Effect of Phthalates and Their Substitutes on the Physiology of Pseudomonas aeruginosa
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. P. aeruginosa Strains, Growth Medium, and Monitoring of P. aeruginosa Growth
2.3. Determination of Minimum Concentration of Phthalates Inhibiting the Growth of P. aeruginosa Clinical Isolates
2.4. Antibiotic Susceptibility Assays
2.5. Virulence Factor Assays
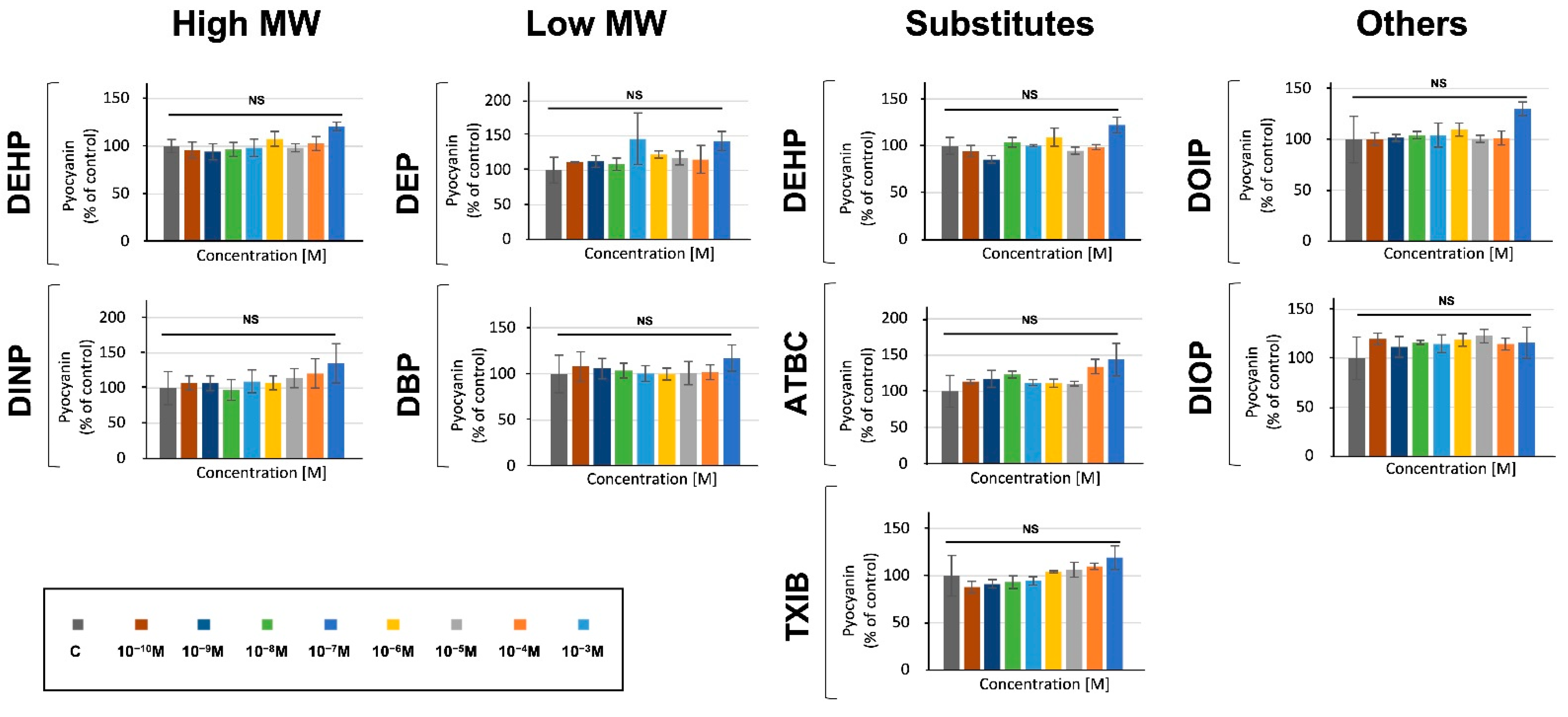
2.5.1. Pyocyanin
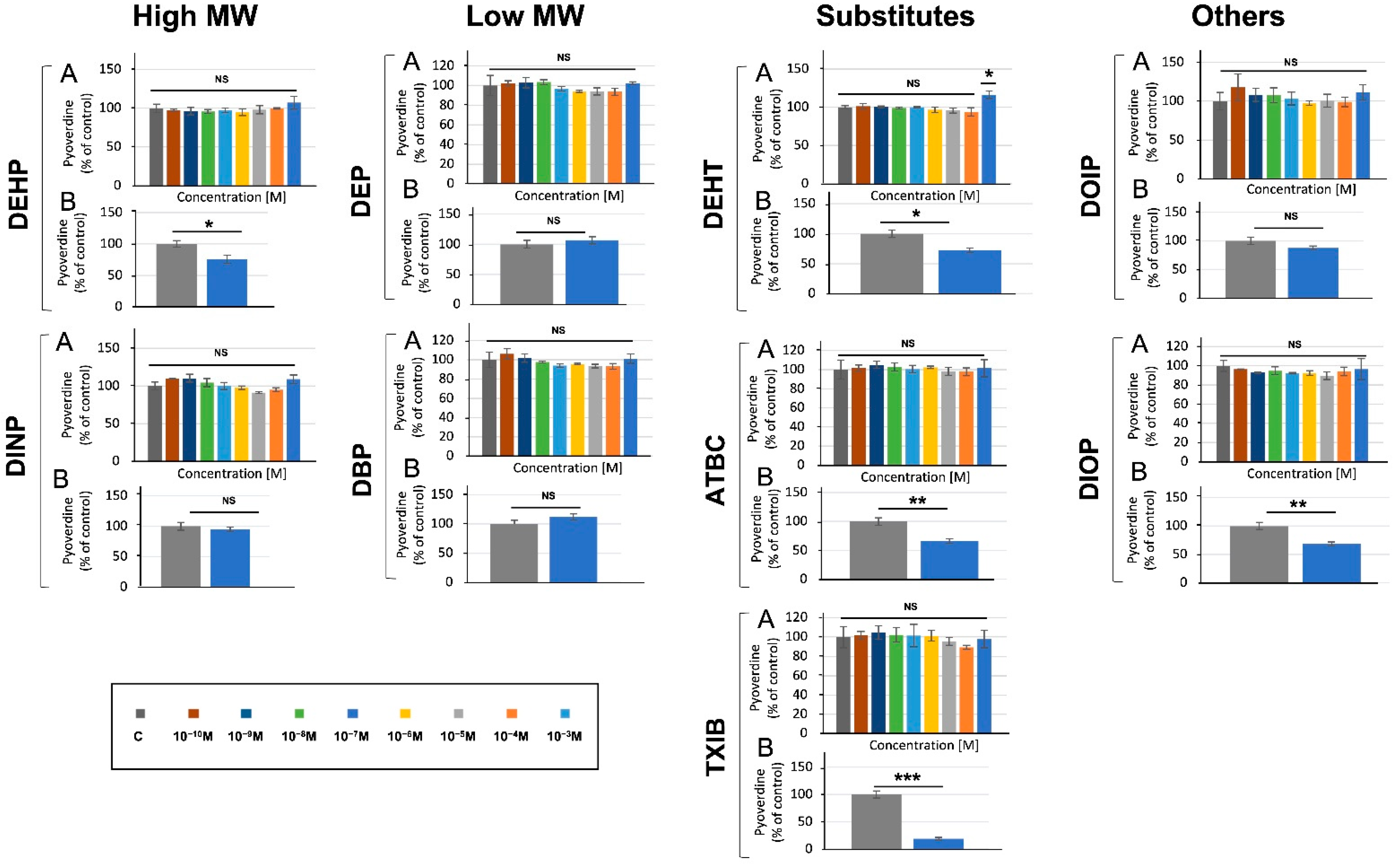
2.5.2. Pyoverdine
2.6. Biofilm Assays
2.6.1. Polystyrene Microtiter Plate Assays
2.6.2. Pellicle Formation
2.6.3. Glass-Bottom Plate Assays and Biofilm Observation with Confocal Laser Scanning Microscopy (CLSM)
2.7. Scanning Electron Microscope Analyses
2.8. Membrane Fluidity Measurement by Fluorescence Anisotropy
2.9. Statistical Analysis
3. Results
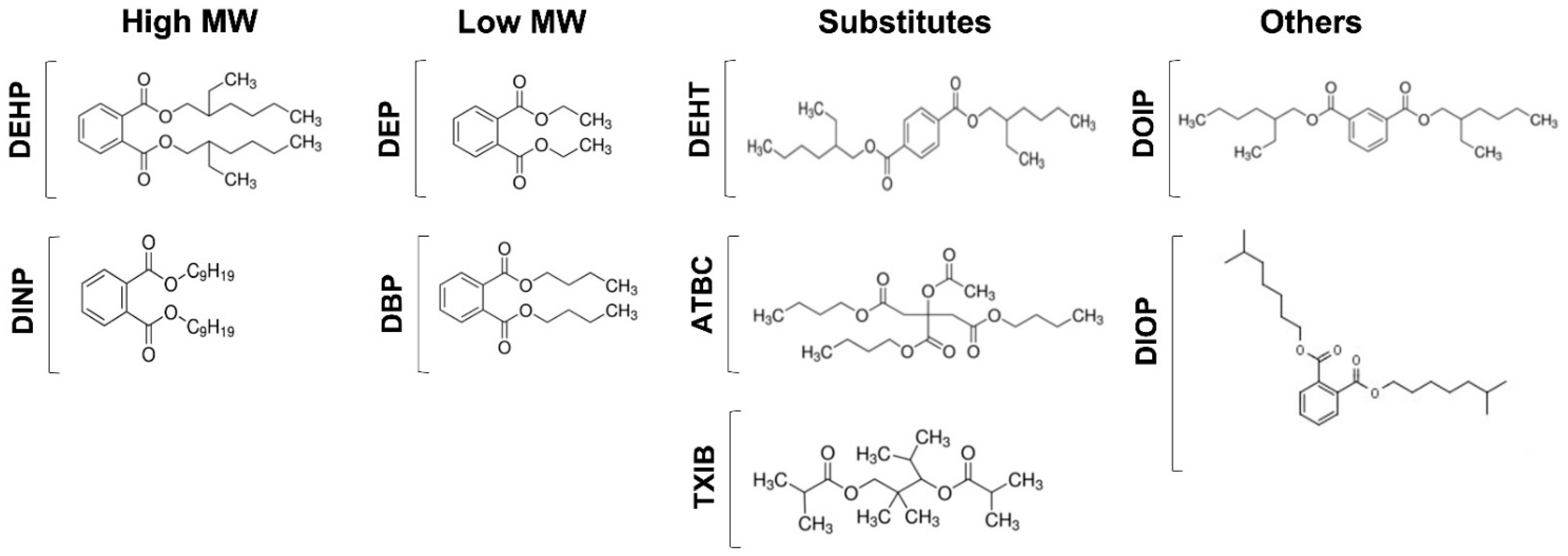
3.1. Phthalates and Substitutes
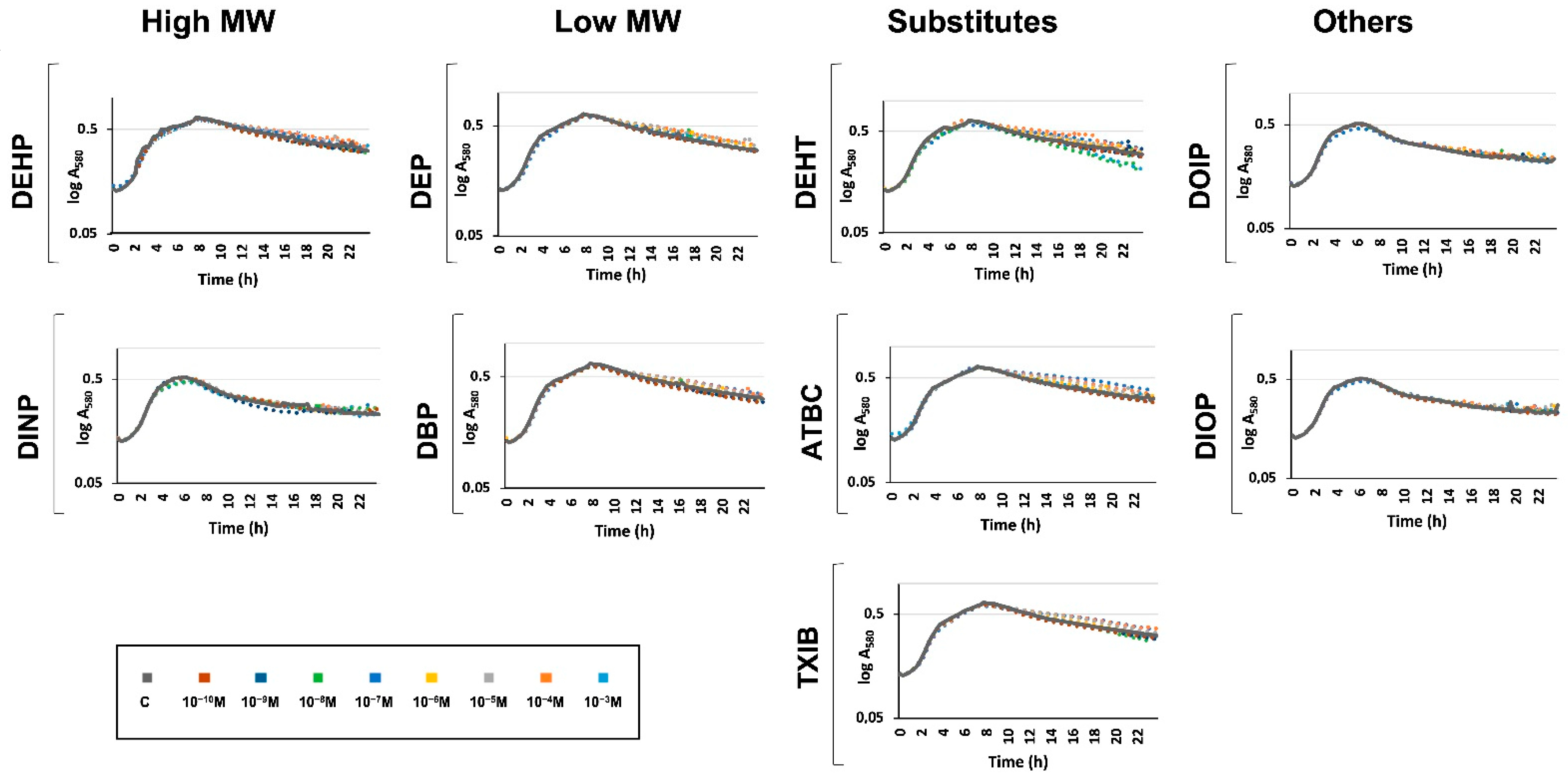
3.2. Exposure to Phthalates and Substitutes Did Not Affect P. aeruginosa Growth Kinetic
3.3. Exposure to Phthalates and Substitutes Did Not Affect Minimal Inhibitory Concentrations of Antibiotics towards P. aeruginosa
3.4. Phthalates and Substitutes Did Not Deeply Alter the Production by P. aeruginosa of Two Major Virulence Factors
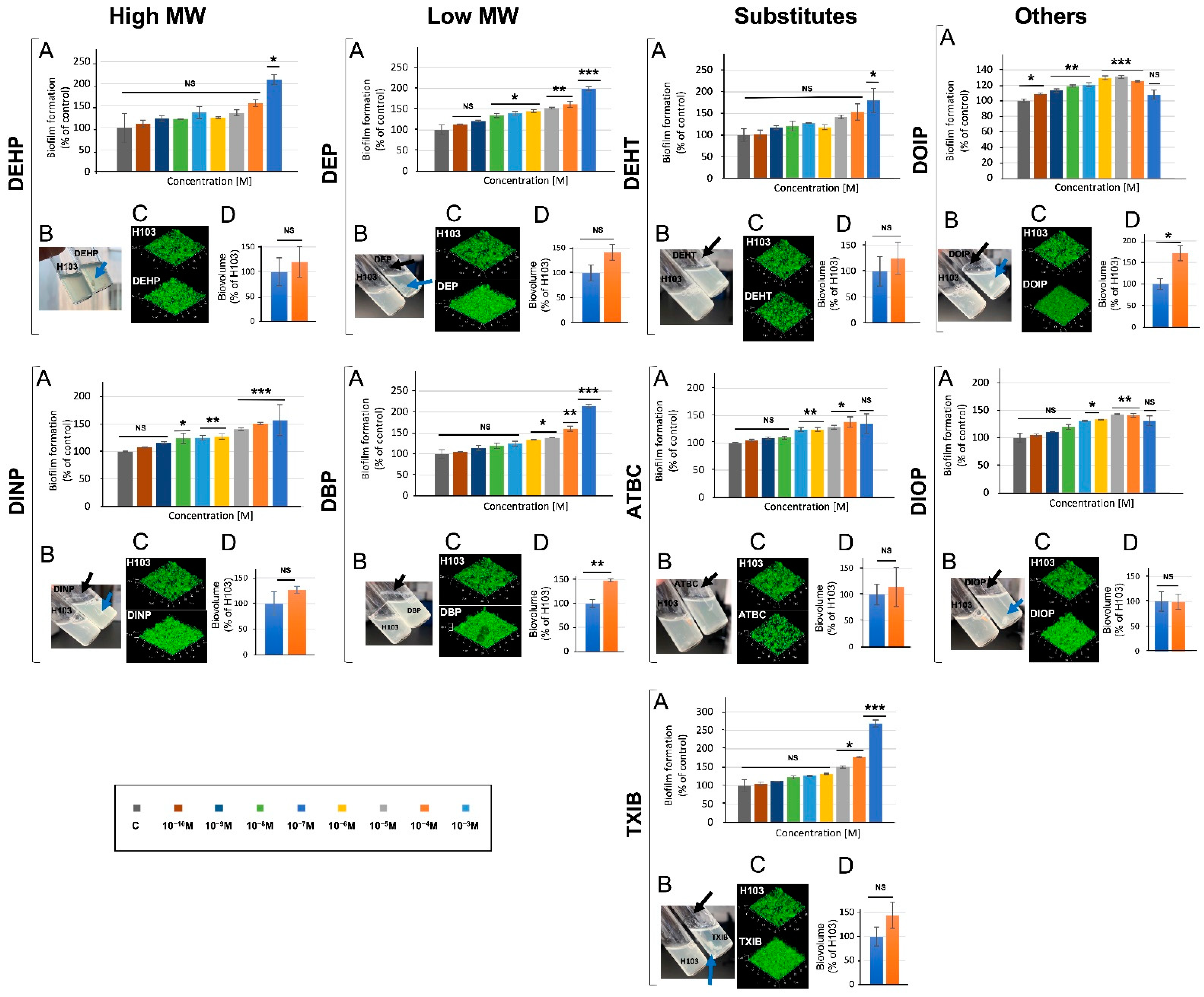
3.5. Phthalates and Substitutes Affect P. aeruginosa Biofilm Formation
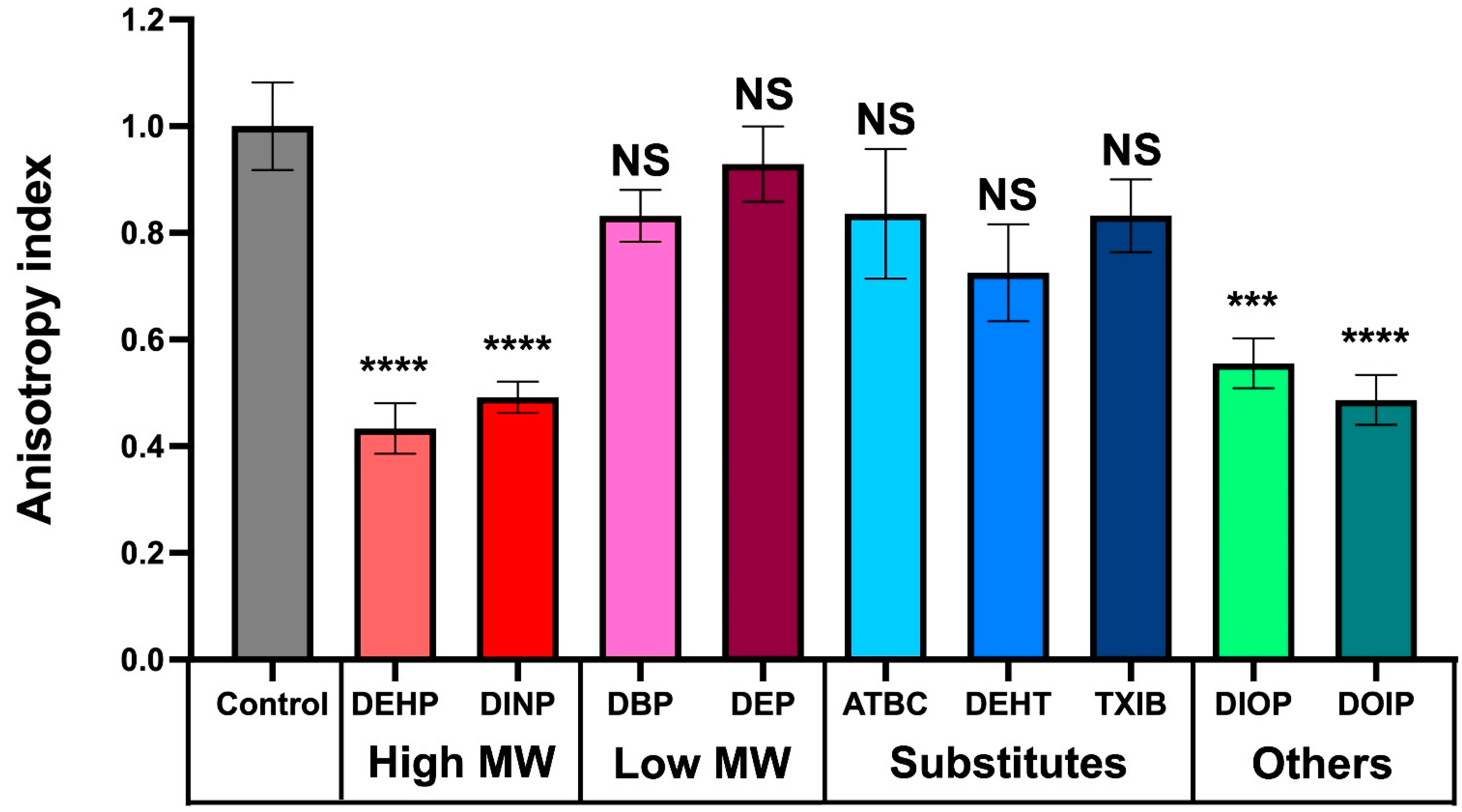
3.6. Phthalate and Substitute Exposure Affect P. aeruginosa Membrane Fluidity
3.7. Effect of Phthalates and Substitutes on P. aeruginosa Morphology
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lyczak, J.B.; Cannon, C.L.; Pier, G.B. Lung infections associated with cystic fibrosis. Clin. Microbiol. Rev. 2002, 15, 194–222. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Publishes List of Bacteria for Which New Antibiotics Are Urgently Needed; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- Crone, S.; Vives-Florez, M.; Kvich, L.; Saunders, A.M.; Malone, M.; Nicolaisen, M.H.; Martinez-Garcia, E.; Rojas-Acosta, C.; Catalina Gomez-Puerto, M.; Calum, H.; et al. The environmental occurrence of Pseudomonas aeruginosa. APMIS 2020, 128, 220–231. [Google Scholar] [CrossRef] [PubMed]
- Spiers, A.J.; Buckling, A.; Rainey, P.B. The causes of Pseudomonas diversity. Microbiology 2000, 146 Pt 10, 2345–2350. [Google Scholar] [CrossRef]
- Valentini, M.; Gonzalez, D.; Mavridou, D.A.; Filloux, A. Lifestyle transitions and adaptive pathogenesis of Pseudomonas aeruginosa. Curr. Opin. Microbiol. 2018, 41, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Cornelis, P.; Dingemans, J. Pseudomonas aeruginosa adapts its iron uptake strategies in function of the type of infections. Front. Cell. Infect. Microbiol. 2013, 3, 75. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.; Camara, M. Quorum sensing and environmental adaptation in Pseudomonas aeruginosa: A tale of regulatory networks and multifunctional signal molecules. Curr. Opin. Microbiol. 2009, 12, 182–191. [Google Scholar] [CrossRef]
- Donlan, R.M. Biofilms: Microbial life on surfaces. Emerg. Infect. Dis. 2002, 8, 881–890. [Google Scholar] [CrossRef]
