Oral Microbiota Profile in Patients with Anti-Neutrophil Cytoplasmic Antibody–Associated Vasculitis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Pre-Symptomatic AAV Individuals and Matched Controls Screened for Immunoglobulin (IgG) Antibodies to Oral Bacteria
2.3. Established AAV Cases Screened for IgG Antibodies to Oral Bacteria
2.4. Acute AAV Cases and Controls for Saliva Microbiota Sequencing
2.5. Plasma/Serum Antibody Screening
2.6. Saliva Microbiota Sequencing
2.7. Statistical Analyses
3. Results
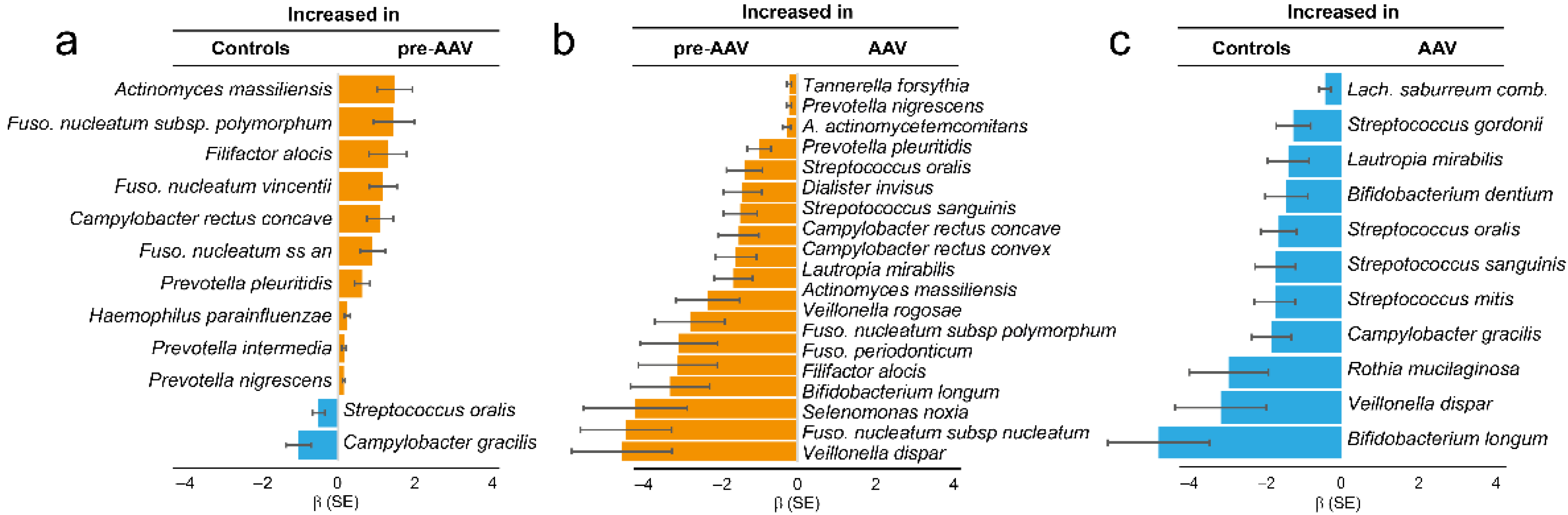
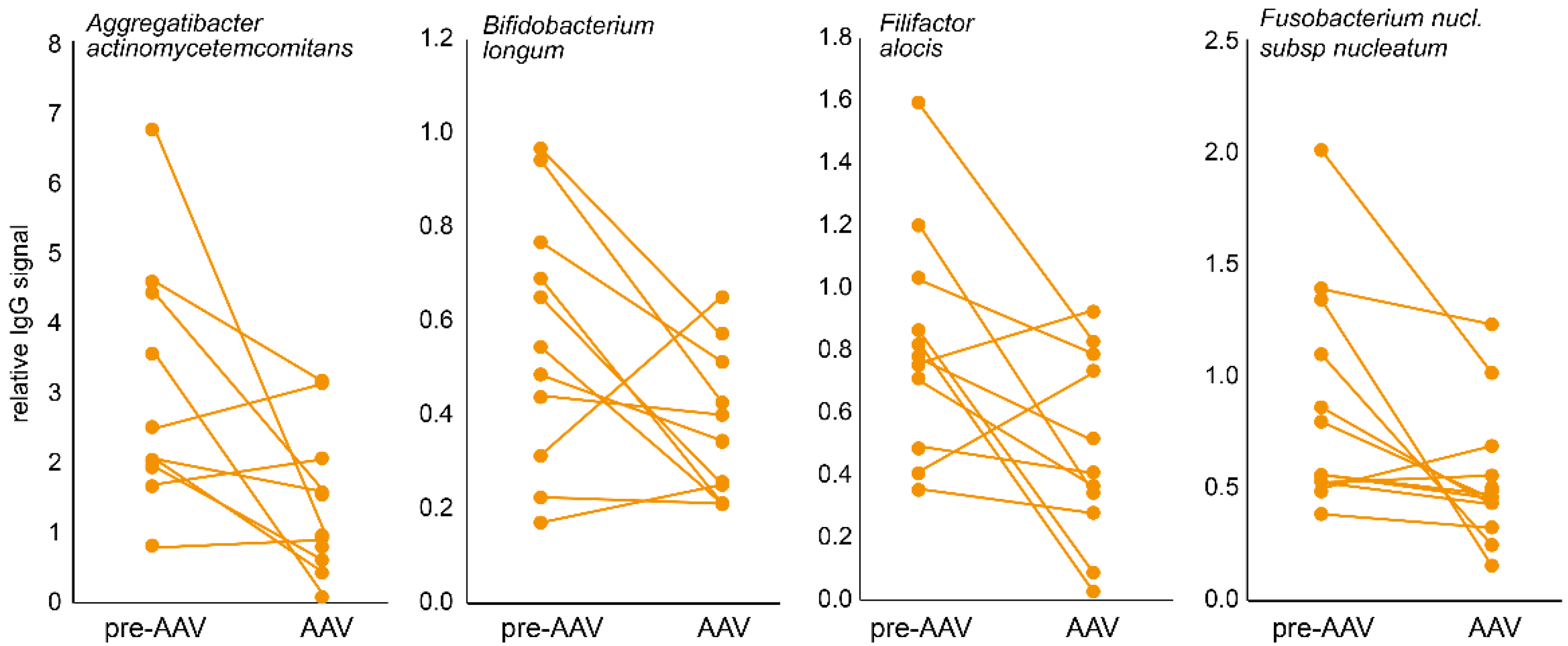
3.1. Immune Response to Oral Bacteria in Pre-Symptomatic versus Established AAV Individuals
3.2. Dental Status in Established AAV
3.3. Illumina Amplicon Sequencing of Saliva Microbiota in Acute AAV Samples
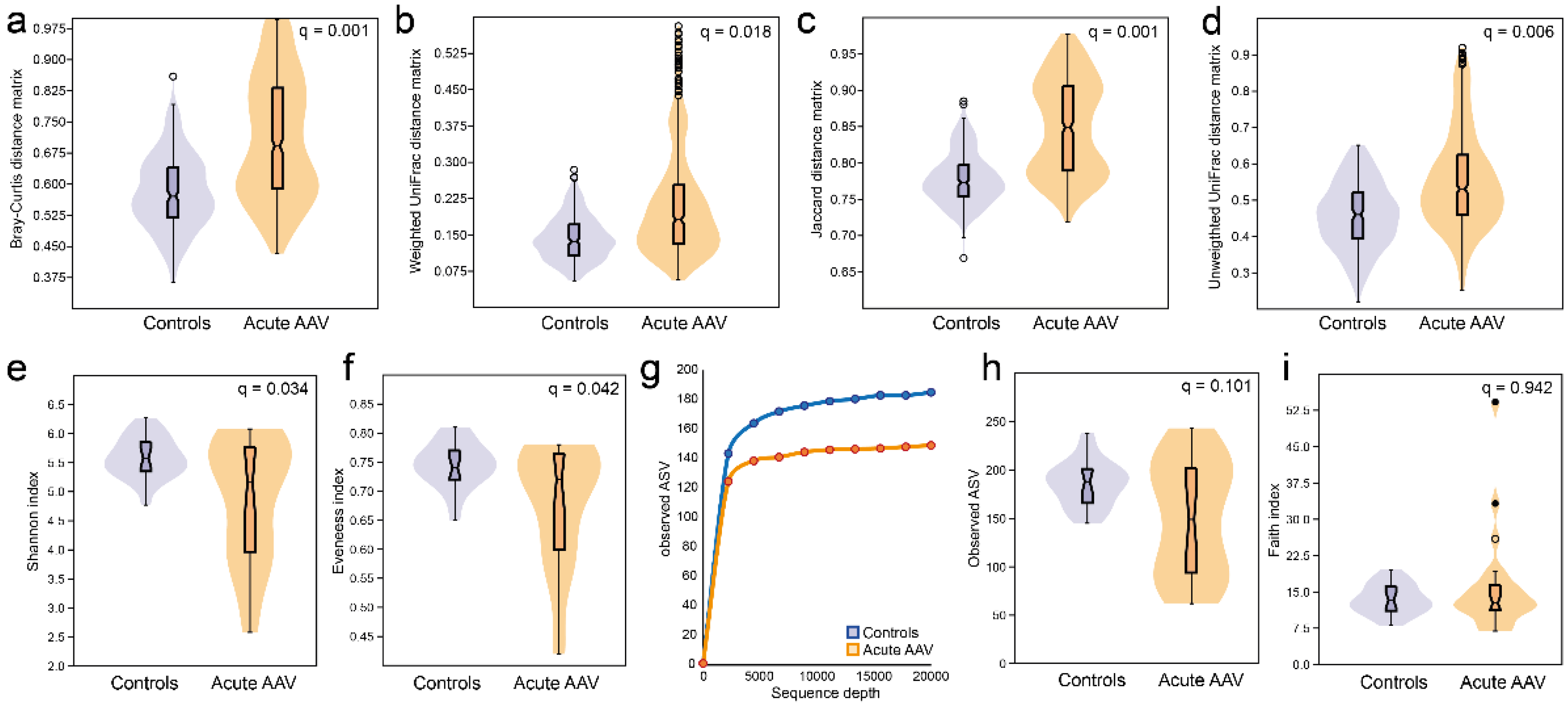
3.4. Microbiota Diversity Characterization in Acute AAV versus Controls
3.5. LEfSe Identified Differences in Saliva Microbiota in Acute AAV versus Control Samples
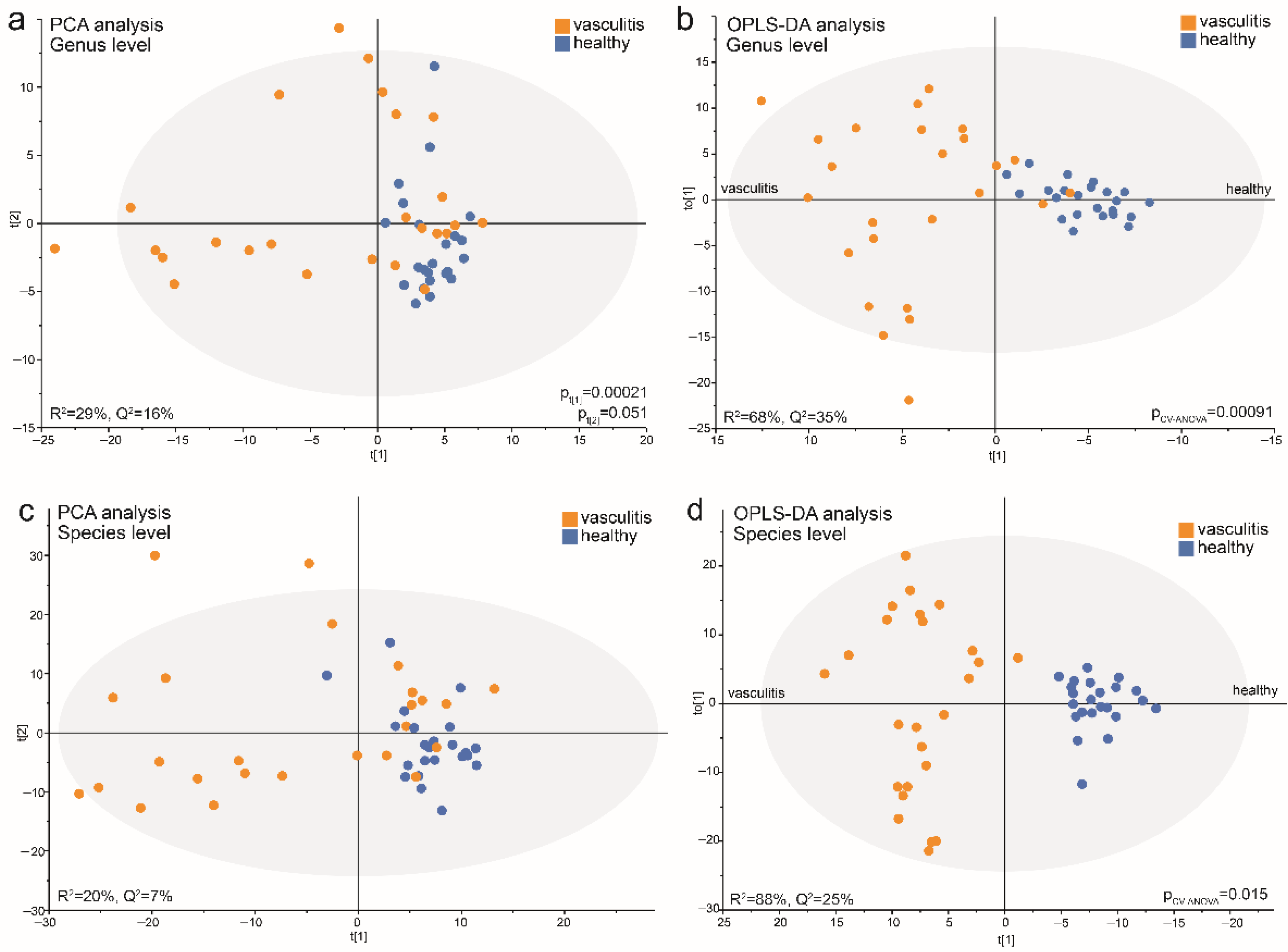
3.6. Data-Driven Profiling of Saliva Microbiota in Acute AAV versus Controls
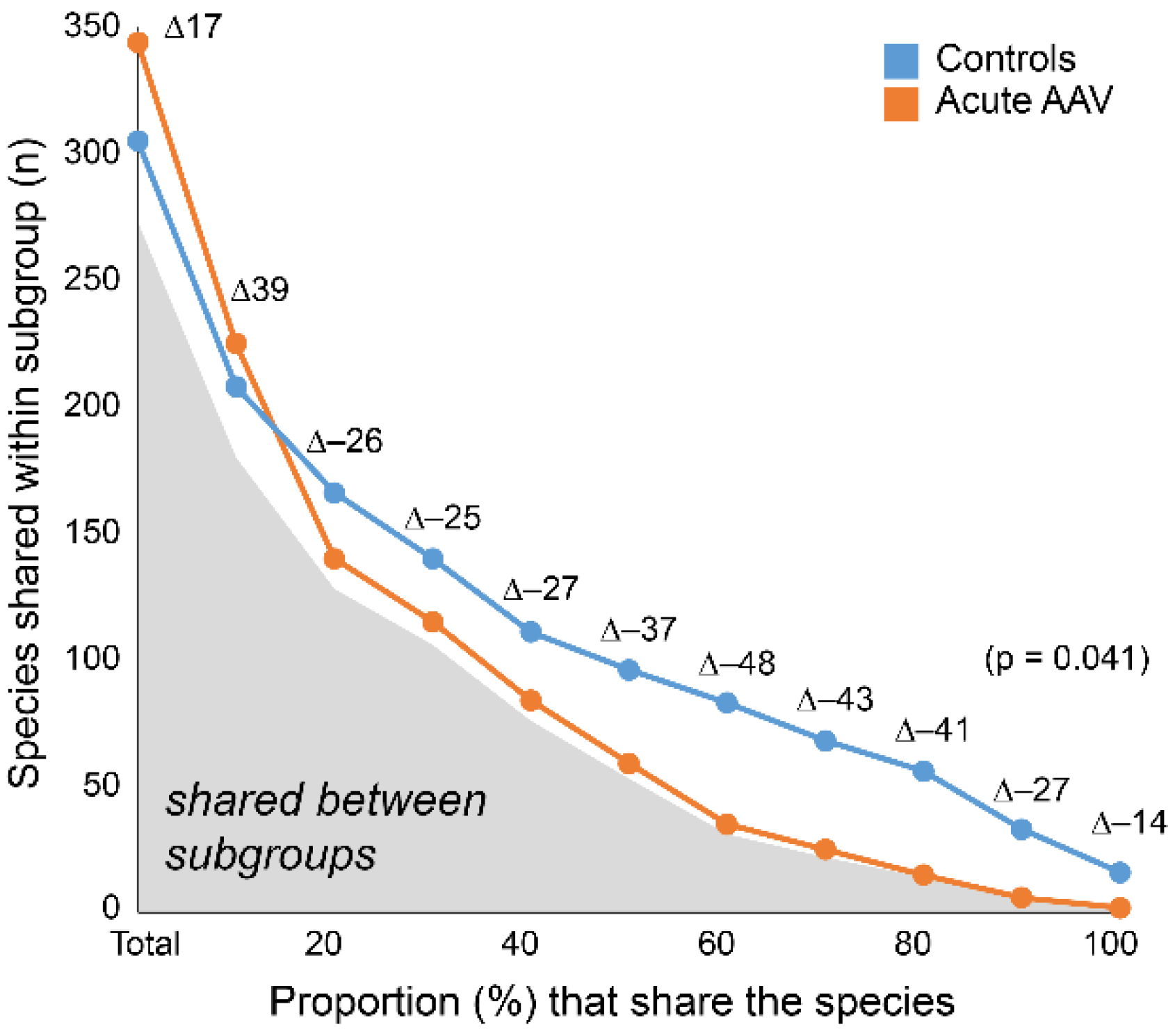
3.7. Acute AAV Subgroup Characterization
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Turnbaugh, P.J.; Ley, R.E.; Hamady, M.; Fraser-Liggett, C.M.; Knight, R.; Gordon, J.I. The human microbiome project. Nature 2007, 449, 804–810. [Google Scholar] [CrossRef]
- Maynard, C.L.; Elson, C.O.; Hatton, R.D.; Weaver, C.T. Reciprocal interactions of the intestinal microbiota and immune system. Nature 2012, 489, 231–241. [Google Scholar] [CrossRef]
- Konig, M.F. The microbiome in autoimmune rheumatic disease. Best Pract. Res. Clin. Rheumatol. 2020, 34, 101473. [Google Scholar] [CrossRef]
- Nishida, A.; Inoue, R.; Inatomi, O.; Bamba, S.; Naito, Y.; Andoh, A. Gut microbiota in the pathogenesis of inflammatory bowel disease. Clin. J. Gastroenterol. 2018, 11, 1–10. [Google Scholar] [CrossRef]
