Pathogenesis of Irritable Bowel Syndrome—Review Regarding Associated Infection and Immune Activation. Digestion 2013, 87, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.A. The Role of Genetics in IBS. Gastroenterol. Clin. N. Am. 2011, 40, 45–67. [Google Scholar] [CrossRef] [PubMed]
- Simrén, M.; Månsson, A.; Langkilde, A.M.; Svedlund, J.; Abrahamsson, H.; Bengtsson, U.; Björnsson, E.S. Food-Related Gastrointestinal Symptoms in the Irritable Bowel Syndrome. Digestion 2001, 63, 108–115. [Google Scholar] [CrossRef]
- Monsbakken, K.W.; Vandvik, P.O.; Farup, P.G. Perceived food intolerance in subjects with irritable bowel syndrome-etiology, prevalence and consequences. Eur. J. Clin. Nutr. 2006, 60, 667–672. [Google Scholar] [CrossRef]
- Lee, Y.J.; Park, K.S. Irritable bowel syndrome: Emerging paradigm in pathophysiology. World J. Gastroenterol. 2014, 20, 2456–2469. [Google Scholar] [CrossRef]
- Mazzawi, T.; El-Salhy, M. Effect of diet and individual dietary guidance on gastrointestinal endocrine cells in patients with irritable bowel syndrome (Review). Int. J. Mol. Med. 2017, 40, 943–952. [Google Scholar] [CrossRef]
- El-Salhy, M.; Gundersen, D.; Gilja, O.H.; Hatlebakk, J.G.; Hausken, T. Is irritable bowel syndrome an organic disorder? World J. Gastroenterol. 2014, 20, 384–400. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.L.; Chen, C.Y.; Chang, F.Y.; Lee, S.D. Characteristics of small bowel motility in patients with irritable bowel syndrome and normal humans: An Oriental study. Clin. Sci. 1998, 95, 165–169. [Google Scholar] [CrossRef]
- Spiller, R. Inflammation as a basis for functional GI disorders. Best Pract. Res. Clin. Gastroenterol. 2004, 18, 641–661. [Google Scholar] [CrossRef] [PubMed]
- Ghoshal, U.C.; Srivastava, D. Irritable bowel syndrome and small intestinal bacterial overgrowth: Meaningful association or unnecessary hype. World J. Gastroenterol. 2014, 20, 2482–2491. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, R.; Braverman, D.; Stankiewicz, H. Carbohydrate malabsorption and the effect of dietary restriction on symptoms of irritable bowel syndrome and functional bowel complaints. Isr. Med Assoc. J. IMAJ 2000, 2, 583–587. [Google Scholar]
- Camilleri, M. Physiological underpinnings of irritable bowel syndrome: Neurohormonal mechanisms. J. Physiol. 2014, 592 Pt 14, 2967–2980. [Google Scholar] [CrossRef]
- Bonetto, S.; Fagoonee, S.; Battaglia, E.; Grassini, M.; Saracco, G.M.; Pellicano, R. Recent advances in the treatment of irritable bowel syndrome. Pol. Arch. Intern. Med. 2021, 131, 709–715. [Google Scholar] [CrossRef]
- Tack, J.; Vanuytsel, T.; Corsetti, M. Modern Management of Irritable Bowel Syndrome: More Than Motility. Dig. Dis. 2016, 34, 566–573. [Google Scholar] [CrossRef]
- Rao, S.; Weber, H.C. New treatment targets for the management of irritable bowel syndrome. Curr. Opin. Endocrinol. Diabetes Obes. 2014, 21, 9–14. [Google Scholar] [CrossRef]
- Collado, M.C.; Rautava, S.; Isolauri, E.; Salminen, S. Gut microbiota: A source of novel tools to reduce the risk of human disease? Pediatr. Res. 2015, 77, 182–188. [Google Scholar] [CrossRef]