- Sauer, K.; Stoodley, P.; Goeres, D.M.; Hall-Stoodley, L.; Burmolle, M.; Stewart, P.S.; Bjarnsholt, T. The biofilm life cycle: Expanding the conceptual model of biofilm formation. Nat. Rev. Microbiol. 2022. [Google Scholar] [CrossRef]
- Lichtenberg, M.; Jakobsen, T.H.; Kuhl, M.; Kolpen, M.; Jensen, P.O.; Bjarnsholt, T. The Structure-Function Relationship of Pseudomonas aeruginosa in Infections and its Influence on the Microenvironment. FEMS Microbiol. Rev. 2022. [Google Scholar] [CrossRef]
- Mann, E.E.; Wozniak, D.J. Pseudomonas biofilm matrix composition and niche biology. FEMS Microbiol. Rev. 2012, 36, 893–916. [Google Scholar] [CrossRef]
- Halden, R.U. Plastics and health risks. Annu. Rev. Public Health 2010, 31, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.W.; Wen, Z.D. Phthalate esters in the environment: A critical review of their occurrence, biodegradation, and removal during wastewater treatment processes. Sci. Total Environ. 2016, 541, 986–1001. [Google Scholar] [CrossRef] [PubMed]
- Lyche, J.L.; Nourizadeh-Lillabadi, R.; Karlsson, C.; Stavik, B.; Berg, V.; Skare, J.U.; Alestrom, P.; Ropstad, E. Natural mixtures of POPs affected body weight gain and induced transcription of genes involved in weight regulation and insulin signaling. Aquat. Toxicol. 2011, 102, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Szewczynska, M.; Dobrzynska, E.; Posniak, M. Determination of phthalates in particulate matter and gaseous phase emitted in indoor air of offices. Environ. Sci. Pollut. Res. Int. 2021, 28, 59319–59327. [Google Scholar] [CrossRef]
- Net, S.; Delmont, A.; Sempere, R.; Paluselli, A.; Ouddane, B. Reliable quantification of phthalates in environmental matrices (air, water, sludge, sediment and soil): A review. Sci. Total Environ. 2015, 515–516, 162–180. [Google Scholar] [CrossRef]
- Net, S.; Rabodonirina, S.; Sghaier, R.B.; Dumoulin, D.; Chbib, C.; Tlili, I.; Ouddane, B. Distribution of phthalates, pesticides and drug residues in the dissolved, particulate and sedimentary phases from transboundary rivers (France-Belgium). Sci. Total Environ. 2015, 521–522, 152–159. [Google Scholar] [CrossRef]
- Schettler, T. Human exposure to phthalates via consumer products. Int. J. Androl. 2006, 29, 134–139; discussion 181–185. [Google Scholar] [CrossRef]
- Martinez-Arguelles, D.B.; Papadopoulos, V. Prenatal phthalate exposure: Epigenetic changes leading to lifelong impact on steroid formation. Andrology 2016, 4, 573–584. [Google Scholar] [CrossRef]
- Wojtowicz, A.K.; Szychowski, K.A.; Wnuk, A.; Kajta, M. Dibutyl Phthalate (DBP)-Induced Apoptosis and Neurotoxicity are Mediated via the Aryl Hydrocarbon Receptor (AhR) but not by Estrogen Receptor Alpha (ERalpha), Estrogen Receptor Beta (ERbeta), or Peroxisome Proliferator-Activated Receptor Gamma (PPARgamma) in Mouse Cortical Neurons. Neurotox. Res. 2017, 31, 77–89. [Google Scholar]
- Gascon, M.; Valvi, D.; Forns, J.; Casas, M.; Martinez, D.; Julvez, J.; Monfort, N.; Ventura, R.; Sunyer, J.; Vrijheid, M. Prenatal exposure to phthalates and neuropsychological development during childhood. Int. J. Hyg. Environ. Health 2015, 218, 550–558. [Google Scholar] [CrossRef]
