Nationwide Seroprevalence Survey of Angiostrongylus vasorum-Derived Antigens and Specific Antibodies in Dogs from Colombia
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koch, J.; Willesen, J.L. Canine pulmonary angiostrongylosis: An update. Vet. J. 2009, 179, 348–359. [Google Scholar] [CrossRef] [PubMed]
- Morgan, E.R.; Modry, D.; Paredes-Esquivel, C.; Foronda, P.; Traversa, D. Angiostrongylosis in animals and humans in Europe. Pathogens 2021, 10, 1236. [Google Scholar] [CrossRef] [PubMed]
- Guilhon, J.; Cens, B. Angiostrongylus vasorum (Baillet, 1866). Etude biologique et morphologique. Ann. Parasitol. Hum. Comparée 1973, 48, 567–596. [Google Scholar] [CrossRef]
- Gillis-Germitsch, N.; Kockmann, T.; Asmis, L.M.; Tritten, L.; Schnyder, M. The Angiostrongylus vasorum excretory/secretory and surface proteome contains putative modulators of the host coagulation. Front. Cell. Infect. Microbiol. 2021, 11, 1052. [Google Scholar] [CrossRef] [PubMed]
- Grob, D.; Conejeros, I.; López-Osorio, S.; Velásquez, Z.D.; Segeritz, L.; Gärtner, U.; Schaper, R.; Hermosilla, C.; Taubert, A. Canine Angiostrongylus vasorum-induced early innate immune reactions based on NETs formation and canine vascular endothelial cell activation in vitro. Biology 2021, 10, 427. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.; Traversa, D.; Grillotti, E.; Pezzuto, C.; De Tommaso, C.; Pampurini, F.; Schaper, R.; Drake, J.; Crisi, P.E.; Russi, I.; et al. Highly variable clinical pictures in dogs naturally infected with Angiostrongylus vasorum. Pathogens 2021, 10, 1372. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.M.R.; Hindenberg, S.; Balzhäuser, L.; Moritz, A.; Hermosilla, C.; Taubert, A. Pneumothorax in a persistent canine Angiostrongylus vasorum infection. Vet. Parasitol. Reg. Stud. Reports 2021, 26, 100650. [Google Scholar] [CrossRef]
- Hurníková, Z.; Čabanová, V.; Karpjak, P.; Kasenčák, M.; Miterpáková, M. Rare case of Angiostrongylus vasorum intraocular infestation in an asymptomatic dog. Helminthologia 2019, 56, 319–322. [Google Scholar] [CrossRef]
- Colella, V.; Lia, R.P.; Premont, J.; Gilmore, P.; Cervone, M.; Latrofa, M.S.; D’Anna, N.; Williams, D.; Otranto, D. Angiostrongylus vasorum in the eye: New case reports and a review of the literature. Parasit. Vectors 2016, 9, 161. [Google Scholar] [CrossRef] [PubMed]
- Negrin, A.; Cherubini, G.B.; Steeves, E. Angiostrongylus vasorum causing meningitis and detection of parasite larvae in the cerebrospinal fluid of a pug dog. J. Small Anim. Pract. 2008, 49, 468–471. [Google Scholar] [CrossRef] [PubMed]
- Gredal, H.; Willesen, J.L.; Jensen, H.E.; Nielsen, O.L.; Kristensen, A.T.; Koch, J.; Kirk, R.K.; Pors, S.E.; Skerritt, G.C.; Berendt, M. Acute neurological signs as the predominant clinical manifestation in four dogs with Angiostrongylus vasorum infections in Denmark. Acta Vet. Scand. 2011, 53, 43. [Google Scholar] [CrossRef]
