1. Introduction
Metabolic syndrome (MS), represented by visceral obesity with dyslipidemia, hyperglycemia, and hypertension, has become one of the major public-health challenges worldwide [
1]. The epidemiological and clinical studies data indicate that eating disorders, sedentary lifestyle, and a fat-rich diet with a low fiber content are the main modifiable factors in the treatment of various variants of MS. [
2]. One of its developmental pathogenetic factors is gut microorganism disorders [
3]. It was shown that dysbiosis could be involved in the MS development by relating the oxidative stress with an inflammatory process [
4]. The methods of gut microbiota correction such as probiotics, prebiotics, nutraceuticals, and fecal microbiota transplantation (FMT) may represent effective forms for improvement in MS course [
5,
6].
Probiotic lactobacilli were mainly used in the treatment of MS [
7], which promote the changes in intestinal microbiome, normalization of the lipid and carbohydrate metabolism, and weight reduction. However, due to the lack of biocompatibility between the host organism and its microbiota to the administered probiotics, they are eliminated rapidly from the organism, and longer treatment periods are needed [
8]. In some studies, dysbiosis condition could be corrected with the use of autoprobiotics, the indigenous beneficial host bacteria which have a high potential for overcoming these problems [
9].
Autoprobiotics are indigenous representatives of the normal microbiota of host organisms (lactobacilli, enterococci, bifidobacteria and their mixture, isolated from the organism and orally consumed by the host after cultivation in the same concentrations as probiotics). Their indisputable advantages are immunological tolerance, compatibility with the host microbiota and the metabolic status, allowing autoprobiotics to persist in the body for a long time [
8]. The efficacy of mono-strain autoprobiotics has been proven in an antibiotic-associated intestinal dysbiosis experimental model [
9,
10] and in treatment of irritable bowel syndrome and pneumonia [
11]. However, the introduction of single components of intestinal microbiota cannot recreate the complete microbiota, characterized by a high biodiversity and the presence of its obligate representatives. Microbial fecal transplantation (FMT) is preferable in this case [
12]. However, FMT has logistical challenges and is associated with several problems: infections, undesirable outcomes (e.g., increased risk of microbiota-associated diseases), and so called ‘yuck’ factor [