- Martins, K.; Hagedorn, B.; Ali, S.; Kennish, J.; Applegate, B.; Leu, M.; Epp, L.; Pallister, C.; Zwollo, P. Tissue Phthalate Levels Correlate with Changes in Immune Gene Expression in a Population of Juvenile Wild Salmon. Arch. Environ. Contam. Toxicol. 2016, 71, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, A.; Ohtsu, S.; Muroi, M.; Tanamoto, K. Effects of possible endocrine disrupting chemicals on bacterial component-induced activation of NF-kappaB. Biol. Pharm. Bull. 2006, 29, 2120–2122. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tseng, H.H.; Li, C.Y.; Wu, S.T.; Su, H.H.; Wong, T.H.; Wu, H.E.; Chang, Y.W.; Huang, S.K.; Tsai, E.M.; Suen, J.L. Di-(2-ethylhexyl) Phthalate Promotes Allergic Lung Inflammation by Modulating CD8alpha(+) Dendritic Cell Differentiation via Metabolite MEHP-PPARgamma Axis. Front. Immunol. 2022, 13, 581854. [Google Scholar] [CrossRef]
- Yoshitake, J.; Kato, K.; Yoshioka, D.; Sueishi, Y.; Sawa, T.; Akaike, T.; Yoshimura, T. Suppression of NO production and 8-nitroguanosine formation by phenol-containing endocrine-disrupting chemicals in LPS-stimulated macrophages: Involvement of estrogen receptor-dependent or -independent pathways. Nitric Oxide 2008, 18, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Ruh, M.F.; Bi, Y.; Cox, L.; Berk, D.; Howlett, A.C.; Bellone, C.J. Effect of environmental estrogens on IL-1beta promoter activity in a macrophage cell line. Endocrine 1998, 9, 207–211. [Google Scholar] [CrossRef]
- Inadera, H. The immune system as a target for environmental chemicals: Xenoestrogens and other compounds. Toxicol. Lett. 2006, 164, 191–206. [Google Scholar] [CrossRef]
- Chalubinski, M.; Kowalski, M.L. Endocrine disrupters—Potential modulators of the immune system and allergic response. Allergy 2006, 61, 1326–1335. [Google Scholar] [CrossRef]
- Teixeira, D.; Marques, C.; Pestana, D.; Faria, A.; Norberto, S.; Calhau, C.; Monteiro, R. Effects of xenoestrogens in human M1 and M2 macrophage migration, cytokine release, and estrogen-related signaling pathways. Environ. Toxicol. 2016, 31, 1496–1509. [Google Scholar] [CrossRef]
- Shafikova, T.N.; Omelichkina, Y.V.; Enikeev, A.G.; Boyarkina, S.V.; Gvildis, D.E.; Semenov, A.A. Ortho-Phthalic Acid Esters Suppress the Phytopathogen Capability for Biofilm Formation. In Doklady Biological Sciences; Pleiades Publishing: New York, NY, USA, 2018; Volume 480, pp. 107–109. [Google Scholar]
- Lin, C.H.; Wu, C.Y.; Kou, H.S.; Chen, C.Y.; Huang, M.C.; Hu, H.M.; Wu, M.C.; Lu, C.Y.; Wu, D.C.; Wu, M.T.; et al. Effect of Di(2-ethylhexyl)phthalate on Helicobacter pylori-Induced Apoptosis in AGS Cells. Gastroenterol. Res. Pract. 2013, 2013, 924769. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, C.; You, Y.; Xu, W.; Lv, Z.; Liu, Z.; Chen, W.; Shi, Y.; Wang, J. Response of Pseudomonas fluorescens to dimethyl phthalate. Ecotoxicol. Environ. Saf. 2019, 167, 36–43. [Google Scholar] [CrossRef]
- Lyte, M. Microbial Endocrinology in the Pathogenesis of Infectious Disease. Microbiol. Spectr. 2016, 4, 137–168. [Google Scholar] [CrossRef] [PubMed]
- Lesouhaitier, O.; Veron, W.; Chapalain, A.; Madi, A.; Blier, A.S.; Dagorn, A.; Connil, N.; Chevalier, S.; Orange, N.; Feuilloley, M. Gram-negative bacterial sensors for eukaryotic signal molecules. Sensors 2009, 9, 6967–6990. [Google Scholar] [CrossRef] [PubMed]
- Lesouhaitier, O.; Clamens, T.; Rosay, T.; Desriac, F.; Louis, M.; Rodrigues, S.; Gannesen, A.; Plakunov, V.K.; Bouffartigues, E.; Tahrioui, A.; et al. Host Peptidic Hormones Affecting Bacterial Biofilm Formation and Virulence. J. Innate Immun. 2019, 11, 227–241. [Google Scholar] [CrossRef]
