Evaluation of Intestinal Microbial Metabolites in Preterm Infants with Different Initial Feeding Methods by In Vitro Fermentation Modeling System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Data Collection
2.3. Fecal Sample Management
2.4. Batch Culture Fermentation
2.5. Thin-Layer Chromatography
2.6. Short Chain Fatty Acids Analysis
2.7. Enzyme-Linked Immunosorbent Assay (ELISA)
2.8. Correlation Coefficients and Statistical Analysis
3. Results
3.1. Volunteer Clinical Characteristics
3.2. Original Fecal Characteristics
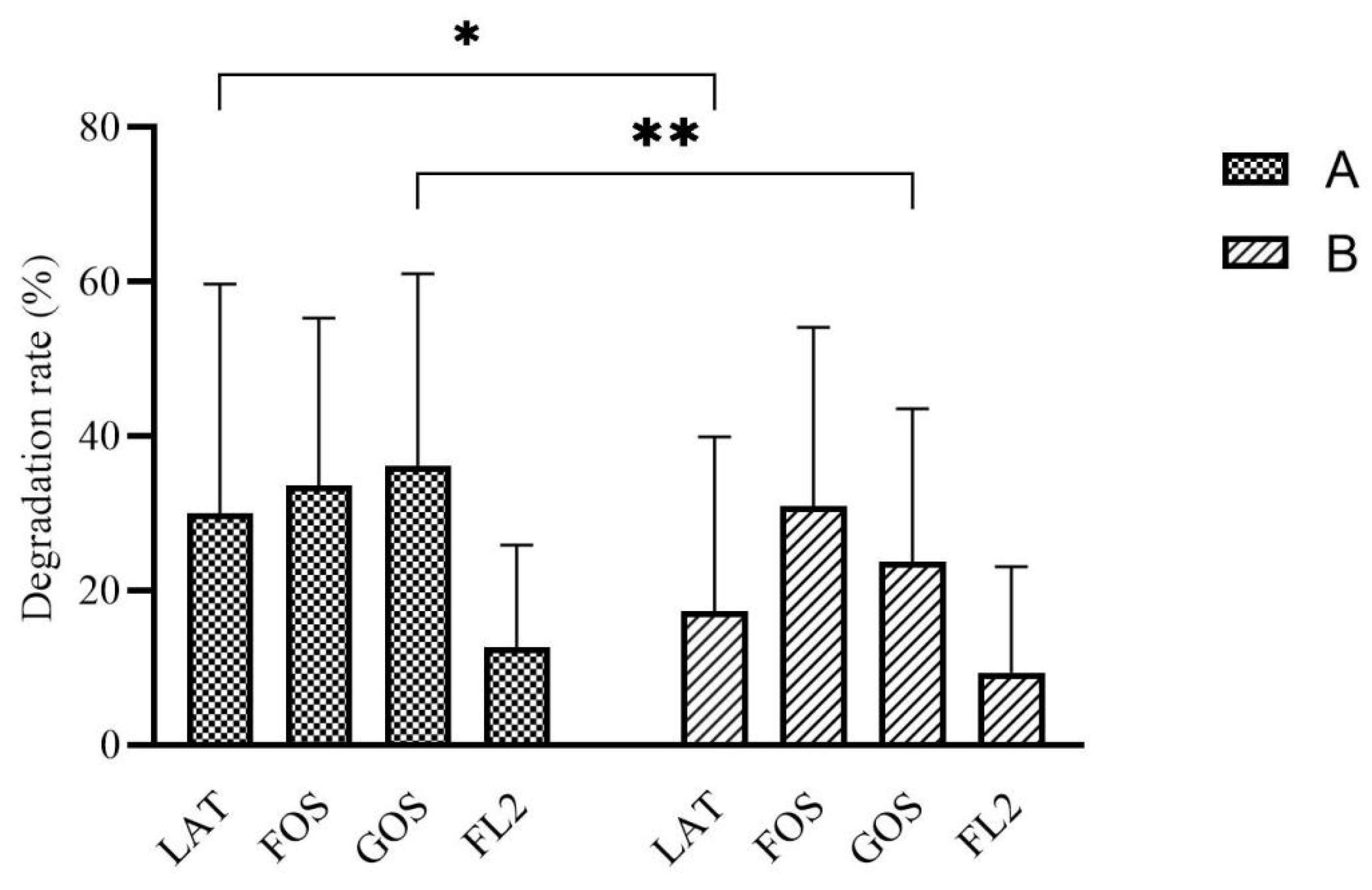
3.3. Carbon Source Degradation Rate
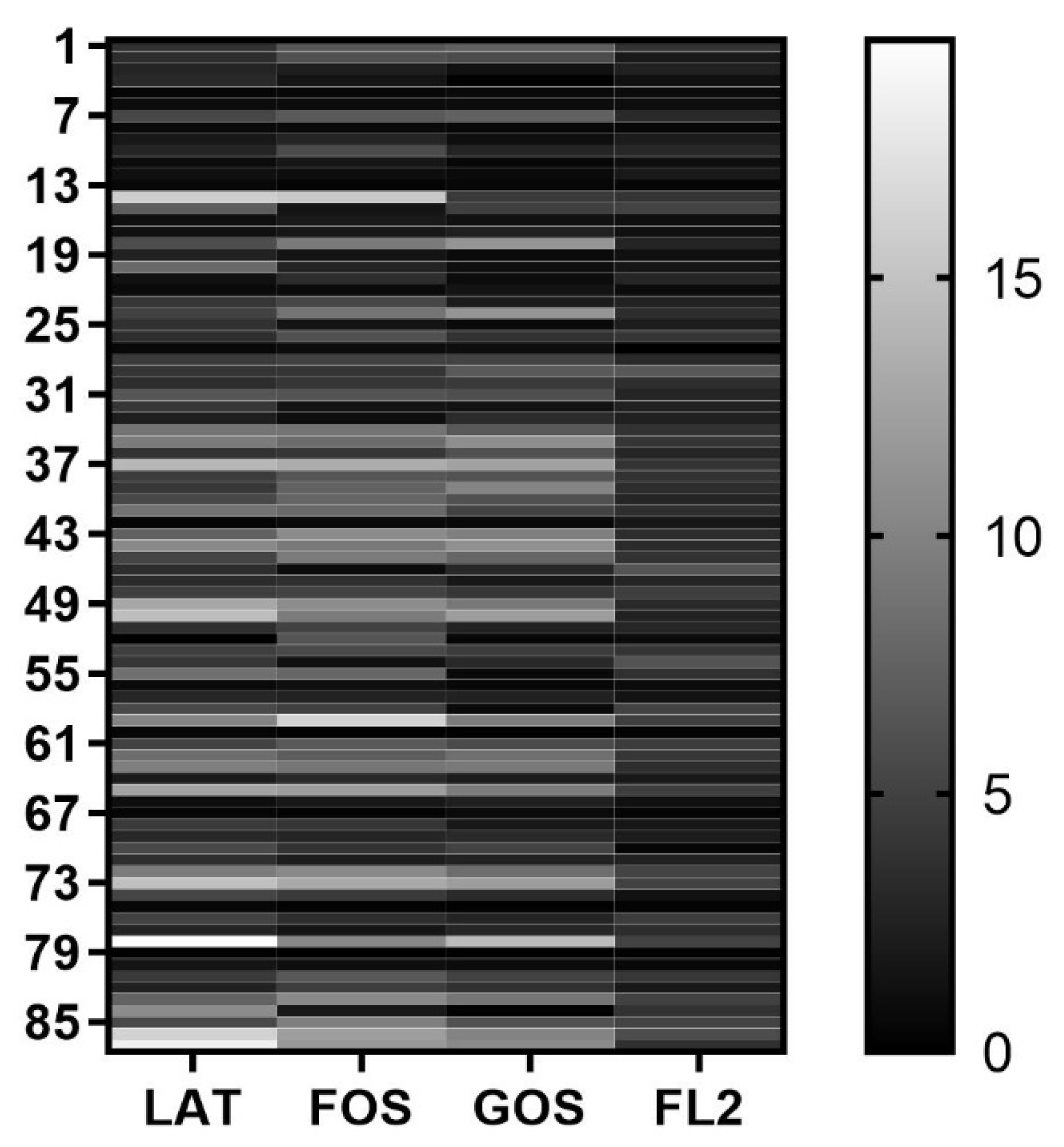
3.4. Gas Production
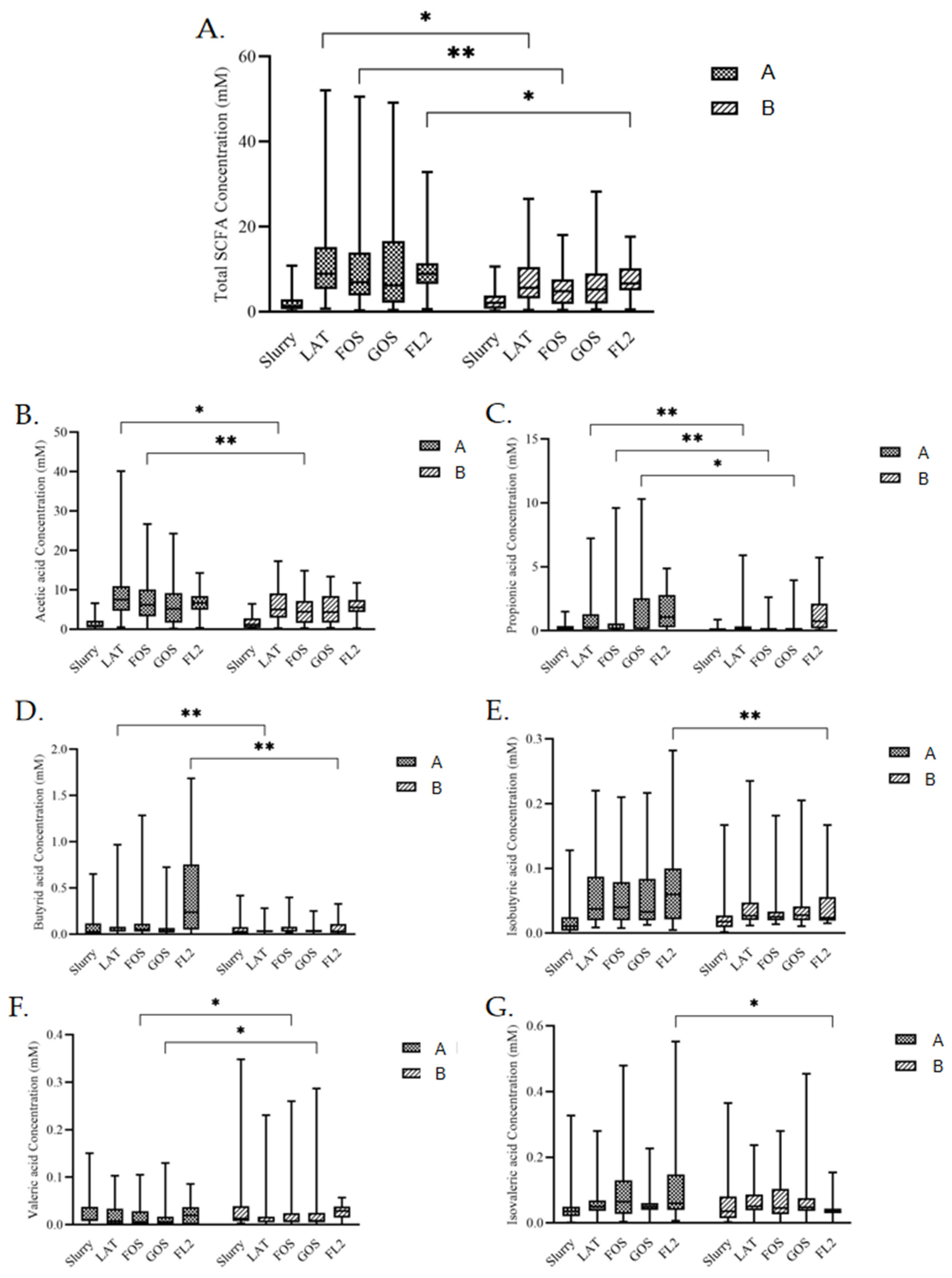
3.5. SCFAs Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Neu, J. Gastrointestinal maturation and implications for infant feeding. Early Hum. Dev. 2007, 83, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Fanaro, S. Feeding intolerance in the preterm infant. Early Hum. Dev. 2013, 89 (Suppl. S2), S13–S20. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Pi, X.; Liu, W.; Chen, H.; Yin, Y.; Yu, H.D.; Wang, X.; Zhu, L. Fermentation properties of isomaltooligosaccharides are affected by human fecal enterotypes. Anaerobe 2017, 48, 206–214. [Google Scholar] [CrossRef]
- Yin, Y.; Fan, B.; Liu, W.; Ren, R.; Chen, H.; Bai, S.; Zhu, L.; Sun, G.; Yang, Y.; Wang, X. Investigation into the stability and culturability of Chinese enterotypes. Sci. Rep. 2017, 7, 7947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schanler, R.J.; Lau, C.; Hurst, N.M.; Smith, E.O. Randomized trial of donor human milk versus preterm formula as substitutes for mothers’ own milk in the feeding of extremely premature infants. Pediatrics 2005, 116, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Lei, F.; Yin, Y.; Wang, Y.; Deng, B.; Yu, H.D.; Li, L.; Xiang, C.; Wang, S.; Zhu, B.; Wang, X. Higher-level production of volatile fatty acids in vitro by chicken gut microbiotas than by human gut microbiotas as determined by functional analyses. Appl. Environ. Microbiol. 2012, 78, 5763–5772. [Google Scholar] [CrossRef] [Green Version]