13]. Efforts are also under way to create artificial microbiota using fecal samples as source of own or donor bacteria. To date, no attempts have been made to use new generation of autoprobiotics, an indigenous consortium based on own fecal bacteria for the treatment of somatic diseases. Previously, its effectiveness was tested on an experimental model of dysbiosis [
9].
The aim of the study was to investigate the effectiveness of an IC of fecal bacteria in treatment of patients with MS analyzing the anthropometric parameters, eating behavior, metabolism, inflammatory markers, and microbiome restoration.
2. Materials and Methods
2.1. Characteristics of the Study Participants
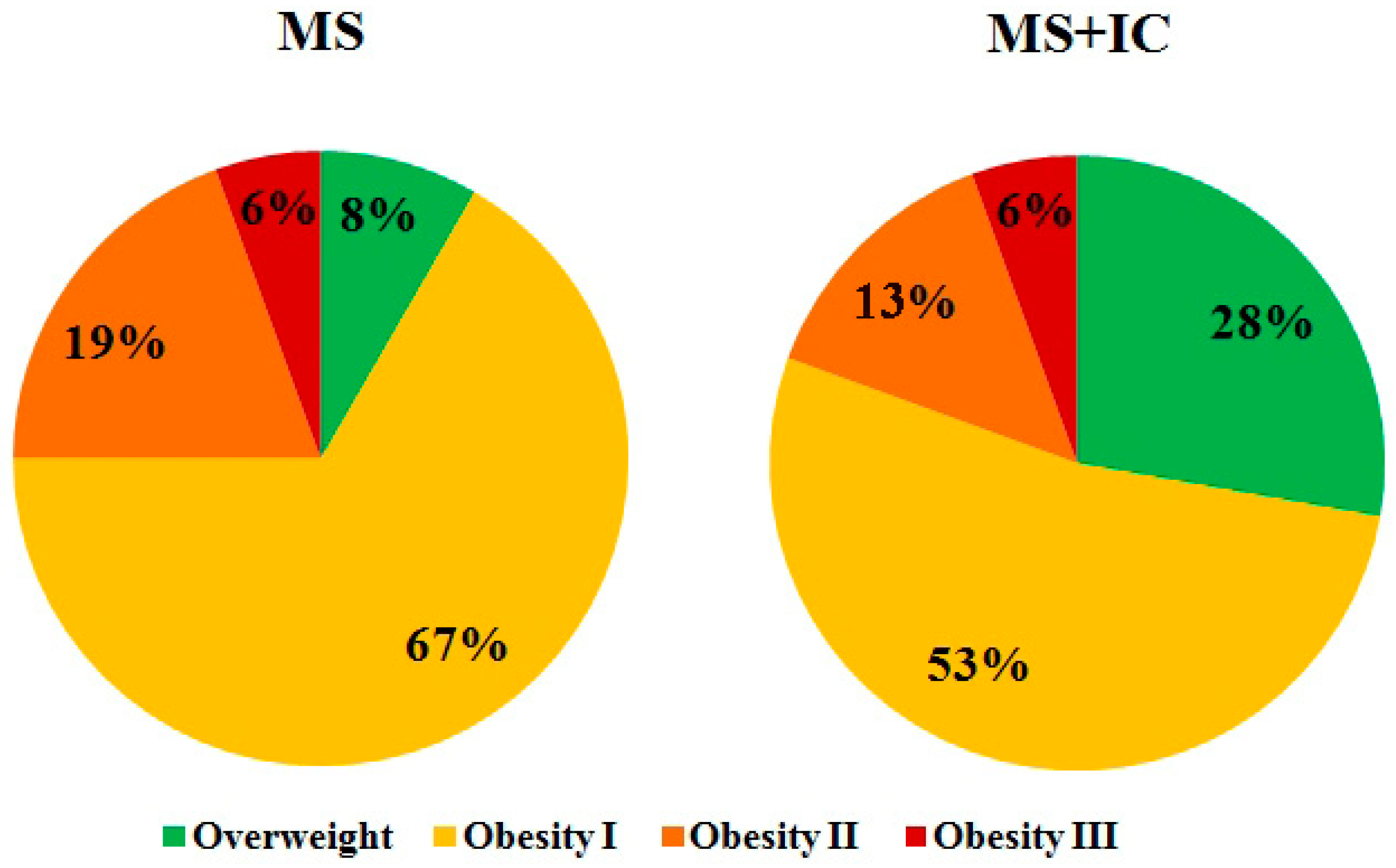
The study participants were patients with MS, manifested in abdominal obesity, dyslipidemia, and arterial hypertension. Patients were followed up in the clinics of the Saint-Petersburg State University and North-Western State Medical University named after I.I. Mechnikov. All patients signed an informed consent. The inclusion criteria in the group of MS were: age of patients from 25 to 60 years (
Figure S1), abdominal obesity, assessed by waist circumference (WC) of more than 80 cm in women and more than 94 cm in men, with arterial hypertension (blood pressure ≥ 130/85 mmHg), and decreased high-density lipoprotein cholesterol (HDL cholesterol < 1, 0 mmol/L in men; <1.2 mmol/L in women) or increased levels of low-density lipoprotein cholesterol (LDL cholesterol > 3.0 mmol/L) or increased triglyceride levels (TG ≥ 1.7 mmol/L).
Exclusion criteria for patients from the study were thyrotoxicosis, hypothyroidism, type 1 diabetes, decompensated type 2 diabetes, oncological diseases, organic intestinal pathology, acute intestinal infections in the past six months, taking antibacterial drugs or probiotics in the last 6 months. Healthy volunteers aged 25–60 with normal body weight (BMI = 18.5–24.9 kg/m
2), with BP ≤ 120–129/80–84 mmHg, with normal lipid profile and normal fasting blood glucose formed the control group. The study was carried out on 36 patients with MS and 20 healthy volunteers (HV). The subjects included 13 men and 23 women (
Figure S1a). The mean age in the group was 47.0 ± 2.8 (20–74) years (
Figure S1b). The control group was formed by HV of similar age and gender, and same proportion of male/female subjects as in the MS group.
2.2. Ethics Approval
All studies were performed with the informed consent of the subjects and in accordance with the Helsinki Declaration of the World Medical Association “Ethical Principles for Medical Research Involving Human Subjects” with amendments from 2013 [
14] and “Rules of Clinical Practice in the Russian Federation” approved by the Order of the Ministry of Health of the Russian Federation 19.06.2003 No. 266.
