Use of Probiotic Bacteria and Bacteriocins as an Alternative to Antibiotics in Aquaculture
Abstract
:1. Introduction
2. Antibiotics and Fish Infection Control
3. Probiotic Use in Aquaculture
4. Mode of Action and Benefits of Probiotic
5. Bacteriocin Use in Aquaculture
6. Safety
7. Conclusions and Future Perspectives
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dawood, M.A.; Koshio, S.; Abdel-Daim, M.M.; van Doan, H.; Mahmoud Dawood, C.A. Probiotic Application for Sustainable Aquaculture. Rev. Aquac. 2018, 11, 907–924. [Google Scholar] [CrossRef]
- Watts, J.E.M.; Schreier, H.J.; Lanska, L.; Hale, M.S. Marine Drugs The Rising Tide of Antimicrobial Resistance in Aquaculture: Sources, Sinks and Solutions. Mar. Drugs 2017, 15, 158. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations. Fisheries and Aquaculture Department. The State of World Fisheries and Aquaculture 2018: Meeting the Sustainable Development Goals; FAO: Rome, Italy, 2018; ISBN 9789251305621. Available online: https://www.fao.org/documents/card/en/c/I9540EN/ (accessed on 10 February 2022).
- FAO Food and Agriculture Organization of the United Nations. The State of World Fisheries and Aquaculture. 2014. Available online: https://www.fao.org/3/i3720e/i3720e.pdf (accessed on 10 February 2022).
- Valladão, G.M.R.; Gallani, S.U.; Pilarski, F. South American Fish for Continental Aquaculture. Rev. Aquac. 2018, 10, 351–369. [Google Scholar] [CrossRef]
- Little, D.C.; Young, J.A.; Zhang, W.; Newton, R.W.; al Mamun, A.; Murray, F.J. Sustainable Intensification of Aquaculture Value Chains between Asia and Europe: A Framework for Understanding Impacts and Challenges. Aquaculture 2018, 493, 338–354. [Google Scholar] [CrossRef]
- Ringø, E. Probiotics in Shellfish Aquaculture. Aquac. Fish. 2020, 5, 1–27. [Google Scholar] [CrossRef]
- Concha, C.; Miranda, C.D.; Hurtado, L.; Romero, J. Characterization of Mechanisms Lowering Susceptibility to Flumequine among Bacteria Isolated from Chilean Salmonid Farms. Microorganisms 2019, 7, 698. [Google Scholar] [CrossRef]
- World Health Organization. Critically Important Antimicrobials for Human Medicine 5th Revision 2016 Ranking of Medically Important Antimicrobials for Risk Management of Antimicrobial Resistance Due to Non-Human Use. 2017. Available online: https://apps.who.int/iris/handle/10665/255027 (accessed on 10 February 2022).
- Romero, J.; Feijoo, C.G.; Navarrete, P. Antibiotics in Aquaculture—Use, Abuse and Alternatives. Health Environ. Aquac. 2012, 159, 159–198. [Google Scholar] [CrossRef]
- Romero, J.; Ringø, E.; Merrifield, D.L. The Gut Microbiota of Fish. In Aquaculture Nutrition: Gut Health, Probiotics, and Prebiotics; John Wiley & Sons Inc.: Chichester, UK, 2014; pp. 75–100. ISBN 9781118897263. [Google Scholar]
- Banerjee, G.; Ray, A.K. The Advancement of Probiotics Research and Its Application in Fish Farming Industries. Res. Veter. Sci. 2017, 115, 66–77. [Google Scholar] [CrossRef]
- Guardabassi, L.; Hilde, K. Princípios Da Utilização Prudente E Racional De Antimicrobianos Em Animais. Artmed: RS. 2010. Available online: https://statics-americanas.b2w.io/sherlock/books/firstChapter/27113326.pdf (accessed on 10 February 2022).
- Pérez-Sánchez, T.; Mora-Sánchez, B.; Balcázar, J.L. Biological Approaches for Disease Control in Aquaculture: Advantages, Limitations and Challenges. Trends Microbiol. 2018, 26, 896–903. [Google Scholar] [CrossRef]
- Love, D.C.; Fry, J.P.; Cabello, F.; Good, C.M.; Lunestad, B.T. Veterinary Drug Use in United States Net Pen Salmon Aquaculture: Implications for Drug Use Policy. Aquaculture 2020, 518, 734820. [Google Scholar] [CrossRef]
- Romero, J.; Díaz, O.; Miranda, C.D.; Rojas, R. Red Cusk-Eel (Genypterus chilensis) Gut Microbiota Description of Wild and Aquaculture Specimens. Microorganisms 2022, 10, 105. [Google Scholar] [CrossRef] [PubMed]
- Lima Junior, D.P.; Magalhães, A.L.B.; Pelicice, F.M.; Vitule, J.R.S.; Azevedo-Santos, V.M.; Orsi, M.L.; Simberloff, D.; Agostinho, A.A. Aquaculture Expansion in Brazilian Freshwaters against the Aichi Biodiversity Targets. Ambio 2018, 47, 427–440. [Google Scholar] [CrossRef] [PubMed]
- Chai, Y.; Cong, B.; Yu, S.; Liu, Y.; Man, X.; Wang, L.; Zhu, Q. Effect of a LECT2 on the Immune Response of Peritoneal Lecukocytes against Vibrio Anguillarum in Roughskin Sculpin. Fish Shellfish Immunol. 2018, 74, 620–626. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Gan, X.; Ao, Q.; Shen, X.; Tan, Y.; Chen, M.; Luo, Y.; Wang, H.; Jiang, H.; Li, C. Basal Polarization of the Immune Responses to Streptococcus Agalactiae Susceptible and Resistant Tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2018, 75, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Kayansamruaj, P.; Dong, H.T.; Hirono, I.; Kondo, H.; Senapin, S.; Rodkhum, C. Comparative Genome Analysis of Fish Pathogen Flavobacterium Columnare Reveals Extensive Sequence Diversity within the Species. Infect. Genet. Evol. 2017, 54, 7–17. [Google Scholar] [CrossRef]
- Munir, M.B.; Hashim, R.; Nor, S.A.M.; Marsh, T.L. Effect of Dietary Prebiotics and Probiotics on Snakehead (Channa Striata) Health: Haematology and Disease Resistance Parameters against Aeromonas Hydrophila. Fish Shellfish Immunol. 2018, 75, 99–108. [Google Scholar] [CrossRef]
- Baldissera, M.D.; Souza, C.F.; Verdi, C.M.; Vizzotto, B.S.; Santos, R.C.V.; Baldisserotto, B. Aeromonas Caviae Alters the Activities of Ecto-Enzymes That Hydrolyze Adenine Nucleotides in Fish Thrombocytes. Microb. Pathog. 2017, 115, 64–67. [Google Scholar] [CrossRef]
- Kharabsheh, H.A.; Han, S.; Allen, S.; Chao, S.L. Metabolism of Chlorpyrifos by Pseudomonas Aeruginosa Increases Toxicity in Adult Zebrafish (Danio rerio). Int. Biodeterior. Biodegrad. 2017, 121, 114–121. [Google Scholar] [CrossRef]
- Novais, C.; Campos, J.; Freitas, A.R.; Barros, M.; Silveira, E.; Coque, T.M.; Antunes, P.; Peixe, L. Water Supply and Feed as Sources of Antimicrobial-Resistant Enterococcus Spp. in Aquacultures of Rainbow Trout (Oncorhyncus mykiss), Portugal. Sci. Total Environ. 2018, 625, 1102–1112. [Google Scholar] [CrossRef]
- Lampe, E.O.; Zingmark, C.; Tandberg, J.I.; Thrane, I.M.P.; Brudal, E.; Sjöstedt, A.; Winther-Larsen, H.C. Francisella Noatunensis Subspecies Noatunensis ClpB Deletion Mutant Impairs Development of Francisellosis in a Zebrafish Model. Vaccine 2017, 35, 7264–7272. [Google Scholar] [CrossRef]
- Ma, J.; Bruce, T.J.; Sudheesh, P.S.; Knupp, C.; Loch, T.P.; Faisal, M.; Cain, K.D. Assessment of Cross-Protection to Heterologous Strains of Flavobacterium Psychrophilum Following Vaccination with a Live-Attenuated Coldwater Disease Immersion Vaccine. J. Fish Dis. 2019, 42, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Soltani, S.; Hammami, R.; Cotter, P.D.; Rebuffat, S.; Said, L.ben; Gaudreau, H.; Bédard, F.; Biron, E.; Drider, D.; Fliss, I. Bacteriocins as a New Generation of Antimicrobials: Toxicity Aspects and Regulations. FEMS Microbiol. Rev. 2021, 45, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations; World Health Organization. Probiotics in Food: Health and Nutritional Properties and Guidelines for Evaluation; Food and Agriculture Organization of the United Nations: Rome, Italy, 2006; ISBN 9251055130. [Google Scholar]
- Verschuere, L.; Rombaut, G.; Sorgeloos, P.; Verstraete, W. Probiotic Bacteria as Biological Control Agents in Aquaculture. Microbiol. Mol. Biol. Rev. 2000, 64, 655–671. [Google Scholar] [CrossRef] [PubMed]
- Rottmann, R.W.; Francis-Floyd, R.; Durborow, R. Southern Regional Aquaculture Center The Role of Stress in Fish Disease; Southern Regional Aquaculture Center: Stoneville, MS, USA, 1992. [Google Scholar]
- van Doan, H.; Hoseinifar, S.H.; Ringø, E.; Ángeles Esteban, M.; Dadar, M.; Dawood, M.A.O.; Faggio, C. Host-Associated Probiotics: A Key Factor in Sustainable Aquaculture. Rev. Fish. Sci. Aquac. 2020, 28, 16–42. [Google Scholar] [CrossRef]
- Ringø, E.; Hoseinifar, S.H.; Ghosh, K.; van Doan, H.; Beck, B.R.; Song, S.K. Lactic Acid Bacteria in Finfish-An Update. Front. Microbiol. 2018, 9, 1818. [Google Scholar] [CrossRef]
- Ariǧ, N.; Suzer, C.; Gökvardar, A.; Başaran, F.; Çoban, D.; Yildirim, Ş.; Kamaci, H.O.; Firat, K.; Saka, Ş. Effects of Probiotic (Bacillus sp.) Supplementation during Larval Development of Gilthead Sea Bream (Sparus Aurata, L.). Turk. J. Fish. Aquat. Sci. 2013, 13, 407–414. [Google Scholar] [CrossRef]
- Lim, H.J.; Kapareiko, D.; Schott, E.J.; Hanif, A.; Wikfors, G.H. Isolation and Evaluation of New Probiotic Bacteria for Use in Shellfish Hatcheries: I. Isolation and Screening for Bioactivity. J. Shellfish Res. 2011, 30, 609–615. [Google Scholar] [CrossRef]