- Child, M.W.; Kennedy, A.; Walker, A.W.; Bahrami, B.; Macfarlane, S.; Macfarlane, G.T. Studies on the effect of system retention time on bacterial populations colonizing a three-stage continuous culture model of the human large gut using FISH techniques. FEMS Microbiol. Ecol. 2006, 55, 299–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.; Li, G.; Zhu, L.; Yin, Y.; Zhao, X.; Xiang, C.; Yu, G.; Wang, X. Isolation and characterization of an agaro-oligosaccharide (AO)-hydrolyzing bacterium from the gut microflora of Chinese individuals. PLoS ONE 2014, 9, e91106. [Google Scholar] [CrossRef] [Green Version]
- Mao, S.; Huo, W.; Zhu, W. Use of pyrosequencing to characterize the microbiota in the ileum of goats fed with increasing proportion of dietary grain. Curr. Microbiol. 2013, 67, 341–350. [Google Scholar] [CrossRef]
- Picaud, J.C. Review highlights the importance of donor human milk being available for very low birth weight infants. Acta Paediatr. 2022, 111, 1127–1133. [Google Scholar] [CrossRef]
- Macfarlane, G.T.; Macfarlane, S. Human colonic microbiota: Ecology, physiology and metabolic potential of intestinal bacteria. Scand. J. Gastroenterol. Suppl. 1997, 222, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Duncan, S.H.; Louis, P.; Thomson, J.M.; Flint, H.J. The role of pH in determining the species composition of the human colonic microbiota. Environ. Microbiol. 2009, 11, 2112–2122. [Google Scholar] [CrossRef] [PubMed]
- Boullier, S.; Tanguy, M.; Kadaoui, K.A.; Caubet, C.; Sansonetti, P.; Corthesy, B.; Phalipon, A. Secretory IgA-mediated neutralization of Shigella flexneri prevents intestinal tissue destruction by down-regulating inflammatory circuits. J. Immunol. 2009, 183, 5879–5885. [Google Scholar] [CrossRef] [Green Version]
- Ben, X.M.; Zhou, X.Y.; Zhao, W.H.; Yu, W.L.; Pan, W.; Zhang, W.L.; Wu, S.M.; Van Beusekom, C.M.; Schaafsma, A. Supplementation of milk formula with galacto-oligosaccharides improves intestinal micro-flora and fermentation in term infants. Chin. Med. J. 2004, 117, 927–931. [Google Scholar] [PubMed]