- Kotwa, J.D.; Schnyder, M.; Jardine, C.M.; Deplazes, P.; Pearl, D.L.; Berke, O.; Mercer, N.; Peregrine, A.S. Investigation of the occurrence of Angiostrongylus vasorum in coyotes in southern Ontario, Canada. J. Vet. Diagn. Investig. 2021, 33, 664–669. [Google Scholar] [CrossRef]
- Schnyder, M.; Jefferies, R.; Schucan, A.; Morgan, E.R.; Deplazes, P. Comparison of coprological, immunological and molecular methods for the detection of dogs infected with Angiostrongylus vasorum before and after anthelmintic treatment. Parasitology 2015, 142, 1270–1277. [Google Scholar] [CrossRef] [PubMed]
- Ianniello, D.; Pepe, P.; Alves, L.C.; Ciuca, L.; Maurelli, M.P.; Amadesi, A.; Bosco, A.; Musella, V.; Cringoli, G.; Rinaldi, L. Why Use the Mini-FLOTAC to detect metastrongyloid larvae in dogs and cats? Acta Parasitol. 2020, 65, 546–549. [Google Scholar] [CrossRef]
- Nagy-Reis, M.; Oshima, J.E.D.F.; Kanda, C.Z.; Palmeira, F.B.L.; Melo, F.R.; Morato, R.G.; Bonjorne, L.; Magioli, M.; Leuchtenberger, C.; Rohe, F.; et al. Neotropical carnivores: A data set on carnivore distribution in the Neotropics. Ecology 2020, 101, e03128. [Google Scholar] [CrossRef] [PubMed]
- Venco, L.; Colaneri, G.; Formaggini, L.; De Franco, M.; Rishniw, M. Utility of thoracic ultrasonography in a rapid diagnosis of angiostrongylosis in young dogs presenting with respiratory distress. Vet. J. 2021, 271, 105649. [Google Scholar] [CrossRef] [PubMed]
- Schucan, A.; Schnyder, M.; Tanner, I.; Barutzki, D.; Traversa, D.; Deplazes, P. Detection of specific antibodies in dogs infected with Angiostrongylus vasorum. Vet. Parasitol. 2012, 185, 216–224. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Helm, J.R.; Morgan, E.R.; Jackson, M.W.; Wotton, P.; Bell, R. Canine angiostrongylosis: An emerging disease in Europe. J. Vet. Emerg. Crit. Care 2010, 20, 98–109. [Google Scholar] [CrossRef]
- Penagos-Tabares, F.; Lange, M.K.; Chaparro-Gutiérrez, J.J.; Taubert, A.; Hermosilla, C. Angiostrongylus vasorum and Aelurostrongylus abstrusus: Neglected and underestimated parasites in South America. Parasit. Vectors 2018, 11, 208. [Google Scholar] [CrossRef]
- Segeritz, L.; Cardona, A.; Taubert, A.; Hermosilla, C.; Ruiz, A. Autochthonous Angiostrongylus cantonensis, Angiostrongylus vasorum and Aelurostrongylus abstrusus infections in native terrestrial gastropods from the Macaronesian Archipelago of Spain. Parasitol. Res. 2021, 120, 2671–2680. [Google Scholar] [CrossRef]
- Idrissi, H.; Khatat, S.E.H.; Duchateau, L.; Kachani, M.; Azrib, R.; Sahibi, H. Canine cardiopulmonary nematodes in Morocco: Prevalence of Dirofilaria immitis and report of the first autochthonous infection with Angiostrongylus vasorum. Moroc. J. Agric. Sci. 2022, 3, 99–105. [Google Scholar]
- Bwangamoi, O. Angiostrongylus vasorum and other worms in dogs in Uganda. Vet. Rec. 1972, 91, 267. [Google Scholar] [CrossRef] [PubMed]