- Gheziel, C.; Russo, P.; Arena, M.P.; Spano, G.; Ouzari, H.I.; Kheroua, O.; Saidi, D.; Fiocco, D.; Kaddouri, H.; Capozzi, V. Evaluating the Probiotic Potential of Lactobacillus Plantarum Strains from Algerian Infant Feces: Towards the Design of Probiotic Starter Cultures Tailored for Developing Countries. Probiotics Antimicrob. Proteins 2019, 11, 113–123. [Google Scholar] [CrossRef]
- Vilander, A.C.; Dean, G.A. Adjuvant Strategies for Lactic Acid Bacterial Mucosal Vaccines. Vaccines 2019, 7, 150. [Google Scholar] [CrossRef]
- Singhal, N.; Singh, N.S.; Mohanty, S.; Singh, P.; Virdi, J.S. Evaluation of Probiotic Characteristics of Lactic Acid Bacteria Isolated from Two Commercial Preparations Available in Indian Market. Indian J. Microbiol. 2019, 59, 112–115. [Google Scholar] [CrossRef]
- Guerreiro, I.; Oliva-Teles, A.; Enes, P. Prebiotics as Functional Ingredients: Focus on Mediterranean Fish Aquaculture. Rev. Aquac. 2018, 10, 800–832. [Google Scholar] [CrossRef]
- Cruz, B.C.S.; Sarandy, M.M.; Messias, A.C.; Gonçalves, R.V.; Ferreira, C.L.L.F.; Peluzio, M.C.G. Preclinical and Clinical Relevance of Probiotics and Synbiotics in Colorectal Carcinogenesis: A Systematic Review. Nutr. Rev. 2020, 78, 667–687. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.M.; Denning, P.W. Therapeutic Use of Prebiotics, Probiotics, and Postbiotics to Prevent Necrotizing Enterocolitis: What Is the Current Evidence? Clin. Perinatol. 2013, 40, 11. [Google Scholar] [CrossRef]
- Ang, C.Y.; Sano, M.; Dan, S.; Leelakriangsak, M.; Lal, T.M. Postbiotics Applications as Infectious Disease Control Agent in Aquaculture. Biocontrol Sci. 2020, 25, 1–7. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; Eweedah, N.M.; Moustafa, E.M.; Farahat, E.M. Probiotic Effects of Aspergillus Oryzae on the Oxidative Status, Heat Shock Protein, and Immune Related Gene Expression of Nile Tilapia (Oreochromis niloticus) under Hypoxia Challenge. Aquaculture 2020, 520, 734669. [Google Scholar] [CrossRef]
- Abdel-Aziz, M.; Bessat, M.; Fadel, A.; Elblehi, S. Responses of Dietary Supplementation of Probiotic Effective Microorganisms (EMs) in Oreochromis niloticus on Growth, Hematological, Intestinal Histopathological, and Antiparasitic Activities. Aquac. Int. 2020, 28, 947–963. [Google Scholar] [CrossRef]
- Ahmadifar, E.; Sadegh, T.H.; Dawood, M.A.O.; Dadar, M.; Sheikhzadeh, N. The Effects of Dietary Pediococcus Pentosaceus on Growth Performance, Hemato-Immunological Parameters and Digestive Enzyme Activities of Common Carp (Cyprinus Carpio). Aquaculture 2020, 516, 734656. [Google Scholar] [CrossRef]
- Arias-Moscoso, J.L.; Espinoza-Barrón, L.G.; Miranda-Baeza, A.; Rivas-Vega, M.E.; Nieves-Soto, M. Effect of Commercial Probiotics Addition in a Biofloc Shrimp Farm during the Nursery Phase in Zero Water Exchange. Aquac. Rep. 2018, 11, 47–52. [Google Scholar] [CrossRef]
- Austin, B.; STuckey, L.F.; Robertson, P.A.W.; Effendi, I.; Griffith, D.R.W. A Probiotic Strain of Vibrio Alginolyticus Effective in Reducing Diseases Caused by Aeromonas salmonicida, Vibrio Anguillarum and Vibrio Ordalii. J. Fish Dis. 1995, 18, 93–96. [Google Scholar] [CrossRef]
- Austin, B.; Baudet, E.; Stobie, M. Inhibition of Bacterial Fish Pathogens by Tetraselmis Suecica. J. Fish Dis. 1992, 15, 55–61. [Google Scholar] [CrossRef]
- Balcázar, J.L.; Vendrell, D.; de Blas, I.; Ruiz-Zarzuela, I.; Múzquiz, J.L. Effect of Lactococcus Lactis CLFP 100 and Leuconostoc Mesenteroides CLFP 196 on Aeromonas salmonicida Infection in Brown Trout (Salmo trutta). Microb. Physiol. 2009, 17, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Banu, M.R.; Akter, S.; Islam, M.R.; Mondol, M.N.; Hossain, M.A. Probiotic Yeast Enhanced Growth Performance and Disease Resistance in Freshwater Catfish Gulsa Tengra, Mystus Cavasius. Aquac. Rep. 2020, 16, 100237. [Google Scholar] [CrossRef]
- Bhujel, R.C.; Jha, D.K.; Anal, A.K. Effects of Probiotic Doses on the Survival and Growth of Hatchlings, Fry, and Advanced Fry of Rohu (Labeoï¿¿rohita Hamilton). J. Appl. Aquac. 2020, 32, 34–52. [Google Scholar] [CrossRef]
- Chien, C.C.; Lin, T.Y.; Chi, C.C.; Liu, C.H. Probiotic, Bacillus Subtilis E20 Alters the Immunity of White Shrimp, Litopenaeus Vannamei via Glutamine Metabolism and Hexosamine Biosynthetic Pathway. Fish Shellfish Immunol. 2020, 98, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Dawood, M.A.O.; Moustafa, E.M.; Gewaily, M.S.; Abdo, S.E.; AbdEl-kader, M.F.; SaadAllah, M.S.; Hamouda, A.H. Ameliorative Effects of Lactobacillus Plantarum L-137 on Nile Tilapia (Oreochromis niloticus) Exposed to Deltamethrin Toxicity in Rearing Water. Aquat. Toxicol. 2020, 219, 105377. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; Koshio, S.; Ishikawa, M.; Yokoyama, S. Effects of Dietary Inactivated Pediococcus Pentosaceus on Growth Performance, Feed Utilization and Blood Characteristics of Red Sea Bream, Pagrus Major Juvenile. Aquac. Nutr. 2016, 22, 923–932. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; Koshio, S.; Ishikawa, M.; Yokoyama, S. Immune Responses and Stress Resistance in Red Sea Bream, Pagrus Major, after Oral Administration of Heat-Killed Lactobacillus Plantarum and Vitamin C. Fish Shellfish Immunol. 2016, 54, 266–275. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; Koshio, S.; Ishikawa, M.; Yokoyama, S.; el Basuini, M.F.; Hossain, M.S.; Nhu, T.H.; Dossou, S.; Moss, A.S. Effects of Dietary Supplementation of Lactobacillus Rhamnosus or/and Lactococcus Lactis on the Growth, Gut Microbiota and Immune Responses of Red Sea Bream, Pagrus Major. Fish Shellfish Immunol. 2016, 49, 275–285. [Google Scholar] [CrossRef]
- Elsabagh, M.; Mohamed, R.; Moustafa, E.M.; Hamza, A.; Farrag, F.; Decamp, O.; Dawood, M.A.O.; Eltholth, M. Assessing the Impact of Bacillus Strains Mixture Probiotic on Water Quality, Growth Performance, Blood Profile and Intestinal Morphology of Nile Tilapia, Oreochromis niloticus. Aquac. Nutr. 2018, 24, 1613–1622. [Google Scholar] [CrossRef]
- Fox, D.M.; Trulove, P.C.; De, H.C.; Sholihuddin, T.D.; Arief, M.; Kenconojati, H. Effect of Different Bacterial Strain in Probiotics on the Growth Performance of Nile Tilapia (Oreochromis niloticus). IOP Conf. Ser. Earth Environ. Sci. 2020, 441, 012072. [Google Scholar] [CrossRef]
- Foysal, M.J.; Alam, M.; Kawser, A.Q.M.R.; Hasan, F.; Rahman, M.M.; Tay, C.Y.; Prodhan, M.S.H.; Gupta, S.K. Meta-Omics Technologies Reveals Beneficiary Effects of Lactobacillus Plantarum as Dietary Supplements on Gut Microbiota, Immune Response and Disease Resistance of Nile Tilapia (Oreochromis niloticus). Aquaculture 2020, 520, 734974. [Google Scholar] [CrossRef]
- Grammes, F.; Reveco, F.E.; Romarheim, O.H.; Landsverk, T.; Mydland, L.T.; Øverland, M. Candida Utilis and Chlorella Vulgaris Counteract Intestinal Inflammation in Atlantic Salmon (Salmo salar L.). PLOS ONE 2013, 8, e83213. [Google Scholar] [CrossRef] [PubMed]
- Gildberg, A.; Johansen, A.; Bøgwald, J. Growth and Survival of Atlantic salmon (Salmo salar) Fry given Diets Supplemented with Fish Protein Hydrolysate and Lactic Acid Bacteria during a Challenge Trial with Aeromonas salmonicida. Aquaculture 1995, 138, 23–34. [Google Scholar] [CrossRef]
- Gildberg, A.; Mikkelsen, H.; Sandaker, E.; Ringø, E. Probiotic Effect of Lactic Acid Bacteria in the Feed on Growth and Survival of Fry of Atlantic (Gadus morhua). Hydrobiologia 1997, 352, 279–285. [Google Scholar] [CrossRef]
- Giri, S.S.; Jun, J.W.; Yun, S.; Kim, H.J.; Kim, S.G.; Kim, S.W.; Woo, K.J.; Han, S.J.; Oh, W.T.; Kwon, J.; et al. Effects of Dietary Heat-Killed Pseudomonas Aeruginosa Strain VSG2 on Immune Functions, Antioxidant Efficacy, and Disease Resistance in Cyprinus Carpio. Aquaculture 2020, 514, 734489. [Google Scholar] [CrossRef]
- Gobi, N.; Vaseeharan, B.; Chen, J.C.; Rekha, R.; Vijayakumar, S.; Anjugam, M.; Iswarya, A. Dietary Supplementation of Probiotic Bacillus Licheniformis Dahb1 Improves Growth Performance, Mucus and Serum Immune Parameters, Antioxidant Enzyme Activity as Well as Resistance against Aeromonas Hydrophila in Tilapia Oreochromis mossambicus. Fish Shellfish Immunol. 2018, 74, 501–508. [Google Scholar] [CrossRef]
- Gobi, N.; Malaikozhundan, B.; Sekar, V.; Shanthi, S.; Vaseeharan, B.; Jayakumar, R.; Khudus Nazar, A. GFP Tagged Vibrio Parahaemolyticus Dahv2 Infection and the Protective Effects of the Probiotic Bacillus Licheniformis Dahb1 on the Growth, Immune and Antioxidant Responses in Pangasius Hypophthalmus. Fish Shellfish Immunol. 2016, 52, 230–238. [Google Scholar] [CrossRef]