2.3. Study Design
The study design is presented in
Table S1 (Supplementary Materials). The experimental group of patients received personalized therapy with IC as a personified functional food product 2 times a day for 10 days. The control group received a similar product without probiotic supplements in a daily dose of 50 mL of fermented soy protein product 2 times a day for 10 days. Before starting therapy, fecal samples were collected from patients to obtain IC. Fecal and blood samples were taken before starting therapy and two weeks after autoprobiotic therapy to study the composition of the intestinal microbiota and biochemical parameters of blood serum. Before and after therapy, patients with MS were examined anthropometrically and surveyed to identify the features of eating behavior. During the experiment, the changes in the main clinical symptoms of MS were analyzed after administration of IC. The patients were examined by a gastroenterologist before and after therapy and filled out gastroenterological diaries daily.
All laboratory tests were carried out on the day of the blood collection. The blood samples were obtained by the peripheral vein puncture and collected in vacuum tubes. Fecal matter was collected from patients before and after therapy, stored in a freezer at 80 °C, and used for IC creation metagenome study.
2.4. Preparation of Indigenous Consortium
A functional personified food product based on IC was prepared in the base of own patients’ fecal samples as described in RF patent No. 2,734,896 C2.
Samples of 50 mg feces (previously frozen −80 °C) were transferred into 10 mL of thioglycol medium (PDB, Conda-Pronadisa Laboratories, Madrid, Spain) with addition of 250 μL of 40% sterile glucose solution and 5 μL of vicasol (Joint Stock Company DALKHIMPHARM, Khabarovsk, Russia), then mixed in the lower part of the test tube and throughout the volume of the medium. The tubes were placed in a thermostat for cultivation at a temperature of 37 °C for 6 days.
Inoculation of IC occurred in 10 mL of medium protein-vitamin soy product Supro Plus 2640 (Monsanto company, Missouri, United States, concentration 40 g/L), 1 mL of the consortium was centrifuged (MiniSpin centrifuge (Eppendorf)) for 15 min at 3.5 thousand rpm, then supernatant was discarded and 10 mL of Supro Plus 2640 were added to the sediment and were mixed. The samples were incubated at a temperature of 37 °C under anaerobic conditions for 1 day. Then, 7 mL of the fermented product were added to the 1 L of Supro 2640 and the bottle was incubated for 48–56 h at 37 °C.
Final personified IC products were tested by bacteriological method and qPCR. Exclusion criterion for admission was the presence of pathogenic and opportunistic bacteria.
2.5. Intestinal Microbiota Study
Intestinal microbiota was studied bacteriologically by the quantitative polymerase chain reaction (qPCR) and by metagenome analysis (16S rRNA).
2.6. Bacteriological Study of Indigenous Consortium
Consortium samples were diluted (diluted 100–10,000 times). The following differential diagnostic media were seeded: saline mannitol agar, Uroselect (Himedya, Thane West, Maharashtra, India). Identification of the grown bacteria was carried out with using MALDI-TOF mass-spectrometry with biotype Bruker Daltonics, Bremen, Germany.
