The Difference in the Bacterial Attachment among Pratylenchus neglectus Populations and Its Effect on the Nematode Infection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Nematode Culture
2.2. Testing the Soil Bacterial Attachment to P. neglectus Populations
2.3. DGGE Fingerprinting of Soil Bacteria Attached to P. neglectus Populations
2.4. Testing the Bacterial Attachment of Rothia sp. to P. neglectus Population
2.5. Comparing the Bacterial Attachment of Rothia sp. among P. neglectus Populations
2.6. Bioassay to Evaluate the Penetration of P. neglectus Populations into Barley Roots with and without Rothia Attachment
2.7. Amplifying far-1 Gene of P. neglectus for Sequence Determination
2.8. Data Analysis
3. Results
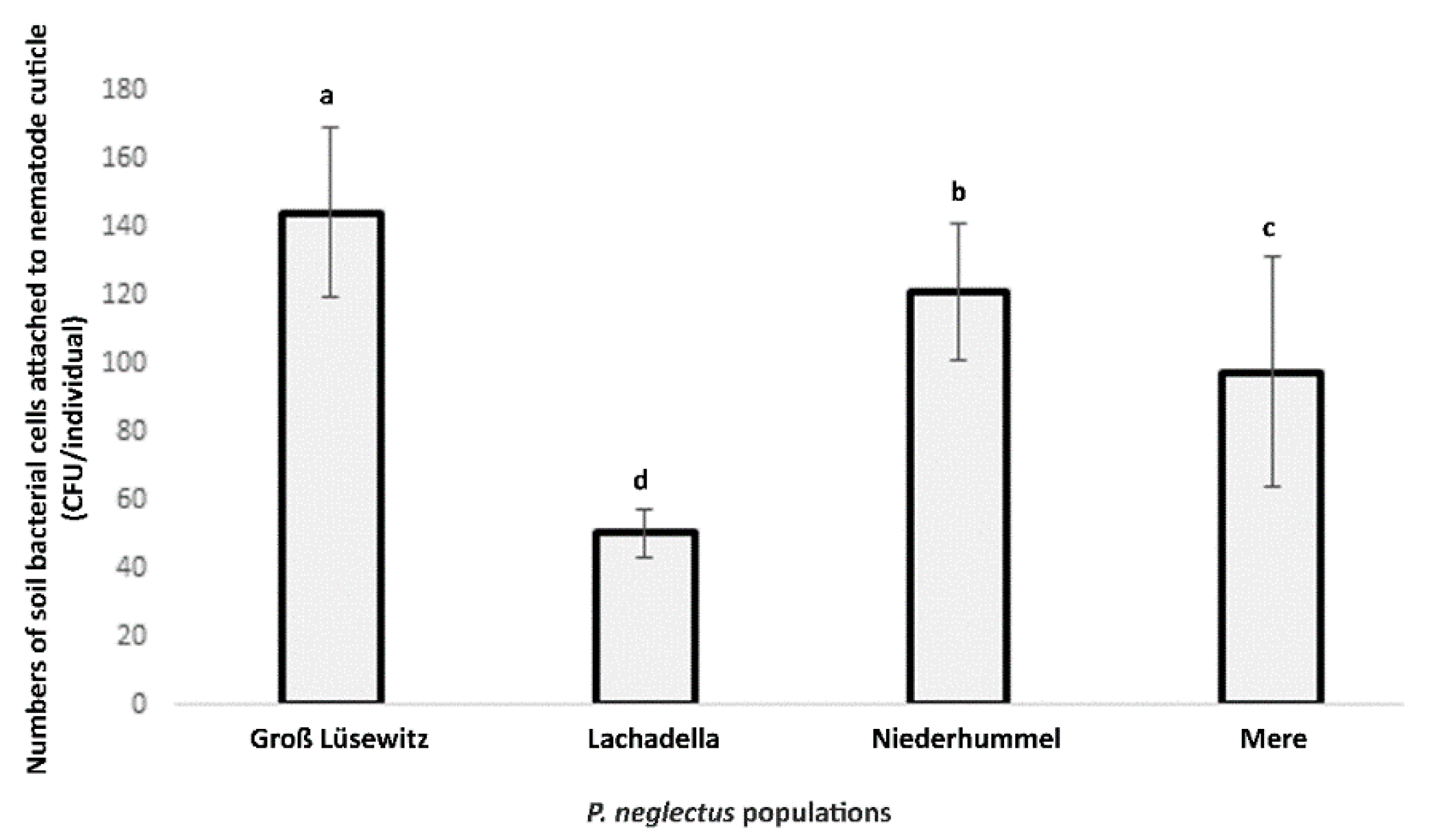
3.1. The Attachment of Soil Bacteria to Nematode Cuticle of P. neglectus Populations
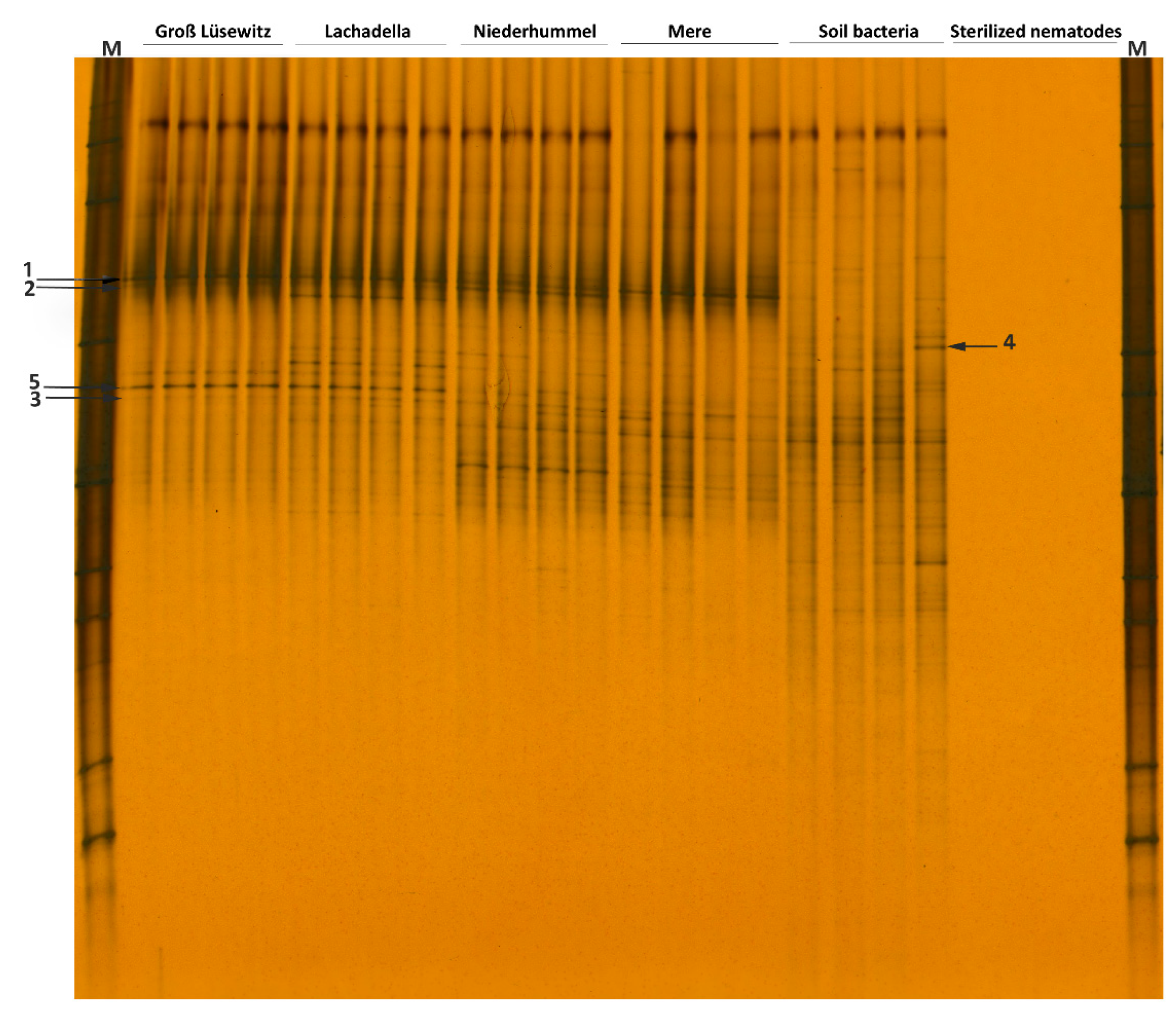
3.2. DGGE Fingerprints of Soil Bacteria Attached to P. neglectus Populations
3.3. The Specificity of the Bacterial Attachment by Rothia sp. to P. neglectus Population
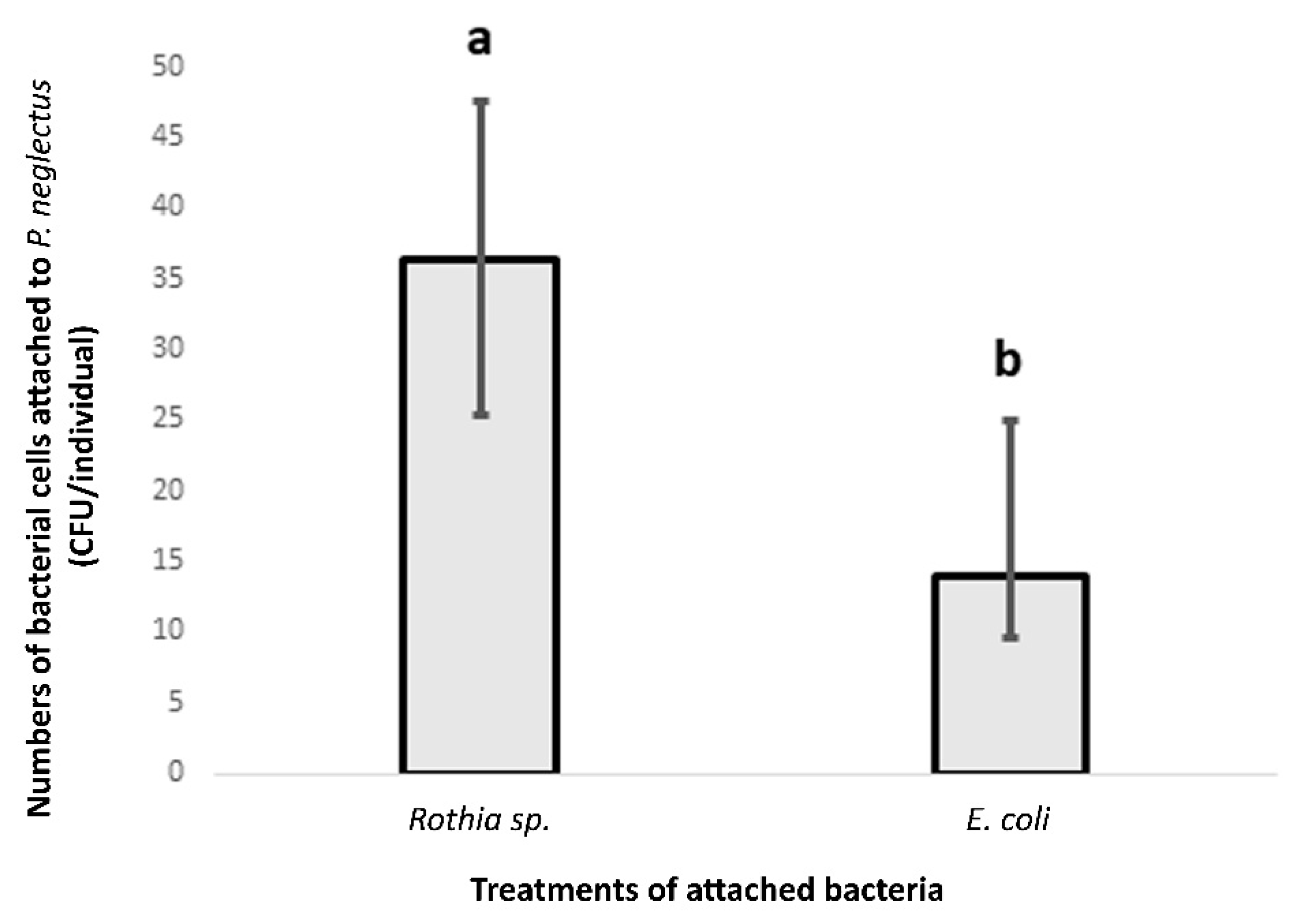
3.4. The Attachment by Rothia sp. to Four Populations of P. neglectus
3.5. Penetration of P. neglectus Populations into Barley Roots with and without an Attachment by Rothia sp.
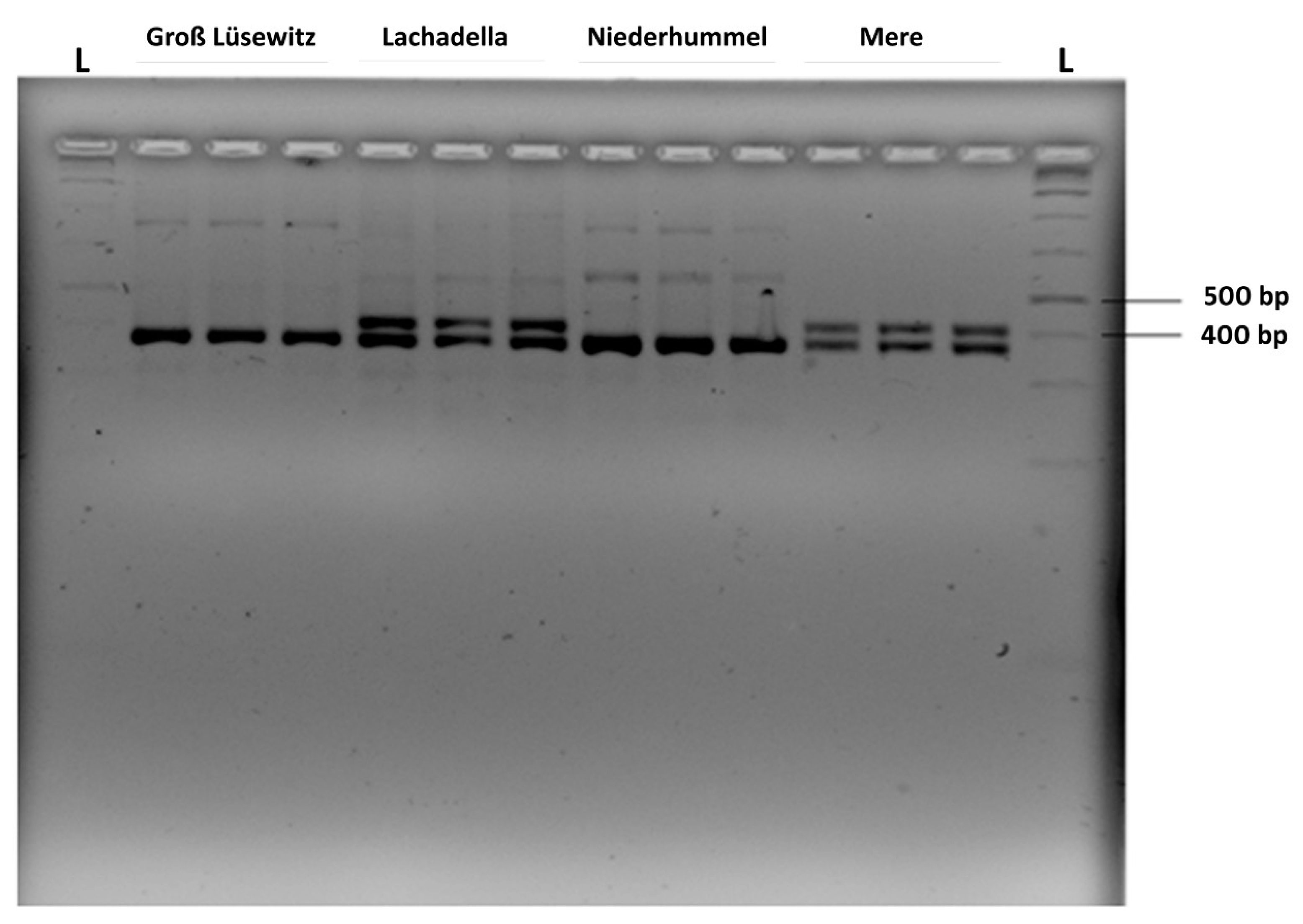
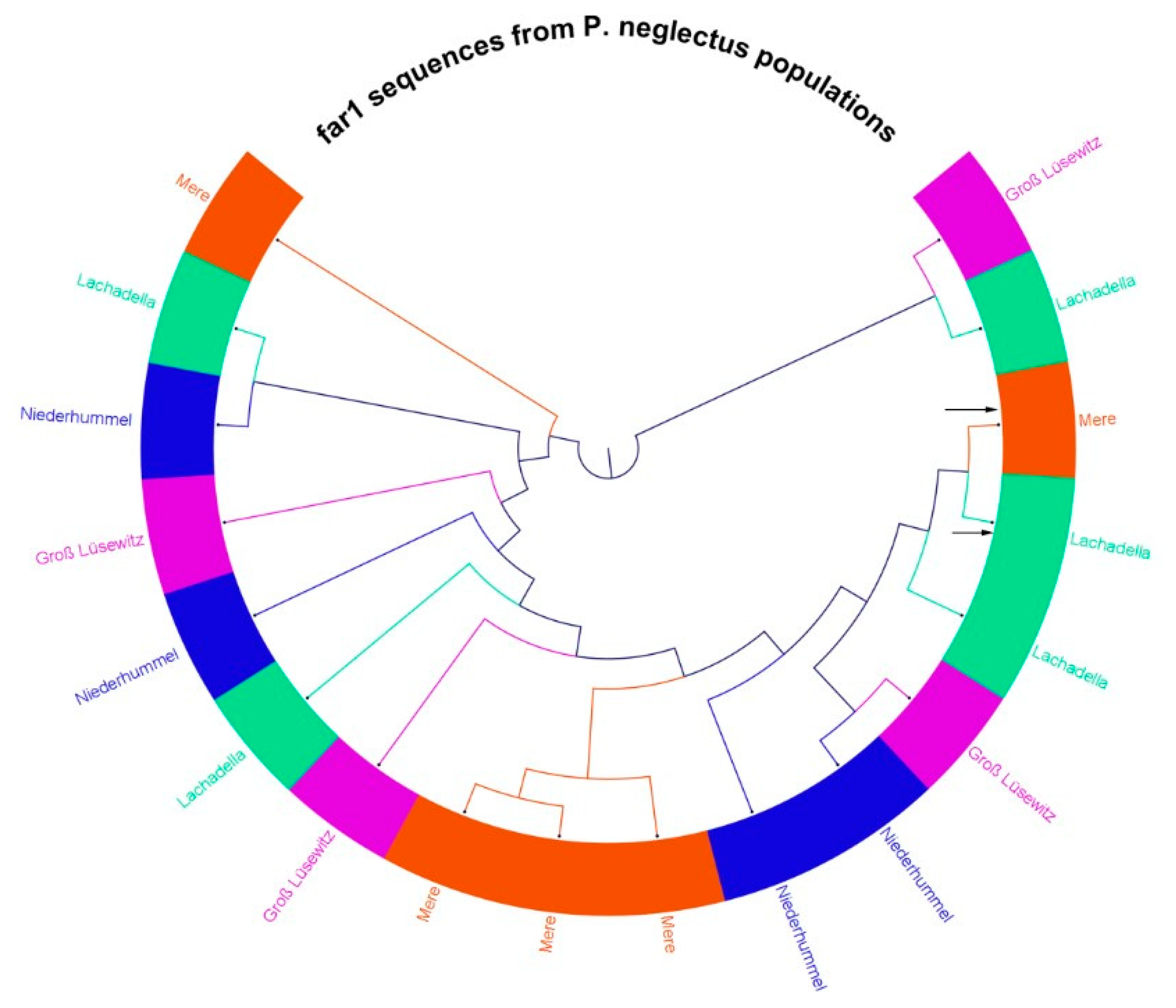
3.6. Amplification of far-1 Gene of P. neglectus Populations for Sequence Determination
4. Discussion
Supplementary Materials
Funding
Acknowledgments
Conflicts of Interest
References
- Adam, M.; Westphal, A.; Hallmann, J.; Heuer, H. Specific microbial attachment to root knot nematodes in suppressive soil. Appl. Environ. Microbiol. 2014, 80, 2679–2686. [Google Scholar] [CrossRef] [Green Version]
