Immunomodulatory Effects of Probiotics on COVID-19 Infection by Targeting the Gut–Lung Axis Microbial Cross-Talk
Abstract
:1. Introduction
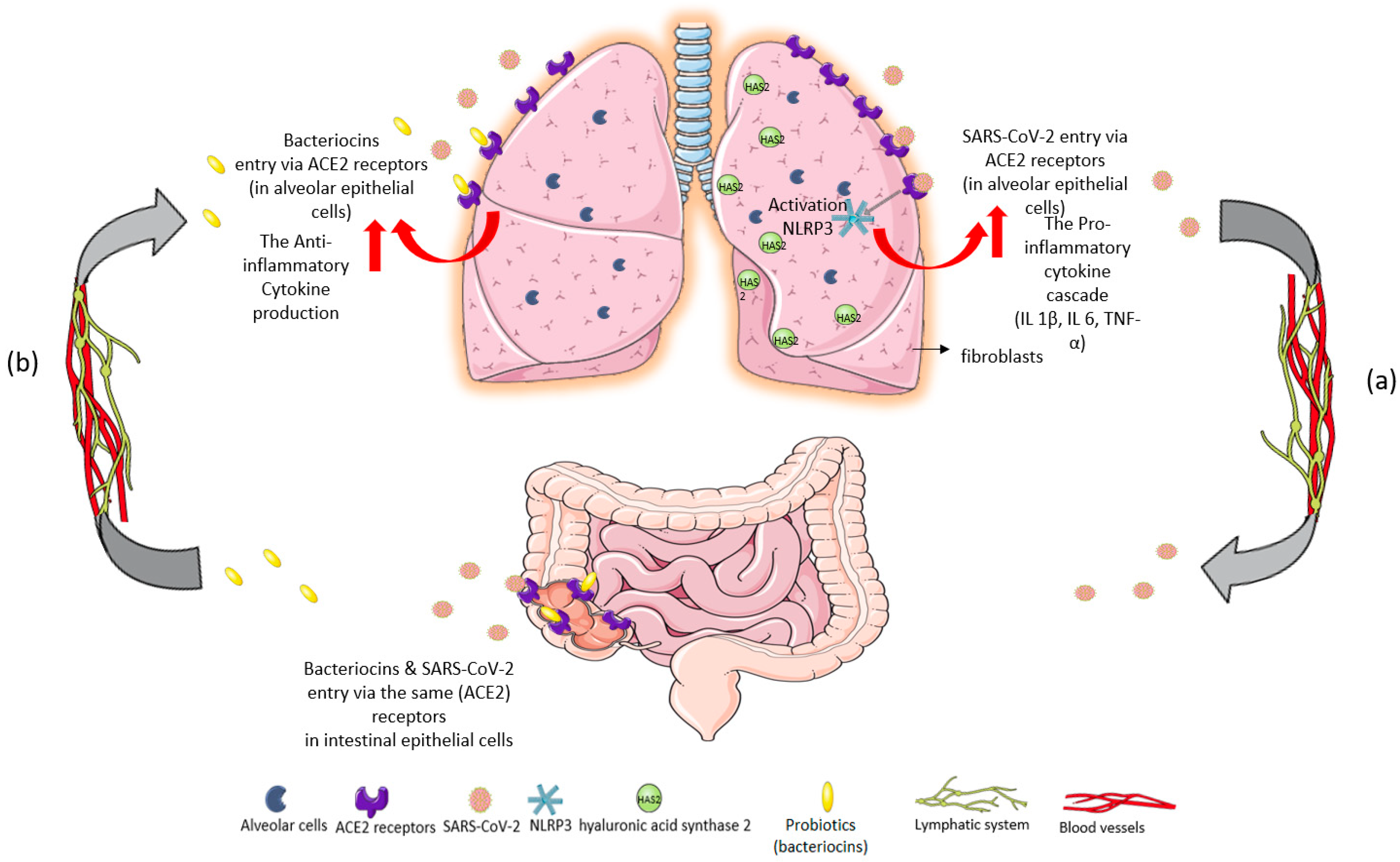
1.1. Preclinical Trials Supporting the Gut–Lung Axis Communication in COVID-19
1.2. Clinical Trials Supporting the Gut–Lung Axis Communication in COVID-19
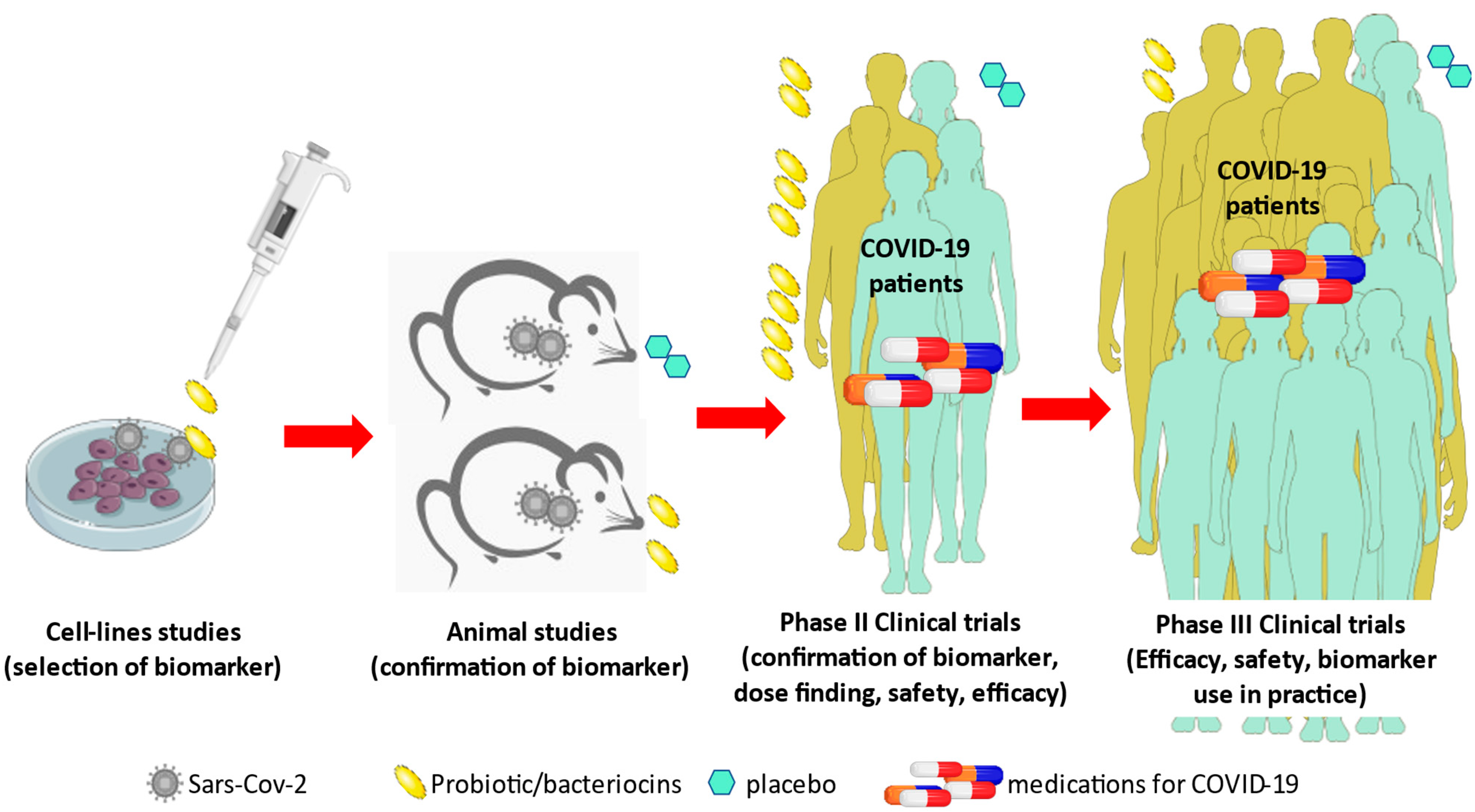
2. Literature Search Strategy
3. Preclinical Studies of Probiotic Administration in COVID-19 Infection
4. Clinical Trials with Probiotic Administration in COVID-19 Infection
5. In Vitro Trials with Probiotic Challenge in Cell Lines
6. Mixed Trials with Probiotic Administration in COVID-19 Infection
7. Discussion
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sokol, H.; Contreras, V.; Maisonnasse, P.; Desmons, A.; Delache, B.; Sencio, V.; Trottein, F. SARS-CoV-2 infection in nonhuman primates alters the composition and functional activity of the gut microbiota. Gut Microbes 2021, 13, 1893113. [Google Scholar] [CrossRef] [PubMed]
- Lynch, S.V.; Pedersen, O. The Human Intestinal Microbiome in Health and Disease. N. Engl. J. Med. 2016, 375, 2369–2379. [Google Scholar] [CrossRef] [PubMed]
- Roquilly, A.; Torres, A.; Villadangos, J.; Netea, M.G.; Dickson, R.; Becher, B.; Asehnoune, K. Pathophysiologial role of respiratory dysbiosis in hospital-acquired pneumonia. Lancet Respir. Med. 2019, 7, 710–720. [Google Scholar] [CrossRef]
- O’Dwyer, D.N.; Dickson, R.P.; Moore, B.B.; O’Dwyer, D.N.; Dickson, R.P.; Moore, B.B. The Lung Microbiome, Immunity, and the Pathogenesis of Chronic Lung Disease. J. Immunol. 2016, 196, 4839–4847. [Google Scholar] [CrossRef] [PubMed]
- d’Ettorre, G.; Ceccarelli, G.; Marazzato, M.; Campagna, G.; Pinacchio, C.; Alessandri, F.; Mastroianni, C.M. Challenges in the Management of SARS-CoV2 Infection: The Role of Oral Bacteriotherapy as Complementary Therapeutic Strategy to Avoid the Progression of COVID-19. Front. Med. 2020, 7, 389. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, G.L.V.; Oliveira, C.N.S.; Pinzan, C.F.; de Salis, L.V.V.; de Cardoso, C.R.B. Microbiota Modulation of the Gut-Lung Axis in COVID-19. Front. Immunol. 2021, 12, 635471. [Google Scholar] [CrossRef]