- Tariq, S.; Clifford, A.H. An update on the microbiome in vasculitis. Curr. Opin. Rheumatol. 2021, 33, 15–23. [Google Scholar] [CrossRef]
- Kitching, A.R.; Anders, H.J.; Basu, N.; Brouwer, E.; Gordon, J.; Jayne, D.R.; Kullman, J.; Lyons, P.A.; Merkel, P.A.; Savage, C.O.S.; et al. ANCA-associated vasculitis. Nat. Rev. Dis. Primers 2020, 6, 71. [Google Scholar] [CrossRef]
- Lamprecht, P.; Fischer, N.; Huang, J.; Burkhardt, L.; Lütgehetmann, M.; Arndt, F.; Rolfs, I.; Kerstein, A.; Iking-Konert, C.; Laudien, M. Changes in the composition of the upper respiratory tract microbial community in granulomatosis with polyangiitis. J. Autoimmun. 2019, 97, 29–39. [Google Scholar] [CrossRef]
- Rhee, R.L.; Sreih, A.G.; Najem, C.E.; Grayson, P.C.; Zhao, C.; Bittinger, K.; Collman, R.G.; Merkel, P.A. Characterisation of the nasal microbiota in granulomatosis with polyangiitis. Ann. Rheum. Dis. 2018, 77, 1448–1453. [Google Scholar] [CrossRef]
- Wagner, J.; Harrison, E.M.; Martinez Del Pero, M.; Blane, B.; Mayer, G.; Leierer, J.; Gopaluni, S.; Holmes, M.A.; Parkhill, J.; Peacock, S.J.; et al. The composition and functional protein subsystems of the human nasal microbiome in granulomatosis with polyangiitis: A pilot study. Microbiome 2019, 7, 137. [Google Scholar] [CrossRef]
- Richter, A.G.; Stockley, R.A.; Harper, L.; Thickett, D.R. Pulmonary infection in Wegener granulomatosis and idiopathic pulmonary fibrosis. Thorax 2009, 64, 692–697. [Google Scholar] [CrossRef]
- Fukui, S.; Morimoto, S.; Ichinose, K.; Nakashima, S.; Ishimoto, H.; Hara, A.; Kakugawa, T.; Sakamoto, N.; Tsuji, Y.; Aramaki, T.; et al. Comparison of lung microbiota between antineutrophil cytoplasmic antibody–associated vasculitis and sarcoidosis. Sci. Rep. 2020, 10, 9466. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, L.; Wang, Y.; Liu, X.; Zhang, H.; Liu, Y.; Shen, N.; Yang, J.; Gai, Z. Gut microbiota dysbiosis is associated with Henoch-Schönlein Purpura in children. Int. Immunopharmacol. 