- Huurre, A.; Kalliomäki, M.; Rautava, S.; Rinne, M.; Salminen, S.; Isolauri, E. Mode of delivery-effects on gut microbiota and humoral immunity. Neonatology 2008, 93, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Neu, J.; Rushing, J. Cesarean Versus Vaginal Delivery: Long-term Infant Outcomes and the Hygiene Hypothesis. Clin. Perinatol. 2011, 38, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Derrien, M.; Alvarez, A.-S.; de Vos, W.M. The Gut Microbiota in the First Decade of Life. Trends Microbiol. 2019, 27, 997–1010. [Google Scholar] [CrossRef]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef]
- Wilson, B.; Vatanen, T.; Cutfield, W.S.; O’Sullivan, J.M. The Super-Donor Phenomenon in Fecal Microbiota Transplantation. Front. Cell. Infect. Microbiol. 2019, 9, 2. [Google Scholar] [CrossRef] [PubMed]
- El-Salhy, M.; Hatlebakk, J.G.; Hausken, T. Diet in Irritable Bowel Syndrome (IBS): Interaction with Gut Microbiota and Gut Hormones. Nutrients 2019, 11, 1824. [Google Scholar] [CrossRef]
- Bennet, S.M.; Ohman, L.; Simren, M. Gut Microbiota as Potential Orchestrators of Irritable Bowel Syndrome. Gut Liver 2015, 9, 318–331. [Google Scholar] [CrossRef]
- Chang, C.; Lin, H. Dysbiosis in gastrointestinal disorders. Best Pract. Res. Clin. Gastroenterol. 2016, 30, 3–15. [Google Scholar] [CrossRef]
- Bartlett, J.G. Narrative Review: The New Epidemic of Clostridium difficile–Associated Enteric Disease. Ann. Intern. Med. 2006, 145, 758–764. [Google Scholar] [CrossRef]
- Jalanka, J.; Salojärvi, J.; Salonen, A.; Immonen, O.; Garsed, K.; Kelly, F.M.; Zaitoun, A.; Palva, A.; Spiller, R.; De Vos, W.M. Faecal microbiota composition and host–microbe cross-talk following gastroenteritis and in postinfectious irritable bowel syndrome. Gut 2014, 63, 1737–1745. [Google Scholar] [CrossRef]
- Mazzawi, T.; Hausken, T.; Hov, J.R.; Valeur, J.; Sangnes, D.A.; El-Salhy, M.; Gilja, O.H.; Hatlebakk, J.G.; Lied, G.A. Clinical response to fecal microbiota transplantation in patients with diarrhea-predominant irritable bowel syndrome is associated with normalization of fecal microbiota composition and short-chain fatty acid levels. Scand. J. Gastroenterol. 2019, 54, 690–699. [Google Scholar] [CrossRef] [PubMed]
- Duan, R.; Zhu, S.; Wang, B.; Duan, L. Alterations of Gut Microbiota in Patients With Irritable Bowel Syndrome Based on 16S rRNA-Targeted Sequencing: A Systematic Review. Clin. Transl. Gastroenterol. 2019, 10, e00012. [Google Scholar] [CrossRef] [PubMed]
- Pittayanon, R.; Lau, J.T.; Yuan, Y.; Leontiadis, G.I.; Tse, F.; Surette, M.; Moayyedi, P. Gut Microbiota in Patients With Irritable Bowel Syndrome—A Systematic Review. Gastroenterology 2019, 157, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Halmos, E.P.; Christophersen, C.T.; Bird, A.R.; Shepherd, S.J.; Gibson, P.R.; Muir, J.G. Diets that differ in their FODMAP content alter the colonic luminal microenvironment. Gut 2015, 64, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Tap, J.; Derrien, M.; Törnblom, H.; Brazeilles, R.; Cools-Portier, S.; Doré, J.; Störsrud, S.; Le Nevé, B.; Öhman, L.; Simrén, M. Identification of an Intestinal Microbiota Signature Associated With Severity of Irritable Bowel Syndrome. Gastroenterology 2017, 152, 111–123.e8. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.-S.; Chang, P.-F.; Liao, C.-H.; Lee, T.-H.; Chen, Y.; Lee, Y.-C.; Wu, M.-S.; Wang, H.-P.; Ni, Y.-H. Differences of microbiota in small bowel and faeces between irritable bowel syndrome patients and healthy subjects. Scand. J. Gastroenterol. 2015, 51, 410–419. [Google Scholar] [CrossRef]
