Abstract
Blastocystis is a common intestinal protist in humans and animals worldwide. Wild and domestic animals are thought to be reservoirs of Blastocystis subtypes that also infect humans. There are limited studies on the prevalence and subtype distribution of Blastocystis in horses. In this study, 185 fecal samples were collected from horses (1 month to 17 years of age) in four regions of Colombia (Sabana de Bogotá, Costa Atlántica, Llanos Orientales, and Bogotá D.C.). Blastocystis presence and subtypes were determined by PCR and next generation amplicon sequencing. Eighty-one (43.8%) horses were positive for Blastocystis, with positive horses in all four regions. Molecular characterization identified 12 Blastocystis subtypes, 10 known subtypes (ST1, ST3–ST6, ST10, ST14, ST25, ST26), and 2 novel subtypes (ST33 and ST34). The validity of the novel subtypes was confirmed via phylogenetic and pairwise distance analyses of the full-length SSU rRNA gene sequences. Mixed subtype infections were common (55.6% of Blastocystis-positive horses). ST10 was the most prevalent subtype, present in 82.8% of Blastocystis-positive horses. Potentially zoonotic subtypes were identified in 88.9% of the Blastocystis-positive horses. This constitutes the most comprehensive study of Blastocystis in horses. Our findings indicate that horses harbor potentially zoonotic subtypes and could contribute to the transmission of Blastocystis to humans.
1. Introduction
Blastocystis is one of the most common intestinal eukaryotes found in humans [1]. Infections have been reported as associated with gastrointestinal symptoms and/or urticaria as well as asymptomatic [2,3,4]. Blastocystis has also been detected in a wide range of animals, suggesting its zoonotic potential and that animals may be a source of human infections [5]. A wide genetic diversity has also been identified within Blastocystis specimens isolated from mammals and birds, which has been described based on polymorphisms in the small subunit ribosomal RNA (SSU rRNA) gene. To date, 28 genetic variants, called subtypes (STs), meet the recommended conditions for unique subtype designation (ST1–ST17, ST21, and ST23–ST32) [6,7,8,9]. Twelve subtypes (ST1–ST10, ST12, and ST14) have been reported in both humans and animals, indicating the possibility of zoonotic and reverse zoonotic transmission [5,10,11,12]. Blastocystis transmission occurs via the fecal–oral route, with consumption of cysts either via direct contact with infected hosts or indirectly by ingestion of contaminated food or water [5].
Equines play an important role within human culture and are used for working purposes in industry, agriculture, and transport and for leisure or sporting activities. The Food and Agriculture Organization of the United Nations (FAOSTAT) estimates that the number of horses in the world is around 60 million [13]. Horses can carry zoonotic pathogens such as Cryptosporidium or Giardia [14,15,16,17]. Data on Blastocystis in horses is scarce. To date, there are eight published studies that tested for Blastocystis in horses and most examined few samples [7,18,19,20,21,22,23]. Only two of these studies detected Blastocystis in horses [7,18]. Of eight horses tested for Blastocystis at an army base in Thailand, one horse was positive [18]. A second study identified Blastocystis in 6 of the 11 horses examined in Colombia [7]. Better knowledge of subtypes of Blastocystis in horses is necessary to understand their potential role in Blastocystis transmission dynamics and as reservoirs for human infections. Therefore, the current study aimed to investigate the presence of Blastocystis subtypes in horses using next generation amplicon sequencing (NGS). Additionally, long-read sequencing using Oxford Nanopore Technology’s MinION was used to obtain the full-length SSU rRNA gene of two novel Blastocystis sequences identified in horses in this study, to confirm their validity as novel subtypes.
2. Materials and Methods
2.1. Source and Collection of Specimens
For this study, 185 fecal samples were collected from horses from four geographic regions in Colombia, South America (Bogotá D.C, Sabana de Bogotá, Llanos Orientales, and Costa Atlántica) from August to October 2007 (Table 1). The horses ranged from 1 month to 17 years of age, included males and females, and generally appeared in good health. Horses from the Sabana de Bogotá were “Silla Argentina” breed. Males were housed in separate stables, and their drinking water was from the nearest municipal aqueduct. Females were pastured with colts, with direct access to natural spring water. Horses from Costa Atlántica were “Paso Fino Colombiano” breed. Males were housed in separate stables, while females were pastured with colts. Both males and females consumed natural spring water. Horses from Bogotá D.C. that belong to the Universidad Nacional de Colombia were used for academic practices and had access to water from the Bogotá D.C. aqueduct. Horses from Los Llanos Orientales were almost wild, with direct access to natural spring water; they are only grouped together once or twice a year for examination and care. Feces were collected directly from the rectum of each horse and were placed into a 50 mL centrifuge tube with records of the date, location, age, gender, and identification and were transferred to an insulated container packed with ice or cold packs. Fecal specimens were shipped overnight to the USDA, ARS (Beltsville, MD, USA).

Table 1.
Number of horses examined for Blastocystis by location with information on age and gender.
2.2. Parasite Concentration from Feces and DNA Extraction
To concentrate parasites, each fecal specimen was sieved and concentrated using a cesium chloride gradient, as previously described [24]. Genomic DNA was extracted using the DNeasy Tissue Kit (Qiagen, Valencia, CA, USA), in accordance with the recommendations of the manufacturer, with minor exceptions. Modifications included incubation with proteinase K (20 mg/mL) at 55 °C overnight and elution in 100 μL of AE buffer to increase the quantity of recovered DNA.