- Vestad, B.; Ueland, T.; Lerum, T.V.; Dahl, T.B.; Holm, K.; Barratt-Due, A.; Kåsine, T.; Dyrhol-Riise, A.M.; Stiksrud, B.; Tonby, K.; et al. Respiratory dysfunction three months after severe COVID-19 is associated with gut microbiota alterations. J. Intern. Med. 2022, 291, 801–812. [Google Scholar] [CrossRef]
- Aishwarya, S.; Gunasekaran, K.; Anita Margret, A. Intermodulation of gut-lung axis microbiome and the implications of biotics to combat COVID-19. J. Biomol. Struct. Dyn. 2021, 26, 1–17. [Google Scholar]
- Kasti, A.N.; Synodinou, K.D.; Pyrousis, I.A.; Nikolaki, M.D.; Triantafyllou, K.D. Probiotics Regulating Inflammation via NLRP3 Inflammasome Modulation: A Potential Therapeutic Approach for COVID-19. Microorganisms 2021, 9, 2376. [Google Scholar] [CrossRef]
- Bermudez-Brito, M.; Plaza-Díaz, J.; Muñoz-Quezada, S.; Gómez-Llorente, C.; Gil, A. Probiotic mechanisms of action. Ann. Nutr. Metab. 2012, 61, 160–174. [Google Scholar] [CrossRef]
- Wilkinson, T.S.; Potter-Perigo, S.; Tsoi, C.; Altman, L.C.; Wight, T.N. Pro- and anti-inflammatory factors cooperate to control hyaluronan synthesis in lung fibroblasts. Am. J. Respir. Cell Mol. Biol. 2004, 31, 92–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bell, T.J.; Brand, O.J.; Morgan, D.J.; Salek-Ardakani, S.; Jagger, C.; Fujimori, T.; Cholewa, L.; Tilakaratna, V.; Östling, J.; Thomas, M.; et al. Defective lung function following influenza virus is due to prolonged, reversible hyaluronan synthesis. Matrix. Biol. 2019, 80, 14–28. [Google Scholar] [CrossRef] [PubMed]
- Sencio, V.; Machelart, A.; Robil, C.; Benech, N.; Hoffmann, E.; Galbert, C.; Trottein, F. Alteration of the gut microbiota following SARS-CoV-2 infection correlates with disease severity in hamsters. Gut Microbes 2022, 14, 2018900. [Google Scholar] [CrossRef] [PubMed]
- Groves, H.T.; Cuthbertson, L.; James, P.; Moffatt, M.F.; Cox, M.J.; Tregoning, J.S. Respiratory Disease following Viral Lung Infection Alters the Murine Gut Microbiota. Front. Immunol. 2018, 9, 182. [Google Scholar] [CrossRef]
- Jin, X.; Lian, J.S.; Hu, J.H.; Gao, J.; Zheng, L.; Zhang, Y.M.; Hao, S.R.; Jia, H.Y.; Cai, H.; Zhang, X.L.; et al. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut 2020, 69, 1002–1009. [Google Scholar] [CrossRef]
- ZZuo, T.; Zhang, F.; Lui, G.C.Y.; Yeoh, Y.K.; Li, A.Y.L.; Zhan, H.; Wan, Y.; Chung, A.C.K.; Cheung, C.P.; Chen, N.; et al. Alterations in Gut Microbiota of Patients with COVID-19 during Time of Hospitalization. Gastroenterology 2020, 159, 944–955.e8. [Google Scholar] [CrossRef]