- Racine, P.J.; Janvier, X.; Clabaut, M.; Catovic, C.; Souak, D.; Boukerb, A.M.; Groboillot, A.; Konto-Ghiorghi, Y.; Duclairoir-Poc, C.; Lesouhaitier, O.; et al. Dialog between skin and its microbiota: Emergence of “Cutaneous Bacterial Endocrinology”. Exp. Dermatol. 2020, 29, 790–800. [Google Scholar] [CrossRef]
- Rosay, T.; Bazire, A.; Diaz, S.; Clamens, T.; Blier, A.S.; Mijouin, L.; Hoffmann, B.; Sergent, J.A.; Bouffartigues, E.; Boireau, W.; et al. Pseudomonas aeruginosa Expresses a Functional Human Natriuretic Peptide Receptor Ortholog: Involvement in Biofilm Formation. mBio 2015, 6, e01033-15. [Google Scholar] [CrossRef]
- Zaborina, O.; Lepine, F.; Xiao, G.; Valuckaite, V.; Chen, Y.; Li, T.; Ciancio, M.; Zaborin, A.; Petrof, E.O.; Turner, J.R.; et al. Dynorphin activates quorum sensing quinolone signaling in Pseudomonas aeruginosa. PLoS Pathog. 2007, 3, e35. [Google Scholar]
- Cambronel, M.; Tortuel, D.; Biaggini, K.; Maillot, O.; Taupin, L.; Rehel, K.; Rince, I.; Muller, C.; Hardouin, J.; Feuilloley, M.; et al. Epinephrine affects motility, and increases adhesion, biofilm and virulence of Pseudomonas aeruginosa H103. Sci. Rep. 2019, 9, 20203. [Google Scholar] [CrossRef] [PubMed]
- Clabaut, M.; Suet, A.; Racine, P.J.; Tahrioui, A.; Verdon, J.; Barreau, M.; Maillot, O.; Le Tirant, A.; Karsybayeva, M.; Kremser, C.; et al. Effect of 17beta-estradiol on a human vaginal Lactobacillus crispatus strain. Sci. Rep. 2021, 11, 7133. [Google Scholar] [CrossRef] [PubMed]
- Rowland, S.S.; Falkler, W.A., Jr.; Bashirelahi, N. Identification of an estrogen-binding protein in Pseudomonas aeruginosa. J. Steroid Biochem. Mol. Biol. 1992, 42, 721–727. [Google Scholar] [CrossRef]
- Chotirmall, S.H.; Smith, S.G.; Gunaratnam, C.; Cosgrove, S.; Dimitrov, B.D.; O’Neill, S.J.; Harvey, B.J.; Greene, C.M.; McElvaney, N.G. Effect of estrogen on Pseudomonas mucoidy and exacerbations in cystic fibrosis. N. Engl. J. Med. 2012, 366, 1978–1986. [Google Scholar] [CrossRef]
- Ramsey, D.M.; Wozniak, D.J. Understanding the control of Pseudomonas aeruginosa alginate synthesis and the prospects for management of chronic infections in cystic fibrosis. Mol. Microbiol. 2005, 56, 309–322. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, S.; Bouffartigues, E.; Bazire, A.; Tahrioui, A.; Duchesne, R.; Tortuel, D.; Maillot, O.; Clamens, T.; Orange, N.; Feuilloley, M.G.J.; et al. Extracytoplasmic function sigma factors in Pseudomonas aeruginosa. Biochim. Biophys. Acta-Gene Regul. Mech. 2019, 1862, 706–721. [Google Scholar] [CrossRef] [PubMed]
- Hancock, R.E.; Carey, A.M. Outer membrane of Pseudomonas aeruginosa: Heat- 2-mercaptoethanol-modifiable proteins. J. Bacteriol. 1979, 140, 902–910. [Google Scholar] [CrossRef] [PubMed]
- Gicquel, G.; Bouffartigues, E.; Bains, M.; Oxaran, V.; Rosay, T.; Lesouhaitier, O.; Connil, N.; Bazire, A.; Maillot, O.; Benard, M.; et al. The extra-cytoplasmic function sigma factor sigX modulates biofilm and virulence-related properties in Pseudomonas aeruginosa. PLoS ONE 2013, 8, e80407. [Google Scholar]