- Lyra, A.; Rinttilä, T.; Nikkilä, J.; Krogius-Kurikka, L.; Kajander, K.; Malinen, E.; Mättö, J.; Mäkelä, L.; Palva, A. Diarrhoea-predominant irritable bowel syndrome distinguishable by 16S rRNA gene phylotype quantification. World J. Gastroenterol. 2009, 15, 5936–5945. [Google Scholar] [CrossRef]
- Chassard, C.; Dapoigny, M.; Scott, K.P.; Crouzet, L.; Del’Homme, C.; Marquet, P.; Martin, J.C.; Pickering, G.; Ardid, D.; Eschalier, A.; et al. Functional dysbiosis within the gut microbiota of patients with constipated-irritable bowel syndrome. Aliment. Pharmacol. Ther. 2012, 35, 828–838. [Google Scholar] [CrossRef]
- Vasant, D.H.; A Paine, P.; Black, C.J.; A Houghton, L.; A Everitt, H.; Corsetti, M.; Agrawal, A.; Aziz, I.; Farmer, A.D.; Eugenicos, M.P.; et al. British Society of Gastroenterology guidelines on the management of irritable bowel syndrome. Gut 2021, 70, 1214–1240. [Google Scholar] [CrossRef]
- Marsh, A.; Eslick, E.M.; Eslick, G.D. Does a diet low in FODMAPs reduce symptoms associated with functional gastrointestinal disorders? A comprehensive systematic review and meta-analysis. Eur. J. Nutr. 2016, 55, 897–906. [Google Scholar] [CrossRef]
- Staudacher, H.M.; Whelan, K. The low FODMAP diet: Recent advances in understanding its mechanisms and efficacy in IBS. Gut 2017, 66, 1517–1527. [Google Scholar] [CrossRef] [PubMed]
- Staudacher, H.M.; Lomer, M.C.E.; Farquharson, F.M.; Louis, P.; Fava, F.; Franciosi, E.; Scholz, M.; Tuohy, K.M.; Lindsay, J.O.; Irving, P.M.; et al. A Diet Low in FODMAPs Reduces Symptoms in Patients With Irritable Bowel Syndrome and A Probiotic Restores Bifidobacterium Species: A Randomized Controlled Trial. Gastroenterology 2017, 153, 936–947. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, K.; Reed, D.E.; Schneider, T.; Dang, F.; Keshteli, A.H.; De Palma, G.; Madsen, K.; Bercik, P.; Vanner, S. FODMAPs alter symptoms and the metabolome of patients with IBS: A randomised controlled trial. Gut 2017, 66, 1241–1251. [Google Scholar] [CrossRef]
- Staudacher, H.M.; Lomer, M.C.; Anderson, J.L.; Barrett, J.S.; Muir, J.G.; Irving, P.M.; Whelan, K. Fermentable Carbohydrate Restriction Reduces Luminal Bifidobacteria and Gastrointestinal Symptoms in Patients with Irritable Bowel Syndrome. J. Nutr. 2012, 142, 1510–1518. [Google Scholar] [CrossRef]
- Staudacher, H.M.; Rossi, M.; Kaminski, T.; Dimidi, E.; Ralph, F.S.E.; Wilson, B.; Martin, L.D.; Louis, P.; Lomer, M.C.; Irving, P.M.; et al. Long-term personalized low FODMAP diet improves symptoms and maintains luminal Bifidobacteria abundance in irritable bowel syndrome. Neurogastroenterol. Motil. 2022, 34, e14241. [Google Scholar] [CrossRef] [PubMed]