- Zuo, T.; Zhan, H.; Zhang, F.; Liu, Q.; Tso, E.Y.; Lui, G.C.; Chen, N.; Li, A.; Lu, W.; Chan, F.K.; et al. Alterations in Fecal Fungal Microbiome of Patients with COVID-19 during Time of Hospitalization until Discharge. Gastroenterology 2020, 159, 1302–1310.e5. [Google Scholar] [CrossRef]
- Zuo, T.; Liu, Q.; Zhang, F.; Lui GC, Y.; Tso, E.Y.; Yeoh, Y.K.; Ng, S.C. Depicting SARS-CoV-2 faecal viral activity in association with gut microbiota composition in patients with COVID-19. Gut 2021, 70, 276–284. [Google Scholar] [CrossRef]
- Yeoh, Y.K.; Zuo, T.; Lui, G.C.-Y.; Zhang, F.; Liu, Q.; Li, A.Y.; Chung, A.C.; Cheung, C.P.; Tso, E.Y.; Fung, K.S.; et al. Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut 2021, 70, 698–706. [Google Scholar] [CrossRef]
- Li, S.; Yang, S.; Zhou, Y.; Disoma, C.; Dong, Z.; Du, A.; Xia, Z. Microbiome Profiling Using Shotgun Metagenomic Sequencing Identified Unique Microorganisms in COVID-19 Patients with Altered Gut Microbiota. Front. Microbiol. 2021, 12, 712081. [Google Scholar] [CrossRef]
- Pham, M.T.; Yang, A.J.; Kao, M.S.; Gankhuyag, U.; Zayabaatar, E.; Jin SL, C.; Huang, C.M. Gut probiotic Lactobacillus rhamnosus attenuates PDE4B-mediated interleukin-6 induced by SARS-CoV-2 membrane glycoprotein. J. Nutr. Biochem. 2021, 98, 108821. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Ren, Z.; Cao, K.; Li, X.; Yang, J.; Luo, X.; Zhu, L.; Wang, X.; Ding, L.; Liang, J.; et al. Boosting Vaccine-Elicited Respiratory Mucosal and Systemic COVID-19 Immunity in Mice With the Oral Lactobacillus plantarum. Front. Nutr. 2021, 8, 789242. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Xu, Q.; Cao, Z.; Pan, D.; Zhu, Y.; Wang, S.; Liu, D.; Song, Z.; Jiang, W.; Ruan, Y.; et al. The volatile and heterogeneous gut microbiota shifts of COVID-19 patients over the course of a probiotics-assisted therapy. Clin. Transl. Med. 2021, 11, e643. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Castrellón, P.; Gandara-Martí, T.; Abreu YAbreu, A.T.; Nieto-Rufino, C.D.; López-Orduña, E.; Jiménez-Escobar, I.; Espadaler-Mazo, J. Probiotic improves symptomatic and viral clearance in Covid19 outpatients: A randomized, quadruple-blinded, placebo-controlled trial. Gut Microbes 2022, 14, 2018899. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Ferreiro, A.; Formigo-Couceiro, F.J.; Veiga-Gutierrez, R.; Maldonado-Lobón, J.A.; Hermida-Cao, A.M.; Rodriguez, C.; Bañuelos, O.; Olivares, M.; Blanco-Rojo, R. Effects of Loigolactobacillus coryniformis K8 CECT 5711 on the Immune Response of Elderly Subjects to COVID-19 Vaccination: A Randomized Controlled Trial. Nutrients 2022, 14, 228. [Google Scholar] [CrossRef]