- Bourque, A.; Conboy, G.; Miller, L.; Whitney, H.; Ralhan, S. Angiostrongylus vasorum infection in 2 dogs from Newfoundland. Can. Vet. J. La. Rev. Vet. Can. 2002, 43, 876–879. [Google Scholar]
- Bourque, A.; Whitney, H.; Conboy, G. Angiostrongylus vasorum infection in a Coyote (Canis latrans) from Newfoundland and Labrador, Canada. J. Wildl. Dis. 2005, 41, 816–819. [Google Scholar] [CrossRef] [PubMed]
- Bourque, A.C.; Conboy, G.; Miller, L.M.; Whitney, H. Pathological findings in dogs naturally infected with Angiostrongylus vasorum in Newfoundland and Labrador, Canada. J. Vet. Diagn. Investig. 2008, 20, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Aziz, N.A.A.; Daly, E.; Allen, S.; Rowson, B.; Greig, C.; Forman, D.; Morgan, E.R. Distribution of Angiostrongylus vasorum and its gastropod intermediate hosts along the rural-urban gradient in two cities in the United Kingdom, using real time PCR. Parasit. Vectors 2016, 9, 56. [Google Scholar] [CrossRef]
- Mahjoub, H.A.; Robbins, W.T.; Galeuzzi, O.; Graham, K.F.; Jones, M.E.B.; Buote, M.A.; Greenwood, S.J.; Conboy, G.A. First report of Angiostrongylus vasorum (French heartworm) in red foxes (Vulpes vulpes) on Prince Edward Island. Can. Vet. J. La Rev. Vet. Can. 2022, 63, 637–640. [Google Scholar]
- Fiorello, C.V.; Robbins, R.G.; Maffei, L.; Wade, S.E. Parasites of free-ranging small canids and felids in the bolivian chaco. J. Zoo Wildl. Med. 2006, 37, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Lima, W.D.S.; Costa, H.M.D.A.; Guimarães, M.P.; Leite, A.C.R. Angiostrongylus vasorum (Baillet, 1866) Nematoda: Prostostrongylidae, em cães de Minas Gerais, Brasil. Mem. Do Inst. Oswaldo Cruz 1985, 80, 233–235. [Google Scholar] [CrossRef]
- Penagos-Tabares, F.; Lange, M.K.; Vélez, J.; Hirzmann, J.; Gutiérrez-Arboleda, J.; Taubert, A.; Hermosilla, C.; Chaparro Gutiérrez, J.J. The invasive giant African snail Lissachatina fulica as natural intermediate host of Aelurostrongylus abstrusus, Angiostrongylus vasorum, Troglostrongylus brevior, and Crenosoma vulpis in Colombia. PLoS Negl. Trop. Dis. 2019, 13, e0007277. [Google Scholar] [CrossRef]
- Spratt, D.M. Species of Angiostrongylus (Nematoda: Metastrongyloidea) in wildlife: A review. Int. J. Parasitol. Parasites Wildl. 2015, 4, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Varela-Arias, N.; Toro-Mesa, D.; Caicedo-Martinez, J.; Ospina-Chiviri, J. Coinfección entre distemper canino Y un verme pulmonar en un Cerdocyon thous en estado silvestre en el municipio de Pereira. In Memorias de la Conferencia Interna en Medicina y Aprovechamiento de Fauna Silvestre, Exótica y no Convencional, Bogotá, Colombia; Memorias de la CIMA: London, UK, 2014; pp. 145–159. [Google Scholar]
- Schnyder, M.; Fahrion, A.; Riond, B.; Ossent, P.; Webster, P.; Kranjc, A.; Glaus, T.; Deplazes, P. Clinical, laboratory and pathological findings in dogs experimentally infected with Angiostrongylus vasorum. Parasitol. Res. 2010, 107, 1471–1480. [Google Scholar] [CrossRef]