2.7. Quantitative Polymerase Chain Reaction
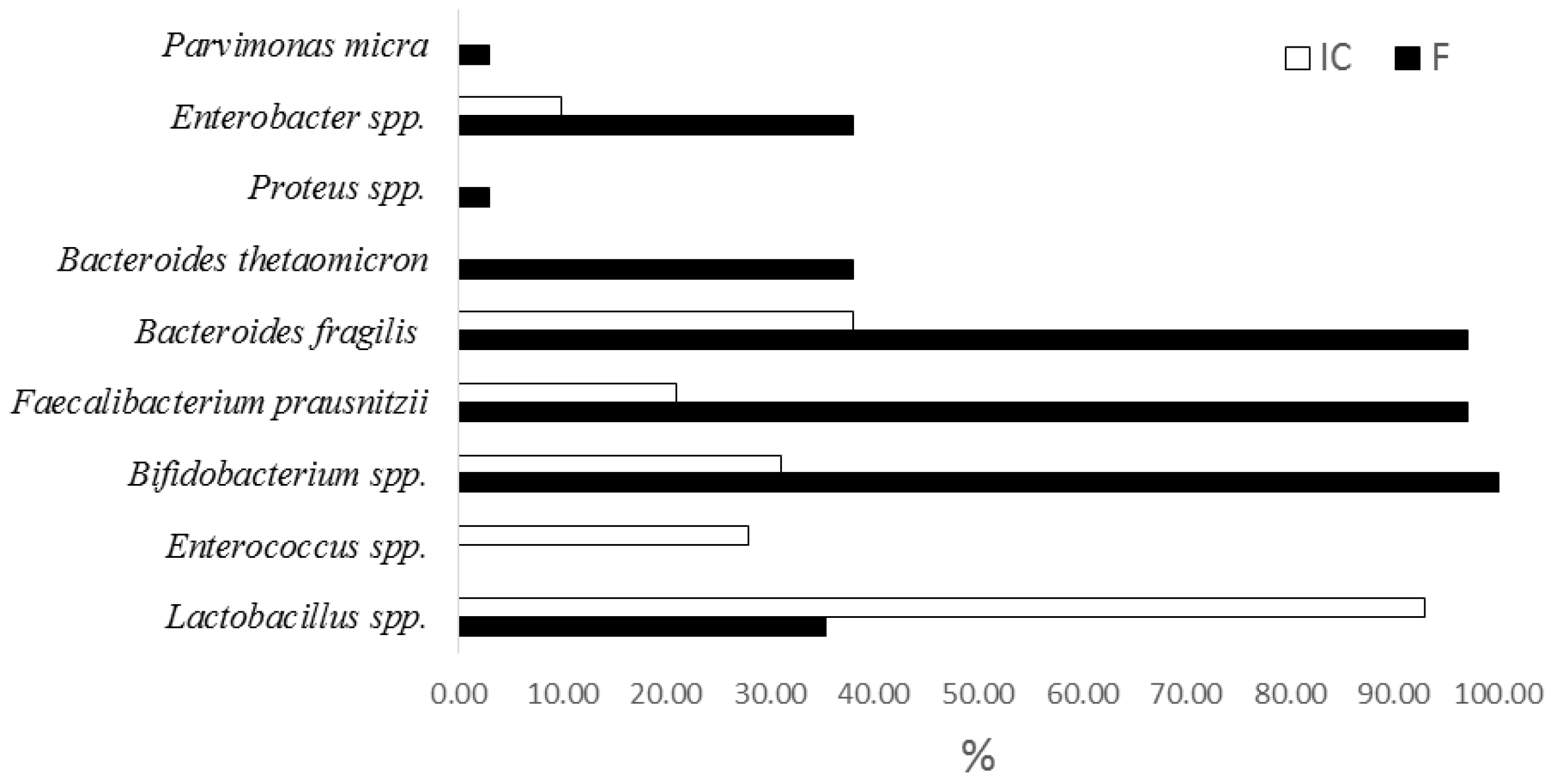
Samples of IC and feces of patients were examined before and after the introduction of the IC by qPCR using the kit Colonoflor (AlphaLab, Saint-Petersburg, Russia) on Mini-Opticon (Applied Biosystems, 850 Lincoln Centre Drive, Foster City, CA, USA). The quantity content of Acinetobacter spp., Citrobacter spp., Escherichia coli and enteropathogenic E. coli, Proteus spp., Lactobacillus spp., Bifidobacterium spp., Bacteroides thetaiotaomicron, Bacteroides fragilis group, Clostridioides difficile, Clostridium perfringens, Enterococcus spp., Faecalibacterium prausnitzii, Fusobacterium nucleatum, Parvimonas micra, Roseburia inilinivorans, and Akkermansia muciniphila content in intestinal microbiota was studied.
2.8. Microbiome (16S rRNA) Study
The IC personified products, fecal samples before and after therapy were investigated by 16S rRNA gene-based metagenomics analysis using a previously described approach [
9]. DNA were isolated from feces using the kit «DNA Express bio» Alkor Bio (Saint Petersburg, Russia). Metagenome study was used for the analysis of Libraries of hypervariable regions V3 and V4 of the 16S RNA genes using MiSeq (Illumina, San Diego, CA, USA). The standard method recommended by Illumina based on employing two rounds of PCR was used to prepare the libraries.