- Bennet, S.M.P.; Böhn, L.; Störsrud, S.; Liljebo, T.; Collin, L.; Lindfors, P.; Törnblom, H.; Öhman, L.; Simrén, M. Multivariate modelling of faecal bacterial profiles of patients with IBS predicts responsiveness to a diet low in FODMAPs. Gut 2017, 67, 872–881. [Google Scholar] [CrossRef] [PubMed]
- El-Salhy, M.; Ystad, S.O.; Mazzawi, T.; Gundersen, D. Dietary fiber in irritable bowel syndrome (Review). Int. J. Mol. Med. 2017, 40, 607–613. [Google Scholar] [CrossRef]
- Dale, H.F.; Lied, G.A. Gut microbiota and therapeutic approaches for dysbiosis in irritable bowel syndrome: Recent developments and future perspectives. Turk. J. Med Sci. 2020, 50, 1632–1641. [Google Scholar] [CrossRef]
- Gibson, G.R.; Probert, H.M.; Van Loo, J.; Rastall, R.A.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota: Updating the concept of prebiotics. Nutr. Res. Rev. 2004, 17, 259–275. [Google Scholar] [CrossRef]
- Bouhnik, Y.; Flourié, B.; Riottot, M.; Bisetti, N.; Gailing, M.; Guibert, A.; Bornet, F.; Rambaud, J. Effects of fructo-oligosaccharides ingestion on fecal bifidobacteria and selected metabolic indexes of colon carcinogenesis in healthy humans. Nutr. Cancer 1996, 26, 21–29. [Google Scholar] [CrossRef]
- Mazzawi, T.; El-Salhy, M. Changes in small intestinal chromogranin A-immunoreactive cell densities in patients with irritable bowel syndrome after receiving dietary guidance. Int. J. Mol. Med. 2016, 37, 1247–1253. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mazzawi, T.; El-Salhy, M. Dietary guidance and ileal enteroendocrine cells in patients with irritable bowel syndrome. Exp. Ther. Med. 2016, 12, 1398–1404. [Google Scholar] [CrossRef] [PubMed]
- Mazzawi, T.; Gundersen, D.; Hausken, T.; El-Salhy, M. Increased gastric chromogranin A cell density following changes to diets of patients with irritable bowel syndrome. Mol. Med. Rep. 2014, 10, 2322–2326. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mazzawi, T.; Hausken, T.; Gundersen, D.; El-Salhy, M. Dietary guidance normalizes large intestinal endocrine cell densities in patients with irritable bowel syndrome. Eur. J. Clin. Nutr. 2016, 70, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Mazzawi, T.; Hausken, T.; Gundersen, D.; El-Salhy, M. Effect of dietary management on the gastric endocrine cells in patients with irritable bowel syndrome. Eur. J. Clin. Nutr. 2015, 69, 519–524. [Google Scholar] [CrossRef]
- Mazzawi, T.; Hausken, T.; El-Salhy, M. Changes in colonic enteroendocrine cells of patients with irritable bowel syndrome following fecal microbiota transplantation. Scand. J. Gastroenterol. 2022, 1–5. [Google Scholar] [CrossRef]
- Silk, D.B.A.; Davis, A.; Vulevic, J.; Tzortzis, G.; Gibson, G.R. Clinical trial: The effects of a trans-galactooligosaccharide prebiotic on faecal microbiota and symptoms in irritable bowel syndrome. Aliment. Pharmacol. Ther. 2009, 29, 508–518. [Google Scholar] [CrossRef]
- Canakis, A.; Haroon, M.; Weber, H.C. Irritable bowel syndrome and gut microbiota. Curr. Opin. Endocrinol. Diabetes Obes. 2020, 27, 28–35. [Google Scholar] [CrossRef]
- Chlebicz-Wójcik, A.; Śliżewska, K. Probiotics, Prebiotics, and Synbiotics in the Irritable Bowel Syndrome Treatment: A Review. Biomolecules 2021, 11, 1154. [Google Scholar] [CrossRef]
