Atypical Presentation of Aspergillus niger Infection in the Oral Cavity as a Prediction of Invasive Pulmonary Aspergillosis in a Patient with COVID-19: Case Report and Literature Review
Abstract
:1. Introduction
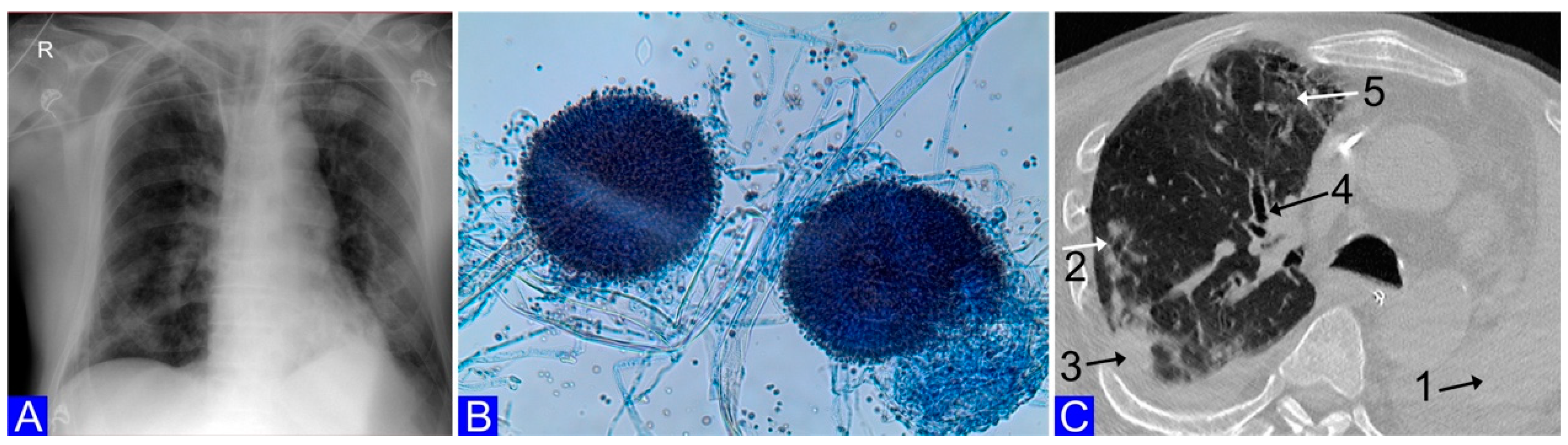
2. Case Report
3. Limitations
4. Discussion and Literature Review
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lai, C.C.; Wang, C.Y.; Wang, Y.H.; Hsueh, S.C.; Ko, W.C.; Hsueh, P.R. Global epidemiology of coronavirus disease 2019 (COVID-19): Disease incidence, daily cumulative index, mortality, and their association with country healthcare resources and economic status. Int. J. Antimicrob. Agents 2020, 55, 105946. [Google Scholar] [CrossRef] [PubMed]
- Lansbury, L.; Lim, B.; Baskaran, V.; Lim, W.S. Co-infections in people with COVID-19: A systematic review and meta-analysis. J. Infect. 2020, 81, 266–275. [Google Scholar] [CrossRef]
- Zhu, X.; Ge, Y.; Wu, T.; Zhao, K.; Chen, Y.; Wu, B.; Zhu, F.; Zhu, B.; Cui, L. Co-infection with respiratory pathogens among COVID-2019 cases. Virus Res. 2020, 285, 198005. [Google Scholar] [CrossRef]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef]
- El-Baba, F.; Gao, Y.; Soubani, A.O. Pulmonary aspergillosis: What the generalist needs to know. Am. J. Med. 2020, 133, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Latgé, J.P.; Chamilos, G. Aspergillus fumigatus and Aspergillosis in 2019. Clin. Microbiol. Rev. 2019, 33, e00140-18. [Google Scholar] [CrossRef]
- Montagna, M.T.; Caggiano, G.; Lovero, G.; De Giglio, O.; Coretti, C.; Cuna, T.; Iatta, R.; Giglio, M.; Dalfino, L.; Bruno, F.; et al. Epidemiology of invasive fungal infections in the intensive care unit: Results of a multicenter Italian survey (AURORA Project). Infection 2014, 42, 141–151. [Google Scholar] [CrossRef]
- Arastehfar, A.; Carvalho, A.; van de Veerdonk, F.L.; Jenks, J.D.; Koehler, P.; Krause, R.; Cornely, A.O.; Perlin, D.S.; Lass-Flörl, C.; Hoenigl, M. COVID-19 Associated Pulmonary Aspergillosis (CAPA)—From Immunology to Treatment. J. Fungi 2020, 6, 91. [Google Scholar] [CrossRef]
- Tudesq, J.J.; Peyrony, O.; Lemiale, V.; Azoulay, E. Invasive pulmonary aspergillosis in nonimmunocompromised hosts. Semin. Respir. Crit. Care Med. 2019, 40, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Patterson, T.F.; Thompson, G.R.; Denning, D.W.; Fishman, J.A.; Hadley, S.; Herbrecht, R.; Kontoyiannis, D.P.; Marr, K.A.; Morrison, V.A.; Nguyen, M.H.; et al. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 63, e1–e60. [Google Scholar] [CrossRef] [PubMed]