- Elhady, A.; Giné, A.; Topalović, O.; Jacquiod, S.; Sørensen, S.J.; Sorribas, F.J.; Heuer, H. Microbiomes associated with infective stages of root-knot and lesion nematodes in soil. PLoS ONE 2017, 12, e0177145. [Google Scholar] [CrossRef] [Green Version]
- Topalović, O.; Elhady, A.; Hallmann, J.; Richert-Pöggeler, K.R.; Heuer, H. Bacteria isolated from the cuticle of plant-parasitic nematodes attached to and antagonized the root-knot nematode Meloidogyne hapla. Sci. Rep. 2019, 9, 11477. [Google Scholar] [CrossRef]
- Tian, B.; Yang, J.; Zhang, K.-Q. Bacteria used in the biological control of plant-parasitic nematodes: Populations, mechanisms of action, and future prospects. FEMS Microbiol. Ecol. 2007, 61, 197–213. [Google Scholar] [CrossRef] [Green Version]
- Stirling, G.R. Biological Control of Plant Parasitic Nematodes, 2nd ed.; CAB International: Wallingford, UK, 2014; ISBN 1780644159. [Google Scholar]
- Davies, K.G.; Fargette, M.; Balla, G.; Daudi, A.; Duponnois, R.; Gowen, S.R.; Mateille, T.; Phillips, M.S.; Sawadogo, S.; Trivino, C.; et al. Cuticle heterogeneity as exhibited by Pasteuria spore attachment is not linked to the phylogeny of parthenogenetic root-knot nematodes (Meloidogyne spp.). Parasitology 2001, 122, 111–120. [Google Scholar] [CrossRef]
- Adam, M.; Heuer, H.; Hallmann, J. Bacterial antagonists of fungal pathogens also control root-knot nematodes by induced systemic resistance of tomato plants. PLoS ONE 2014, 9, e90402. [Google Scholar] [CrossRef] [Green Version]
- Davies, K.G.; Opperman, C.H. A potential role for collagen in the attachment of Pasteuria penetrans to nematode cuticle. In Multitrophic Interactions in Soil and Integrated Control, Proceedings of the Meeting, Wageningen, The Netherlands, 5–8 June 2005; Ryyijmakers, J.M., Sikora, R.A., Eds.; IOBC/WPRS: Dijon, France, 2006; pp. 11–15. ISBN 92-9067-185-6. [Google Scholar]