- O’Mahony, L.; McCarthy, J.; Kelly, P.; Hurley, G.; Luo, F.; Chen, K.; O’Sullivan, G.C.; Kiely, B.; Collins, J.K.; Shanahan, F.; et al. Lactobacillus and bifidobacterium in irritable bowel syndrome: Symptom responses and relationship to cytokine profiles. Gastroenterology 2005, 128, 541–551. [Google Scholar] [CrossRef]
- Ford, A.C.; Harris, L.A.; Lacy, B.E.; Quigley, E.M.M.; Moayyedi, P. Systematic review with meta-analysis: The efficacy of prebiotics, probiotics, synbiotics and antibiotics in irritable bowel syndrome. Aliment. Pharmacol. Ther. 2018, 48, 1044–1060. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.S.; Sohn, W.; Lee, O.Y.; Lee, S.P.; Lee, K.N.; Jun, D.W.; Lee, H.L.; Yoon, B.C.; Choi, H.S.; Chung, W.-S.; et al. Effect of multispecies probiotics on irritable bowel syndrome: A randomized, double-blind, placebo-controlled trial. J. Gastroenterol. Hepatol. 2014, 29, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.; Park, Y.S.; Lee, D.H.; Seo, J.-G.; Shin, C.M.; Kim, N. Effect of administering a multi-species probiotic mixture on the changes in fecal microbiota and symptoms of irritable bowel syndrome: A randomized, double-blind, placebo-controlled trial. J. Clin. Biochem. Nutr. 2015, 57, 129–134. [Google Scholar] [CrossRef]
- Cha, B.K.; Jung, S.M.; Choi, C.H.; Song, I.D.; Lee, H.W.; Kim, H.J.; Hyuk, J.; Chang, S.K.; Kim, K.; Chung, W.S.; et al. The effect of a multispecies probiotic mixture on the symptoms and fecal microbiota in diarrhea-dominant irritable bowel syndrome: A randomized, double-blind, placebo-controlled trial. J. Clin. Gastroenterol. 2012, 46, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef] [PubMed]
- Chong, P.P.; Chin, V.K.; Looi, C.Y.; Wong, W.F.; Madhavan, P.; Yong, V.C. The Microbiome and Irritable Bowel Syndrome—A Review on the Pathophysiology, Current Research and Future Therapy. Front. Microbiol. 2019, 10, 1136. [Google Scholar] [CrossRef] [PubMed]
- Shayto, R.H.; Mrad, R.A.; I Sharara, A. Use of rifaximin in gastrointestinal and liver diseases. World J. Gastroenterol. 2016, 22, 6638–6651. [Google Scholar] [CrossRef] [PubMed]
- Lembo, A.; Pimentel, M.; Rao, S.S.; Schoenfeld, P.; Cash, B.; Weinstock, L.B.; Paterson, C.; Bortey, E.; Forbes, W.P. Repeat Treatment With Rifaximin Is Safe and Effective in Patients With Diarrhea-Predominant Irritable Bowel Syndrome. Gastroenterology 2016, 151, 1113–1121. [Google Scholar] [CrossRef]
- Pimentel, M.; Chang, C.; Chua, K.S.; Mirocha, J.; DiBaise, J.; Rao, S.; Amichai, M. Antibiotic Treatment of Constipation-Predominant Irritable Bowel Syndrome. Am. J. Dig. Dis. 2014, 59, 1278–1285. [Google Scholar] [CrossRef]
- Mazzawi, T.; Lied, G.A.; Sangnes, D.A.; El-Salhy, M.; Hov, J.R.; Gilja, O.H.; Hatlebakk, J.G.; Hausken, T. The kinetics of gut microbial community composition in patients with irritable bowel syndrome following fecal microbiota transplantation. PLoS ONE 2018, 13, e0194904. [Google Scholar] [CrossRef]
- Kelly, C.R.; Ihunnah, C.; Fischer, M.; Khoruts, A.; Surawicz, C.; Afzali, A.; Aroniadis, O.; Barto, A.; Borody, T.; Giovanelli, A.; et al. Fecal Microbiota Transplant for Treatment of Clostridium difficile Infection in Immunocompromised Patients. Am. J. Gastroenterol. 2014, 109, 1065–1071. [Google Scholar] [CrossRef] [PubMed]