2.3. Molecular Detection and Subtype Identification Using Next Generation Amplicon Sequencing
A next generation amplicon sequencing strategy was used to detect Blastocystis, as previously described [25]. Briefly, a PCR using primers ILMN_Blast505_532F and ILMN_Blast998_1017R was used to screen all 185 horse samples. These primers amplify a fragment of the SSU rRNA gene (ca. 500 bp) and are identical to Blast505_532F/Blast998_1017R [26], with the exception of containing Illumina overhang adapter sequences on the 5′ end. Final libraries were quantified by Qubit fluorometric quantitation (Invitrogen, Carlsbad, CA, USA) prior to normalization. A final pooled library concentration of 8 pM with 20% PhiX control was sequenced using an Illumina MiSeq and a 600 cycle v3 kit (Illumina, San Diego, CA, USA). Paired end reads were processed and analyzed with an in-house pipeline that uses the BBTools package v38.82 [27], VSEARCH v2.15.1 [28], and BLAST+ 2.11.0 [29]. Briefly, read pairs were merged, filtered for quality and length, denoised, and checked for chimeric sequences. Clustering and the assignment of centroid sequences to operational taxonomic units (OTU) was performed within each sample at a 98% identity threshold. Only those OTUs with a minimum of 100 sequences were retained and then checked for chimeras once more. OTUs were then blasted against Blastocystis references from NCBI. All hits below an alignment length of 400 bp were removed. Raw FASTQ files were submitted to NCBI’s sequence read archive under project PRJNA856306 and accession numbers SRR20005885–SRR20005965. The nucleotide sequences generated using NGS in this study were deposited in GenBank under the accession numbers ON932503–ON932559.
2.4. PCR Amplification and Sequencing of the Full-Length SSU rRNA Gene
DNA from three horse samples (d31, d43, and d45) were used to obtain full-length SSU rRNA gene sequence to validate novel subtypes ST33 and ST34. A previously described PCR and Nanopore sequencing strategy was used to generate sequences of the approximately 1800 base pair SSU rRNA gene [30], with the following updates. Briefly, a PCR using the MinION-tailed primers SSU-F1 (5′–TTT CTG TTG GTG CTG ATA TTG C AAC CTG GTT GAT CCT GCC AGT AGT C−3′) and SSU-R1 (5′–ACT TGC CTG TCG CTC TAT CTT C TGA TCC TTC TGC AGG TTC ACC TAC G−3′), which amplify most eukaryotic organisms’ full-length SSU rRNA gene, was performed using the high-fidelity proofreading polymerase contained in KAPA HiFi HotStart ReadyMix (KAPABioSystems, Cape Town, South Africa). Initial denaturation was performed at 98 °C for 5 min followed by 35 cycles of amplification: 20 s at 98 °C, 45 s at 60 °C, and 90 s at 72 °C. The final extension continued for 5 min. PCR amplicons were purified using a 0.5X AMPure XP beads (Beckman Coulter, Brea, CA, USA) to sample ratio and quantified on a Qubit Fluorometer (ThermoFisher Scientific, Waltham, MA, USA). To prepare the nanopore sequencing library, the Oxford Nanopore Technologies (ONT) SQK-LSK110 Ligation Sequencing Kit was used in accordance with the protocol of the manufacturer for PCR Barcoding Amplicons (PBAC12_9112_v110_revB_10Nov2020) and loading guidelines for R10.3 flow cells. The EXP-PBC001 PCR Barcoding Kit (ONT, Oxford, UK) was used in combination with the ligation kit for barcoding each sample. A modification to the barcoding PCR protocol included the use of the KAPA HiFi polymerase, as described above, instead of the NEB LongAmp Taq. Amplicons were purified with 0.5X XP beads once more, quantified, and diluted to ensure 100 fmol of library in 12 µL was loaded onto a flow cell and run aboard an Mk1C MinION using MinKNOW v21.11.7 software.
Basecalling was performed using Guppy v6.1.1 (gpu) and the Super Accuracy model available for R10 flow cells in the following configuration files dna_r10.3_450bps_sup.cfg. A minimum quality score cutoff of 10 was used for filtering reads. FASTQ reads were then length-filtered to include only reads between 1600 and 2000 nucleotides. Filtered reads were corrected using canu v2.2 and then length-filtered again to retain reads between 1600 and 2000 nt. Next, MinION PCR adapters were trimmed and only reads with intact forward and reverse eukaryotic primers were retained (bbduk.sh k = 18 restrictleft/right = 150 mm = f edist = 2; BBTools v38.94). Primer orientation was used to ensure all reads were converted to the plus strand, before combining them into a single FASTA file. Reads were then clustered using the vsearch –cluster_fast command (vsearch v2.15.1) with a 98% identity threshold and checked for chimeras using the vsearch –uchime_denovo command. The chimera-free clusters were then polished using Racon v1.4.20. The SAM file needed for racon polishing was generated by first extracting non-chimeric reads from pre-clustered reads and mapping them back to the chimera-free clusters using Minimap2 v2.22 and the flags -ax asm5 –secondary = no. Racon-polished consensus sequences were clustered again at 98% identity using the vsearch –cluster_size command and sequences with <10 supporting reads were removed. Another round of polishing was performed using Medaka v1.4.3 (medaka_consensus –m r103_sup_g507). Raw FASTQ files were submitted to NCBI’s sequence read archive under project PRJNA856306 and accession numbers SRR29543362 (d31), SRR29543364 (d43), and SRR29543365 (d45).