2.9. Biochemical Analysis
The biochemical study of serum blood samples was performed automatically by Abbot ARCHITECT si 8200 (333 Fiske Street, Holliston, MA, USA). The following parameters were studied: total cholesterol (TC), triglycerides (TG), high-density lipoproteins (HDL), low-density lipoproteins (LDL), alanine aminotransferase (ALT), aspartate aminotransferase (AST), glucose, C-reactive protein (CRP) concentrations in blood serum were determined.
2.10. Physical Examination
Patients of both groups were examined by a physician to assess eligibility for inclusion in the study. Waist circumference, height, weight, and blood pressure were measured. Body mass index was calculated to evaluate the severity of overweight.
2.11. Eating Behavior
The patients’ eating behaviors were assessed according to the Dutch DEBQ Eating Behavior Questionnaire [
17]. Patients completed a 33-question questionnaire on their own prior to taking the IC. According to the results of the survey, the patient could be assigned to one of three types of eating behavior: restrictive, emotional, or external. Evaluation of differences in eating behavior in the compared groups was carried out.
2.12. Gastro Diary
During 10 days of taking IC, patients kept gastro diaries, which included the following indicators: stool frequency, Bristol Stool Form Scale, the presence and severity of abdominal pain and tympanites (0—no pain, tympanites; 1—moderate pain, tympanites; 2—severe pain, tympanites) [
18].
2.13. Statistical Analysis
Statistical analysis was performed using the software package Statistica 8.0. (StatSoft, Tulsa, OK, USA). Differences between the groups were obtained using Kruskal–Wallis tests and ANOVA with post-hoc HSD test for unequal n, p < 0.05 was considered significant. The normality of the data distribution was determined using the Kolmogorov–Smirnov criterion. The nonparametric criteria were used on this basis. Statistic data processing was performed using the software package IBM SPSS Statistics-22 (IBM, Armonk, NY, USA) and Statistica-10 (Statsoft, Tulsa, OK, USA). The presence of statistically significant differences among groups were determined using the Mann–Whitney U-criterion, adjusted for multiple comparisons by the Benjamini–Hochberg method. Wilcoxon’s t-test was also used for paired samples. Comparative analysis was conducted using the a posteriori test honestly significant difference (HSD) for unequal N in the Statistica-8 software. A search for correlations between the studied parameters was performed using Spearman’s test using the software package Statistica 10.0. (StatSoft, Tulsa, OK, USA). χ2 tests with Yates correction were used. Differences in p < 0.05 were considered significant.
4. Discussion
The present paper is the first study devoted to the influence of the personified functional food product prepared by cultivation of indigenous fecal bacteria on the gut microbiota and clinical and laboratory parameters of patients with MS. Previously, we analyzed the effectiveness of IC on the animal experimental model [
9].
A positive metabolic influence of microbiota employing the usage of bacteria as probiotics or by fecal microbiota transplantation is well documented in scientific literature [
19]. The advantage of autoprobiotic microbial therapy is based on the usage for the indigenous bacteria which were adopted to the immune system of the host due the mechanisms of immune tolerance. This allows long conflict-free propagation of the bacteria in the gut in contrast to probiotics, which are usually rapidly eliminated from the gut [
20]. Another advantage of autoprobiotics relative to the fecal microbiota transplantation is their safety profile and the ability to avoid the usage of bacteria with multiple antibiotic resistances or eukaryotic viruses that might be acquired from the donor [
21]. Monostrain autoprobiotcs allowing the genome study of the bacteria used for microbial therapy seems the least dangerous but they are good for the recipients with relatively developed microbiota which need proper microbiota modulation. In the present study, we were evaluating another approach of making autoprobiotics based on fecal microbiota cultivation outside the host on selective media for several days. After 6 days of cultivation of the indigenous bacteria microbiota richness decreased to a certain degree but still it was represented by about 30 bacterial families with predominance of lactobacilli.
The study included patients with MS without serious disorders of carbohydrate metabolism. MS is usually manifested with abdominal type of obesity, violation of the lipid profile and arterial hypertension. Analysis of eating behavior of the group of patients under study revealed significant disorders, which could be a trigger for the development of severe MS associated with type II diabetes or cardio-vascular diseases and the course of the pathology being examined. These patients had elevated levels of ALT, AST, and triglycerides, which indicated liver dysfunction and lipid metabolism disorders. It attracted an increase in CRP which could indicate the presence of small inflammation, metabolic endotoxemia associated with an increase in the population of Gram-negative bacteria containing lipopolysaccharides (LPS), which might be a cause of metabolic endotoxemia [
22].