- Islam, M.A.; Albarracin, L.; Tomokiyo, M.; Valdez, J.C.; Sacur, J.; Vizoso-Pinto, M.G.; Villena, J. Immunobiotic Lactobacilli Improve Resistance of Respiratory Epithelial Cells to SARS-CoV-2 Infection. Pathogens 2021, 10, 1197. [Google Scholar] [CrossRef]
- Kageyama, Y.; Nishizaki, Y.; Aida, K.; Yayama, K.; Ebisui, T.; Akiyama, T.; Nakamura, T. Lactobacillus plantarum induces innate cytokine responses that potentially provide a protective benefit against COVID-19: A single-arm, double-blind, prospective trial combined with an in vitro cytokine response assay. Exp. Ther. Med. 2022, 23, 20. [Google Scholar] [CrossRef]
- Mak, J.W.Y.Y.; Chan, F.K.L.L.; Ng, S.C. Probiotics and COVID-19: One size does not fit all. Lancet Gastroenterol. Hepatol. 2020, 5, 644–645. [Google Scholar] [CrossRef]
- Hung, Y.-P.; Lee, C.-C.; Lee, J.-C.; Tsai, P.-J.; Ko, W.-C. Gut Dysbiosis during COVID-19 and Potential Effect of Probiotics. Microorganisms 2021, 9, 1605. [Google Scholar] [CrossRef]
- King, S.; Glanville, J.; Sanders, M.E.; Fitzgerald, A.; Varley, D. Effectiveness of probiotics on the duration of illness in healthy children and adults who develop common acute respiratory infectious conditions: A systematic review and meta-analysis. Br. J. Nutr. 2014, 112, 41–54. [Google Scholar] [CrossRef]
- Hao, Q.; Dong, B.R.; Wu, T. Probiotics for preventing acute upper respiratory tract infections. Cochrane Database Syst. Rev. 2015, 2, CD006895. [Google Scholar] [CrossRef] [PubMed]
- Mullish, B.H.; Marchesi, J.R.; McDonald, J.A.; Pass, D.A.; Masetti, G.; Michael, D.R.; Wang, D. Probiotics reduce self-reported symptoms of upper respiratory tract infection in overweight and obese adults: Should we be considering probiotics during viral pandemics? Gut Microbes 2021, 13, 1900997. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Qu, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef] [PubMed]