- Guo, X.; Chen, D.D.; Peng, K.S.; Cui, Z.W.; Zhang, X.J.; Li, S.; Zhang, Y.A. Identification and Characterization of Bacillus Subtilis from Grass Carp (Ctenopharynodon Idellus) for Use as Probiotic Additives in Aquatic Feed. Fish Shellfish Immunol. 2016, 52, 74–84. [Google Scholar] [CrossRef]
- Gupta, A.; Gupta, P.; Dhawan, A. Paenibacillus Polymyxa as a Water Additive Improved Immune Response of Cyprinus Carpio and Disease Resistance against Aeromonas Hydrophila. Aquac. Rep. 2016, 4, 86–92. [Google Scholar] [CrossRef]
- Guzmán-Villanueva, L.T.; Escobedo-Fregoso, C.; Barajas-Sandoval, D.R.; Gomez-Gil, B.; Peña-Rodríguez, A.; Martínez-Diaz, S.F.; Balcázar, J.L.; Quiroz-Guzmán, E. Assessment of Microbial Dynamics and Antioxidant Enzyme Gene Expression Following Probiotic Administration in Farmed Pacific White Shrimp (Litopenaeus Vannamei). Aquaculture 2020, 519, 734907. [Google Scholar] [CrossRef]
- Hamed Sayed Hassani, M.; Yousefi Jourdehi, A.; Hosseinpour Zelti, A.; Shenavar Masouleh, A.; Bagherzadeh Lakani, F. Effects of Commercial Superzist Probiotic on Growth Performance and Hematological and Immune Indices in Fingerlings Acipenser Baerii. Aquac. Int. 2020, 28, 377–387. [Google Scholar] [CrossRef]
- Han, B.; Long, W.Q.; He, J.Y.; Liu, Y.J.; Si, Y.Q.; Tian, L.X. Effects of Dietary Bacillus Licheniformis on Growth Performance, Immunological Parameters, Intestinal Morphology and Resistance of Juvenile Nile Tilapia (Oreochromis niloticus) to Challenge Infections. Fish Shellfish Immunol. 2015, 46, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Haniffa, M.M. Effect of Probiotic on Microbiological and Haematological Responsiveness of Cat Fish (Heteropnuestes Fossilis) Challenged with Bacteria Aeromonas Hydrophila and Fungi Aphanomyces Invadans. J. Aquac. Res. Dev. 2015, 6, 384. [Google Scholar] [CrossRef]
- Hooshyar, Y.; Abedian Kenari, A.; Paknejad, H.; Gandomi, H. Effects of Lactobacillus Rhamnosus ATCC 7469 on Different Parameters Related to Health Status of Rainbow Trout (Oncorhynchus mykiss) and the Protection Against Yersinia Ruckeri. Probiotics Antimicrob. Proteins 2020, 12, 1370–1384. [Google Scholar] [CrossRef] [PubMed]
- Huynh, T.G.; Hu, S.Y.; Chiu, C.S.; Truong, Q.P.; Liu, C.H. Bacterial Population in Intestines of White Shrimp, Litopenaeus Vannamei Fed a Synbiotic Containing Lactobacillus Plantarum and Galactooligosaccharide. Aquac. Res. 2019, 50, 807–817. [Google Scholar] [CrossRef]
- Jöborn, A.; Olsson, J.C.; Westerdahl, A.; Conway, P.L.; Kjelleberg, S. Colonization in the Fish Intestinal Tract and Production of Inhibitory Substances in Intestinal Mucus and Faecal Extracts by Carnobacterium Sp. Strain K1. J. Fish Dis. 1997, 20, 383–392. [Google Scholar] [CrossRef]
- Kaktcham, P.M.; Temgoua, J.B.; Zambou, F.N.; Diaz-Ruiz, G.; Wacher, C.; de Pérez-Chabela, M.L. In Vitro Evaluation of the Probiotic and Safety Properties of Bacteriocinogenic and Non-Bacteriocinogenic Lactic Acid Bacteria from the Intestines of Nile Tilapia and Common Carp for Their Use as Probiotics in Aquaculture. Probiotics Antimicrob. Proteins 2018, 10, 98–109. [Google Scholar] [CrossRef]
- Klakegg, Ø.; Myhren, S.; Juell, R.A.; Aase, M.; Salonius, K.; Sørum, H. Improved Health and Better Survival of Farmed Lumpfish (Cyclopterus Lumpus) after a Probiotic Bath with Two Probiotic Strains of Aliivibrio. Aquaculture 2019, 518, 734810. [Google Scholar] [CrossRef]
- Kuebutornye, F.K.A.; Wang, Z.; Lu, Y.; Abarike, E.D.; Sakyi, M.E.; Li, Y.; Xie, C.X.; Hlordzi, V. Effects of Three Host-Associated Bacillus Species on Mucosal Immunity and Gut Health of Nile Tilapia, Oreochromis niloticus and Its Resistance against Aeromonas Hydrophila Infection. Fish Shellfish Immunol. 2019, 97, 83–95. [Google Scholar] [CrossRef]
- Lee, C.; Cha, J.H.; Kim, M.G.; Shin, J.; Woo, S.H.; Kim, S.H.; Kim, J.W.; Ji, S.C.; Lee, K.J. The Effects of Dietary Bacillus Subtilis on Immune Response, Hematological Parameters, Growth Performance, and Resistance of Juvenile Olive Flounder (Paralichthys Olivaceus) against Streptococcus Iniae. J. World Aquac. Soc. 2020, 51, 551–562. [Google Scholar] [CrossRef]
- Li, M.; Bao, P.; Song, J.; Ding, J.; Liu, Y.; Ma, Y. Colonization and Probiotic Effect of Metschnikowia Sp. C14 in the Intestine of Juvenile Sea Cucumber, Apostichopus Japonicus. J. Ocean Univ. China 2020, 19, 225–231. [Google Scholar] [CrossRef]
- Lin, H.L.; Shiu, Y.L.; Chiu, C.S.; Huang, S.L.; Liu, C.H. Screening Probiotic Candidates for a Mixture of Probiotics to Enhance the Growth Performance, Immunity, and Disease Resistance of Asian Seabass, Lates Calcarifer (Bloch), against Aeromonas Hydrophila. Fish Shellfish Immunol. 2017, 60, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-H.; Wu, K.; Chu, T.-W.; Wu, T.-M. Dietary Supplementation of Probiotic, Bacillus Subtilis E20, Enhances the Growth Performance and Disease Resistance against Vibrio Alginolyticus in Parrot Fish (Oplegnathus fasciatus). Aquac. Int. 2017, 26, 63–74. [Google Scholar] [CrossRef]
- Marzinelli, E.M.; Dadar, M.; Fiocco, D.; Jaramillo-Torres, A.; Rawling, M.D.; Rodiles, A.; Mikalsen, H.E.; Johansen, L.-H.; Tinsley, J.; Forberg, T.; et al. Influence of Dietary Supplementation of Probiotic Pediococcus Acidilactici MA18/5M During the Transition From Freshwater to Seawater on Intestinal Health and Microbiota of Atlantic Salmon (Salmo salar L.). Front. Microbiol. 2019, 10, 2243. [Google Scholar] [CrossRef]
- Meidong, R.; Khotchanalekha, K.; Doolgindachbaporn, S.; Nagasawa, T.; Nakao, M.; Sakai, K.; Tongpim, S. Evaluation of Probiotic Bacillus Aerius B81e Isolated from Healthy Hybrid Catfish on Growth, Disease Resistance and Innate Immunity of Pla-Mong Pangasius Bocourti. Fish Shellfish Immunol. 2018, 73, 1–10. [Google Scholar] [CrossRef]
- Mohammadi, G.; Rafiee, G.; Abdelrahman, H.A. Effects of Dietary Lactobacillus Plantarum (KC426951) in Biofloc and Stagnant-Renewal Culture Systems on Growth Performance, Mucosal Parameters, and Serum Innate Responses of Nile Tilapia Oreochromis niloticus. Fish Physiol. Biochem. 2020, 46, 1167–1181. [Google Scholar] [CrossRef]
- Mohammadian, T.; Nasirpour, M.; Tabandeh, M.R.; Heidary, A.A.; Ghanei-Motlagh, R.; Hosseini, S.S. Administrations of Autochthonous Probiotics Altered Juvenile Rainbow Trout Oncorhynchus mykiss Health Status, Growth Performance and Resistance to Lactococcus Garvieae, an Experimental Infection. Fish Shellfish Immunol. 2019, 86, 269–279. [Google Scholar] [CrossRef]
- Mohammadian, T.; Nasirpour, M.; Tabandeh, M.R.; Mesbah, M. Synbiotic Effects of β-Glucan, Mannan Oligosaccharide and Lactobacillus Casei on Growth Performance, Intestine Enzymes Activities, Immune-Hematological Parameters and Immune-Related Gene Expression in Common Carp, Cyprinus Carpio: An Experimental Infection with Aeromonas Hydrophila. Aquaculture 2019, 511, 634197. [Google Scholar] [CrossRef]
- Moxley, K.; Coyne, V.E. Improved Growth and Survival of Post-Larval Haliotis Midae in Response to Probiotic Biofilm Diets. Aquaculture 2020, 519, 734929. [Google Scholar] [CrossRef]
- Nandi, A.; Banerjee, G.; Dan, S.K.; Ghosh, K.; Ray, A.K. Probiotic Efficiency of Bacillus sp. in Labeo Rohita Challenged by Aeromonas Hydrophila: Assessment of Stress Profile, Haemato-Biochemical Parameters and Immune Responses. Aquac. Res. 2017, 48, 4334–4345. [Google Scholar] [CrossRef]
- Nofouzi, K.; Sheikhzadeh, N.; Varshoie, H.; Sharabyani, S.K.; Jafarnezhad, M.; Shabanzadeh, S.; Ahmadifar, E.; Stanford, J.; Shahbazfar, A.A. Beneficial Effects of Killed Tsukamurella Inchonensis on Rainbow Trout (Oncorhynchus mykiss) Growth, Intestinal Histology, Immunological, and Biochemical Parameters. Fish Physiol. Biochem. 2019, 45, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Ock Kim, Y.; Mahboob, S.; Viayaraghavan, P.; Biji, D.; Abdullah Al-Ghanim, K.; Al-Misned, F.; Ahmed, Z.; Kwon, J.T.; Won Na, S.; Kim, H.J. Growth Promoting Activity of Penaeus Indicus by Secondary Metabolite Producing Probiotic Bacterium Bacillus Subtilis Isolated from the Shrimp Gut. J. King Saud Univ. Sci. 2020, 32, 1641–1646. [Google Scholar] [CrossRef]
- Ringø, E.; Salinas, I.; Olsen, R.E.; Nyhaug, A.; Myklebust, R.; Mayhew, T.M. Histological Changes in Intestine of Atlantic Salmon (Salmo salar L.) Following in Vitro Exposure to Pathogenic and Probiotic Bacterial Strains. Cell Tissue Res. 2007, 328, 109–116. [Google Scholar] [CrossRef]