- Heydorn, A.; Nielsen, A.T.; Hentzer, M.; Sternberg, C.; Givskov, M.; Ersboll, B.K.; Molin, S. Quantification of biofilm structures by the novel computer program COMSTAT. Microbiology 2000, 146 Pt 10, 2395–2407. [Google Scholar] [CrossRef]
- Verdon, J.; Coutos-Thevenot, P.; Rodier, M.H.; Landon, C.; Depayras, S.; Noel, C.; La Camera, S.; Moumen, B.; Greve, P.; Bouchon, D.; et al. Armadillidin H, a Glycine-Rich Peptide from the Terrestrial Crustacean Armadillidium vulgare, Displays an Unexpected Wide Antimicrobial Spectrum with Membranolytic Activity. Front. Microbiol. 2016, 7, 1484. [Google Scholar] [CrossRef]
- Flechard, M.; Duchesne, R.; Tahrioui, A.; Bouffartigues, E.; Depayras, S.; Hardouin, J.; Lagy, C.; Maillot, O.; Tortuel, D.; Azuama, C.O.; et al. The absence of SigX results in impaired carbon metabolism and membrane fluidity in Pseudomonas aeruginosa. Sci. Rep. 2018, 8, 17212. [Google Scholar] [CrossRef]
- Frery, N.; Santonen, T.; Porras, S.P.; Fucic, A.; Leso, V.; Bousoumah, R.; Duca, R.C.; El Yamani, M.; Kolossa-Gehring, M.; Ndaw, S.; et al. Biomonitoring of occupational exposure to phthalates: A systematic review. Int. J. Hyg. Environ. Health 2020, 229, 113548. [Google Scholar] [CrossRef]
- Koch, H.M.; Kolossa-Gehring, M.; Schroter-Kermani, C.; Angerer, J.; Bruning, T. Bisphenol A in 24 h urine and plasma samples of the German Environmental Specimen Bank from 1995 to 2009: A retrospective exposure evaluation. J. Expo. Sci. Environ. Epidemiol. 2012, 22, 610–616. [Google Scholar] [CrossRef]
- Sonnleitner, E.; Abdou, L.; Haas, D. Small RNA as global regulator of carbon catabolite repression in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 2009, 106, 21866–21871. [Google Scholar] [CrossRef]
- CLSI. 2018. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 11th ed.; Clinical and Laboratory Standards Institute: Annapolis Junction, MD, USA, 2018. [Google Scholar]
- King, E.O.; Ward, M.K.; Raney, D.E. Two simple media for the demonstration of pyocyanin and fluorescin. J. Lab. Clin. Med. 1954, 44, 301–307. [Google Scholar] [PubMed]
- Tortuel, D.; Tahrioui, A.; Rodrigues, S.; Cambronel, M.; Boukerb, A.M.; Maillot, O.; Verdon, J.; Bere, E.; Nusser, M.; Brenner-Weiss, G.; et al. Activation of the Cell Wall Stress Response in Pseudomonas aeruginosa Infected by a Pf4 Phage Variant. Microorganisms 2020, 8, 1700. [Google Scholar] [CrossRef] [PubMed]
- Bouffartigues, E.; Si Hadj Mohand, I.; Maillot, O.; Tortuel, D.; Omnes, J.; David, A.; Tahrioui, A.; Duchesne, R.; Azuama, C.O.; Nusser, M.; et al. The Temperature-Regulation of Pseudomonas aeruginosa cmaX-cfrX-cmpX Operon Reveals an Intriguing Molecular Network Involving the Sigma Factors AlgU and SigX. Front. Microbiol. 2020, 11, 579495. [Google Scholar] [CrossRef] [PubMed]
- Boll, M.; Geiger, R.; Junghare, M.; Schink, B. Microbial degradation of phthalates: Biochemistry and environmental implications. Environ. Microbiol. Rep. 2020, 12, 3–15. [Google Scholar] [CrossRef]
- Kumar, V.; Sharma, N.; Maitra, S.S. Comparative study on the degradation of dibutyl phthalate by two newly isolated Pseudomonas sp. V21b and Comamonas sp. 51F. Biotechnol. Rep. 2017, 15, 1–10. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, X.; Li, Y.; Liu, Y.; Tao, L. Exposure to Dibutyl Phthalate and Reproductive-Related Outcomes in Animal Models: Evidence from Rodents Study. Front. Physiol. 2021, 12, 684532. [Google Scholar] [CrossRef]