- Yao, C.K.; Muir, J.G.; Gibson, P.R. Review article: Insights into colonic protein fermentation, its modulation and potential health implications. Aliment. Pharmacol. Ther. 2016, 43, 181–196. [Google Scholar] [CrossRef] [Green Version]
- Verbeke, K.A.; Boobis, A.R.; Chiodini, A.; Edwards, C.A.; Franck, A.; Kleerebezem, M.; Nauta, A.; Raes, J.; van Tol, E.A.; Tuohy, K.M. Towards microbial fermentation metabolites as markers for health benefits of prebiotics. Nutr. Res. Rev. 2015, 28, 42–66. [Google Scholar] [CrossRef] [Green Version]
- Molinaro, A.; Wahlstrom, A.; Marschall, H.U. Role of Bile Acids in Metabolic Control. Trends Endocrinol. Metab. 2018, 29, 31–41. [Google Scholar] [CrossRef]
- Shapiro, H.; Kolodziejczyk, A.A.; Halstuch, D.; Elinav, E. Bile acids in glucose metabolism in health and disease. J. Exp. Med. 2018, 215, 383–396. [Google Scholar] [CrossRef]
- Ajouz, H.; Mukherji, D.; Shamseddine, A. Secondary bile acids: An underrecognized cause of colon cancer. World J. Surg. Oncol. 2014, 12, 164. [Google Scholar] [CrossRef] [Green Version]
- Bernstein, H.; Bernstein, C.; Payne, C.M.; Dvorak, K. Bile acids as endogenous etiologic agents in gastrointestinal cancer. World J. Gastroenterol. 2009, 15, 3329–3340. [Google Scholar] [CrossRef]
- Di Ciaula, A.; Garruti, G.; Lunardi Baccetto, R.; Molina-Molina, E.; Bonfrate, L.; Wang, D.Q.; Portincasa, P. Bile Acid Physiology. Ann. Hepatol. 2017, 16 (Suppl. S1), S4–S14. [Google Scholar] [CrossRef] [PubMed]
- Ridlon, J.M.; Kang, D.J.; Hylemon, P.B.; Bajaj, J.S. Bile acids and the gut microbiome. Curr. Opin. Gastroenterol. 2014, 30, 332–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ridlon, J.M.; Kang, D.J.; Hylemon, P.B. Bile salt biotransformations by human intestinal bacteria. J. Lipid Res. 2006, 47, 241–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Keefe, S.J. Diet, microorganisms and their metabolites, and colon cancer. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 691–706. [Google Scholar] [CrossRef]