- Morrow, L.E.; Kollef, M.H.; Casale, T.B. Probiotic prophylaxis of ventilator-associated pneumonia: A blinded, randomized, controlled trial. Am. J. Respir. Crit. Care Med. 2010, 182, 1058–1064. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Wang, C.-T.; Zhang, F.-S.; Qi, F.; Wang, S.-F.; Ma, S.; Wu, T.-J.; Tian, H.; Tian, Z.-T.; Zhang, S.-L.; et al. Effect of probiotics on the incidence of ventilator-associated pneumonia in critically ill patients: A randomized controlled multicenter trial. Intensive Care Med. 2016, 42, 1018–1028. [Google Scholar] [CrossRef]
- Kullberg, R.F.J.; Wiersinga, W.J.; Haak, B.W. Gut microbiota and sepsis: From pathogenesis to novel treatments. Curr. Opin. Gastroenterol. 2021, 37, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Tripathi, A.S.; Sharma, N.; Singh, G.; Mohapatra, L. Is Regular Probiotic Practice Safe for Management of Sepsis? Chin. J. Integr. Med. 2022, 28, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, K.; Yamada, T.; Ogura, H.; Mohri, T.; Kiguchi, T.; Fujimi, S.; Asahara, T.; Ojima, M.; Ikeda, M.; Shimazu, T. Synbiotics modulate gut microbiota and reduce enteritis and ventilator-associated pneumonia in patients with sepsis: A randomized controlled trial. Crit. Care 2018, 22, 239. [Google Scholar] [CrossRef]
- Anwar, F.; Altayb, H.N.; Al-Abbasi, F.A.; Al-Malki, A.L.; Kamal, M.A.; Kumar, V. Antiviral effects of probiotic metabolites on COVID-19. J. Biomol. Struct. Dyn. 2020, 39, 4175–4184. [Google Scholar] [CrossRef]


| Clinical Trials with Probiotic Administration in COVID-19 Infection | ||||||
|---|---|---|---|---|---|---|
| Reference | Study Group (SG) | Intervention | Control Group (CG) | Intervention | Biological Samples | Results |
| Wu et al. [23] | COVID-19 patients (n = 13) | B. lactis HNO19 L. casei Lc-11 L. plantarum Lp-15 B. lactis B420 B. longum BL05 L. format Lg-36 L. rhamnosus Lr-32 L. paracasei Lpc-37 L. salivarius | Patients with community-acquired pneumonia (n = 15) | B. lactis HNO19 L. casei Lc-11 L. plantarum Lp-15 B. lactis B420 B. longum BL05 L. format Lg-36 L. rhamnosus Lr-32 L. paracasei Lpc-37 L. salivarius | Feces | Restoration of intestinal dysbiosis and pulmonary dysfunction. Reduced inflammatory biomarkers: ↓TNF-α, ↓ IL-1β, ↓IL-4, and ↓IL-12P70. |
| Healthy controls (n = 15) | --- | |||||