- Schnyder, M.; Schaper, R.; Bilbrough, G.; Morgan, E.R.; Deplazes, P. Seroepidemiological survey for canine angiostrongylosis in dogs from Germany and the UK using combined detection of Angiostrongylus vasorum antigen and specific antibodies. Parasitology 2013, 140, 1442–1450. [Google Scholar] [CrossRef] [PubMed]
- Beck, H.E.; Zimmermann, N.E.; McVicar, T.R.; Vergopolan, N.; Berg, A.; Wood, E.F. Present and future Köppen-Geiger climate classification maps at 1-km resolution. Sci. Data 2018, 5, 180214. [Google Scholar] [CrossRef]
- Schnyder, M.; Tanner, I.; Webster, P.; Barutzki, D.; Deplazes, P. An ELISA for sensitive and specific detection of circulating antigen of Angiostrongylus vasorum in serum samples of naturally and experimentally infected dogs. Vet. Parasitol. 2011, 179, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Lange, M.K.; Penagos-Tabares, F.; Vélez, J.; Gutiérrez, J.; Hirzmann, J.; Chaparro-Gutiérrez, J.J.; Piedrahita, D.; Taubert, A.; Hermosilla, C. Regional report on Angiostrongylus vasorum in Colombia: Genetic similarity to European lineage. Vet. Parasitol. Reg. Stud. Reports 2018, 13, 21–23. [Google Scholar] [CrossRef] [PubMed]
- Traversa, D.; Di Cesare, A.; Conboy, G. Canine and feline cardiopulmonary parasitic nematodes in Europe: Emerging and underestimated. Parasit. Vectors 2010, 3, 62. [Google Scholar] [CrossRef] [PubMed]
- Alho, A.M.; Schnyder, M.; Schaper, R.; Meireles, J.; Belo, S.; Deplazes, P.; de Carvalho, L.M. Seroprevalence of circulating Angiostrongylus vasorum antigen and parasite-specific antibodies in dogs from Portugal. Parasitol. Res. 2016, 115, 2567–2572. [Google Scholar] [CrossRef]
- Schnyder, M.; Schaper, R.; Pantchev, N.; Kowalska, D.; Szwedko, A.; Deplazes, P. Serological detection of circulating Angiostrongylus vasorum antigen- and parasite-specific antibodies in dogs from Poland. Parasitol. Res. 2013, 112, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Schnyder, M.; Schaper, R.; Lukács, Z.; Hornok, S.; Farkas, R. Combined serological detection of circulating Angiostrongylus vasorum antigen and parasite-specific antibodies in dogs from Hungary. Parasitol. Res. 2015, 114, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Deak, G.; Gillis-Germitsch, N.; Ionică, A.M.; Mara, A.; Păstrav, I.R.; Cazan, C.D.; Ioniță, M.; Mitrea, I.L.; Răileanu, C.; Bărburaș, D.; et al. The first seroepidemiological survey for Angiostrongylus vasorum in domestic dogs from Romania. Parasit. Vectors 2019, 12, 224. [Google Scholar] [CrossRef] [PubMed]
- Vieira, F.M.; Luque, J.L.; Muniz-Pereira, L.C. Checklist of helminth parasites in wild carnivore mammals from Brazil. Zootaxa 2008, 1721, 1–23. [Google Scholar] [CrossRef]