2018, 58, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Wang, J.; Wang, Y.; Zhang, J.; Zhao, C.; Shen, N.; Yang, J.; Gai, Z.; Zhang, L. Oral microbiota dysbiosis and its association with Henoch-Schönlein Purpura in children. Int. Immunopharmacol. 2018, 65, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Stegeman, C.A.; Tervaert, J.W.; Sluiter, W.J.; Manson, W.L.; de Jong, P.E.; Kallenberg, C.G. Association of chronic nasal carriage of Staphylococcus aureus and higher relapse rates in Wegener granulomatosis. Ann. Intern. Med. 1994, 120, 12–17. [Google Scholar] [CrossRef]
- Laudien, M.; Gadola, S.D.; Podschun, R.; Hedderich, J.; Paulsen, J.; Reinhold-Keller, E.; Csernok, E.; Ambrosch, P.; Hellmich, B.; Moosig, F.; et al. Nasal carriage of Staphylococcus aureus and endonasal activity in Wegener s granulomatosis as compared to rheumatoid arthritis and chronic Rhinosinusitis with nasal polyps. Clin. Exp. Rheumatol. 2010, 28, 51–55. [Google Scholar]
- Ooi, J.D.; Jiang, J.H.; Eggenhuizen, P.J.; Chua, L.L.; van Timmeren, M.; Loh, K.L.; O’Sullivan, K.M.; Gan, P.Y.; Zhong, Y.; Tsyganov, K.; et al. A plasmid-encoded peptide from Staphylococcus aureus induces anti-myeloperoxidase nephritogenic autoimmunity. Nat. Commun. 2019, 10, 3392. [Google Scholar] [CrossRef] [PubMed]
- Cohen Tervaert, J.W. Trimethoprim-sulfamethoxazole and antineutrophil cytoplasmic antibodies-associated vasculitis. Curr. Opin. Rheumatol. 2018, 30, 388–394. [Google Scholar] [CrossRef]
- Van Timmeren, M.M.; Heeringa, P.; Kallenberg, C.G. Infectious triggers for vasculitis. Curr. Opin. Rheumatol. 2014, 26, 416–423. [Google Scholar] [CrossRef]
- Willmann, M.; Vehreschild, M.; Biehl, L.M.; Vogel, W.; Dörfel, D.; Hamprecht, A.; Seifert, H.; Autenrieth, I.B.; Peter, S. Distinct impact of antibiotics on the gut microbiome and resistome: A longitudinal multicenter cohort study. BMC Biol. 2019, 17, 76. [Google Scholar] [CrossRef]