For comparison purposes, full-length sequences and partial sequences obtained with MinION and MiSeq, respectively, were aligned using ClustalW in MegAlign 15 (DNASTAR Lasergene 15, Madison, WI, USA), and pairwise distances between consensus sequences were calculated. The full-length SSU rRNA gene nucleotide sequences generated in this study were deposited in GenBank under the accession numbers ON932569–ON932571.
2.5. Phylogenetic and Pairwise Distance Analysis
The full-length SSU rRNA gene nucleotide sequences obtained in this study and appropriate full-length Blastocystis reference nucleotide sequences that include all currently accepted subtypes were aligned to generate a phylogenetic tree, which was rooted using Proteromonas lacertae, a Stramenopile that is closely related to Blastocystis, as an outgroup. Nucleotide sequences were aligned with the Clustal W algorithm, and the phylogenetic analysis was performed using the neighbor-joining (NJ) method, and genetic distances were calculated with the Kimura 2-parameter model using MEGA X [31,32]. A total of 1958 positions were included in the final dataset, which included 74 nucleotide sequences. Bootstrapping with 1000 replicates was used to determine support for the clades generated. Additionally, evolutionary analysis was conducted to establish divergence between nucleotide sequences (pairwise distance) using the Kimura 2-parameter model in MEGA X.
2.6. Statistical Analysis
The prevalence of Blastocystis was compared between localities, sexes, and age groups via either Fisher’s exact test or chi-squared test using GraphPad Software 9.4.1 (San Diego, CA, USA). Differences were considered significant when p < 0.05.
3. Results
3.1. Prevalence of Blastocystis in Horses
Of the 185 horse fecal samples examined in this study, 81 (43.8%) were found to be Blastocystis-positive using PCR and confirmed by NGS (Table 2; Supplementary Table S1). Blastocystis was found in all four geographic regions where horses were tested, with prevalence ranging from 41.1% in Costa Atlántica to 56.0% in Bogotá D.C. No difference in overall prevalence was observed among the four localities (p = 0.6203).

Table 2.
Number of horses examined, number of horses positive, prevalence (%), and subtypes of Blastocystis by location. Subtypes in bold denote those previously identified in humans.
No difference in overall prevalence was observed between male (42.6%; 29/68) and female horses (44.4%; 52/117) (p = 0.8783) or between horses <1 year of age (40.6%; 28/70) and >1 year of age (46.1%; 53/115) (p = 0.4478).
3.2. Subtypes of Blastocystis in Horses
Twelve STs of Blastocystis were detected, ten previously reported (ST1, ST3, ST4, ST5, ST6, ST10, ST14, ST24, ST25, and ST26), and two proposed novel STs, named ST33 and ST34 (Table 2). ST33 was identified in two horses from Llanos Orientales, and ST34 was found in three horses, one from Sabana de Bogotá, and two from Llanos Orientales.
A wide genetic diversity was observed in horses from all regions included in the study, with the lowest diversity of STs in Bogotá D.C. with six STs, and the highest in Llanos Orientales with nine STs detected in 14 and 19 Blastocystis-positive horses, respectively (Table 2). Similar genetic diversity was found in females (11 STs) and males (10 STs) (Table 4) as well as in horses younger than 1 year of age (11 STs) and older than 1 year of age (9 STs) (Table 3). ST3, ST4, and ST24 were only present in horses younger than 1 year of age, while ST6 was only found in animals older than 1 year of age. ST6 and ST14 were detected in females but not in males, while ST24 was present in males but not in females.

Table 4.
Number of horses examined, number of horses positive, prevalence (%), and subtypes of Blastocystis by gender at each location. Subtypes in bold denote those previously identified in humans.

Table 3.
Number of horses examined, number of horses positive, prevalence (%), and subtypes of Blastocystis by age at each location. Subtypes in bold denote those previously identified in humans.
Mixed ST infections were frequently observed (Table 5; Supplementary Table S1). A single ST was identified in 36 (44.4%) Blastocystis-positive horses, while two or more STs were detected in 45 (55.6%) of the Blastocystis-positive horses. Multiple combinations of STs were observed, including combinations of two STs and up to six STs observed in three horses from Llanos Orientales. Of these mixed subtype combinations, co-infection with ST10/ST25 (with and without presence of additional STs) was the most observed combination and represented 37.8% (17/45) of all mixed-subtype infections.

Table 5.
Combination of STs observed in horses in Colombia at each location. Subtypes in bold denote those previously identified in humans.