- Spiegel, Y.; Mor, M.; Sharon, E. Attachment of Pasteuria penetrans endospores to the surface of Meloidogyne javanica second-stage juveniles. J. Nematol. 1996, 28, 328–334. [Google Scholar]
- Stirling, G.R. Host specificity of Pasteuria penetrans within the genus Meloidogyne. Nematologica 1985, 31, 203–209. [Google Scholar] [CrossRef]
- Davies, K.G.; Opperman, C.H. A potential role for collagen in the attachment of Pasteuria penetrans to nematode cuticle. Bull. Oilb Srop Iobc Wprs Bull. 2006, 29, 11–15. [Google Scholar]
- Davies, K.G.; Redden, M. Diversity and partial characterization of putative virulence determinants in Pasteuria penetrans, the hyperparasitic bacterium of root-knot nematodes (Meloidogyne spp.). J. Appl. Microbiol. 1997, 83, 227–235. [Google Scholar] [CrossRef] [Green Version]
- Hicks, S.J.; Theodoropoulos, G.; Carrington, S.D.; Corfield, A.P. The Role of Mucins in Host–Parasite Interactions. Part I—Protozoan Parasites. Parasitol. Today 2000, 16, 476–481. [Google Scholar] [CrossRef]
- Theodoropoulos, G.; Hicks, S.J.; Corfield, A.P.; Miller, B.G.; Carrington, S.D. The role of mucins in host–parasite interactions: Part II—Helminth parasites. Trends Parasitol. 2001, 17, 130–135. [Google Scholar] [CrossRef]
- Koltai, H.; Chejanovsky, N.; Raccah, B.; Spiegel, Y. The first isolated collagen gene of the root-knot nematode Meloidogyne javanica is developmentally regulated. Braz. J. Plant Physiol. 1997, 196, 191–199. [Google Scholar] [CrossRef]
- Liu, J.; Koltai, H.; Chejanovsky, N.; Spiegel, Y. Isolation of a Novel Collagen Gene (MJ-COL-5) in Meloidogyne javanica and Analysis of Its Expression Pattern. J. Parasitol. 2001, 87, 801. [Google Scholar] [CrossRef]
- Castillo, P.; Vovlas, N. Pratylenchus (Nematoda, Pratylenchidae): Diagnosis, Biology, Pathogenicity and Management; Brill: Leiden, The Netherlands, 2007; ISBN 9789004155640. [Google Scholar]