- Atherly, T.; Rossi, G.; White, R.; Seo, Y.J.; Wang, C.; Ackermann, M.; Breuer, M.; Allenspach, K.; Mochel, J.P.; Jergens, A.E. Glucocorticoid and dietary effects on mucosal microbiota in canine inflammatory bowel disease. PLoS ONE 2019, 14, e0226780. [Google Scholar] [CrossRef]
- Iwase, T.; Uehara, Y.; Shinji, H.; Tajima, A.; Seo, H.; Takada, K.; Agata, T.; Mizunoe, Y. Staphylococcus epidermidis Esp inhibits Staphylococcus aureus biofilm formation and nasal colonization. Nature 2010, 465, 346–349. [Google Scholar] [CrossRef] [PubMed]
- Scher, J.U.; Nayak, R.R.; Ubeda, C.; Turnbaugh, P.J.; Abramson, S.B. Pharmacomicrobiomics in inflammatory arthritis: Gut microbiome as modulator of therapeutic response. Nat. Rev. Rheumatol. 2020, 16, 282–292. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, D.; Jia, H.; Feng, Q.; Wang, D.; Liang, D.; Wu, X.; Li, J.; Tang, L.; Li, Y.; et al. The oral and gut microbiomes are perturbed in rheumatoid arthritis and partly normalized after treatment. Nat. Med. 2015, 21, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Watts, R.; Lane, S.; Hanslik, T.; Hauser, T.; Hellmich, B.; Koldingsnes, W.; Mahr, A.; Segelmark, M.; Cohen-Tervaert, J.W.; Scott, D. Development and validation of a consensus methodology for the classification of the ANCA-associated vasculitides and polyarteritis nodosa for epidemiological studies. Ann. Rheum. Dis. 2007, 66, 222–227. [Google Scholar] [CrossRef]
- Berglin, E.; Mohammad, A.J.; Dahlqvist, J.; Johansson, L.; Eriksson, C.; Sjöwall, J.; Rantapää-Dahlqvist, S. Anti-neutrophil cytoplasmic antibodies predate symptom onset of ANCA-associated vasculitis. A case-control study. J. Autoimmun. 2021, 117, 102579. [Google Scholar] [CrossRef]
- Yates, M.; Watts, R.A.; Bajema, I.M.; Cid, M.C.; Crestani, B.; Hauser, T.; Hellmich, B.; Holle, J.U.; Laudien, M.; Little, M.A.; et al. EULAR/ERA-EDTA recommendations for the management of ANCA-associated vasculitis. Ann. Rheum. Dis. 2016, 75, 1583–1594. [Google Scholar] [CrossRef]