Of the 12 STs identified in the horses, 7 have been previously reported in humans and are potentially zoonotic STs (ST1, ST3, ST4, ST5, ST6, ST10, and ST14). Most Blastocystis-positive horses harbored one of those STs (88.9%; 72/81). ST1, ST3, ST4, ST5, ST6, ST10, and ST14 were found in 20, 5, 6, 9, 1, 60, and 3 horses, respectively, as single or mixed STs infections. By location, ST4 (1 horse), ST10 (21 horses), and ST14 (1 horse) were identified in horses from Sabana de Bogotá; ST1 (9 horses), ST5 (1 horse), ST6 (1 horse), ST10 (16 horses), and ST14 (2 horses) in horses from Costa Atlántica; ST1 (1 horse), ST4 (2 horses), ST5 (1 horse), and ST10 (12 horses) in horses from Bogotá D.C.; and ST1 (10 horses), ST3 (5 horses), ST4 (3 horses), ST5 (7 horses), and ST10 (11 horses) in horses from Llanos Orientales. In most Blastocystis-positive horses, potentially zoonotic STs were observed as mixed-STs infection (65.4%; 53/81). Only ST1 and ST10 were observed in single ST infections in 28 horses (34.6%; 28/81), ST1 in 3 horses from Costa Atlántica, and ST10 in 11, 7, 5, and 2 horses from Sabana de Bogotá, Costa Atlántica, Bogotá D.C, and Llanos Orientales, respectively.
3.3. Blastocystis Intra-Subtype Variation
Fifty-eight unique Blastocystis sequences were identified in the 81 Blastocystis-positive horses (Table 6). A high intra-subtype variation was observed for ST1 (16 unique sequences among the 50 ST1-positive horses), ST10 (10 unique sequences among the 67 ST10-positive horses), ST26 (9 unique sequences among the 16 ST26-positive horses), ST5 (6 unique sequences among the 9 ST5-positive horses), and ST25 (6 unique sequences among the 29 ST25-positive horses). Fewer sequence variants were observed for the rest of the subtypes, with three unique sequences for ST3, two unique sequences for ST4 and ST14, and one unique sequence for ST6, ST24, ST33, and ST34.

Table 6.
Blastocystis subtypes identified in horses including number of positive horses in which each subtype was identified and number of unique sequences among each subtype.
3.4. Validation of Novel Subtypes ST33 and ST34
Two sequences generated by Illumina sequencing were suspected of being novel subtypes when compared to nucleotide sequences available in the GenBank database. The sequence similarity between the sequences of the novel subtypes and any nucleotide sequence available in the GenBank database with a subtype designation was approximately 84%. BLAST analysis also identified a sequence from tortoises in the Czech Republic (EF209017) as having approximately 85% sequence similarity with ST33 and ST34. Although both novel subtypes clustered together, their similarity was only 82%.
To confirm validity of the novel subtypes, a nanopore sequencing strategy was used in three horse samples. Samples #d31 and #d43 were used to obtain the full-length sequence of ST33, and sample #d45 was used for ST34. Full-length sequences were successfully obtained for both novel subtypes. There was 100% agreement between the Illumina sequence and the same region within the MinION sequence for both ST33 and ST34.
Phylogenetic analysis of full-length sequences using the NJ method showed both ST33 and ST34 clustering with ST29, with a low bootstrap support of 57 (Figure 1). To evaluate the percentage of sequence similarity between ST33 and ST34 and known subtypes, pairwise distance comparisons were used. An 86% similarity was observed for ST33 and ST5 and for ST34 and ST25. The percentage of similarity for both novel STs was 85% or lower when compared with the rest of the known STs (including ST29 at 84%). When full-length sequences of ST33 and ST34 were compared with each other, sequence similarity was 95%.
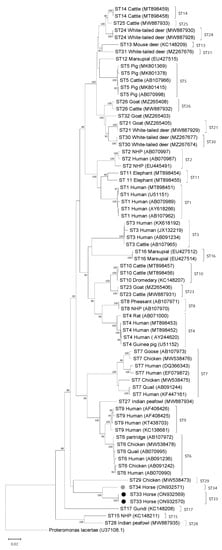
Figure 1.
Phylogenetic relationships among Blastocystis full-length sequences generated in the present study (novel subtypes are new subtypes ST33 and ST34, which are denoted with black- and gray-filled circles, respectively) and representative reference sequences of all accepted subtypes. Proteromonas lacertae was used as outgroup taxon to root the tree. Analysis was conducted by a neighbor-joining method. Genetic distances were calculated using the Kimura two-parameter model. This analysis involved 74 nucleotide sequences, and there were a total of 1953 positions in the final dataset. Bootstrap values lower than 75% are not displayed.
4. Discussion
Horses live in close proximity to humans and are commonly used for companion and agricultural purposes. Yet, data on the subtypes and prevalence of Blastocystis in horses is sparse [5]. In this study, 185 samples were screened for Blastocystis by PCR, and subtyping was performed using an NGS strategy that can distinguish mixed-subtype infections within individual samples. Using this strategy, we have conducted the largest study of Blastocystis ever performed in horses, providing much-needed data to understand prevalence and subtype diversity in this understudied host.
An overall Blastocystis prevalence of 43.8% was observed among the 185 horses included in this study, and positive horses were observed in all four collection sites. The high prevalence of Blastocystis observed among horses is within the range of other reports from large domestic mammals such as cattle, sheep, goats, and pigs [5]. Presence of Blastocystis in horses has only been described in two studies that reported prevalences of 12.5% (1/8) and 54.5% (6/11) in Thailand and Colombia, respectively [7,18]. Five other studies screened horses for Blastocystis in Bangladesh, China, Italy, Iran, and Turkey but did not detect it in any of the samples examined [19,20,21,22,23]. Differences in prevalence among studies could be related to many factors, such as those related to the host (breed, age, gender) or diagnostic method used to detect Blastocystis. In the study conducted in Thailand, the presence of Blastocystis was determine by in vitro cultivation, while in Colombia, PCR was used to detect Blastocystis positives. It is noteworthy that out of the six Blastocystis-positive horses detected by PCR (6/11; 54.5%), in the Colombian study only two were confirmed as positive using NGS analysis (2/11; 18.2%), thus, 54.5% may be an overestimation of the prevalence in horses in that study [7]. Although more studies from horses are needed to fully describe the prevalence of Blastocystis in this host, observations from the present study support Blastocystis being common in horses in Colombia. Data from other regions of the world will assist in determining if regional differences in Blastocystis prevalence for horses exist.