- Ridlon, J.M.; Wolf, P.G.; Gaskins, H.R. Taurocholic acid metabolism by gut microbes and colon cancer. Gut Microbes 2016, 7, 201–215. [Google Scholar] [CrossRef] [Green Version]
- Miñana, I.V. Oligosacáridos en nutrición infantil: Fórmula infantil, alimentación complementaria y del adolescente. Acta Pediátrica Española 2007, 65, 175–178. [Google Scholar]
- Koletzko, B.; Baker, S.; Cleghorn, G.; Neto, U.F.; Gopalan, S.; Hernell, O.; Hock, Q.S.; Jirapinyo, P.; Lonnerdal, B.; Pencharz, P.; et al. Global standard for the composition of infant formula: Recommendations of an ESPGHAN coordinated international expert group. J. Pediatr. Gastroenterol. Nutr. 2005, 41, 584–599. [Google Scholar] [CrossRef] [Green Version]
- Francavilla, R.; Calasso, M.; Calace, L.; Siragusa, S.; Ndagijimana, M.; Vernocchi, P.; Brunetti, L.; Mancino, G.; Tedeschi, G.; Guerzoni, E.; et al. Effect of lactose on gut microbiota and metabolome of infants with cow’s milk allergy. Pediatr. Allergy Immunol. 2012, 23, 420–427. [Google Scholar] [CrossRef]
- Zivkovic, A.M.; German, J.B.; Lebrilla, C.B.; Mills, D.A. Human milk glycobiome and its impact on the infant gastrointestinal microbiota. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. S1), 4653–4658. [Google Scholar] [CrossRef] [Green Version]
- Thongaram, T.; Hoeflinger, J.L.; Chow, J.; Miller, M.J. Human milk oligosaccharide consumption by probiotic and human-associated bifidobacteria and lactobacilli. J. Dairy Sci. 2017, 100, 7825–7833. [Google Scholar] [CrossRef] [Green Version]
- Bode, L. Human milk oligosaccharides: Every baby needs a sugar mama. Glycobiology 2012, 22, 1147–1162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vandenplas, Y.; Berger, B.; Carnielli, V.P.; Ksiazyk, J.; Lagstrom, H.; Sanchez Luna, M.; Migacheva, N.; Mosselmans, J.M.; Picaud, J.C.; Possner, M.; et al. Human Milk Oligosaccharides: 2’-Fucosyllactose (2’-FL) and Lacto-N-Neotetraose (LNnT) in Infant Formula. Nutrients 2018, 10, 2651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levitt, M.D.; Ingelfinger, F.J. Hydrogen