- Von Bültzingslöwen, I.; Östholm, H.; Gahnberg, L.; Ericson, D.; Wennström, J.L.; Paulander, J. Swedish Quality Registry for Caries and Periodontal Diseases—A framework for quality development in dentistry. Int. Dent. J. 2019, 69, 361–368. [Google Scholar] [CrossRef]
- Esberg, A.; Johansson, L.; Johansson, I.; Dahlqvist, S.R. Oral Microbiota Identifies Patients in Early Onset Rheumatoid Arthritis. Microorganisms 2021, 9, 1657. [Google Scholar] [CrossRef]
- Haworth, S.; Esberg, A.; Kuja-Halkola, R.; Lundberg, P.; Magnusson, P.K.E.; Johansson, I. Using national register data to estimate the heritability of periodontitis. J. Clin. Periodontol. 2021, 48, 756–764. [Google Scholar] [CrossRef]
- Arnett, F.C.; Edworthy, S.M.; Bloch, D.A.; McShane, D.J.; Fries, J.F.; Cooper, N.S.; Healey, L.A.; Kaplan, S.R.; Liang, M.H.; Luthra, H.S.; et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988, 31, 315–324. [Google Scholar] [CrossRef]
- Sakellari, D.; Socransky, S.S.; Dibart, S.; Eftimiadi, C.; Taubman, M.A. Estimation of serum antibody to subgingival species using checkerboard immunoblotting. Oral Microbiol. Immunol. 1997, 12, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Esberg, A.; Johansson, A.; Claesson, R.; Johansson, I. 43-Year Temporal Trends in Immune Response to Oral Bacteria in a Swedish Population. Pathogens 2020, 9, 544. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Huntley, J.; Fierer, N.; Owens, S.M.; Betley, J.; Fraser, L.; Bauer, M.; et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 2012, 6, 1621–1624. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Chen, T.; Yu, W.H.; Izard, J.; Baranova, O.V.; Lakshmanan, A.; Dewhirst, F.E. The Human Oral Microbiome Database: A web accessible resource for investigating oral microbe taxonomic and genomic information. Database 2010, 2010, baq013. [Google Scholar] [CrossRef]
- Wang, H.Y.; Kim, S.; Kim, J.; Park, S.D.; Uh, Y.; Lee, H. Multiplex real-time PCR assay for rapid detection of methicillin-resistant staphylococci directly from positive blood cultures. J. Clin. Microbiol. 2014, 52, 1911–1920. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]