Horses were host to 12 different STs in this study, and mixed infections were common and observed in 55.6% of positive samples (Table 2 and Table 5). Subtypes observed included ST1, ST3, ST4, ST5, ST6, ST10, ST14, ST24, ST25, ST26, and two proposed novel STs, named ST33 and ST34. Such diversity is similar to what has been reported in livestock such as cattle, goats, and sheep [7,33,34]. However, cattle are ruminants, while horses are monogastric, so the presence of such comparable diversity is quite intriguing. Pigs, another large monogastric domestic mammal, are most frequently host to ST1, ST3, and ST5, although ST2, ST4, ST7, ST8, ST10, and ST15 have also been reported in pigs, albeit with less frequency [35,36,37,38,39,40]. The overlap of subtypes between horses and ruminants could support factors such as an herbivorous diet having as important a role, as physiology influences the colonization of a host with some subtypes of Blastocystis. Given that horses, like domestic ruminants, are grazing livestock, it is also possible that shared environmental exposure plays an important role in colonization by individual Blastocystis STs. Data on dietary differences and potential reservoir wildlife species that may facilitate transmission to domestic animals are needed to understand such relationships.
Subtype 10 was by far the most common ST among horses in this study and was observed in 60 of the 81 Blastocystis-positive horses (Table 6). Subtypes 25 and 26 were also among the most common STs observed and were found in 29 and 16 horses, respectively. Subtypes 10, 25, and 26 are all common STs reported among domestic and wild ruminant hosts [7,9,34,41,42]. Yet, ST14 and ST24, which are also common among ruminants, were quite rare among horses and were observed in only three and one samples, respectively (Table 6). It has been suggested that ST10 and ST14 may be cattle-adapted STs because of their high prevalence among cattle [43]. However, the distribution of these STs among horses may suggest that more than just parasite genetics influence host suitability for a given ST. While more data are needed to confirm associations between horses and any STs, the present study suggests that other factors should be considered in future studies of host specificity of Blastocystis STs.
Intra-subtype variability differed between the subtypes identified in horses in this study (Table 6). The most OTUs were observed in ST1 and ST10, with 16 and 10 unique sequences identified in infected horses, respectively. No intra-subtype variation was observed for subtypes ST6, ST24, ST33, and ST34. However, these subtypes were only identified in a few animals, exactly one, one, two, and three horse samples for ST6, ST24, ST33, and ST34, respectively. It is interesting that for ST3, which has usually been found in previous studies to be rather homogeneous [3,44,45], three unique sequences were identified within the five horses infected with ST3. The intra-subtype variability observed in horses in this study may indicate that horses acquire Blastocystis from diverse sources in the environment.
Zoonotic infections were common in horses, and 88.9% of Blastocystis-positive specimens contained one or more subtypes that have also been reported in humans. Of the potentially zoonotic STs observed in horses in the present study, ST1 was remarkably common and was present in 25% (20/81) of Blastocystis-positive horses (Table 6). ST1 was absent from Sabana de Bogotá but was observed in horses from the other three locations (Table 2). It was more common in horses >1 year old, in which it was present in three locations, while it was only observed in Llanos Orientales in <1 year old horses (Table 3). In previous reports of subtype diversity in horses, a single horse from Thailand that was Blastocystis positive was reported as having an ST1 infection [18], and in horses from Colombia, ST10, ST14, and ST24 were observed in two horses with ST information [7]. Thus, whether horses are commonly infected with ST1 in other regions of the world remains to be clarified. However, the high rates of ST1 infection among horses in this study indicate they could be an important reservoir of this zoonotic subtype in Colombia. Indeed, ST1 has been reported at high rates among humans in Colombia and is one of the most common STs reported in the Americas [46,47]. Lastly, the high rate of zoonotic ST infection among horses overall indicates more studies on the role of horses in Blastocystis transmission to humans are warranted.
Two new STs were observed in this study and following validation through sequencing of the full-length SSU rRNA genes and comparison to other named STs, these were given the designations ST33 and ST34. Comparison of the Santin region of the SSU rRNA gene for these STs to references available in GenBank did not return any similar sequence matches. However, the barcoding region, which covers approximately the first 600 bp of the gene, did return a BLAST hit with a shared sequence identity of >98% between ST33 and a nucleotide sequence under the GenBank accession number MT039570, which came from a horse in the Czech Republic (unpublished). This same sequence (MT039570) shares 96% sequence identity with ST34 within the same region. The observation of ST33 in a horse from another region of the world further supports the designation of this new ST and may indicate that ST33 and ST34 are part of a horse-specific clade (Figure 1). However, more data from horses and other related hosts would be needed to support such a conclusion.