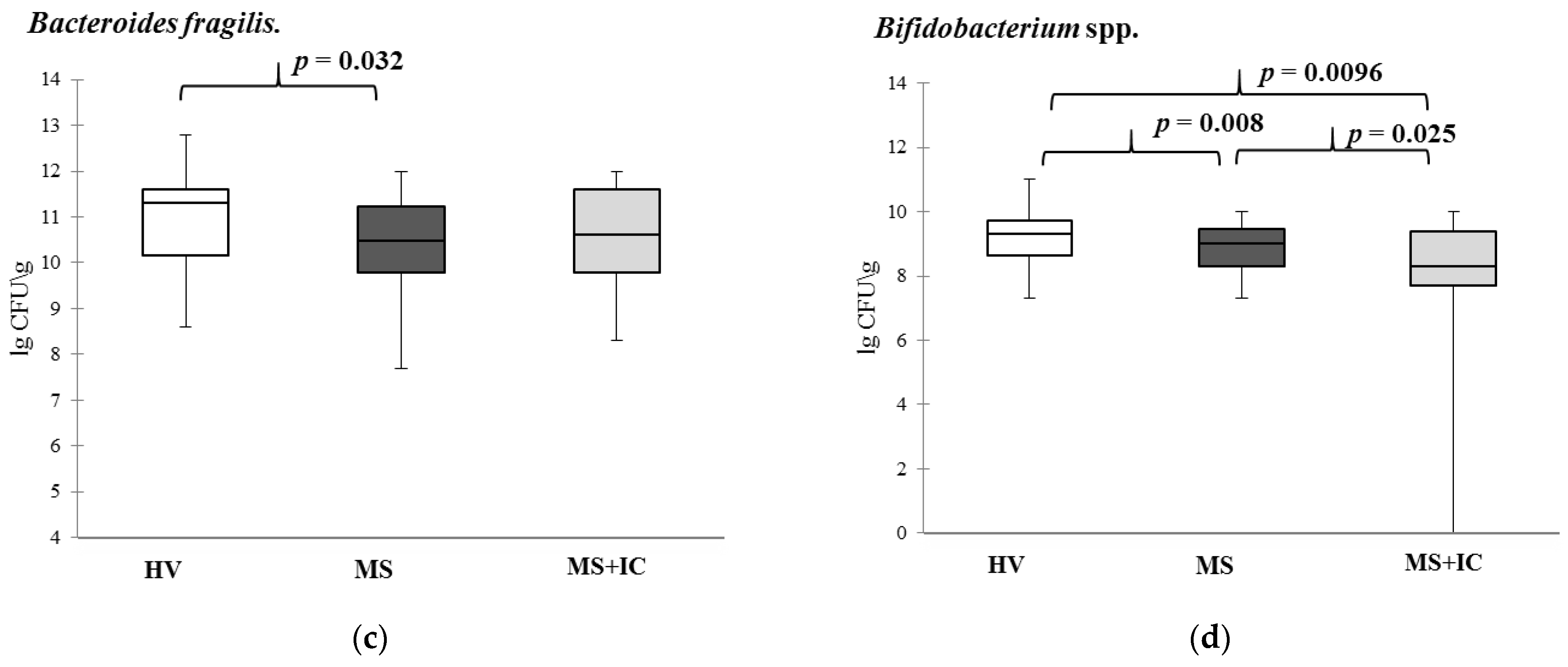
As shown by comparative studies of the composition of fecal samples from patients with MS and the consortiums of bacteria obtained during their cultivation, the selected cultivation conditions allow for selection of bacteria strains, as a result of which mainly beneficial bacteria, belonging to the genera of Lactobacilli, Enterococci, and Bifidobacteria used to create probiotics can be accumulated. At the same time, a number of opportunistic Gram-negative bacteria Campylobacter, Citrobacter, Desulfovibrio, Haemophilus, Bilophila, Paraprevotella, Prevotella, and Burkholderia disappear from samples or their number and representation decreases. The presence of these LPS in the fecal samples of patients could be one of the reasons for the development of the so-called minor inflammation that accompanies MS and was confirmed by an increase in the level of CRP. The approach which favors mainly lactic acid bacteria allows to abolish most of intestinal pathogens and eukaryotic virus contamination. The introduction of IC practically did not cause significant changes in digestive functions (frequency and characteristics of stool, dyspeptic symptoms) and did not affect the eating behavior of patients.