- Hajishengallis, G.; Lamont, R.J. Beyond the red complex and into more complexity: The polymicrobial synergy and dysbiosis (PSD) model of periodontal disease etiology. Mol. Oral Microbiol. 2012, 27, 409–419. [Google Scholar] [CrossRef]
- Szafrański, S.P.; Deng, Z.L.; Tomasch, J.; Jarek, M.; Bhuju, S.; Meisinger, C.; Kühnisch, J.; Sztajer, H.; Wagner-Döbler, I. Functional biomarkers for chronic periodontitis and insights into the roles of Prevotella nigrescens and Fusobacterium nucleatum; a metatranscriptome analysis. NPJ Biofilms Microbiomes 2015, 1, 15017. [Google Scholar] [CrossRef]
- Teles, R.P.; Haffajee, A.D.; Socransky, S.S. Microbiological goals of periodontal therapy. Periodontol. 2000 2006, 42, 180–218. [Google Scholar] [CrossRef]
- Ogrendik, M. Rheumatoid arthritis is linked to oral bacteria: Etiological association. Mod. Rheumatol. 2009, 19, 453–456. [Google Scholar] [CrossRef] [PubMed]
- Wolff, B.; Boutin, S.; Lorenz, H.-M.; Ueffing, H.; Dalpke, A.; Wolff, D. FRI0698 Prevotella and Alloprevotella species characterize the oral microbiome of early rheumatoid arthritis. Ann. Rheum. Dis. 2017, 76, 754. [Google Scholar] [CrossRef]
- Fuggle, N.R.; Smith, T.O.; Kaul, A.; Sofat, N. Hand to Mouth: A Systematic Review and Meta-Analysis of the Association between Rheumatoid Arthritis and Periodontitis. Front. Immunol. 2016, 7, 80. [Google Scholar] [CrossRef]
- Ponniah, I.; Shaheen, A.; Shankar, K.A.; Kumaran, M.G. Wegener’s granulomatosis: The current understanding. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2005, 100, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Lilly, J.; Juhlin, T.; Lew, D.; Vincent, S.; Lilly, G. Wegener’s granulomatosis presenting as oral lesions: A case report. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1998, 85, 153–157. [Google Scholar] [CrossRef]