5. Conclusions
This study represents the most comprehensive analysis of Blastocystis to ever be conducted in horses. Prevalence and subtype data from this study demonstrate that Blastocystis can be common in horses and that many subtypes are able to colonize this host. Our findings have expanded the host range of ST3, ST4, ST5, ST6, ST25, and ST26, as this study represents the first report of these STs in horses. We have identified and validated two new subtypes, ST33 and ST34. Furthermore, horses had subtypes that are frequently observed in both humans and other domestic animals, indicating horses may be reservoirs of these subtypes for other hosts. Given the scarcity of data for Blastocystis in horses, this study is an important first step in clarifying the role of horses in the epidemiology of Blastocystis and provides essential data for understanding the transmission of different STs between domestic animals and humans in Colombia and beyond.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microorganisms10091693/s1.
Author Contributions
Conceptualization, J.G.M. and M.S.; data curation, J.G.M., A.M. and M.S.; formal analysis, J.G.M., A.M. and M.S.; funding acquisition, J.G.M. and M.S.; investigation, S.B., J.G.M., A.M., N.S.G., J.A.C.V. and M.S.; methodology, J.G.M., A.M., N.S.G. and M.S.; project administration, M.S.; resources, J.G.M. and M.S.; software, A.M. and M.S.; supervision, M.S.; validation, J.G.M. and M.S.; visualization, J.G.M. and M.S.; writing—original draft, S.B., J.G.M. and M.S.; Writing—review and editing, J.G.M., A.M., N.S.G., J.A.C.V. and M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by USDA-ARS Project No: 8042-32000-112-00-D.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All relevant data are within the article and its additional files. The raw FASTQ files were submitted to NCBI’s sequence read archive under project PRJNA856306. The sequences data were submitted to the GenBank database under the accession numbers ON932503–ON932559 (NGS) and ON932569–ON932571 (MinION).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Andersen, L.O.; Stensvold, C.R. Blastocystis in health and disease: Are we moving from a clinical to a public health perspective? J. Clin. Microbiol. 2016, 54, 524–528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salvador, F.; Sulleiro, E.; Sánchez-Montalvá, A.; Alonso, C.; Santos, J.; Fuentes, I.; Molina, I. Epidemiological and clinical profile of adult patients with Blastocystis sp. infection in Barcelona, Spain. Parasites Vectors 2016, 9, 548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rojas-Velázquez, L.; Maloney, J.G.; Molokin, A.; Morán, P.; Serrano-Vázquez, A.; González, E.; Pérez-Juárez, H.; Ximénez, C.; Santin, M. Use of next-generation amplicon sequencing to study Blastocystis genetic diversity in a rural human population from Mexico. Parasites Vectors 2019, 12, 566. [Google Scholar] [CrossRef] [Green Version]
- Aykur, M.; Camyar, A.; Türk, B.G.; Sin, A.Z.; Dagci, H. Evaluation of association with subtypes and alleles of Blastocystis with chronic spontaneous urticaria. Acta Trop. 2022, 231, 106455. [Google Scholar] [CrossRef]
- Hublin, J.S.Y.; Maloney, J.G.; Santin, M. Blastocystis in domesticated and wild mammals and birds. Res. Vet. Sci. 2021, 135, 260–282. [Google Scholar] [CrossRef] [PubMed]
- Stensvold, C.R.; Clark, C.G. Pre-empting Pandora’s Box: Blastocystis subtypes revisited. Trends Parasitol. 2020, 36, 229–232. [Google Scholar] [CrossRef]
- Higuera, A.; Herrera, G.; Jimenez, P.; García-Corredor, D.; Pulido-Medellín, M.; Bulla-Castañeda, D.M.; Pinilla, J.C.; Moreno-Pérez, D.A.; Maloney, J.G.; Santín, M.; et al. Identification of multiple Blastocystis subtypes in domestic animals from Colombia using amplicon-based next generation sequencing. Front. Vet. Sci. 2021, 8, 732129. [Google Scholar] [CrossRef]
- Maloney, J.G.; Santin, M. Mind the gap: New full-length sequences of Blastocystis subtypes generated via Oxford Nanopore Minion sequencing allow for comparisons between full-length and partial sequences of the small subunit of the ribosomal RNA gene. Microorganisms 2021, 9, 997. [Google Scholar] [CrossRef]
- Maloney, J.G.; Jang, Y.; Molokin, A.; George, N.S.; Santin, M. Wide genetic diversity of Blastocystis in white-tailed deer (Odocoileus virginianus) from Maryland, USA. Microorganisms 2021, 9, 1343. [Google Scholar] [CrossRef]
- Alfellani, M.A.; Stensvold, C.R.; Vidal-Lapiedra, A.; Onuoha, E.S.; Fagbenro-Beyioku, A.F.; Clark, C.G. Variable geographic distribution of Blastocystis subtypes and its potential implications. Acta Trop. 2013, 126, 11–18. [Google Scholar] [CrossRef] [Green Version]
- Ramírez, J.D.; Sánchez, A.; Hernández, C.; Flórez, C.; Bernal, M.C.; Giraldo, J.C.; Reyes, P.; López, M.C.; García, L.; Cooper, P.J.; et al. Geographic distribution of human Blastocystis subtypes in South America. Infect. Genet. Evol. 2016, 41, 32–35. [Google Scholar] [CrossRef] [PubMed]
- Khaled, S.; Gantois, N.; Ly, A.T.; Senghor, S.; Even, G.; Dautel, E.; Dejager, R.; Sawant, M.; Baydoun, M.; Benamrouz-Vanneste, S.; et al. Prevalence and subtype distribution of Blastocystis sp. in senegalese school children. Microorganisms 2020, 8, 1408. [Google Scholar] [CrossRef]
- FAOSTAT FAO, Data. Domains–Production–Crops livestock products–Regions–World–Live Animals. 2022. Available online: http://www.fao.org/faostat/en/#data/QCL (accessed on 27 May 2022).