- Jefferies, R.; Shaw, S.E.; Viney, M.E.; Morgan, E.R. Angiostrongylus vasorum from South America and Europe represent distinct lineages. Parasitology 2009, 136, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Grisi, L. Ocorrência de Angiocaulus raillieti (Travassos, 1927) Comb. n. em Canis familiaris L. (Hematoda, Protostrongylidae). Rev. Bras. Biol 1971, 31, 27–32. [Google Scholar]
- Travassos, L. Nematódeos novos. Bol. Biol. São Paulo. 1927, 6, 52–61. [Google Scholar]
- Gomez-Puerta, L.A.; Flores, V.; Vega, R.; Brugni, N.; Viozzi, G.; Lopez-Urbina, M.T.; Gonzalez, A.E. Morphological and molecular evidence of Oslerus osleri (Nematoda: Filaroididae) in the Andean fox (Lycalopex culpaeus). Vet. Parasitol. Reg. Stud. Rep. 2021, 23, 100532. [Google Scholar] [CrossRef] [PubMed]
- Segeritz, L.; Westhoff, K.M.; Schaper, R.; Hermosilla, C.; Taubert, A. Angiostrongylus vasorum, Aelurostrongylus abstrusus, Crenosoma vulpis and Troglostrongylus brevior infections in native slug populations of Bavaria and Baden-Wuerttemberg in Germany. Pathogens 2022, 11, 747. [Google Scholar] [CrossRef]
- Robbins, W.; Conboy, G.; Greenwood, S.; Schaper, R. Infectivity of gastropod-shed third-stage larvae of Angiostrongylus vasorum and Crenosoma vulpis to dogs. Parasit. Vectors 2021, 14, 307. [Google Scholar] [CrossRef]
- Pereira, C.A.d.J.; Coaglio, A.L.; Capettini, L.S.; Becattini, R.; Ferreira, A.P.P.N.; Costa, A.; Soares, L.M.; Oliveira, L.L.; Lima, W.d.S. New approaches to studying morphological details of intramolluscan stages of Angiostrongylus vasorum. Rev. Bras. Parasitol. Veterinária 2020, 29, 32609238. [Google Scholar] [CrossRef]
- Maksimov, P.; Hermosilla, C.; Taubert, A.; Staubach, C.; Sauter-Louis, C.; Conraths, F.J.; Vrhovec, M.G.; Pantchev, N. GIS-supported epidemiological analysis on canine Angiostrongylus vasorum and Crenosoma vulpis infections in Germany. Parasit. Vectors 2017, 10, 108. [Google Scholar] [CrossRef]
- Schug, K.; Krämer, F.; Schaper, R.; Hirzmann, J.; Failing, K.; Hermosilla, C.; Taubert, A. Prevalence survey on lungworm (Angiostrongylus vasorum, Crenosoma vulpis, Eucoleus aerophilus) infections of wild red foxes (Vulpes vulpes) in central Germany. Parasit. Vectors 2018, 11, 85. [Google Scholar] [CrossRef] [PubMed]
- Gillis-Germitsch, N.; Tritten, L.; Hegglin, D.; Deplazes, P.; Schnyder, M. Conquering Switzerland: The emergence of Angiostrongylus vasorum in foxes over three decades and its rapid regional increase in prevalence contrast with the stable occurrence of lungworms. Parasitology 2020, 147, 1071–1079. [Google Scholar] [CrossRef] [PubMed]
- Lemming, L.; Jørgensen, A.C.; Nielsen, L.B.; Nielsen, S.T.; Mejer, H.; Chriél, M.; Petersen, H.H. Cardiopulmonary nematodes of wild carnivores from Denmark: Do they serve as reservoir hosts for infections in domestic animals? Int. J. Parasitol. Parasites Wildl. 2020, 13, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Pantchev, N.; Schnyder, M.; Vrhovec, M.G.; Schaper, R.; Tsachev, I. Current surveys of the seroprevalence of Borrelia burgdorferi, Ehrlichia canis, Anaplasma phagocytophilum, Leishmania infantum, Babesia canis, Angiostrongylus vasorum and Dirofilaria immitis in dogs in Bulgaria. Parasitol. Res. 2015, 114, 117–130. [Google Scholar] [CrossRef] [PubMed]