- Romarheim, O.H.; Øverland, M.; Mydland, L.T.; Skrede, A.; Landsverk, T. Bacteria Grown on Natural Gas Prevent Soybean Meal-Induced Enteritis in Atlantic Salmon. J. Nutr. 2011, 141, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Safari, R.; Adel, M.; Lazado, C.C.; Caipang, C.M.A.; Dadar, M. Host-Derived Probiotics Enterococcus Casseliflavus Improves Resistance against Streptococcus Iniae Infection in Rainbow Trout (Oncorhynchus mykiss) via Immunomodulation. Fish Shellfish Immunol. 2016, 52, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Salinas, I.; Myklebust, R.; Esteban, M.A.; Olsen, R.E.; Meseguer, J.; Ringø, E. In Vitro Studies of Lactobacillus delbrueckii Subsp. Lactis in Atlantic salmon (Salmo salar L.) Foregut: Tissue Responses and Evidence of Protection against Aeromonas salmonicida Subsp. Salmonicida Epithelial Damage. Veter. Microbiol. 2008, 128, 167–177. [Google Scholar] [CrossRef]
- Samson, J.S.; Choresca, C.H.; Quiazon, K.M.A. Selection and Screening of Bacteria from African Nightcrawler, Eudrilus eugeniae (Kinberg, 1867) as Potential Probiotics in Aquaculture. World J. Microbiol. Biotechnol. 2020, 36, 16. [Google Scholar] [CrossRef]
- Sankar, H.; Philip, B.; Philip, R.; Singh, I.S.B. Effect of Probiotics on Digestive Enzyme Activities and Growth of Cichlids, Etroplus suratensis (Pearl Spot) and Oreochromis mossambicus (Tilapia). Aquac. Nutr. 2017, 23, 852–864. [Google Scholar] [CrossRef]
- Santos, K.O.; Costa-Filho, J.; Spagnol, K.L.; Nornberg, B.F.; Lopes, F.M.; Tesser, M.B.; Marins, L.F. The Inclusion of a Transgenic Probiotic Expressing Recombinant Phytase in a Diet with a High Content of Vegetable Matter Markedly Improves Growth Performance and the Expression of Growth-Related Genes and Other Selected Genes in Zebrafish. Aquaculture 2020, 519, 734878. [Google Scholar] [CrossRef]
- Schaeck, M.; Reyes-López, F.E.; Vallejos-Vidal, E.; van Cleemput, J.; Duchateau, L.; van den Broeck, W.; Tort, L.; Decostere, A. Cellular and Transcriptomic Response to Treatment with the Probiotic Candidate Vibrio Lentus in Gnotobiotic Sea Bass (Dicentrarchus labrax) Larvae. Fish Shellfish Immunol. 2017, 63, 147–156. [Google Scholar] [CrossRef]
- Tan, H.Y.; Chen, S.W.; Hu, S.Y. Improvements in the Growth Performance, Immunity, Disease Resistance, and Gut Microbiota by the Probiotic Rummeliibacillus Stabekisii in Nile Tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2019, 92, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Sha, Y.; Wang, L.; Liu, M.; Jiang, K.; Xin, F.; Wang, B. Effects of Lactic Acid Bacteria and the Corresponding Supernatant on the Survival, Growth Performance, Immune Response and Disease Resistance of Litopenaeus Vannamei. Aquaculture 2016, 452, 28–36. [Google Scholar] [CrossRef]
- Soltani, M.; Pakzad, K.; Taheri-Mirghaed, A.; Mirzargar, S.; Shekarabi, S.P.H.; Yosefi, P.; Soleymani, N. Dietary Application of the Probiotic Lactobacillus Plantarum 426951 Enhances Immune Status and Growth of Rainbow Trout (Oncorhynchus mykiss) Vaccinated Against Yersinia Ruckeri. Probiotics Antimicrob. Proteins 2019, 11, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, L.; Telli, G.S.; de Carla Dias, D.; Gonçalves, G.S.; Ishikawa, C.M.; Cavalcante, R.B.; Natori, M.M.; Hamed, S.B.; Ranzani-Paiva, M.J.T. Effect of Feeding Strategy of Probiotic Enterococcus Faecium on Growth Performance, Hematologic, Biochemical Parameters and Non-Specific Immune Response of Nile Tilapia. Aquac. Rep. 2020, 16, 100277. [Google Scholar] [CrossRef]
- Tamamdusturi, R.; Widanarni; Yuhana, M. Administration of Microencapsulated Probiotic Bacillus sp. NP5 and Prebiotic Mannan Oligosaccharide for Prevention of Aeromonas Hydrophila Infection on Pangasianodon Hypophthalmus. J. Fish. Aquat. Sci. 2016, 11, 67–76. [Google Scholar] [CrossRef]
- Tarkhani, R.; Imani, A.; Hoseinifar, S.H.; Ashayerizadeh, O.; Sarvi Moghanlou, K.; Manaffar, R.; van Doan, H.; Reverter, M. Comparative Study of Host-Associated and Commercial Probiotic Effects on Serum and Mucosal Immune Parameters, Intestinal Microbiota, Digestive Enzymes Activity and Growth Performance of Roach (Rutilus rtilus caspicus) Fingerlings. Fish Shellfish Immunol. 2020, 98, 661–669. [Google Scholar] [CrossRef]
- Thurlow, C.M.; Williams, M.A.; Carrias, A.; Ran, C.; Newman, M.; Tweedie, J.; Allison, E.; Jescovitch, L.N.; Wilson, A.E.; Terhune, J.S.; et al. Bacillus Velezensis AP193 Exerts Probiotic Effects in Channel Catfish (Ictalurus punctatus) and Reduces Aquaculture Pond Eutrophication. Aquaculture 2019, 503, 347–356. [Google Scholar] [CrossRef]
- Tsai, C.Y.; Chi, C.C.; Liu, C.H. The Growth and Apparent Digestibility of White Shrimp, Litopenaeus Vannamei, Are Increased with the Probiotic, Bacillus Subtilis. Aquac. Res. 2019, 50, 1475–1481. [Google Scholar] [CrossRef]
- Vadassery, D.H.; Pillai, D. Quorum Quenching Potential of Enterococcus Faecium QQ12 Isolated from Gastrointestinal Tract of Oreochromis niloticus and Its Application as a Probiotic for the Control of Aeromonas Hydrophila Infection in Goldfish Carassius auratus (Linnaeus 1758). Braz. J. Microbiol. 2020, 51, 1333–1343. [Google Scholar] [CrossRef]
- Vasanth, G.K.; Kiron, V.; Kulkarni, A.; Dahle, D.; Lokesh, J.; Kitani, Y. A Microbial Feed Additive Abates Intestinal Inflammation in Atlantic Salmon. Front. Immunol. 2015, 6, 409. [Google Scholar] [CrossRef]
- Vazirzadeh, A.; Roosta, H.; Masoumi, H.; Farhadi, A.; Jeffs, A. Long-Term Effects of Three Probiotics, Singular or Combined, on Serum Innate Immune Parameters and Expressions of Cytokine Genes in Rainbow Trout during Grow-Out. Fish Shellfish Immunol. 2020, 98, 748–757. [Google Scholar] [CrossRef] [PubMed]
- Vinoj, G.; Jayakumar, R.; Chen, J.C.; Withyachumnarnkul, B.; Shanthi, S.; Vaseeharan, B. N-Hexanoyl-L-Homoserine Lactone-Degrading Pseudomonas Aeruginosa PsDAHP1 Protects Zebrafish against Vibrio Parahaemolyticus Infection. Fish Shellfish Immunol. 2015, 42, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yi, M.; Lu, M.; Gao, F.; Liu, Z.; Huang, Q.; Li, Q.; Zhu, D. Effects of Probiotics Bacillus Cereus NY5 and Alcaligenes Faecalis Y311 Used as Water Additives on the Microbiota and Immune Enzyme Activities in Three Mucosal Tissues in Nile Tilapia Oreochromis niloticus Reared in Outdoor Tanks. Aquac. Rep. 2020, 17, 100309. [Google Scholar] [CrossRef]
- Wu, Z.Q.; Jiang, C.; Ling, F.; Wang, G.X. Effects of Dietary Supplementation of Intestinal Autochthonous Bacteria on the Innate Immunity and Disease Resistance of Grass Carp (Ctenopharyngodon idellus). Aquaculture 2015, 438, 105–114. [Google Scholar] [CrossRef]
- Yamashita, M.M.; Ferrarezi, J.V.; do Pereira, G.V.; Bandeira, G.; Côrrea da Silva, B.; Pereira, S.A.; Martins, M.L.; Pedreira Mouriño, J.L. Autochthonous vs Allochthonous Probiotic Strains to Rhamdia Quelen. Microb. Pathog. 2020, 139, 103897. [Google Scholar] [CrossRef]
- Yi, Y.; Zhang, Z.; Zhao, F.; Liu, H.; Yu, L.; Zha, J.; Wang, G. Probiotic Potential of Bacillus Velezensis JW: Antimicrobial Activity against Fish Pathogenic Bacteria and Immune Enhancement Effects on Carassius auratus. Fish Shellfish Immunol. 2018, 78, 322–330. [Google Scholar] [CrossRef]
- Yu, L.; Qiao, N.; Li, T.; Yu, R.; Zhai, Q.; Tian, F.; Zhao, J.; Zhang, H.; Chen, W. Dietary Supplementation with Probiotics Regulates Gut Microbiota Structure and Function in Nile Tilapia Exposed to Aluminum. PeerJ 2019, 7, e6963. [Google Scholar] [CrossRef]
- Yu, L.; Zhai, Q.; Zhu, J.; Zhang, C.; Li, T.; Liu, X.; Zhao, J.; Zhang, H.; Tian, F.; Chen, W. Dietary Lactobacillus Plantarum Supplementation Enhances Growth Performance and Alleviates Aluminum Toxicity in Tilapia. Ecotoxicol. Environ. Saf. 2017, 143, 307–314. [Google Scholar] [CrossRef]
- Zhao, D.; Wu, S.; Feng, W.; Jakovlić, I.; Tran, N.T.; Xiong, F. Adhesion and Colonization Properties of Potentially Probiotic Bacillus Paralicheniformis Strain FA6 Isolated from Grass Carp Intestine. Fish. Sci. 2020, 86, 153–161. [Google Scholar] [CrossRef]
- Qin, C.; Zhang, Z.; Wang, Y.; Li, S.; Ran, C.; Hu, J.; Xie, Y.; Li, W.; Zhou, Z. EPSP of L. Casei BL23 Protected against the Infection Caused by Aeromonas Veronii via Enhancement of Immune Response in Zebrafish. Front. Microbiol. 2017, 8, 2406. [Google Scholar] [CrossRef]
- Yousefi, B.; Eslami, M.; Ghasemian, A.; Kokhaei, P.; Salek Farrokhi, A.; Darabi, N. Probiotics Importance and Their Immunomodulatory Properties. J. Cell. Physiol. 2019, 234, 8008–8018. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Zhang, F.; Zhou, K.; Zhao, Y.; Zhao, Q.; Sun, H. Isolation, Identification and Fermentation Optimization of Lactic Acid Bacteria for Aquaculture Water Purification. Acta Microbiol. Sin. 2017, 57, 304–314. Available online: https://europepmc.org/article/med/29750493 (accessed on 10 February 2022).