and methane production in man. Ann. N. Y. Acad. Sci. 1968, 150, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Hopper, C.P.; De La Cruz, L.K.; Lyles, K.V.; Wareham, L.K.; Gilbert, J.A.; Eichenbaum, Z.; Magierowski, M.; Poole, R.K.; Wollborn, J.; Wang, B. Role of Carbon Monoxide in Host-Gut Microbiome Communication. Chem. Rev. 2020, 120, 13273–13311. [Google Scholar] [CrossRef] [PubMed]
- Wareham, L.K.; Southam, H.M.; Poole, R.K. Do nitric oxide, carbon monoxide and hydrogen sulfide really qualify as ‘gasotransmitters’ in bacteria? Biochem. Soc. Trans. 2018, 46, 1107–1118. [Google Scholar] [CrossRef] [Green Version]
- Hasler, W.L. Irritable bowel syndrome and bloating. Best Pract. Res. Clin. Gastroenterol. 2007, 21, 689–707. [Google Scholar] [CrossRef] [PubMed]
- Carbonero, F.; Benefiel, A.C.; Alizadeh-Ghamsari, A.H.; Gaskins, H.R. Microbial pathways in colonic sulfur metabolism and links with health and disease. Front. Physiol. 2012, 3, 448. [Google Scholar] [CrossRef] [Green Version]
- Pimentel, M.; Mayer, A.G.; Park, S.; Chow, E.J.; Hasan, A.; Kong, Y. Methane production during lactulose breath test is associated with gastrointestinal disease presentation. Dig. Dis. Sci. 2003, 48, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Flint, H.J.; Scott, K.P.; Duncan, S.H.; Louis, P.; Forano, E. Microbial degradation of complex carbohydrates in the gut. Gut Microbes 2012, 3, 289–306. [Google Scholar] [CrossRef] [Green Version]
- Stumpff, F. A look at the smelly side of physiology: Transport of short chain fatty acids. Pflugers Arch. 2018, 470, 571–598. [Google Scholar] [CrossRef]
- Kelly, C.J.; Zheng, L.; Campbell, E.L.; Saeedi, B.; Scholz, C.C.; Bayless, A.J.; Wilson, K.E.; Glover, L.E.; Kominsky, D.J.; Magnuson, A.; et al. Crosstalk between Microbiota-Derived Short-Chain Fatty Acids and Intestinal Epithelial HIF Augments Tissue Barrier Function. Cell Host Microbe 2015, 17, 662–671. [Google Scholar] [CrossRef] [Green Version]