- Santín, M.; Cortés Vecino, J.A.; Fayer, R. A large scale molecular study of Giardia duodenalis in horses from Colombia. Vet. Parasitol. 2013, 196, 31–36. [Google Scholar] [CrossRef]
- Li, F.; Wang, R.; Guo, Y.; Li, N.; Feng, Y.; Xiao, L. Zoonotic potential of Enterocytozoon bieneusi and Giardia duodenalis in horses and donkeys in northern China. Parasitol. Res. 2020, 119, 1101–1108. [Google Scholar] [CrossRef]
- Paruch, L.; Paruch, A.M. Molecular identification of infectious enteropathogens in faeces of fealthy horses. Microbiol. Insights 2022, 15, 11786361221089005. [Google Scholar] [CrossRef] [PubMed]
- Tuemmers, C.; Fellenberg, C.; Pérez, E.J.; Paillaqueo, J. Prevalence of Cryptosporidium spp. in horses from communities of the Mapuche native people, Araucanía Region, Chile. Equine Vet. J. 2022. [Google Scholar] [CrossRef] [PubMed]
- Thathaisong, U.; Worapong, J.; Mungthin, M.; Tan-Ariya, P.; Viputtigul, K.; Sudatis, A.; Noonai, A.; Leelayoova, S. Blastocystis isolates from a pig and a horse are closely related to Blastocystis hominis. J. Clin. Microbiol. 2003, 41, 967–975. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Gong, B.; Liu, X.; Zhao, W.; Bu, T.; Zhang, W.; Liu, A.; Yang, F. Distribution and genetic diversity of Blastocystis subtypes in various mammal and bird species in Northeastern China. Parasites Vectors 2018, 11, 522. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Karim, M.R.; Li, D.; Rahaman Sumon, S.; Siddiki, S.; Rume, F.I.; Sun, R.; Jia, Y.; Zhang, L. Molecular characterization of Blastocystis sp. in captive wildlife in Bangladesh National Zoo: Non-human primates with high prevalence and zoonotic significance. Int. J. Parasitol. Parasites Wildl. 2019, 10, 314–320. [Google Scholar] [CrossRef]
- Gabrielli, S.; Palomba, M.; Furzi, F.; Brianti, E.; Gaglio, G.; Napoli, E.; Rinaldi, L.; Alburqueque, R.A.; Mattiucci, S. Molecular subtyping of Blastocystis sp. isolated from farmed animals in southern Italy. Microorganisms 2021, 9, 1656. [Google Scholar] [CrossRef]
- Onder, Z.; Yildirim, A.; Pekmezci, D.; Duzlu, O.; Pekmezci, G.Z.; Ciloglu, A.; Simsek, E.; Kokcu, N.D.; Yetismis, G.; Ercan, N.; et al. Molecular identification and subtype distribution of Blastocystis sp. in farm and pet animals in Turkey. Acta Trop. 2021, 220, 105939. [Google Scholar] [CrossRef] [PubMed]
- Mohammad Rahimi, H.; Mirjalali, H.; Zali, M.R. Molecular epidemiology and genotype/subtype distribution of Blastocystis sp., Enterocytozoon bieneusi, and Encephalitozoon spp. in livestock: Concern for emerging zoonotic infections. Sci. Rep. 2021, 11, 17467. [Google Scholar] [CrossRef] [PubMed]
- Santín, M.; Trout, J.M.; Xiao, L.; Zhou, L.; Greiner, E.; Fayer, R. Prevalence and age-related variation of Cryptosporidium species and genotypes in dairy calves. Vet. Parasitol. 2004, 122, 103–117. [Google Scholar] [CrossRef] [PubMed]
- Maloney, J.G.; Molokin, A.; Santin, M. Next generation amplicon sequencing improves detection of Blastocystis mixed subtype infections. Infect. Genet. Evol. 2019, 73, 119–125. [Google Scholar] [CrossRef]
- Santín, M.; Gómez-Muñoz, M.T.; Solano-Aguilar, G.; Fayer, R. Development of a new PCR protocol to detect and subtype Blastocystis spp. from humans and animals. Parasitol. Res. 2011, 109, 205–212. [Google Scholar] [CrossRef]
- Bushnell, B.; BBMap Download. SourceForge.net. 2014. Available online: https://sourceforge.net/projects/bbmap/ (accessed on 27 May 2022).