- Wang, A.; Ran, C.; Wang, Y.; Zhang, Z.; Ding, Q.; Yang, Y.; Olsen, R.E.; Ringø, E.; Bindelle, J.; Zhou, Z. Use of Probiotics in Aquaculture of China—A Review of the Past Decade. Fish Shellfish Immunol. 2019, 86, 734–755. [Google Scholar] [CrossRef]
- Cotter, P.D.; Ross, R.P.; Hill, C. Bacteriocins—A Viable Alternative to Antibiotics? Nat. Rev. Microbiol. 2012, 11, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Amenyogbe, E.; Chen, G.; Wang, Z.; Huang, J.S.; Huang, B.; Li, H. The Exploitation of Probiotics, Prebiotics and Synbiotics in Aquaculture: Present Study, Limitations and Future Directions: A Review. Aquac. Int. 2020, 28, 1017–1041. [Google Scholar] [CrossRef]
- Chauhan, A.; Singh, R. Probiotics in Aquaculture: A Promising Emerging Alternative Approach. Symbiosis 2019, 77, 99–113. [Google Scholar] [CrossRef]
- Merrifield, D.L.; Bradley, G.; Harper, G.M.; Baker, R.T.M.; Munn, C.B.; Davies, S.J. Assessment of the Effects of Vegetative and Lyophilized Pediococcus Acidilactici on Growth, Feed Utilization, Intestinal Colonization and Health Parameters of Rainbow Trout (Oncorhynchus mykiss Walbaum). Aquac. Nutr. 2011, 17, 73–79. [Google Scholar] [CrossRef]
- Ramakrishna, B. Probiotic-Induced Changes in the Intestinal Epithelium: Implications in Gastrointestinal Disease. Trop. Gastroenterol. 2009, 30, 76–85. [Google Scholar] [CrossRef]
- Lugert, V.; Thaller, G.; Tetens, J.; Schulz, C.; Krieter, J. A Review on Fish Growth Calculation: Multiple Functions in Fish Production and Their Specific Application. Rev. Aquac. 2016, 8, 30–42. [Google Scholar] [CrossRef]
- Saurabh, S.; Sahoo, P.K. Lysozyme: An Important Defence Molecule of Fish Innate Immune System. Aquac. Res. 2008, 39, 223–239. [Google Scholar] [CrossRef]
- Verheul, A.; Russell, N.J.; van ’T Hof, R.; Rombouts, F.M.; Abee, T. Modifications of Membrane Phospholipid Composition in Nisin-Resistant Listeria Monocytogenes Scott A. Appl. Environ. Microbiol. 1997, 63, 3451–3457. [Google Scholar] [CrossRef] [PubMed]
- Ogaki, M.B.; Furlaneto, M.C.; Maia, L.F. Revisão: Aspectos Gerais Das Bacteriocinas. Braz. J. Food Technol. 2015, 18, 267–276. [Google Scholar] [CrossRef]
- Chen, Y.; Ludescher, R.D.; Montville, T.J. Electrostatic Interactions, but Not the YGNGV Consensus Motif, Govern the Binding of Pediocin PA-1 and Its Fragments to Phospholipid Vesicles. Appl. Environ. Microbiol. 1997, 63, 4770–4777. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.C.; Lin, C.H.; Sung, C.T.; Fang, J.Y. Antibacterial Activities of Bacteriocins: Application in Foods and Pharmaceuticals. Front. Microbiol. 2014, 5, 241. [Google Scholar] [CrossRef]
- Klaenhammer, T.R. Genetics of Bacteriocins Produced by Lactic Acid Bacteria. FEMS Microbiol. Rev. 1993, 12, 39–85. [Google Scholar] [CrossRef]
- Riley, M.A.; Wertz, J.E. Bacteriocins: Evolution, Ecology, and Application. Annu. Rev. Microbiol. 2003, 56, 117–137. [Google Scholar] [CrossRef]
- Cleveland, J.; Montville, T.J.; Nes, I.F.; Chikindas, M.L. Bacteriocins: Safe, Natural Antimicrobials for Food Preservation. Int. J. Food Microbiol. 2001, 71, 1–20. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, L.; Zhao, L.; He, Y.; Yang, H. Antimicrobial Kinetics of Nisin and Grape Seed Extract against Inoculated Listeria Monocytogenes on Cooked Shrimps: Survival and Residual Effects. Food Control 2020, 115, 107278. [Google Scholar] [CrossRef]
- Cohen, P.A. Probiotic Safety—No Guarantees. JAMA Intern. Med. 2018, 178, 1577–1578. [Google Scholar] [CrossRef]
- An, J.; Zhu, W.; Liu, Y.; Zhang, X.; Sun, L.; Hong, P.; Wang, Y.; Xu, C.; Xu, D.; Liu, H. Purification and Characterization of a Novel Bacteriocin CAMT2 Produced by Bacillus Amyloliquefaciens Isolated from Marine Fish Epinephelus Areolatus. Food Control 2015, 51, 278–282. [Google Scholar] [CrossRef]
- Banerjee, G.; Nandi, A.; Ray, A.K. Assessment of Hemolytic Activity, Enzyme Production and Bacteriocin Characterization of Bacillus Subtilis LR1 Isolated from the Gastrointestinal Tract of Fish. Arch. Microbiol. 2017, 199, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Baños, A.; Ariza, J.J.; Nuñez, C.; Gil-Martínez, L.; García-López, J.D.; Martínez-Bueno, M.; Valdivia, E. Effects of Enterococcus Faecalis UGRA10 and the Enterocin AS-48 against the Fish Pathogen Lactococcus Garvieae. Studies in Vitro and in Vivo. Food Microbiol. 2019, 77, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Feliatra, F.; Muchlisin, Z.A.; Teruna, H.Y.; Utamy, W.R.; Nursyirwani, N.; Dahliaty, A. Potential of Bacteriocins Produced by Probiotic Bacteria Isolated from Tiger Shrimp and Prawns as Antibacterial to Vibrio, Pseudomonas, and Aeromonas Species on Fish. F1000Research 2018, 7, 415. [Google Scholar] [CrossRef]
- Fu, L.; Wang, C.; Ruan, X.; Li, G.; Zhao, Y.; Wang, Y. Preservation of Large Yellow Croaker (Pseudosciaena crocea) by Coagulin L1208, a Novel Bacteriocin Produced by Bacillus coagulans L1208. Int. J. Food Microbiol. 2018, 266, 60–68. [Google Scholar] [CrossRef]
- Lv, X.; Du, J.; Jie, Y.; Zhang, B.; Fengling, B.; Zhao, H.; Li, J. Purification and Antibacterial Mechanism of Fish-Borne Bacteriocin and Its Application in Shrimp (Penaeus vannamei) for Inhibiting Vibrio Parahaemolyticus. World J. Microbiol. Biotechnol. 2017, 33, 156. [Google Scholar] [CrossRef] [PubMed]
- Sarika, A.; Lipton, A.; Aishwarya, M.; Mol, R.R. Lactic Acid Bacteria from Marine Fish: Antimicrobial Resistance and Production of Bacteriocin Effective Against L. monocytogenes In Situ. J. Food Microbiol. Saf. Hyg. 2018, 3, 1–6. [Google Scholar] [CrossRef]
- Schelegueda, L.I.; Vallejo, M.; Gliemmo, M.F.; Marguet, E.R.; Campos, C.A. Synergistic Antimicrobial Action and Potential Application for Fish Preservation of a Bacteriocin Produced by Enterococcus Mundtii Isolated from Odontesthes Platensis. LWT-Food Sci. Technol. 2015, 64, 794–801. [Google Scholar] [CrossRef]
- Sequeiros, C.; Garcés, M.E.; Vallejo, M.; Marguet, E.R.; Olivera, N.L. Potential Aquaculture Probiont Lactococcus Lactis TW34 Produces Nisin Z and Inhibits the Fish Pathogen Lactococcus Garvieae. Arch. Microbiol. 2015, 197, 449–458. [Google Scholar] [CrossRef]
- Du Toit, M.; Franz, C.M.A.P.; Dicks, L.M.T.; Holzapfel, W.H. Preliminary Characterization of Bacteriocins Produced by Enterococcus Faecium and Enterococcus Faecalis Isolated from Pig Faeces. J. Appl. Microbiol. 2000, 88, 482–494. [Google Scholar] [CrossRef]
- Ennara Sudarsanan, S.; Thangappan, B. Antimicrobial Activity and Anti-Aflatoxigenic Activity of Bacteriocin Isolated from Pediococcus Acidilactici from Fish Wastes. Biotechnol. Res. 2017, 3, 104–125. [Google Scholar] [CrossRef]
- Wang, Y.; Qin, Y.; Xie, Q.; Zhang, Y.; Hu, J.; Li, P. Purification and Characterization of Plantaricin LPL-1, a Novel Class IIa Bacteriocin Produced by Lactobacillus Plantarum LPL-1 Isolated From Fermented Fish. Front. Microbiol. 2018, 9, 2276. [Google Scholar] [CrossRef] [PubMed]
- Woraprayote, W.; Pumpuang, L.; Tosukhowong, A.; Zendo, T.; Sonomoto, K.; Benjakul, S.; Visessanguan, W. Antimicrobial Biodegradable Food Packaging Impregnated with Bacteriocin 7293 for Control of Pathogenic Bacteria in Pangasius Fish Fillets. LWT Food Sci. Technol. 2018, 89, 427–433. [Google Scholar] [CrossRef]
- Wright, E.E.; Nguyen, H.U.; Owens, L. Oceanogr Fish Open Access J Preliminary Characterization of a Nisin Z Bacteriocin with Activity Against the Fish Pathogen Streptococcus Iniae. Oceanogr. Fish. Open Access J. 2017, 3. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, J.; Liu, Y.; Wu, Y.; Fang, Z.; Wang, Y.; Sun, L.; Deng, Q.; Gooneratne, R.; Xiao, L. A Novel Bacteriocin PE-ZYB1 Produced by Pediococcus Pentosaceus Zy-B Isolated from Intestine of Mimachlamys Nobilis: Purification, Identification and Its Anti-Listerial Action. LWT 2020, 118, 108760. [Google Scholar] [CrossRef]
- Egervärn, M.; Lindmark, H.; Olsson, J.; Roos, S. Transferability of a Tetracycline Resistance Gene from Probiotic Lactobacillus Reuteri to Bacteria in the Gastrointestinal Tract of Humans. Antonie Leeuwenhoek Int. J. General Mol. Microbiol. 2010, 97, 189–200. [Google Scholar] [CrossRef]
- Shanahan, F. A Commentary on the Safety of Probiotics. Gastroenterol. Clin. N. Am. 2012, 41, 869–876. [Google Scholar] [CrossRef]
- FAO Food and Agriculture Organization of the United Nations. Probiotics in Animal Nutrition: Production, Impact and Regulation. Available online: https://agris.fao.org/agris-search/search.do?recordID=XF2017001765 (accessed on 12 February 2022).