- Moayyedi, P.; Surette, M.G.; Kim, P.T.; Libertucci, J.; Wolfe, M.; Onischi, C.; Armstrong, D.; Marshall, J.K.; Kassam, Z.; Reinisch, W.; et al. Fecal Microbiota Transplantation Induces Remission in Patients With Active Ulcerative Colitis in a Randomized Controlled Trial. Gastroenterology 2015, 149, 102–109.e6. [Google Scholar] [CrossRef] [PubMed]
- Aroniadis, O.C.; Brandt, L.J. Fecal microbiota transplantation: Past, present and future. Curr. Opin. Gastroenterol. 2013, 29, 79–84. [Google Scholar] [CrossRef]
- Fretheim, H.; Chung, B.K.; Didriksen, H.; Bækkevold, E.S.; Midtvedt, Ø.; Brunborg, C.; Holm, K.; Valeur, J.; Tennøe, A.H.; Garen, T.; et al. Fecal microbiota transplantation in systemic sclerosis: A double-blind, placebo-controlled randomized pilot trial. PLoS ONE 2020, 15, e0232739. [Google Scholar] [CrossRef]
- Cammarota, G.; Ianiro, G.; Tilg, H.; Rajilic-Stojanovic, M.; Kump, P.; Satokari, R.; Sokol, H.; Arkkila, P.; Pintus, C.; Hart, A.; et al. European consensus conference on faecal microbiota transplantation in clinical practice. Gut 2017, 66, 569–580. [Google Scholar] [CrossRef] [PubMed]
- DeFilipp, Z.; Bloom, P.P.; Torres Soto, M.; Mansour, M.K.; Sater, M.R.; Huntley, M.H.; Turbett, S.; Chung, R.T.; Chen, Y.B.; Hohmann, E.L. Drug-Resistant E. coli Bacteremia Transmitted by Fecal Microbiota Transplant. N. Engl. J. Med. 2019, 381, 2043–2050. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Lv, L.; Wang, C. Efficacy of Fecal Microbiota Transplantation in Irritable Bowel Syndrome: A Meta-Analysis of Randomized Controlled Trials. Front. Cell. Infect. Microbiol. 2022, 12, 827395. [Google Scholar] [CrossRef]
- Lahtinen, P.; Jalanka, J.; Hartikainen, A.; Mattila, E.; Hillilä, M.; Punkkinen, J.; Koskenpato, J.; Anttila, V.-J.; Tillonen, J.; Satokari, R.; et al. Randomised clinical trial: Faecal microbiota transplantation versus autologous placebo administered via colonoscopy in irritable bowel syndrome. Aliment. Pharmacol. Ther. 2020, 51, 1321–1331. [Google Scholar] [CrossRef] [PubMed]
- Holvoet, T.; Joossens, M.; Vázquez-Castellanos, J.F.; Christiaens, E.; Heyerick, L.; Boelens, J.; Verhasselt, B.; van Vlierberghe, H.; De Vos, M.; Raes, J.; et al. Fecal Microbiota Transplantation Reduces Symptoms in Some Patients With Irritable Bowel Syndrome With Predominant Abdominal Bloating: Short- and Long-term Results From a Placebo-Controlled Randomized Trial. Gastroenterology 2021, 160, 145–157.e8. [Google Scholar] [CrossRef]
- Holster, S.; Lindqvist, C.M.; Repsilber, D.; Salonen, A.; de Vos, W.M.; König, J.; Brummer, R.J. The Effect of Allogenic Versus Autologous Fecal Microbiota Transfer on Symptoms, Visceral Perception and Fecal and Mucosal Microbiota in Irritable Bowel Syndrome: A Randomized Controlled Study. Clin. Transl. Gastroenterol. 2019, 10, e00034. [Google Scholar] [CrossRef]
- El-Salhy, M.; Hatlebakk, J.G.; Gilja, O.H.; Kristoffersen, A.B.; Hausken, T. Efficacy of faecal microbiota transplantation for patients with irritable bowel syndrome in a randomised, double-blind, placebo-controlled study. Gut 2019, 69, 859–867. [Google Scholar] [CrossRef]