- Smiley, R.W.; Machado, S. Pratylenchus neglectus reduces yield of winter wheat in dryland cropping systems. Plant Dis. 2009, 93, 263–271. [Google Scholar] [CrossRef] [Green Version]
- Taylor, S.P.; Vanstone, V.A.; Ware, A.H.; McKay, A.C.; Szot, D.; Russ, M.H. Measuring yield loss in cereals caused by root lesion nematodes (Pratylenchus neglectus and P. thornei) with and without nematicide. Aust. J. Agric. Res. 1999, 50, 617. [Google Scholar] [CrossRef]
- Roman, J.; Triantaphyllou, A.C. Gametogenesis and Reproduction of Seven Species of Pratylenchus. J. Nematol. 1969, 1, 357–362. [Google Scholar] [PubMed]
- Griffin, G.D. Differential pathogenicity of four Pratylenchus neglectus populations on alfalfa. J. Nematol. 1991, 23, 380–385. [Google Scholar]
- Nuaima, R.H.; Roeb, J.; Hallmann, J.; Daub, M.; Otte, S.; Heuer, H. Effector gene vap1 based DGGE fingerprinting to assess variation within and among Heterodera schachtii populations. J. Nematol. 2018, 50, 517–528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, X.; Xiang, Y.; Xie, H.; Xu, C.-L.; Xie, T.-F.; Zhang, C.; Li, Y. Molecular characterization and functions of fatty acid and retinoid binding protein gene (Ab-far-1) in Aphelenchoides besseyi. PLoS ONE 2013, 8, e66011. [Google Scholar] [CrossRef]
- Phani, V.; Shivakumara, T.N.; Davies, K.G.; Rao, U. Meloidogyne incognita fatty acid- and retinol- binding protein (Mi-FAR-1) affects nematode infection of plant roots and the attachment of Pasteuria penetrans endospores. Front. Microbiol. 2017, 8, 2122. [Google Scholar] [CrossRef] [PubMed]
- Boisseau, M.; Sarah, J.-L. In vitro rearing of Pratylenchidae nematodes on carrot discs. Fruits 2008, 63, 307–310. [Google Scholar] [CrossRef] [Green Version]