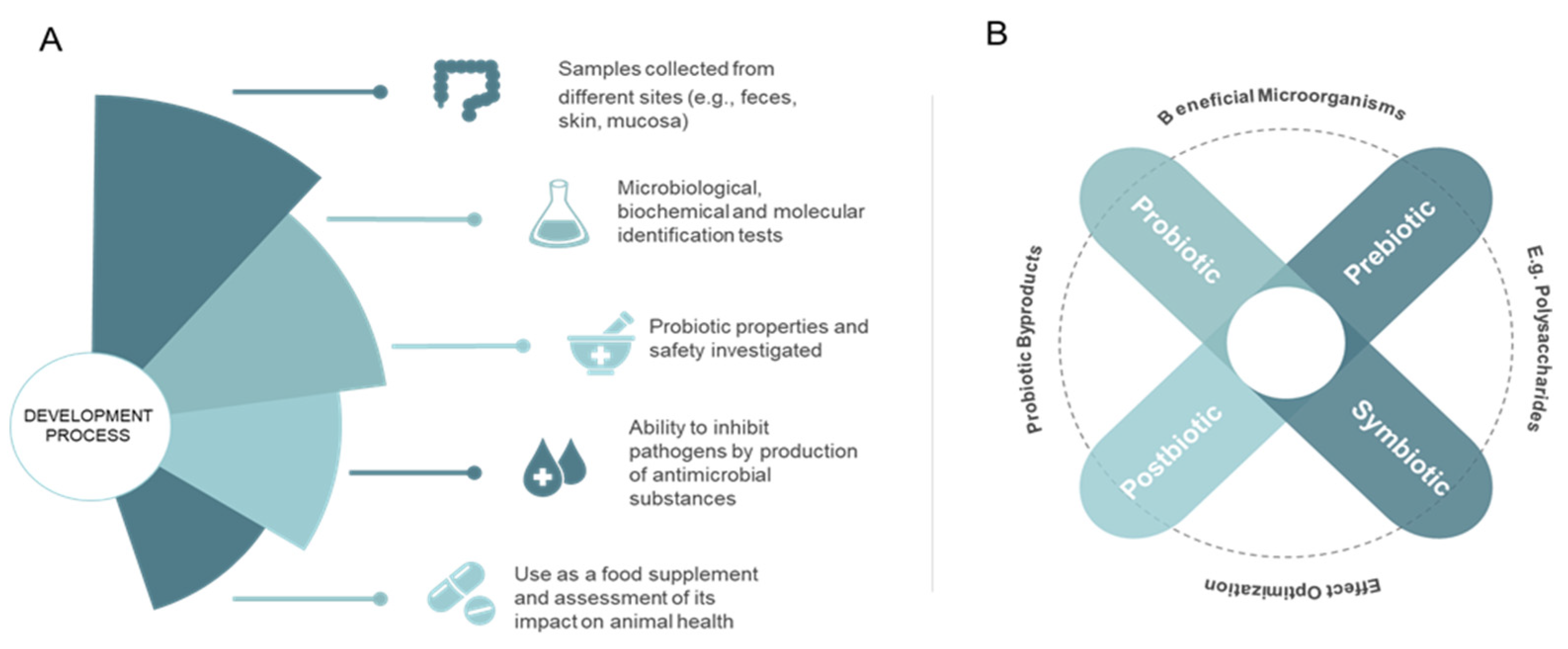


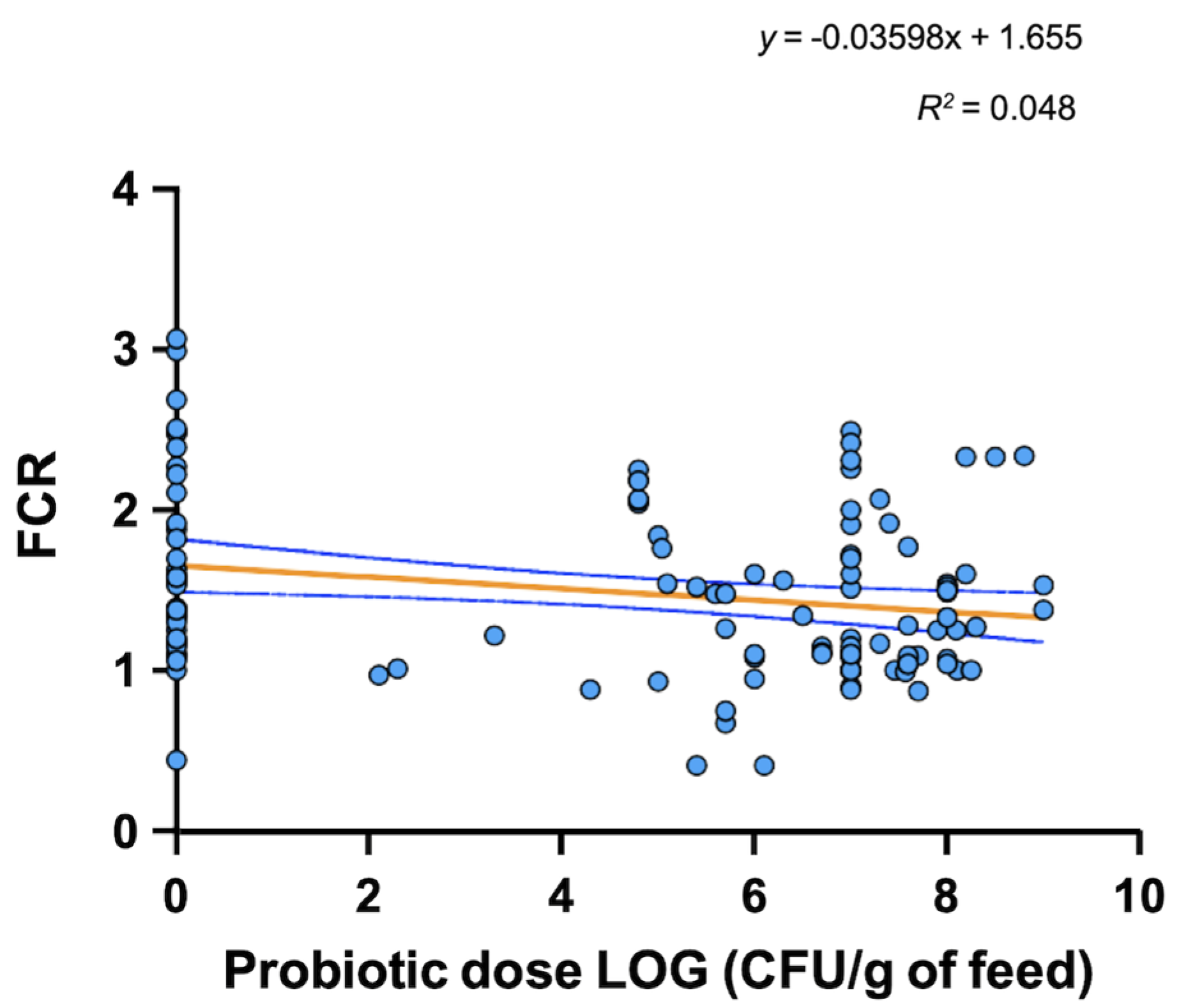
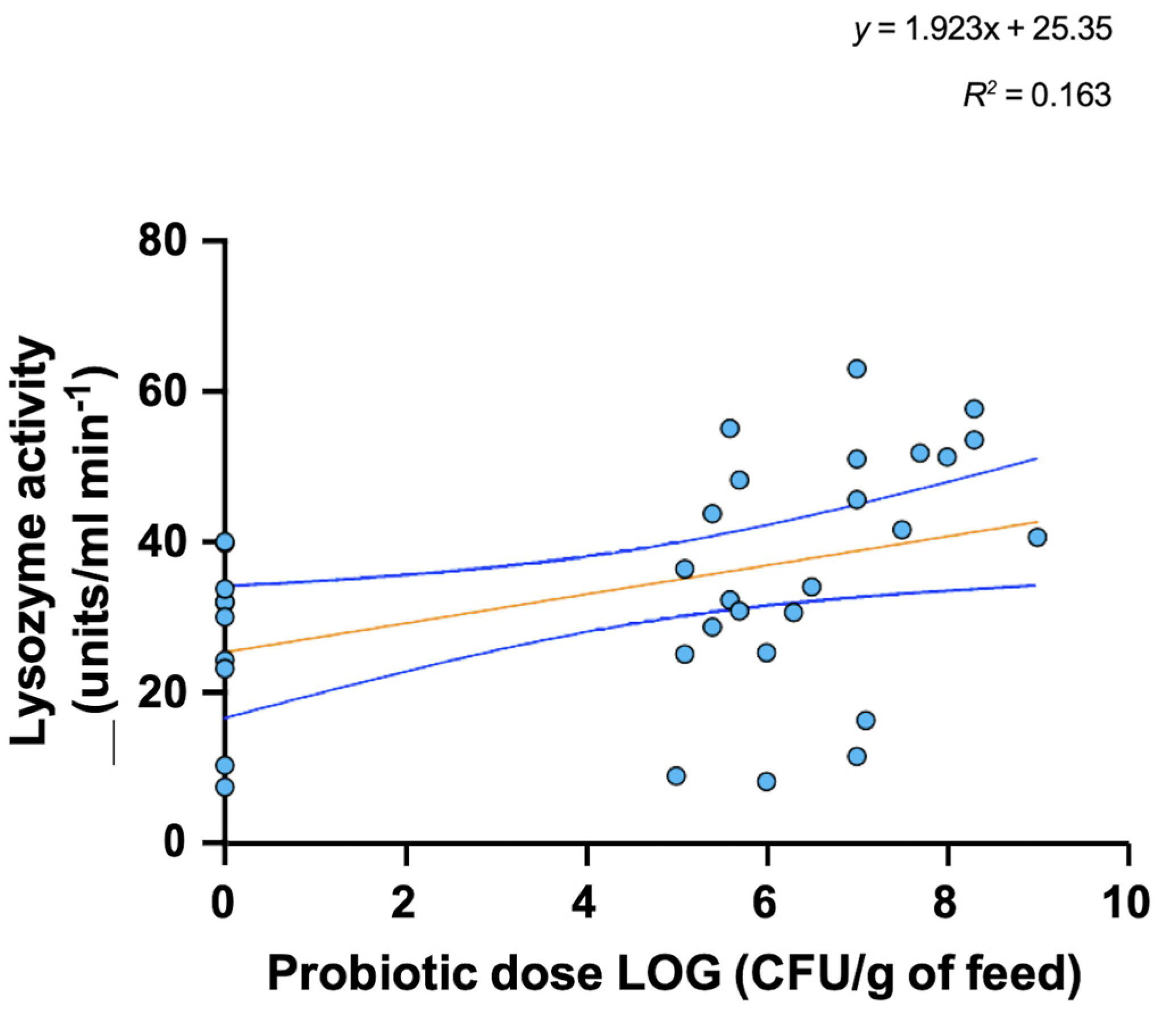
| Aquatic Specie | Probiotic | Pathogen or Challenge | Clinical Impact | Reference |
|---|---|---|---|---|
| Oreochromis niloticus | Mixture of LAB | Trichodina sp. | Improved growth rate and antiparasitic activity | [43] |
| Cyprinus carpio | Pediococcus pentosaceus | Aeromonas hydrophila | Probiotic increases digestive enzyme activity; enhancement of growth rate and immune response; resistance against bacterial infection | [44] |
| Litopenaeus vannamei | Mix of commercial probiotics (e.g., Bacillus spp., Lactobacillus spp., Saccharomyces spp.) | Not evaluated | The probiotics did not change water quality or growth parameters when compared with control group | [45] |
| Salmonids | Vibrio alginolyticus | A. salmonicida, V. anguillariim, V. ordalii | Pathogen inhibition | [46] |
| Salmo salar | Tetraselmis suecica | A. salmonicida, S. liquefaciens, V. anguillariim, V. salmonicida, Y. ruckeri | Suppress pathogen growth | [47] |
| Salmo tutta | Lactococcus lactis, Leuconostoc mesenteroides | Aeromonas salmonicida | Higher survival rate | [48] |
| Mystus cavasius | Saccharomyces cerevisiae | Pseudomonas fluorescens | Better weight gain, low mortality; resistance against tested pathogen | [49,50] |
| Labeo rohita | Probiotic mixture (Bacillus subtilis, Pediococcus acidilactici, yeast Saccharomyces cerevisiae) and symbiotics (Bifidobacterium, Lactobacilli, Saccharomyces cerevisiae, microalgae Spirulina sp., phytase) | Not evaluated | Better survival and growth rate; probiotic action is best if administered to developing fish in their first days | [50] |
| Litopenaeus vannamei | Bacillus subtilis | Not evaluated | Significant secretion of hepatopancreatic metabolites; expression of genes linked to antioxidant enzymes | [51] |
| Oreochromis niloticus | Aspergillus oryzae | Aeromonas hydrophila | Improvement of immune response and growth rate | [52] |
| Oreochromis niloticus | Lactobacillus plantarum L-137 | Exposition to deltamethrin toxicity | Reduction of the toxicity | [52] |
| Pagrus major | Pediococcus pentosaceus | Not evaluated | Increased weight gain, mucus secretion, growth rate, bacterial resistance, and blood parameters | [53] |
| Pagrus major | Lactobacillus plantarum | Not evaluated | Immunostimulant property (innate defenses) | [54] |
| Pagrus major | Lactobacillus rhamnosus and Lactococcus lactis | Not evaluated | Better growth, feed utilization, serum lysozyme activity, bactericidal property, and lower triglycerides and cholesterol | [55] |
| Oreochromis niloticus | Bacillus subtilis and Bacillus licheniformis | Not evaluated | Enhanced immunological parameters (hematocrit, total leukocytes count, monocytes, and globulin), improved growth and feed utilization | [56] |
| Oreochromis niloticus | Lactobacillus sp., Bacillus sp., Bifidobacterium sp. (probiotic mixture) | Not evaluated | Antimicrobial activity, better growth rate | [57] |
| Oreochromis niloticus | Lactobacillus plantarum | Enterococcus faecalis | Modulation of gut microbiota, immune response, and resistance against pathogenic bacteria | [58] |
| Atlantic salmon | Candida utilis | Chlorella vulgaris | Counteracts intestinal inflammation | [59] |
| Salmon salar | Lactic acid bacteria | Aeromonas salmonicida | Higher mortality | [60] |
| Gadus morhua (Atlantic cod), | Carnobacterium divergens | V. anguillarum | Disease resistance | [61] |
| Cyprinus carpio | Pseudomonas aeruginosa | Aeromonas hydrophila | Antioxidant and immune action; better infection control with probiotic treatment | [62] |
| Oreochromis mossambicus | Bacillus licheniformis Dahb1 (105 and 107) | Aeromonas hydrophilain | Weight and specific growth rate improvement; high mucosal activity of enzymes; resistance to the infection | [63] |
| Pangasius hypophthalmus | Bacillus licheniformis | Vibrio parahaemolyticus | Increased immune, antioxidant and growth parameters; protected against infection | [64] |
| Ctenopharynodon idellus | Bacillus subtilis | Aeromonas hydrophila, Aeromonas punctata, Edwardsiella ictaluri, Aeromonas punctate, Vibrio flurialis and Streptococcus agalactiae | Inhibitory activity against all pathogenic bacteria tested | [65] |
| Cyprinus carpio | Paenibacillus polymyxa | Aeromonas hydrophila | Improved survival rate and immune response; disease resistance against pathogenic bacteria tested | [66] |
| Litopenaeus vannamei | Bacillus subtilis, Bacillus pumilus, Bacillus tequilensis, Enterococcus faecalis | Not evaluated | Significant difference in growth rate, weight gain, and survival | [67] |
| Acipenser baerii | Lactobacillus spp. Bacillus subtilis, Bifidobacterium bifidum (probiotics mixture) | Not evaluated | Immunity and growth improvement | [68] |
| Oreochromis niloticus | Bacillus licheniformis | Streptococcus iniae | Better survival rate | [69] |
| Heteropnuestes fossilis | Bacillus subtilis | Aeromonas hydrophila and Aphanomyces invadans | Bacterial treatment leads to a health improvement; fungi treatment does not | [70] |
| Oncorhynchus mykiss | Lactobacillus rhamnosus | Yersinia ruckeri | Improved growth rate, immune response, and antioxidant activity; pathogen inhibition | [71] |
| Litopenaeus vannamei | Lactobacillus plantarum and galactooligosaccharide (symbiotic) | Vibrio harveyi and Photobacterium damselae | Improvement in growth and health parameters; infection control; significant changes in intestinal microbiota of shrimp | [72] |
| Salmonids | Carnobacterium Inhibens K1 | Vibrio anguillarum, Aeromonas salmonicida | Suppress pathogen growth | [73] |