- Fang, T.; Cui, Q.; Huang, Y.; Dong, P.; Wang, H.; Liu, W.T.; Ye, Q. Distribution comparison and risk assessment of free-floating and particle-attached bacterial pathogens in urban recreational water: Implications for water quality management. Sci. Total Environ. 2018, 613–614, 428–438. [Google Scholar] [CrossRef]
- Yu, H.; Wang, L.; Lin, Y.; Liu, W.; Tuyiringire, D.; Jiao, Y.; Zhang, L.; Meng, Q.; Zhang, Y. Complete metabolic study by dibutyl phthalate degrading Pseudomonas sp. DNB-S1. Ecotoxicol. Environ. Saf. 2020, 194, 110378. [Google Scholar] [CrossRef]
- Mavrodi, D.V.; Bonsall, R.F.; Delaney, S.M.; Soule, M.J.; Phillips, G.; Thomashow, L.S. Functional analysis of genes for biosynthesis of pyocyanin and phenazine-1-carboxamide from Pseudomonas aeruginosa PAO1. J. Bacteriol. 2001, 183, 6454–6465. [Google Scholar] [CrossRef]
- Goncalves, T.; Vasconcelos, U. Colour Me Blue: The History and the Biotechnological Potential of Pyocyanin. Molecules 2021, 26, 927. [Google Scholar] [CrossRef]
- Manago, A.; Becker, K.A.; Carpinteiro, A.; Wilker, B.; Soddemann, M.; Seitz, A.P.; Edwards, M.J.; Grassme, H.; Szabo, I.; Gulbins, E. Pseudomonas aeruginosa pyocyanin induces neutrophil death via mitochondrial reactive oxygen species and mitochondrial acid sphingomyelinase. Antioxid. Redox Signal. 2015, 22, 1097–1110. [Google Scholar] [CrossRef] [PubMed]
- Allen, L.; Dockrell, D.H.; Pattery, T.; Lee, D.G.; Cornelis, P.; Hellewell, P.G.; Whyte, M.K. Pyocyanin production by Pseudomonas aeruginosa induces neutrophil apoptosis and impairs neutrophil-mediated host defenses in vivo. J. Immunol. 2005, 174, 3643–3649. [Google Scholar] [CrossRef] [PubMed]
- Cornelis, P. Iron uptake and metabolism in pseudomonads. Appl. Microbiol. Biotechnol. 2010, 86, 1637–1645. [Google Scholar] [CrossRef] [PubMed]
- Ochsner, U.A.; Johnson, Z.; Lamont, I.L.; Cunliffe, H.E.; Vasil, M.L. Exotoxin A production in Pseudomonas aeruginosa requires the iron-regulated pvdS gene encoding an alternative sigma factor. Mol. Microbiol. 1996, 21, 1019–1028. [Google Scholar] [CrossRef]
- Wilderman, P.J.; Vasil, A.I.; Johnson, Z.; Wilson, M.J.; Cunliffe, H.E.; Lamont, I.L.; Vasil, M.L. Characterization of an endoprotease (PrpL) encoded by a PvdS-regulated gene in Pseudomonas aeruginosa. Infect. Immun. 2001, 69, 5385–5394. [Google Scholar] [CrossRef]
- Rashiya, N.; Padmini, N.; Ajilda, A.A.K.; Prabakaran, P.; Durgadevi, R.; Veera Ravi, A.; Ghosh, S.; Sivakumar, N.; Selvakumar, G. Inhibition of biofilm formation and quorum sensing mediated virulence in Pseudomonas aeruginosa by marine sponge symbiont Brevibacterium casei strain Alu 1. Microb. Pathog. 2021, 150, 104693. [Google Scholar] [CrossRef]
- Schooling, S.R.; Beveridge, T.J. Membrane vesicles: An overlooked component of the matrices of biofilms. J. Bacteriol. 2006, 188, 5945–5957. [Google Scholar] [CrossRef]
- Chevalier, S.; Bouffartigues, E.; Bodilis, J.; Maillot, O.; Lesouhaitier, O.; Feuilloley, M.G.J.; Orange, N.; Dufour, A.; Cornelis, P. Structure, function and regulation of Pseudomonas aeruginosa porins. FEMS Microbiol. Rev. 2017, 41, 698–722. [Google Scholar] [CrossRef]
- Flemming, H.C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef]