- Hay, F.S. Surface sterilisation of Heterodera trifolii Goffart (Nematoda: Tylenchida) and its monoxenic culture on root cultures of white clover (Trifolium repens L.). N. Z. J. Zool. 1994, 21, 209–212. [Google Scholar] [CrossRef] [Green Version]
- Heuer, H.; Wieland, G.; Schönfeld, J.; Schönwälder, A.; Gomes, N.C.M.; Smalla, K. Bacterial community profiling using DGGE or TGGE analysis. In Environmental Molecular Microbiology: Protocols and Applications; Rochelle, P.A., Ed.; Horizon Scientific Press: Wymondham, UK, 2001; pp. 177–190. ISBN 1898486298. [Google Scholar]
- Williamson, V.M.; Čepulytė, R. Assessing Attraction of Nematodes to Host Roots Using Pluronic Gel Medium. Methods Mol. Biol. 2017, 1573, 261–268. [Google Scholar] [CrossRef]
- Nuaima, R.H.; Heuer, H.; Westphal, A. Effects of cover cropping on microbial communities associated with Heterodera schachtii and nematode virulence. Soil Syst. 2019, 3, 67. [Google Scholar] [CrossRef] [Green Version]
- Bird, A.F.; Stynes, B.A. The life cycle of Anguina agrostis: Development in host plant. Int. J. Parasitol. 1981, 11, 431–440. [Google Scholar] [CrossRef]
- Decraemer, W.; Karanastasi, E.; Brown, D.; Backeljau, T. Review of the ultrastructure of the nematode body cuticle and its phylogenetic interpretation. Biol. Rev. Camb. Philos. Soc. 2003, 78, 465–510. [Google Scholar] [CrossRef] [PubMed]
- Abad, P.; Gouzy, J.; Aury, J.-M.; Castagnone-Sereno, P.; Danchin, E.G.J.; Deleury, E.; Perfus-Barbeoch, L.; Anthouard, V.; Artiguenave, F.; Blok, V.C.; et al. Genome sequence of the metazoan plant-parasitic nematode Meloidogyne incognita. Nat. Biotechnol. 