| Oreochromis niloticus and Cyprinus carpio | Lactococcus lactis subsp. lactis, Lactobacillus plantarum, Lactobacillus brevi | Vibrio sp., Staphylococcus sp., Pseudomonas aeruginosa, Salmonella enterica, Listeria monocytogenes | Antimicrobial action | [74] |
| Cyclopterus lumpus | Aliivibrio sp. | Moritella viscosa (contamination) | Resistance against infection caused by M. viscosa; low incidence of mortality and ulcers | [75] |
| Oreochromis niloticus | Bacillus velezensis, Bacillus subtilis, Bacillus amyloliquefaciens | Aeromonas hydrophila | Improvement of immune response; antimicrobial activity | [76] |
| Paralichthys olivaceus | Bacillus sp. and β-glucan (symbiotic) | Edwardsiella tarda | Strain has significant antimicrobial activity; symbiotic effect improved growth performance; resistance against tested pathogen (antibiotic replacement) | [77] |
| Apostichopus japonicus | Metschnikowia sp. | Not evaluated | High activity of lysozyme, total nitric oxide synthase, trypsin, and phenoloxidase | [78] |
| Lates calcarifer | Lactobacillus casei, Lactobacillus plantarum, Lactobacillus pentosus, Lactobacillus fermentum, Enterococcus faecium, Bacillus subtilis, and Saccharomyces cerevisiae | Aeromonas hydrophila | The probiotic mixture improved growth and health status of Asian Seabass | [79] |
| Oplegnathus fasciatus | Bacillus subtilis E20 | Vibrio alginolyticus | Better growth rate and immune response; pathogen resistance | [80] |
| Salmon salar | Pediococcus acidilactici | IPN virus | Antiviral response | [81] |
| Pangasius bocourti | Bacillus aerius B81 | Aeromonas hydrophila, Streptococcus agalactiae | Antimicrobial effect against tested pathogens, high immune response | [82] |
| Oreochromis niloticus | Lactobacillus plantarum | Environmental challenges | High mucosal immune response | [83] |
| Oncorhynchus mykiss | Lactobacillus acidophilus | Lactococcus garvieae | Better growth rate, digestive enzyme production, resistance against tested pathogen | [84] |
| Cyprinus carpio | Lactobacillus casei, β-glucan and mannan oligosaccharide (symbiotic) | Aeromonas hydrophila | Symbiotic improves the digestibility; elevation in important enzymes (lipase, amylase, trypsin, and protease); low mortality | [85] |
| Haliotis midae | Vibrio midae | Not evaluated | Increase in growth performance and survival rate | [86] |
| Labeo rohita | Bacillus sp. | Aeromonas hydrophila | Improved hematological serum an immunological parameter | [87] |
| Oncorhynchus mykiss | Gordonia bronchialis | Not evaluated | Enhanced growth performance | [88] |
| Penaeus indicus | Bacillus subtilis | Bacillus sp., Pseudomonas sp., Vibrio sp., Micrococcus sp. | High bacteriocin production; diet with bacteriocin enhances shrimp growth; antibiotic potentials (well diffusion method) | [89] |
| Salmon salar | Carnobacterium divergens | Aeromonas salmonicida, Vibrio anguillarum | Prevent pathogen-induced damage | [90] |
| Salmon salar | Methylococcus capsulatus | Not evaluated | No inflammation with soybean meal | [91] |
| Oncorhynchus mykiss | Enterococcus casseliflavus | Streptococcus iniae | Elevated digestive enzyme activity, humoral immunity (IgM), total serum protein, and albumin production | [92] |
| Salmon salar | Lactobacillus delbruckii | Aeromonas salmonicida | Prevent pathogen damage | [93] |
| Oreochromis niloticus | Bacillus sp. | Aeromonas hydrophila, Micrococcus luteus, Pseudomonas fuorescence, Enterococcus faecalis, and Streptococcus agalactiae | Probiotic potential (resistance to adverse stomach condition, production of important enzymes) | [94] |
| Etroplus suratensis and Oreochromis Mossambicus | Bacillus sp., Micrococcus sp. | Not evaluated | Better growth performance and nutritional efficiency | [95] |
| Danio rerio | Bacillus subtilis (transgenic probiotic) | Not evaluated | The transgenic probiotic (phytase) can improve fish nutrition | [96] |
| Dicentrarchus labrax | Vibrio lentus | Not evaluated | Immunomodulation and activation of genes associated to cell proliferation | [97] |
| Oreochromis niloticus | Bacillus amyloliquefaciens | Yersinia ruckeri, Clostridium perfringens | Improved immune status (IL-1 and TNF-α mRNA) and disease resistance | [98] |
| Litopenaeus vannamei | Enterococcus faecium and Lactobacillus pentosus | Vibrio harveyi, Vibrio parahaemolyticus | High antibacterial activity and survival rate; improved humoral immune response | [99] |
| Oncorhynchus mykiss | Lactobacillus plantarum | Yersinia ruckeri | High activity of lysozyme and alkaline phosphatase; no interference in the production of immunological proteins | [100] |
| Oreochromis niloticus | Enterococcus faecium | Aeromonas hydrophila | Better growth rate and immune defenses | [101] |
| Oreochromis niloticus | Bacillus sp. | Streptococcosis (Streptococcus agalactiae) | Controlled the Streptococcosis caused by pathogenic bacteria tested | [102] |
| Rutilus caspicus | Enterococcus faecium | Aeromonas hydrophila, Yersinia ruckeri | Better growth rate, immune response, and pathogen resistance | [103] |
| Ictalurus punctatus | Bacillus velezensis | Not evaluated | Induction of growth in fingerling and water quality improvement | [104] |
| Litopenaeus vannamei | Bacillus subtilis | Not evaluated | Better growth performance and feed utilization | [105] |
| Carassius auratus | Enterococcus faecium | Aeromonas hydrophila | High survival rate as a result of E. faecium probiotic proprieties; quorum sense potential | [106] |
| Atlantic salmon | Pediococcus acidilactici | Improvements in the gut health | [107] | |