- Johnsen, P.H.; Hilpüsch, F.; Cavanagh, J.P.; Leikanger, I.S.; Kolstad, C.; Valle, P.C.; Goll, R. Faecal microbiota transplantation versus placebo for moderate-to-severe irritable bowel syndrome: A double-blind, randomised, placebo-controlled, parallel-group, single-centre trial. Lancet Gastroenterol. Hepatol. 2018, 3, 17–24. [Google Scholar] [CrossRef]
- Aroniadis, O.C.; Brandt, L.J.; Oneto, C.; Feuerstadt, P.; Sherman, A.; Wolkoff, A.W.; Kassam, Z.; Sadovsky, R.G.; Elliott, R.J.; Budree, S.; et al. Faecal microbiota transplantation for diarrhoea-predominant irritable bowel syndrome: A double-blind, randomised, placebo-controlled trial. Lancet Gastroenterol. Hepatol. 2019, 4, 675–685. [Google Scholar] [CrossRef]
- Halkjær, S.I.; Christensen, A.H.; Lo, B.Z.S.; Browne, P.; Günther, S.; Hansen, L.H.; Petersen, A.M. Faecal microbiota transplantation alters gut microbiota in patients with irritable bowel syndrome: Results from a randomised, double-blind placebo-controlled study. Gut 2018, 67, 2107–2115. [Google Scholar] [CrossRef] [PubMed]
- El-Salhy, M.; Mazzawi, T.; Hausken, T.; Hatlebakk, J.G. Irritable bowel syndrome patients who are not likely to respond to fecal microbiota transplantation. Neurogastroenterol. Motil. 2022, e14353. [Google Scholar] [CrossRef]
- El-Salhy, M.; Kristoffersen, A.B.; Valeur, J.; Casen, C.; Hatlebakk, J.G.; Gilja, O.H.; Hausken, T. Long-term effects of fecal microbiota transplantation (FMT) in patients with irritable bowel syndrome. Neurogastroenterol. Motil. 2021, 34, e14200. [Google Scholar] [CrossRef] [PubMed]
- Holvoet, T.; Joossens, M.; Wang, J.; Boelens, J.; Verhasselt, B.; Laukens, D.; van Vlierberghe, H.; Hindryckx, P.; De Vos, M.; De Looze, D.; et al. Assessment of faecal microbial transfer in irritable bowel syndrome with severe bloating. Gut 2016, 66, 980–982. [Google Scholar] [CrossRef]
- El-Salhy, M.; Hausken, T.; Hatlebakk, J.G. Increasing the Dose and/or Repeating Faecal Microbiota Transplantation (FMT) Increases the Response in Patients with Irritable Bowel Syndrome (IBS). Nutrients 2019, 11, 1415. [Google Scholar] [CrossRef]
- Mazzawi, T.; Eikrem, Ø.; Lied, G.A.; Hausken, T. Abnormal Uroguanylin Immunoreactive Cells Density in the Duodenum of Patients with Diarrhea-Predominant Irritable Bowel Syndrome Changes following Fecal Microbiota Transplantation. Gastroenterol. Res. Pract. 2020, 2020, 3520686. [Google Scholar] [CrossRef]
- Mazzawi, T.; El-Salhy, M.; Lied, G.A.; Hausken, T. The Effects of Fecal Microbiota Transplantation on the Symptoms and the Duodenal Neurogenin 3, Musashi 1, and Enteroendocrine Cells in Patients With Diarrhea-Predominant Irritable Bowel Syndrome. Front. Cell. Infect. Microbiol. 2021, 11, 403. [Google Scholar] [CrossRef]
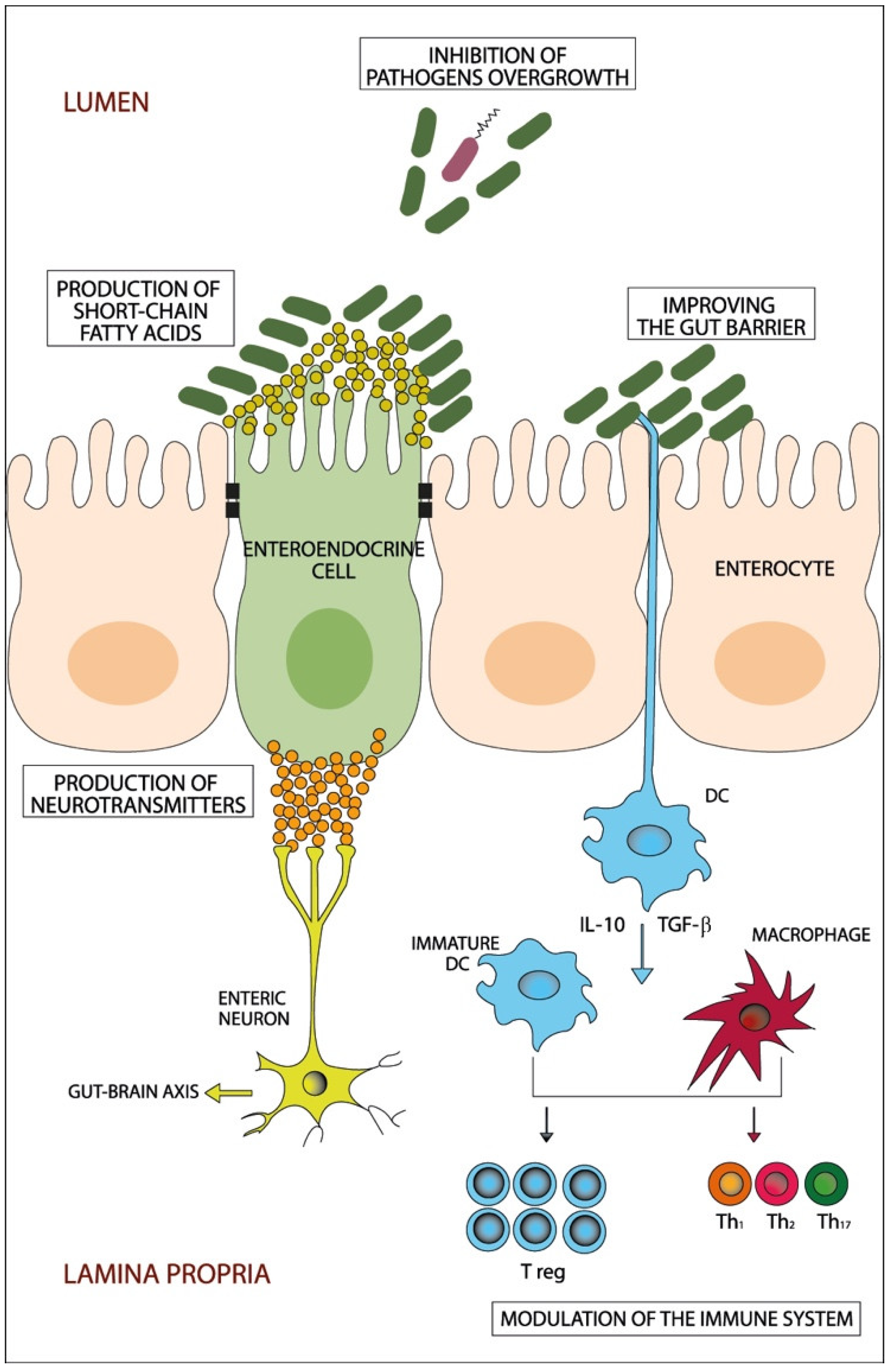
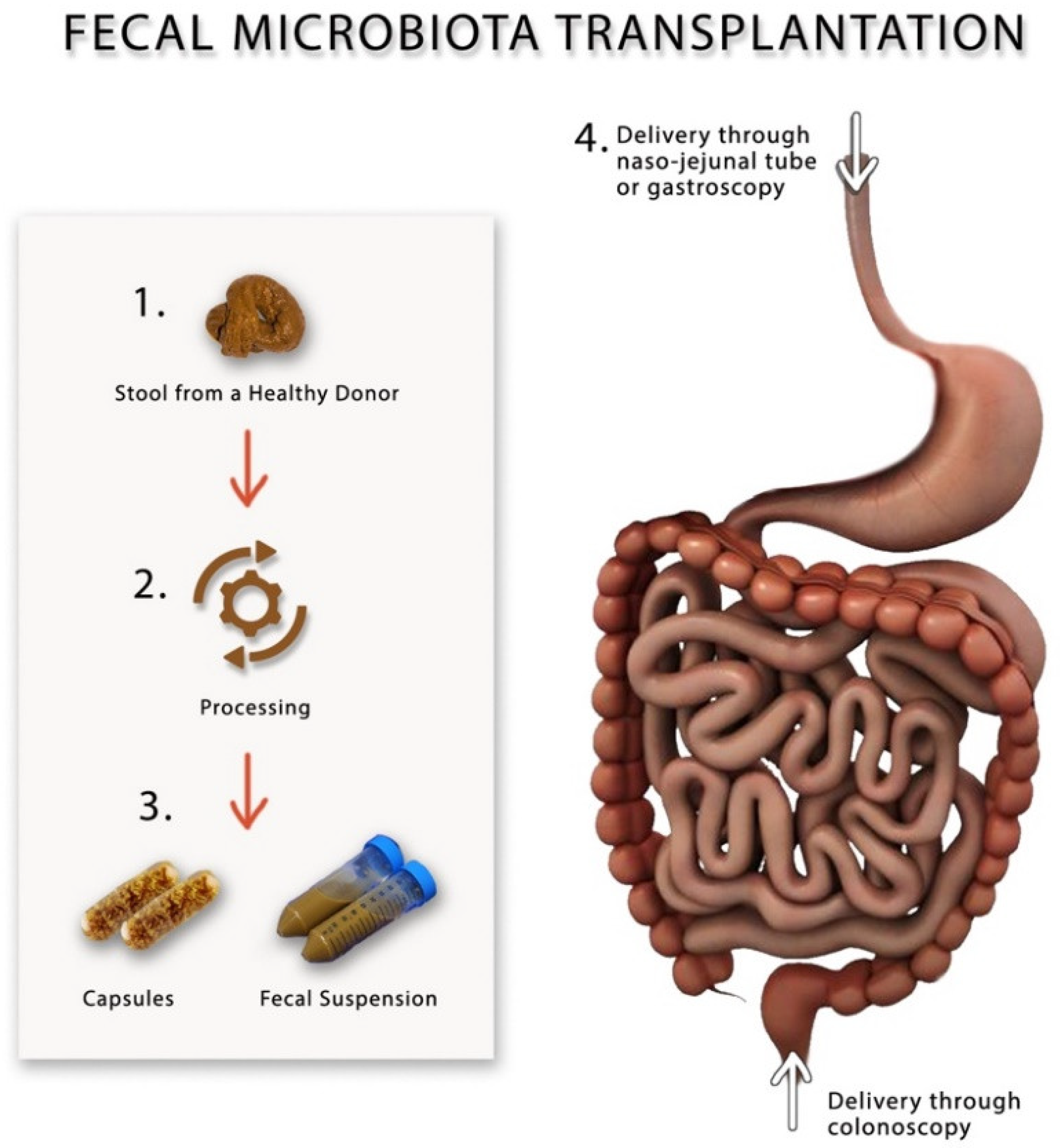
| FODMAPs | Water Insoluble Fibers | Water Soluble Fibers |
|---|---|---|
| Fructose: fruits, honey, corn syrup, agave | Bran | Psyllium |
| Lactose: milk and dairy | Flax seed | Methylcellulose |
| Fructans: wheat, onions, garlic | Rye | Calcium polycarbophil |