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahé, F. VSEARCH: A versatile open source tool for metagenomics. Peer J. 2016, 4, e2584. [Google Scholar] [CrossRef]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and applications. BMC Bioinform. 2009, 10, 421. [Google Scholar] [CrossRef] [Green Version]
- Maloney, J.G.; Molokin, A.; Santin, M. Use of Oxford Nanopore MinION to generate full-length sequences of the Blastocystis small subunit (SSU) rRNA gene. Parasites Vectors 2020, 13, 595. [Google Scholar] [CrossRef]
- Kimura, M. A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Maloney, J.G.; Lombard, J.E.; Urie, N.J.; Shivley, C.B.; Santin, M. Zoonotic and genetically diverse subtypes of Blastocystis in US pre-weaned dairy heifer calves. Parasitol. Res. 2019, 118, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Abarca, N.; Santín, M.; Ortega, S.; Maloney, J.G.; George, N.S.; Molokin, A.; Cardona, G.A.; Dashti, A.; Köster, P.C.; Bailo, B.; et al. Molecular Detection and Characterization of Blastocystis sp. and Enterocytozoon bieneusi in Cattle in Northern Spain. Vet. Sci. 2021, 8, 191. [Google Scholar] [CrossRef] [PubMed]
- Roberts, T.; Stark, D.; Harkness, J.; Ellis, J. Subtype distribution of Blastocystis isolates from a variety of animals from New South Wales, Australia. Vet. Parasitol. 2013, 196, 85–89. [Google Scholar] [CrossRef]
- Yoshikawa, H.; Tokoro, M.; Nagamoto, T.; Arayama, S.; Asih, P.B.; Rozi, I.E.; Syafruddin, D. Molecular survey of Blastocystis sp. from humans and associated animals in an Indonesian community with poor hygiene. Parasitol. Int. 2016, 65, 780–784. [Google Scholar] [CrossRef]
- Song, J.K.; Hu, R.S.; Fan, X.C.; Wang, S.S.; Zhang, H.J.; Zhao, G.H. Molecular characterization of Blastocystis from pigs in Shaanxi province of China. Acta Trop. 2017, 173, 130–135. [Google Scholar] [CrossRef]
- Valença-Barbosa, C.; do Bomfim, T.; Teixeira, B.R.; Gentile, R.; Neto, S.; Magalhães, B.; Balthazar, D.A.; da Silva, F.A.; Biot, R.; d’Avila Levy, C.M.; et al. Molecular epidemiology of Blastocystis isolated from animals in the state of Rio de Janeiro, Brazil. PLoS ONE 2019, 14, e0210740. [Google Scholar] [CrossRef] [Green Version]
- Wylezich, C.; Belka, A.; Hanke, D.; Beer, M.; Blome, S.; Höper, D. Metagenomics for broad and improved parasite detection: A proof-of-concept study using swine faecal samples. Int. J. Parasitol. 2019, 49, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Han, J.Q.; Li, Z.; Zou, Y.; Pu, L.H.; Zhu, X.Q.; Zou, F.C.; Huang, C.Q. Prevalence, molecular characterization and risk factors of Blastocystis sp. from farmed pigs in Yunnan Province, Southwestern China. Acta Parasitol. 2020, 65, 1005–1010. [Google Scholar] [CrossRef]
- Wang, X.; Xue, N.Y.; Qin, L.T.; Liu, Y.Y.; Wang, H.X.; Zhao, Q.; Ni, H.B.; Lyu, C. Molecular characterization of Blastocystis from beef cattle in Northeastern China. Vector Borne Zoonotic Dis. 2021, 21, 955–960. [Google Scholar] [CrossRef]
- Ni, F.; Yu, F.; Yang, X.; An, Z.; Ge, Y.; Liu, X.; Qi, M. Identification and genetic characterization of Blastocystis subtypes in Père David’s deer (Elaphurus davidianus) from Shishou, China. Vet. Res. Commun. 2022. [Google Scholar] [CrossRef]
- Greige, S.; El Safadi, D.; Khaled, S.; Gantois, N.; Baydoun, M.; Chemaly, M.; Benamrouz-Vanneste, S.; Chabé, M.; Osman, M.; Certad, G.; et al. First report on the prevalence and subtype distribution of Blastocystis sp. in dairy cattle in Lebanon and assessment of zoonotic transmission. Acta Trop. 2019, 194, 23–29. [Google Scholar] [CrossRef]
- Beghini, F.; Pasolli, E.; Truong, T.D.; Putignani, L.; Cacciò, S.M.; Segata, N. Large-scale comparative metagenomics of Blastocystis, a common member of the human gut microbiome. ISME J. 2017, 11, 2848–2863. [Google Scholar] [CrossRef]
- Sarzhanov, F.; Dogruman-Al, F.; Santin, M.; Maloney, J.G.; Gureser, A.S.; Karasartova, D.; Taylan-Ozkan, A. Investigation of neglected protists Blastocystis sp. And Dientamoeba fragilis in immunocompetent and immunodeficient diarrheal patients using both conventional and molecular methods. PloS Negl. Trop. Dis. 2021, 15, e0009779. [Google Scholar] [CrossRef]
- Vega, L.; Herrera, G.; Muñoz, M.; Patarroyo, M.A.; Maloney, J.G.; Santín, M.; Ramírez, J.D. Gut microbiota profiles in diarrheic patients with co-occurrence of Clostridioides difficile and Blastocystis. PLoS ONE 2021, 16, e0248185. [Google Scholar] [CrossRef]
- Jiménez, P.A.; Jaimes, J.E.; Ramírez, J.D. A summary of Blastocystis subtypes in North and South America. Parasites Vectors. 2019, 12, 376. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).