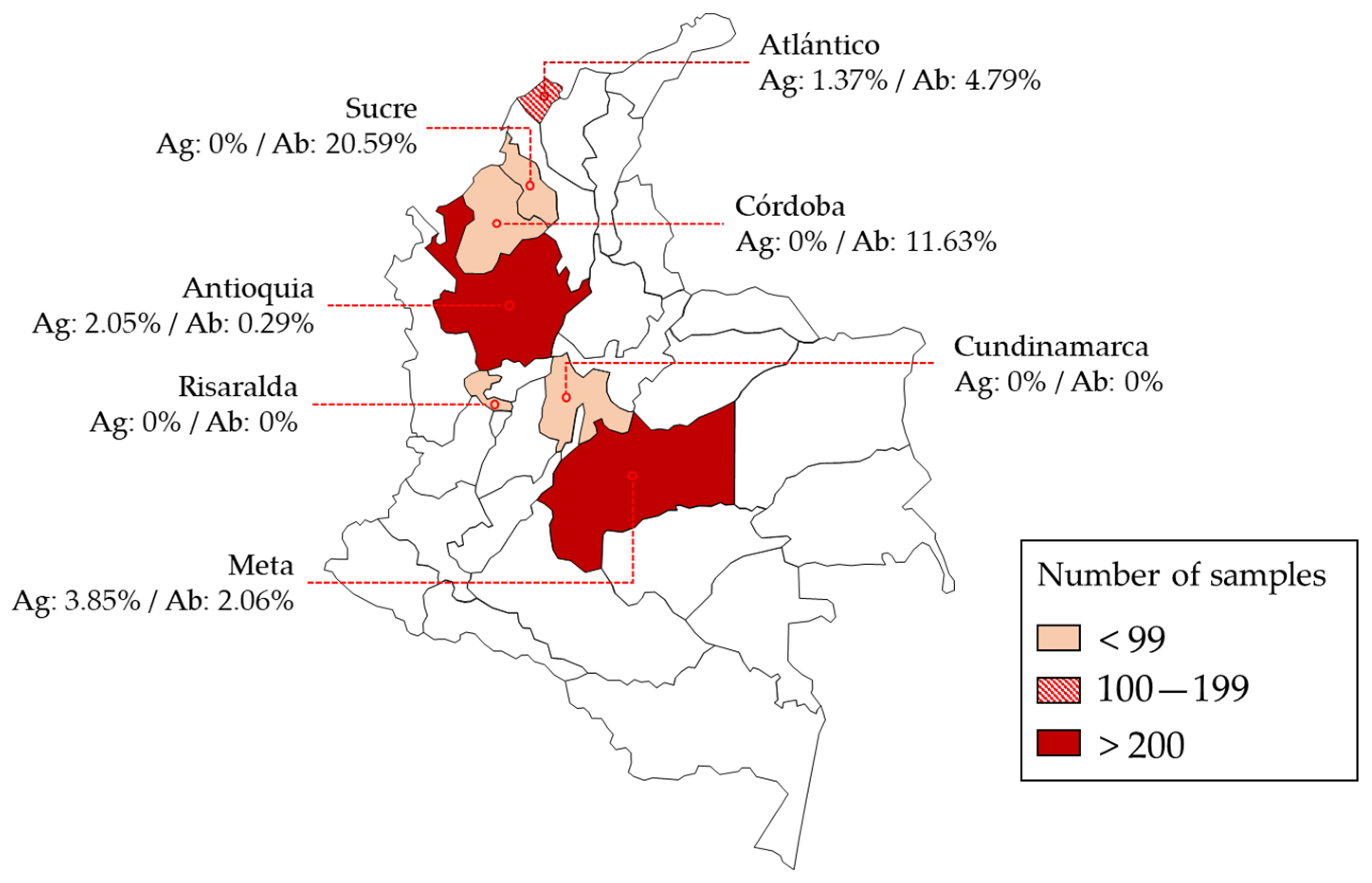
| Departments | Municipalities | n | Antigen + | % | CI 1 | Antibody + | % | CI 1 |
|---|---|---|---|---|---|---|---|---|
| Antioquia | Bello | 6 | 0 | 0.00% | 0 | 0.00% | ||
| Caldas | 1 | 0 | 0.00% | 0 | 0.00% | |||
| Copacabana | 5 | 0 | 0.00% | 0 | 0.00% | |||
| El Retiro | 1 | 0 | 0.00% | 0 | 0.00% | |||
| Itagüi | 5 | 0 | 0.00% | 0 | 0.00% | |||
| La Ceja | 3 | 0 | 0.00% | 0 | 0.00% | |||
| Medellín | 341 | 7 | 2.05% | 0.99–4.17 | 1 | 0.29% | 0.05–1.64 | |
| Rionegro | 3 | 0 | 0.00% | 0 | 0.00% | |||
| Sabaneta | 4 | 0 | 0.00% | 0 | 0.00% | |||
| Atlántico | Barranquilla | 146 | 2 | 1.37% | 0.37–4.85 | 7 | 4.79% | 2.34–9.56 |
| Puerto Colombia | 20 | 0 | 0.00% | 0 | 0.00% | |||
| Córdoba | Montería | 43 | 0 | 0.00% | 5 | 11.63% | 5.07–24.47 | |
| Cundinamarca | NR 2 | 39 | 0 | 0.00% | 0 | 0.00% | ||
| Risaralda | Pereira | 31 | 0 | 0.00% | 0 | 0.00% | ||
| Sucre | Sincelejo | 34 | 0 | 0.00% | 7 | 20.59% | 10.34–36.79 | |
| Meta | Cumaral | 243 | 0 | 0.00% | 5 | 2.06% | 0.88–4.72 | |
| Villavicencio | 26 | 1 | 3.85% | 0.68–18.89 | 0 | 0.00% | ||
| NR 2 | 4 | 0 | 0 | 0 | 0 | |||
| Total | 955 | 10 | 1.05% | 0.569–1.916 | 25 | 2.62% | 1.77–3.83 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uribe, M.; Segeritz, L.; Schnyder, M.; Taubert, A.; Hermosilla, C.; López-Osorio, S.; Góngora-Orjuela, A.; Chaparro-Gutiérrez, J.J. Nationwide Seroprevalence Survey of Angiostrongylus vasorum-Derived Antigens and Specific Antibodies in Dogs from Colombia. Microorganisms 2022, 10, 1565. https://doi.org/10.3390/microorganisms10081565
Uribe M, Segeritz L, Schnyder M, Taubert A, Hermosilla C, López-Osorio S, Góngora-Orjuela A, Chaparro-Gutiérrez JJ. Nationwide Seroprevalence Survey of Angiostrongylus vasorum-Derived Antigens and Specific Antibodies in Dogs from Colombia. Microorganisms. 2022; 10(8):1565. https://doi.org/10.3390/microorganisms10081565
Chicago/Turabian StyleUribe, Manuel, Lisa Segeritz, Manuela Schnyder, Anja Taubert, Carlos Hermosilla, Sara López-Osorio, Agustín Góngora-Orjuela, and Jenny J. Chaparro-Gutiérrez. 2022. "Nationwide Seroprevalence Survey of Angiostrongylus vasorum-Derived Antigens and Specific Antibodies in Dogs from Colombia" Microorganisms 10, no. 8: 1565. https://doi.org/10.3390/microorganisms10081565
APA StyleUribe, M., Segeritz, L., Schnyder, M., Taubert, A., Hermosilla, C., López-Osorio, S., Góngora-Orjuela, A., & Chaparro-Gutiérrez, J. J. (2022). Nationwide Seroprevalence Survey of Angiostrongylus vasorum-Derived Antigens and Specific Antibodies in Dogs from Colombia. Microorganisms, 10(8), 1565. https://doi.org/10.3390/microorganisms10081565