| Galactans: legumes (lentils, beans, soybeans) | Non-digestible seeds and vegetables | Inulin |
| Polyols (sugar alcohols): Xylitol, sorbitol, maltitol, mannitol | Wheat dextrin |
| Authors, Years | Study Design and Duration | Diagnostic Criteria and Materials | Gut Microbiota | Microbiota Metabolites | ||
|---|---|---|---|---|---|---|
| Microbial Analysis | Findings | Methods | Findings | |||
| Halmos EP et al., 2015 [44] | RCT, crossover (single blind), 3 weeks | Rome III IBS and healthy controls. LFD vs. ordinary diet. IBS n = 27, Healthy controls n = 6 | qPCR | Lower absolute abundance of Bifidobacteria, F. prausnitzii, Clostridium Cluster IV and lower relative abundance Akkermansia muciniphila in LFD than ordinary diet. Lower total bacteria in LFD at baseline. Greater diversity Clostridium Cluster XIV in LFD than ordinary diet at baseline | Gas liquid chromatography | No difference in total or individual stool SCFAs in LFD compared to ordinary diet, baseline. |
| McIntosh K, et al., 2017 [53] | RCT (single blind), 3 weeks | Rome III IBS. LFD n = 19, HFD n = 18 | 16S rRNA sequencing (Illumina) | Higher richness of Actinobacteria, Firmicutes, Clostridiales in LFD than HFD. No difference in α- or β-diversity after LFD vs. baseline. Higher richness in LFD than HFD. Higher abundance of Clostridiales family XIII Incertae sedis spp. and Porphyromonas spp. in LFD than baseline. Lower abundance of Propionibacteriaceae, Bifidobacteria in LFD than baseline. | Mass spectroscopy | Urinary metabolomic profile at baseline in LFD vs. HFD showed no difference but separated after intervention. Three metabolites (histamine, p-hydroxybenzoic acid and azelaic acid) discriminated groups. Correlations between metabolite concentrations and abundance of various taxa. |
| Staudacher HM et al., 2012 [54] | RCT (unblind), 4 weeks | Rome III IBS. LFD n = 19, Habitual diet n = 22 | Fluorescence in situ hybridization | Lower abundance of Bifidobacteria in LFD than habitual diet. No difference in total abundance of other groups (F. prausnitzii) | Gas liquid chromatography | No difference in total or individual stool SCFAs in LFD compared to habitual diet |
| Staudacher HM et al., 2017 [52] | RCT (single blind), 4 weeks | Rome III IBS. LFD n = 51, Sham n = 53 | qPCR | Lower abundance of Bifidobacteria in LFD compared to sham | Gas liquid chromatography | Lower stool acetate concentration in LFD compared to control |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazzawi, T. Gut Microbiota Manipulation in Irritable Bowel Syndrome. Microorganisms 2022, 10, 1332. https://doi.org/10.3390/microorganisms10071332
Mazzawi T. Gut Microbiota Manipulation in Irritable Bowel Syndrome. Microorganisms. 2022; 10(7):1332. https://doi.org/10.3390/microorganisms10071332
Chicago/Turabian StyleMazzawi, Tarek. 2022. "Gut Microbiota Manipulation in Irritable Bowel Syndrome" Microorganisms 10, no. 7: 1332. https://doi.org/10.3390/microorganisms10071332
APA StyleMazzawi, T. (2022). Gut Microbiota Manipulation in Irritable Bowel Syndrome. Microorganisms, 10(7), 1332. https://doi.org/10.3390/microorganisms10071332