- Wang, H.; Yu, P.; Schwarz, C.; Zhang, B.; Huo, L.; Shi, B.; Alvarez, P.J.J. Phthalate Esters Released from Plastics Promote Biofilm Formation and Chlorine Resistance. Environ. Sci. Technol. 2022, 56, 1081–1090. [Google Scholar] [CrossRef]
- Suraju, M.O.; Lalinde-Barnes, S.; Sanamvenkata, S.; Esmaeili, M.; Shishodia, S.; Rosenzweig, J.A. The effects of indoor and outdoor dust exposure on the growth, sensitivity to oxidative-stress, and biofilm production of three opportunistic bacterial pathogens. Sci. Total Environ. 2015, 538, 949–958. [Google Scholar] [CrossRef]
- Hemery, G.; Chevalier, S.; Bellon-Fontaine, M.N.; Haras, D.; Orange, N. Growth temperature and OprF porin affect cell surface physicochemical properties and adhesive capacities of Pseudomonas fluorescens MF37. J. Ind. Microbiol. Biotechnol. 2007, 34, 49–54. [Google Scholar] [CrossRef]
- Wang, Z.; Zhu, X.; Su, Y.; Xu, W.; Liu, H.; Liu, Z.; Chen, W.; Wang, J. Dimethyl phthalate damaged the cell membrane of Escherichia coli K12. Ecotoxicol. Environ. Saf. 2019, 180, 208–214. [Google Scholar] [CrossRef]
- Fan, Y.H.; Shen, Y.L.; Lin, Z.W.; Zhou, Y.; Ye, B.C. Key role of exopolysaccharide on di-butyl phthalate adsorbing by Lactobacillus plantarum CGMCC18980. Appl. Microbiol. Biotechnol. 2021, 105, 2587–2595. [Google Scholar]
- Dubois-Brissonnet, F.; Trotier, E.; Briandet, R. The Biofilm Lifestyle Involves an Increase in Bacterial Membrane Saturated Fatty Acids. Front. Microbiol. 2016, 7, 1673. [Google Scholar] [CrossRef]
- Vidaillac, C.; Yong, V.F.L.; Aschtgen, M.S.; Qu, J.; Yang, S.; Xu, G.; Seng, Z.J.; Brown, A.C.; Ali, M.K.; Jaggi, T.K.; et al. Sex Steroids Induce Membrane Stress Responses and Virulence Properties in Pseudomonas aeruginosa. mBio 2020, 11, e02809-20. [Google Scholar] [CrossRef]
- Cepas, V.; Soto, S.M. Relationship between Virulence and Resistance among Gram-Negative Bacteria. Antibiotics 2020, 9, 719. [Google Scholar] [CrossRef]
- Gajdacs, M.; Barath, Z.; Karpati, K.; Szabo, D.; Usai, D.; Zanetti, S.; Donadu, M.G. No Correlation between Biofilm Formation, Virulence Factors, and Antibiotic Resistance in Pseudomonas aeruginosa: Results from a Laboratory-Based In Vitro Study. Antibiotics 2021, 10, 1134. [Google Scholar] [CrossRef]
- Nassar, O.; Desouky, S.E.; El-Sherbiny, G.M.; Abu-Elghait, M. Correlation between phenotypic virulence traits and antibiotic resistance in Pseudomonas aeruginosa clinical isolates. Microb. Pathog. 2022, 162, 105339. [Google Scholar] [CrossRef]
- Louis, M.; Clamens, T.; Tahrioui, A.; Desriac, F.; Rodrigues, S.; Rosay, T.; Harmer, N.; Diaz, S.; Barreau, M.; Racine, P.J.; et al. Pseudomonas aeruginosa Biofilm Dispersion by the Human Atrial Natriuretic Peptide. Adv. Sci. 2022, 9, e2103262. [Google Scholar] [CrossRef]
- Knecht, L.D.; O’Connor, G.; Mittal, R.; Liu, X.Z.; Daftarian, P.; Deo, S.K.; Daunert, S. Serotonin Activates Bacterial Quorum Sensing and Enhances the Virulence of Pseudomonas aeruginosa in the Host. eBioMedicine 2016, 9, 161–169. [Google Scholar] [CrossRef] [PubMed]

| Bacterial Strains | Tobramycin (TM) | TM/DEHP | TM/DEHT | TM/DEP | TM/TXIB | TM/DBP | Ceftazidime CAZ | CAZ/DEHP | CAZ/DEHT | CAZ/DEP | CAZ/TXIB | CAZ/DBP |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P40 | 1 | 2 | 2 | 2 | 2 | 2 | 1 | 1–2 | 2–4 | 2–4 | 2–4 | 2–4 |