- Dellière, S.; Dudoignon, E.; Voicu, S.; Collet, M.; Fodil, S.; Plaud, B.; Chousterman, B.; Bretagne, S.; Azoulay, E.; Mebazaa, A.; et al. Combination of Mycological Criteria: A Better Surrogate to Identify COVID-19-Associated Pulmonary Aspergillosis Patients and Evaluate Prognosis? J. Clin. Microbiol. 2022, 60, e0216921. [Google Scholar] [CrossRef]
- van der Torre, M.H.; Shen, H.; Rautemaa-Richardson, R.; Richardson, M.D.; Novak-Frazer, L. Molecular Epidemiology of Aspergillus fumigatus in Chronic Pulmonary Aspergillosis Patients. J. Fungi 2021, 7, 152. [Google Scholar] [CrossRef] [PubMed]
- Luvanda, M.K.; Posch, W.; Noureen, A.; Lafon, E.; Zaderer, V.; Lass-Flörl, C.; Wilflingseder, D. Dexamethasone creates a suppressive microenvironment and promotes Aspergillus fumigatus invasion in a human 3D epithelial/immune respiratory model. J. Fungi 2021, 18, 221. [Google Scholar] [CrossRef] [PubMed]
- Trovato, L.; Calvoa, M.; Migliorisia, G.; Astutoc, M.; Oliveric, F.; Oliveri, S. Fatal VAP-related pulmonary aspergillosis by Aspergillus niger in a positive COVID-19 patient. Respir. Med. Case Rep. 2021, 32, 101367. [Google Scholar] [CrossRef] [PubMed]
- Mirchin, R.; Czeresnia, J.M.; Orner, E.P.; Chaturvedi, S.; Murphy, K.; Nosanchuk, J.D. The Continuing Emergence of Candida blankii as a Pathogenic Fungus: A New Case of Fungemia in a Patient Infected with SARS-CoV-2. J. Fungi 2022, 8, 166. [Google Scholar] [CrossRef] [PubMed]
- Pasula, S.; Chandrasekar, P. Spontaneous Hemoptysis in a Patient with COVID-19. Chest 2021, 160, e39–e44. [Google Scholar] [CrossRef]
- Katsiari, M.; Mavroidi, A.; Palla, E.; Zourla, K.; Alonistiotis, T.; Ntorlis, K.; Nikolaou, C.; Vrioni, G.; Tsakris, A. Possible COVID-19-Associated Pulmonary Aspergillosis Due to Aspergillus niger in Greece. Antibiotics 2022, 11, 300. [Google Scholar] [CrossRef]
- Tabarsi, P.; Sharifynia, S.; Toutkaboni, M.P.; Abtahian, Z.; Rahdar, M.; Mirahmadian, A.S.; Hakamifarda, A. Mixed etiology COVID-19 associated acute rhinosinusitis caused by two Aspergillus species. Ann. Med. Surg. 2022, 75, 103365. [Google Scholar] [CrossRef]
- Singh, N.; Husain, S. Aspergillus infections after lung transplantation: Clinical differences in type of transplant and implications for management. J. Heart Lung. Transplant. 2003, 22, 258–266. [Google Scholar] [CrossRef]
- Darwazeh, A.M.G.; Al-Dosari, A.; Al-bagieh, N.H. Oral Candida and nasal Aspergillus flora in a group of Saudi healthy dentate subjects. Int. Dent. J. 2002, 52, 273–277. [Google Scholar] [CrossRef]
- Peters, B.A.; Wu, J.; Hayes, R.B.; Ahn, J. The oral fungal mycobiome: Characteristics and relation to periodontitis in a pilot study. BMC Microbiol. 2017, 17, 157. [Google Scholar] [CrossRef] [PubMed]