- Almouhawis, H.A.; Leao, J.C.; Fedele, S.; Porter, S.R. Wegener’s granulomatosis: A review of clinical features and an update in diagnosis and treatment. J. Oral Pathol. Med. 2013, 42, 507–516. [Google Scholar] [CrossRef]
- Kronbichler, A.; Harrison, E.M.; Wagner, J. Nasal microbiome research in ANCA-associated vasculitis: Strengths, limitations, and future directions. Comput. Struct. Biotechnol. J. 2021, 19, 415–423. [Google Scholar] [CrossRef]
- Rantapää-Dahlqvist, S.; De Jong, B.A.; Berglin, E.; Hallmans, G.; Wadell, G.; Stenlund, H.; Sundin, U.; van Venrooij, W.J. Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis. Arthritis Rheum. 2003, 48, 2741–2749. [Google Scholar] [CrossRef]
- Johansson, L.; Berglin, E.; Eriksson, O.; Mohammad, A.; Dahlqvist, J.; Rantapää-Dahlqvist, S. Complement activation prior to symptom onset in myeloperoxidase ANCA-associated vasculitis but not proteinase 3 ANCA associated vasculitis-A Swedish biobank study. Scand. J. Rheumatol. 2022, 51, 214–219. [Google Scholar] [CrossRef]
- Tadema, H.; Heeringa, P.; Kallenberg, C.G. Bacterial infections in Wegener’s granulomatosis: Mechanisms potentially involved in autoimmune pathogenesis. Curr. Opin. Rheumatol. 2011, 23, 366–371. [Google Scholar] [CrossRef] [PubMed]


| Pre-AAV n = 85 | Established AAV n = 78 | Controls n = 85 | Acute AAV n = 25 | Controls n = 23 | |
|---|---|---|---|---|---|
| Women, n (%) | 49 (57.6) | 41 (52.6) | 49 (57.6) | 15 (60) | 12 (52) |
| Age at diagnosis, mean (SD), years | - | 53.2 (19.7) | - | ||
| Age at sampling, mean (SD), years | 52.3 (16.7) | 64.3 (19.1) | 52.0 (16.7) | 60.9 (18.0) | 61.6 (18.2) |
| Ever smoker, n (%) | 10/21 (47.6) | 31/74 (41.9) | 8/17 (47.1) | 8/22 (36.4) | 7/23 (30.4) |
| Current smoker, n (%) | 2/21 (9.5) | 6/74 (8.1) | 5/17 (29.4) | 1 (4) | 0 |
| Diabetes, n (%) | - | 13 (19.7) | - | 4 (16.0) | 0 |
| Pre-dating time, mean (SD) years | 4.4 (3.1) | - | - | - | - |
| Disease duration, mean (SD) years | - | 9.7 (7.1) | - | - | |
| MPO-ANCA+, n (%) | 9 (10.6) | 24 (30.8) | 2 (2.4) | 5 (20) | - |