| Oncorhynchus mykiss | Lactobacillus fermentum, Lactobacillus buchneri, Saccharomyces cerevisiae (probiotics mixture) | Not evaluated | Immunity improvement | [108] |
| Danio rerio | Pseudomonas aeruginosa | Vibrio parahaemolyticus | Reduced mortality, inhibited biofilm, high level of phagocytic cells, superoxide dismutase activity, and lysozyme | [109] |
| Oreochromis niloticus | Bacillus cereus, Alcaligenes faecalis | Environmental challenges | High production of immune proteins and decrease of phosphorus water concentration | [110] |
| Ctenopharyngodon idellus | Shewanella xiamenensis and Aeromonas veronii | Aeromonas hydrophila | Enhancement of phagocytic, lysozyme activity, and expression of immune genes | [111] |
| Rhamdia quelen | Lactococcus lactis | Aeromonas hydrophila, Streptococcus agalactiae | Antimicrobial activity against tested pathogens | [112] |
| Carassius auratus | Bacillus velezensis | Aeromonas hydrophila | Improved survival rate and immune response | [113] |
| Nile tilapia | Probiotic mixture | Aluminum exposition | Probiotics regulated gut microbiota structure and function | [114] |
| Oreochromis niloticus | Lactobacillus plantarum | Aluminum intoxication | Enhanced feed utilization and growth; decreased deaths caused by aluminum and its accumulation | [115] |
| Ctenopharyngodon idellus | Bacillus paralicheniformis | Not evaluated | High adhesion and colonization capacity | [116] |
| Aquatic Specie | Bacteriocin | Pathogen or Challenge | Clinical Impact | Reference |
|---|---|---|---|---|
| Epinephelus areolatus | CAMT2 | Listeria monocytogenes, Staphylococcus aureus | Antimicrobial activity against tested pathogens | [137] |
| Labeo rohita | Bacteriocin produced by Bacillus subtilis LR1 | Aeromonas hydrophila, Aeromonas salmonicida, Bacillus mycoides, Pseudomonas fluorescens | In vitro antimicrobial activity against tested pathogens | [138] |
| Oncorhynchus tshawytscha | Enterocina AS-48 | Lactococcus garvieae | Antimicrobial activity against tested pathogen (in vitro and in vivo) | [139] |
| Penaeus monodon | Bacteriocin 99% homologous to that produced by Bacillus sp. | Vibrio alginolyticus, Aeromonas hydrophila, Pseudomonas stutzeri | In vitro inhibitory activity against tested pathogens | [140] |
| Pseudosciaena croce | Coagulina L1208 | Escherichia coli, Shewanella putrefaciens, Staphylococcus aureus | Bacteriostatic antimicrobial activity against tested pathogens | [141] |
| Litopenaeus vannamei | Bacteriocin produced by Lactobacillus plantarum FGC-12 | Vibrio parahaemolyticus | Pathogen inhibition | [142] |
| Perca sp., Tuna sp., Platax sp. | PSY2 | Listeria monocytogenes | In vitro pathogen inhibition; possible biopreservative against degradation | [143] |
| Odontesthes platensis | Mundticin KS | Pseudomonas aeruginosa, S. putrefaciens | In vitro antimicrobial activity against tested pathogen and Gram-positive bacteria | [144] |
| Odontesthes platensis | Nisin Z | Lactococcus garvieae | Pathogen growth inhibition | [145] |
| Fermented fish roe | Bacteriocin produced by Enterococcus faecium CN-25 | Listeria monocytogenes | In vitro pathogen inhibition | [146] |
| Tilapia sp., Catla catla, Cyprinus carpio | Bacteriocin isolated from Pediococcus acidilactici | Listeria monocytogenes | In vitro antimicrobial activity against tested pathogen | [147] |
| Acipenseridae, Oncorhynchus clarkii | Plantaricin LPL-1 | Listeria monocytogenes | In vitro antimicrobial activity against tested pathogen and Gram-positive bacteria | [148] |
| Pangasius bocourti | 7293 | Listeria monocytogenes, Staphylococcus aureus, Aeromonas hydrophila, Escherichia coli, Pseudomonas aeruginosa, Salmonella Typhimurium | Gram-positive and Gram-negative growth inhibition | [149] |
| Oxyeleotris lineolata | L49 | Streptococcus iniae | In vitro antimicrobial activity against tested pathogen | [150] |
| Mimachlamys nobilis | PE-ZYB1 | Listeria monocytogenes | In vitro antimicrobial activity against Gram-positive and Gram-negative bacteria; pathogen inhibition | [151] |
| Litopenaeus vannamei | Nisin | Listeria monocytogenes | Antimicrobial activity against tested pathogen (in vitro and in vivo) | [135] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, W.A.; Mendonça, C.M.N.; Urquiza, A.V.; Marteinsson, V.Þ.; LeBlanc, J.G.; Cotter, P.D.; Villalobos, E.F.; Romero, J.; Oliveira, R.P.S. Use of Probiotic Bacteria and Bacteriocins as an Alternative to Antibiotics in Aquaculture. Microorganisms 2022, 10, 1705. https://doi.org/10.3390/microorganisms10091705
Pereira WA, Mendonça CMN, Urquiza AV, Marteinsson VÞ, LeBlanc JG, Cotter PD, Villalobos EF, Romero J, Oliveira RPS. Use of Probiotic Bacteria and Bacteriocins as an Alternative to Antibiotics in Aquaculture. Microorganisms. 2022; 10(9):1705. https://doi.org/10.3390/microorganisms10091705
Chicago/Turabian StylePereira, Wellison Amorim, Carlos Miguel N. Mendonça, Alejandro Villasante Urquiza, Viggó Þór Marteinsson, Jean Guy LeBlanc, Paul D. Cotter, Elías Figueroa Villalobos, Jaime Romero, and Ricardo P. S. Oliveira. 2022. "Use of Probiotic Bacteria and Bacteriocins as an Alternative to Antibiotics in Aquaculture" Microorganisms 10, no. 9: 1705. https://doi.org/10.3390/microorganisms10091705
APA StylePereira, W. A., Mendonça, C. M. N., Urquiza, A. V., Marteinsson, V. Þ., LeBlanc, J. G., Cotter, P. D., Villalobos, E. F., Romero, J., & Oliveira, R. P. S. (2022). Use of Probiotic Bacteria and Bacteriocins as an Alternative to Antibiotics in Aquaculture. Microorganisms, 10(9), 1705. https://doi.org/10.3390/microorganisms10091705