| P41 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| P42 | 16 | 16 | 16 | 16 | 16 | 16 | >16 | 16 | >16 | >16 | >16 | >16 |
| P43 | >16 | >16 | >16 | >16 | >16 | >16 | >16 | >16 | >16 | >16 | >16 | >16 |
| P44 | 2–4 | 2 | 2–4 | 2–4 | 2–4 | 2–4 | 2 | 2–4 | 2–4 | 2–4 | 2–4 | 2–4 |
| P45 | 2 | 2 | 2 | 2 | 2 | 2 | 4 | 2–4 | 2–4 | 2–4 | 2–4 | 2–4 |
| P46 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| P47 | 0.5–1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| P48 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| P49 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| P50 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2–4 | 2–4 | 2–4 | 2–4 |
| P51 | 2 | 2 | 2–4 | 2 | 2 | 2 | 2 | 2 | 2–4 | 2–4 | 2–4 | 2–4 |
| P52 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0.5–1 | 1 | 1 | 0.5–1 | 0.5–1 |
| P53 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0.5–1 | 1 | 1 | 0.5–1 | 1 |
| P54 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0.5–1 | 0.5–1 | 1 | 0.5–1 | 0.5–1 |
| P55 | 2 | 2 | 2–4 | 2 | 2 | 2 | 2 | 2 | 2–4 | 2 | 4 | 2–4 |
| P56 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| P57 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| P58 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2–4 | 2–4 | 2–4 | 2–4 |
| P59 | >16 | >16 | >16 | >16 | >16 | >16 | >16 | >16 | >16 | >16 | >16 | >16 |
| P60 | 8 | 8 | 8 | 8 | 8 | 8 | >16 | >16 | >16 | >16 | >16 | >16 |
| P61 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| P66 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1–2 | 2–4 | 2–4 | 2–4 |
| P67 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 1 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| P68 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 1 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| ATCC27 853 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 1 | 1 | 1 | 1 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Louis, M.; Tahrioui, A.; Verdon, J.; David, A.; Rodrigues, S.; Barreau, M.; Manac’h, M.; Thiroux, A.; Luton, B.; Dupont, C.; et al. Effect of Phthalates and Their Substitutes on the Physiology of Pseudomonas aeruginosa. Microorganisms 2022, 10, 1788. https://doi.org/10.3390/microorganisms10091788
Louis M, Tahrioui A, Verdon J, David A, Rodrigues S, Barreau M, Manac’h M, Thiroux A, Luton B, Dupont C, et al. Effect of Phthalates and Their Substitutes on the Physiology of Pseudomonas aeruginosa. Microorganisms. 2022; 10(9):1788. https://doi.org/10.3390/microorganisms10091788
Chicago/Turabian StyleLouis, Mélissande, Ali Tahrioui, Julien Verdon, Audrey David, Sophie Rodrigues, Magalie Barreau, Maëliss Manac’h, Audrey Thiroux, Baptiste Luton, Charly Dupont, and et al. 2022. "Effect of Phthalates and Their Substitutes on the Physiology of Pseudomonas aeruginosa" Microorganisms 10, no. 9: 1788. https://doi.org/10.3390/microorganisms10091788
APA StyleLouis, M., Tahrioui, A., Verdon, J., David, A., Rodrigues, S., Barreau, M., Manac’h, M., Thiroux, A., Luton, B., Dupont, C., Calvé, M. L., Bazire, A., Crépin, A., Clabaut, M., Portier, E., Taupin, L., Defontaine, F., Clamens, T., Bouffartigues, E., ... Chevalier, S. (2022). Effect of Phthalates and Their Substitutes on the Physiology of Pseudomonas aeruginosa. Microorganisms, 10(9), 1788. https://doi.org/10.3390/microorganisms10091788