- Cani, P.D.; Bibiloni, R.; Knauf, C.; Waget, A.; Neyrinck, A.M.; Delzenne, N.M.; Burcelin, R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 2008, 57, 1470–1481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vieira, A.T.; Macia, L.; Galvao, I.; Martins, F.S.; Canesso, M.C.; Amaral, F.A.; Garcia, C.C.; Maslowski, K.M.; De Leon, E.; Shim, D.; et al. A Role for Gut Microbiota and the Metabolite-Sensing Receptor GPR43 in a Murine Model of Gout. Arthritis Rheumatol. 2015, 67, 1646–1656. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly, Y.M.; Glickman, J.N.; Garrett, W.S. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 2013, 341, 569–573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De la Cuesta-Zuluaga, J.; Mueller, N.T.; Alvarez-Quintero, R.; Velasquez-Mejia, E.P.; Sierra, J.A.; Corrales-Agudelo, V.; Carmona, J.A.; Abad, J.M.; Escobar, J.S. Higher Fecal Short-Chain Fatty Acid Levels Are Associated with Gut Microbiome Dysbiosis, Obesity, Hypertension and Cardiometabolic Disease Risk Factors. Nutrients 2018, 11, 2214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Item | Results | ||
|---|---|---|---|
| Group A | Group B | Difference | |
| n | 90 | 70 | |
| Gender | 0.015 | ||
| Male | 62.2% | 42.9% | |
| Female | 37.8% | 57.1% | |
| Gestational age (GA, weeks) | 28.5 (25–29) | 29.5 (28–31) | <0.0001 |
| Birth weight (g) | 950 (860–1173) | 1255 (1005–1420) | <0.0001 |
| Preterm premature rupture of membranes (PPROM) | 37.8% | 52.9% | 0.057 |
| Apgar score 1-Min | 0.009 | ||
| >7 | 82.2% | 80% | |
| 4–7 | 17.8% | 20% | |
| Age (days) | 28 (16–42) | 22 (13–32) | 0.011 |
| Milk volume (ml/kg) | 148 (95–164) | 103 (65–151) | 0.005 |
| Exclusive Enteral Nutrition (EEN) | 56.7% | 28.6% | 0.001 |
| Weight (g) | 1508 (1190–1831) | 1748 (1491–2033) | 0.004 |
| Feeding intolerance | 17.8% | 14.3% | 0.553 |
| Using antibiotic | 53.3% | 64.3% | 0.164 |
| Using probiotics | 38.9% | 42.9% | 0.612 |
| History of sepsis | 27.8% | 42.9% | 0.046 |
| Mother clinical characteristics | |||
| Mother age (years) | 34 (31–36) | 33 (32–38) | 0.505 |
| Diabetes | <0.0001 | ||
| No | 76.7% | 48.6% | |