2008, 26, 909–915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohan, S.; Fould, S.; Davies, K.G. The interaction between the gelatin-binding domain of fibronectin and the attachment of Pasteuria penetrans endospores to nematode cuticle. Parasitology 2001, 123, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Persidis, A.; Lay, J.G.; Manousis, T.; Bishop, A.H.; Ellar, D.J. Characterisation of potential adhesins of the bacterium Pasteuria penetrans, and of putative receptors on the cuticle of Meloidogyne incognita, a nematode host. J. Cell Sci. 1991, 100, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, Y.; Inbar, J.; Kahane, I.; Sharon, E. Carbohydrate-recognition domains on the surface of phytophagous nematodes. Exp. Parasitol. 1995, 80, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, Y.; Koltai, H.; Sharon, E. Root-Nematode Interactions. In Plant Roots; Kafkafi, U., Waisel, Y., Eshel, A., Eds.; CRC Press: Boca Raton, FL, USA, 2002; pp. 933–947. ISBN 978-0-8247-0631-9. [Google Scholar]
- Davies, K.G.; Redden, M.; Pearson, T.K. Endospore heterogeneity in Pasteuria penetrans related to adhesion to plant-parasitic nematodes. Lett. Appl. Microbiol. 1994, 19, 370–373. [Google Scholar] [CrossRef]
- Davies, K.G. In vitro recognition of a 190 kda putative attachment receptor from the cuticle of Meloidogyne javanica by Pasteuria penetrans spore extract. Biocontrol Sci. Technol. 1994, 4, 367–374. [Google Scholar] [CrossRef]
- Davies, K.G.; Danks, C. Interspecific differences in the nematode surface coat between Meloidogyne incognita and M. arenaria related to the adhesion of the bacterium Pasteuria penetrans. Parasitology 1992, 105, 475–480. [Google Scholar] [CrossRef]
- Curtis, R.H.C.; Jones, J.T.; Davies, K.G.; Sharon, E.; Spiegel, Y. Plant nematode surfaces. In Biological Control of Plant-Parasitic Nematodes: Building Coherence between Microbial Ecology and Molecular Mechanisms; Davies, K.G., Spiegel, Y., Eds.; Springer Science + Business Media: Dordrecht, The Netherlands, 2011; pp. 115–144. ISBN 978-1-4020-9648-8. [Google Scholar]