The administration of this IC in patients with MS caused a significant health effect, which was reflected in weight loss, improvement in biochemical parameters of the blood serum, and decrease in symptoms of the low grade inflammation.
The positive changes in anthropometric and biochemical parameters obtained in our work correlate with several previous studies devoted to the effect of probiotics or synbiotics on the patients with MS. A randomized placebo-controlled study evaluating the effect of a two-month intake of a synbiotic (including
Lactobacillus plantarum PBS067,
Lactobacillus acidophilus PBS066, and
Lactobacillus reuteri PBS072 with a prebiotic) showed a significant effect of taking a synbiotic on carbohydrate, lipid metabolism, and obesity criteria. In the group of patients treated with the synbiotic, there was a statistically significant decrease in weight coefficient, insulin levels, total cholesterol, HDL cholesterol, non-HDL cholesterol, fasting plasma triglycerides, as well as a decrease in the value of the visceral obesity index. There was also a significant decrease in blood pressure (BP) and fasting plasma glucose levels in the synbiotic group [
23].
In the other randomized study of 46 obese patients treated with a synbiotic containing Lactobacillus casei, Lactobacillus rhamnosus, Streptococcus thermophilus, Bifidobacterium breve, Lactobacillus acidophilus, Bifidobacterium longum, Lactobacillus bulgaricus, and fructo-oligosaccharides), and following a low-calorie diet, the positive effect of taking the synbiotic was also demonstrated. There was a greater decrease in body weight, BMI, fasting plasma glucose, insulin concentration, and insulin resistance index (HOMA-IR) in the synbiotic group compared to the placebo group. At the same time, the level of glucagon-like peptide-1 and tyrosine–tyrosine peptide, which suppress appetite and promotes weight loss, increased when taking the synbiotic [
24].
The partial compensation of lipid profile disorders revealed by us in this work after the use of IC was manifested in a decrease in the level of triglycerides in the blood serum. Previously, such changes were noted after the introduction of probiotic lactobacilli [
7].
The elevated levels of liver enzymes in the blood sera of patients with MS prior to IC administration most likely reflected formation of metabolic-associated liver disease (MAFLD) in this group of patients. We believe that decrease in ALT and AST levels after taking IC may be associated with a decrease in Gram-negative bacteria (
Enterobacter spp.,
Escherichia coli) quantity. Another group of researchers evaluating the association of MAFLD with changes in the gut microbiota composition in 52 obese patients found more
Enterobacteriaceae and
Escherichia coli in patients with greater severity of MAFLD associated with higher levels of AST [
25]. Similar data were obtained in 2017 in the study comparing the gut microbiota of patients with MAFLD and healthy individuals: more severe fibrosis in MAFLD was associated with a greater number of
Enterobacteriaceae and
Escherichia/
Shigella bacteria.