| Gutiérrez-Castrellón et al. (RCT) [24] | COVID-19 ICU patients (n = 150) | L. plantarum KABP022 L. plantarum KABP023 L. plantarum KABP033 P. acidilactici KABP021 | COVID-19 ICU patients (n = 150) | Placebo | Nasopharyngeal specimens, blood, and feces | Complete remission in 53.1% of patients in SG and 28.1% in CG. Reduced viral load, lung infiltrates, and symptoms duration in SG. Significant increase in IgM and IgG against SARS-CoV-2 and faster reduction of D-Dimers in SG. |
| D’Ettorre et al. [5] | COVID-19 patients (n = 28) | S. thermophilus DSM 32345 L. acidophilus DSM 32241 L. helveticus DSM 32242 L. paracasei DSM 32243 L. plantarum DSM 32244 L. brevis DSM 27961 B. lactis DSM 32246 B. lactis DSM 32247 | COVID-19 patients (n = 42) | --- | --- | SG: remission of diarrhea and other symptoms 72 h after oral bacteriotherapy. Estimated risk of developing respiratory failure: eight-fold lower in SG. CG: Higher ICU admission and mortality rates. |
| Fernández-Ferreiro et al. (RCT) [25] | Elderly people vaccinated with mRNA-based vaccine against SARS-CoV-2 (n = 98) | L. coryniformis K8 CECT 5711 | Elderly people vaccinated with mRNA-based vaccine against SARS-CoV-2 (n = 100) | Placebo | Blood | L. coryniformis K8: enhances vaccine-specific immune responses against SARS-CoV-2 in elderly populations. ↑IgG, ↑IgA in SG. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Synodinou, K.D.; Nikolaki, M.D.; Triantafyllou, K.; Kasti, A.N. Immunomodulatory Effects of Probiotics on COVID-19 Infection by Targeting the Gut–Lung Axis Microbial Cross-Talk. Microorganisms 2022, 10, 1764. https://doi.org/10.3390/microorganisms10091764
Synodinou KD, Nikolaki MD, Triantafyllou K, Kasti AN. Immunomodulatory Effects of Probiotics on COVID-19 Infection by Targeting the Gut–Lung Axis Microbial Cross-Talk. Microorganisms. 2022; 10(9):1764. https://doi.org/10.3390/microorganisms10091764
Chicago/Turabian StyleSynodinou, Kalliopi D., Maroulla D. Nikolaki, Konstantinos Triantafyllou, and Arezina N. Kasti. 2022. "Immunomodulatory Effects of Probiotics on COVID-19 Infection by Targeting the Gut–Lung Axis Microbial Cross-Talk" Microorganisms 10, no. 9: 1764. https://doi.org/10.3390/microorganisms10091764
APA StyleSynodinou, K. D., Nikolaki, M. D., Triantafyllou, K., & Kasti, A. N. (2022). Immunomodulatory Effects of Probiotics on COVID-19 Infection by Targeting the Gut–Lung Axis Microbial Cross-Talk. Microorganisms, 10(9), 1764. https://doi.org/10.3390/microorganisms10091764