- Kumari, C.; Dutta, T.K.; Banakar, P.; Rao, U. Comparing the defence-related gene expression changes upon root-knot nematode attack in susceptible versus resistant cultivars of rice. Sci. Rep. 2016, 6, 22846. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Lower, S.; Williamson, V.M. Application of Pluronic gel to the study of root-knot nematode behaviour. Nematology 2009, 11, 453–464. [Google Scholar] [CrossRef]
- Trudgill, D.L.; Bala, G.; Blok, V.C.; Daudi, A.; Davies, K.G.; Gowen, S.R.; Fargette, M.; Madulu, J.D.; Mateille, T.; Mwageni, W.; et al. The importance of tropical root-knot nematodes (Meloidogyne spp.) and factors affecting the utility of Pasteuria penetrans as a biocontrol agent. Nematology 2000, 2, 823–845. [Google Scholar] [CrossRef] [Green Version]
- Imbriani, J.L.; Mankau, R. Ultrastructure of the nematode pathogen, Bacillus penetrans. J. Invertebr. Pathol. 1977, 30, 337–347. [Google Scholar] [CrossRef]
- Liu, C.; Gibson, A.K.; Timper, P.; Morran, L.T.; Tubbs, R.S. Rapid change in host specificity in a field population of the biological control organism Pasteuria penetrans. Evol. Appl. 2019, 12, 744–756. [Google Scholar] [CrossRef]
- Pujol, N.; Zugasti, O.; Wong, D.; Couillault, C.; Kurz, C.L.; Schulenburg, H.; Ewbank, J.J. Anti-fungal innate immunity in C. elegans is enhanced by evolutionary diversification of antimicrobial peptides. PLoS Pathog. 2008, 4, e1000105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bano, A.; Muqarab, R. Plant defence induced by PGPR against Spodoptera litura in tomato (Solanum lycopersicum L.). Plant Biol. 2017, 19, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Al-Khafaji, R.T.; Gunnink Troth, E.E.; Lambert, K.N.; Johnston, J.A.; Dyer, A.T. Pathotypes detected among populations of Pratylenchus neglectus collected from Montana. Plant Dis. 2019, 103, 3259–3264. [Google Scholar] [CrossRef]