| PR3-ANCA+, n (%) | 21 (24.7) | 54 (69.2) | 2 (2.4) | 20 (80) | - |
| GPA diagnosis 1, n (%) | - | 53 (67.9) | - | 20 (80) | - |
| MPA diagnosis 1, n (%) | - | 23 (29.5) | - | 4 (16) | - |
| EGPA diagnosis 1, n (%) | - | - | - | 1 (4.0) | - |
| Serum/plasma, n (%)/n (%) | 68(80)/17(20) | -/78(100) | 68(80)/17(20) | - | - |
| Treatment during sampling | |||||
| Corticosteroids, n (%) | - | 42/73 (57.5) | - | 7 (28.0) 2 | - |
| Cytotoxic drugs, n (%) | - | 65/73 (89.0) 3 | - | 5/25 4 | - |
| Antibiotics (long term) 5, n (%) | - | 19 (27.9) | - | 14 (56.0) | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esberg, A.; Johansson, L.; Berglin, E.; Mohammad, A.J.; Jonsson, A.P.; Dahlqvist, J.; Stegmayr, B.; Johansson, I.; Rantapää-Dahlqvist, S. Oral Microbiota Profile in Patients with Anti-Neutrophil Cytoplasmic Antibody–Associated Vasculitis. Microorganisms 2022, 10, 1572. https://doi.org/10.3390/microorganisms10081572
Esberg A, Johansson L, Berglin E, Mohammad AJ, Jonsson AP, Dahlqvist J, Stegmayr B, Johansson I, Rantapää-Dahlqvist S. Oral Microbiota Profile in Patients with Anti-Neutrophil Cytoplasmic Antibody–Associated Vasculitis. Microorganisms. 2022; 10(8):1572. https://doi.org/10.3390/microorganisms10081572
Chicago/Turabian StyleEsberg, Anders, Linda Johansson, Ewa Berglin, Aladdin J. Mohammad, Andreas P. Jonsson, Johanna Dahlqvist, Bernd Stegmayr, Ingegerd Johansson, and Solbritt Rantapää-Dahlqvist. 2022. "Oral Microbiota Profile in Patients with Anti-Neutrophil Cytoplasmic Antibody–Associated Vasculitis" Microorganisms 10, no. 8: 1572. https://doi.org/10.3390/microorganisms10081572
APA StyleEsberg, A., Johansson, L., Berglin, E., Mohammad, A. J., Jonsson, A. P., Dahlqvist, J., Stegmayr, B., Johansson, I., & Rantapää-Dahlqvist, S. (2022). Oral Microbiota Profile in Patients with Anti-Neutrophil Cytoplasmic Antibody–Associated Vasculitis. Microorganisms, 10(8), 1572. https://doi.org/10.3390/microorganisms10081572