- Daly, C.G.; Mitchell, D.H.; Highfield, J.E.; Grossberg, D.E.; Stewart, D. Bacteremia due to periodontal probing: A clinical and microbiological investigation. J. Periodontol. 2001, 72, 210–214. [Google Scholar] [CrossRef] [PubMed]
- Charles, M.P.; Kali, A.; Easow, J.M.; Joseph, N.M.; Ravishankar, M.; Srinivasan, S.; Kumar, S.; Umadevi, S. Ventilator-associated pneumonia. Australas. Med. J. 2014, 7, 334–344. [Google Scholar] [CrossRef] [PubMed]
- Torres, A.; Martin-Loeches, I. Invasive pulmonary aspergillosis in ventilator-associated pneumonia: The hidden enemy? Am. J. Respir. Crit. Care Med. 2020, 202, 1071–1073. [Google Scholar] [CrossRef] [PubMed]
| Specimen | Diagnosis | Culture n (%) | PCR n (%) | References |
|---|---|---|---|---|
| BA * | IPA | 1/1 (100%) | 1/1 (100%) | Trovato, L.; Calvoa, M.; Migliorisia, G.; et al. [14] |
| TA * | IPA | 1/1 (100%) | NP | Mirchin, R.; Czeresnia, J.M.; Orner, E.P.; et al. [15] |
| TA * | IPA | 1/1 (100%) | NP | Pasula, S.; Chandrasekar, P. [16] |
| TA * | IPA | 1/1 (100%) | 1/1 (100%) | Singh, N.; Husain, S. [17] |
| FESS * | Acute rhinosinusitis | 1/1 (100%) | NP | Tabarsi, P.; Sharifynia, S.; Pourabdollah, Toutkaboni, M.; et al. [18] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fiema, M.; Wlodarczyk, A.; Wojkowska-Mach, J.; Garlicki, J.; Gregorczyk-Maga, I. Atypical Presentation of Aspergillus niger Infection in the Oral Cavity as a Prediction of Invasive Pulmonary Aspergillosis in a Patient with COVID-19: Case Report and Literature Review. Microorganisms 2022, 10, 1630. https://doi.org/10.3390/microorganisms10081630
Fiema M, Wlodarczyk A, Wojkowska-Mach J, Garlicki J, Gregorczyk-Maga I. Atypical Presentation of Aspergillus niger Infection in the Oral Cavity as a Prediction of Invasive Pulmonary Aspergillosis in a Patient with COVID-19: Case Report and Literature Review. Microorganisms. 2022; 10(8):1630. https://doi.org/10.3390/microorganisms10081630
Chicago/Turabian StyleFiema, Mateusz, Aleksandra Wlodarczyk, Jadwiga Wojkowska-Mach, Jaroslaw Garlicki, and Iwona Gregorczyk-Maga. 2022. "Atypical Presentation of Aspergillus niger Infection in the Oral Cavity as a Prediction of Invasive Pulmonary Aspergillosis in a Patient with COVID-19: Case Report and Literature Review" Microorganisms 10, no. 8: 1630. https://doi.org/10.3390/microorganisms10081630
APA StyleFiema, M., Wlodarczyk, A., Wojkowska-Mach, J., Garlicki, J., & Gregorczyk-Maga, I. (2022). Atypical Presentation of Aspergillus niger Infection in the Oral Cavity as a Prediction of Invasive Pulmonary Aspergillosis in a Patient with COVID-19: Case Report and Literature Review. Microorganisms, 10(8), 1630. https://doi.org/10.3390/microorganisms10081630