- Nuaima, R.H.; Roeb, J.; Hallmann, J.; Daub, M.; Heuer, H. Significant genetic differences among Heterodera schachtii populations within and among sugar beet production areas. Nematology 2019, 22, 165–177. [Google Scholar] [CrossRef]
- Zhang, C.; Xie, H.; Cheng, X.; Wang, D.-W.; Li, Y.; Xu, C.-L.; Huang, X. Molecular identification and functional characterization of the fatty acid- and retinoid-binding protein gene Rs-far-1 in the burrowing nematode Radopholus similis (Tylenchida: Pratylenchidae). PLoS ONE 2015, 10, e0118414. [Google Scholar] [CrossRef] [Green Version]
- Iberkleid, I.; Vieira, P.; de Almeida Engler, J.; Firester, K.; Spiegel, Y.; Horowitz, S.B. Fatty acid-and retinol-binding protein, Mj-FAR-1 induces tomato host susceptibility to root-knot nematodes. PLoS ONE 2013, 8, e64586. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nuaima, R.H. The Difference in the Bacterial Attachment among Pratylenchus neglectus Populations and Its Effect on the Nematode Infection. Microorganisms 2022, 10, 1524. https://doi.org/10.3390/microorganisms10081524
Nuaima RH. The Difference in the Bacterial Attachment among Pratylenchus neglectus Populations and Its Effect on the Nematode Infection. Microorganisms. 2022; 10(8):1524. https://doi.org/10.3390/microorganisms10081524
Chicago/Turabian StyleNuaima, Rasha Haj. 2022. "The Difference in the Bacterial Attachment among Pratylenchus neglectus Populations and Its Effect on the Nematode Infection" Microorganisms 10, no. 8: 1524. https://doi.org/10.3390/microorganisms10081524
APA StyleNuaima, R. H. (2022). The Difference in the Bacterial Attachment among Pratylenchus neglectus Populations and Its Effect on the Nematode Infection. Microorganisms, 10(8), 1524. https://doi.org/10.3390/microorganisms10081524