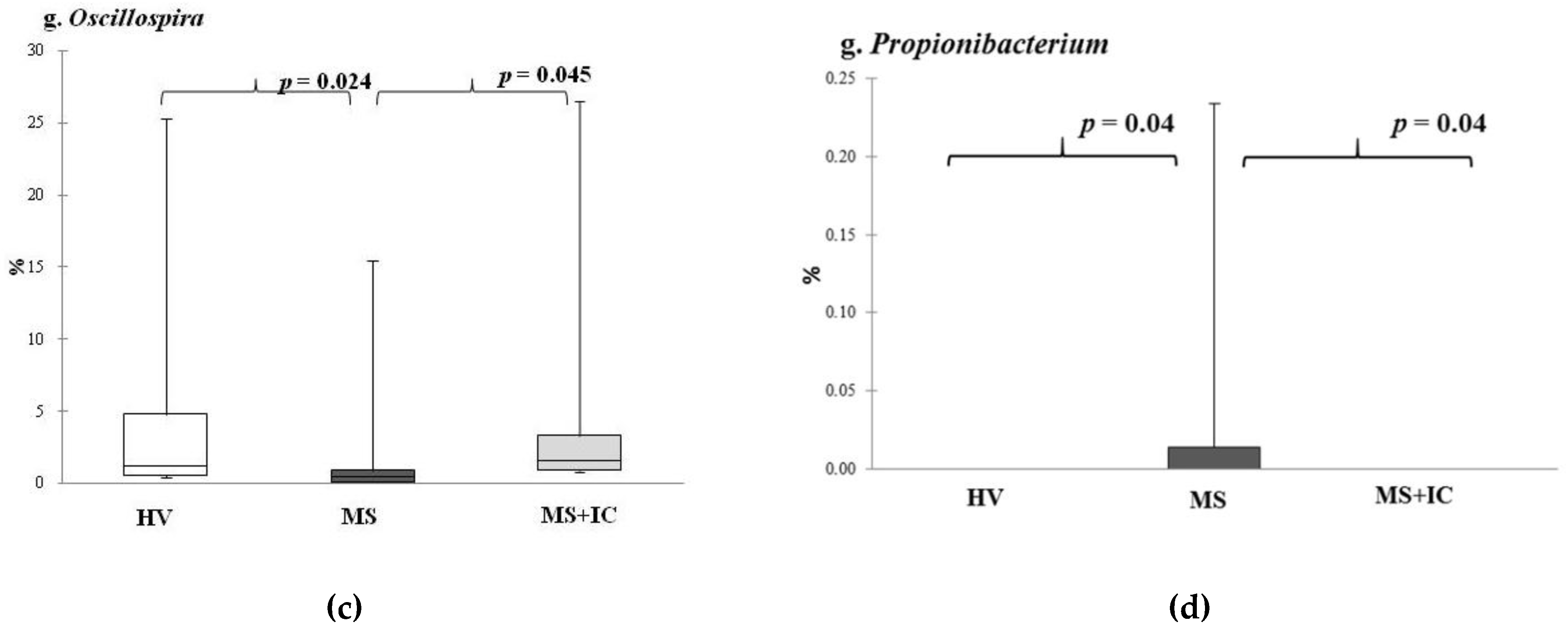
A relative increase in the representation of the genus
Oscillospira after IC consumption might also have contributed to the positive effect of IC administration. Bioinformatic analysis demonstrated that the abundance of this taxon in the intestinal microbiota was in strong association between representation of this bacteria and diseases associated with obesity-related chronic inflammatory and metabolic diseases. Some studies confirm the inverse correlation of this bacteria genus with body weight [
26]. Oscillospira species from the
Clostridial cluster belonging to the
Lachnospiraceae family are butyrate producers, and at least some of them have the ability to utilize glucuronate, a common animal-derived sugar. The presence of this genus is reduced in diseases that involve inflammation [
27]. In addition, several studies have confirmed that
Oscillospira spp. is strongly associated with leanness or lower BMI in children and adults [
28,
29].
The positive effects of
Oscillospira in obesity-related s down by other microbes. These bacteria also produce fatty acid as a byproduct of carbohydrate fermentation which can then be used as energy by the host. It was discovered that Cytochrome
bd oxidase is essential to oxygen consumption. This can allow other obligate anaerobes to survive in the now oxygen-reduced microenvironment [
30,
31]. Polysaccharide A or outer membrane vesicles from nontoxigenic
B. fragilis may mediate beneficial interactions with the host [
32]. The intestinal microbial competition of
B. fragilis is supported by two pervasive ecological drivers: non-contact-dependent secretory antimicrobial proteins and the contact-dependent Type VI secretion system (T6SSs). The first known
Bacteroidales secreted antimicrobial protein-1 (BSAP-1) is released in
B. fragilis OMVs, which contain membrane attack/perforin (MACPF) domains that are used to lyse other bacterial cells or infect host cells via pore formation. BSAP-1 is an important competitive factor that affects the composition of the human intestinal microbiota [
33]. Stimulation of innate immunity, antibacterial activity due to bacteriocins, creation of a favorable microenvironment for beneficial microbes, and neutralization of LPS make it possible to consider it as a potential genus for searching for a probiotic strain. This is confirmed in our work by a negative correlation with the majority of disorders identified in the group of patients with MS under consideration.
It is necessary to note the potential influence of lactobacilli, the leading taxon in IC, whose antibacterial immunomodulatory anti-inflammatory effect is described in the literature but was overshadowed in this study. Changes in the composition, including species composition, are beyond the scope of this work.