| Gestational diabetes mellitus (GDM) | 23.3% | 51.4% | |
| History of antibiotic in perinatal stage | 96.7% | 98.6% | 0.444 |
| Diagnosis and treatment | |||
| Respiratory distress syndrome of newborn (NRDS) | 94.4% | 59% | 0.034 |
| History of invasive ventilator | 60% | 37.1% | 0.001 |
| Bronchopulmonary dysplasia (BPD) | 67.8% | 30% | <0.0001 |
| Necrotizing enterocolitis (NEC) | 10% | 4.3% | 0.173 |
| Age reach EEN (days) | 22 (16–25) | 25 (18–33) | 0.077 |
| Discharge age (days) | 64 (40–75) | 42 (32–49) | <0.0001 |
| Discharge weight (g) | 2160 (2035–2545) | 2120 (2020–2366) | 0.115 |
| Item | Initial Feeding Methods | Difference | |||
|---|---|---|---|---|---|
| Group A | Group B | ||||
| n | Value | n | Value | ||
| Fecal pH | 80 | 6.24 (5.62–6.52) | 45 | 6.37 (5.83–6.89) | 0.023 |
| SigA (OD) | 82 | 0.375 (0.286–0.752) | 38 | 0.670 (0.340–1.108) | 0.046 |
| SigA (concentration) | 82 | 0.000110 (0.00000000218–0.000569) | 38 | 0.00132 (0.00045–0.00243) | <0.0001 |
| Fecal ammonia (μmol/g) | 82 | 4.31 (2.28–8.76) | 38 | 9.08 (4.45–13.57) | 0.001 |
| Fecal bile acids (μmol/g) | 82 | 0.34 (0.31–0.38) | 38 | 0.36 (0.34–0.40) | 0.025 |
| Air Pressure Difference (kpa) | Initial Feeding Methods | Difference | |||
|---|---|---|---|---|---|
| Group A | Group B | ||||
| n | Value | n | Value | ||
| LAT | 89 | 3.8 (1.25–5.75) | 70 | 4.0 (1.2–6.3) | 0.812 |
| FOS | 89 | 4.10 (1.65–7.35) | 70 | 3.3 (1.2–7.5) | 0.207 |
| GOS | 89 | 2.80 (0.95–6.20) | 70 | 1.9 (0.7–7.0) | 0.378 |
| FL2 | 80 | 2.85 (1.53–4.00) | 45 | 2.8 (2.2–4.0) | 0.934 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Jiang, J.; Zhu, L.; Wang, X.; Wan, W.; Wang, D.; Li, Z. Evaluation of Intestinal Microbial Metabolites in Preterm Infants with Different Initial Feeding Methods by In Vitro Fermentation Modeling System. Microorganisms 2022, 10, 1453. https://doi.org/10.3390/microorganisms10071453
Li Y, Jiang J, Zhu L, Wang X, Wan W, Wang D, Li Z. Evaluation of Intestinal Microbial Metabolites in Preterm Infants with Different Initial Feeding Methods by In Vitro Fermentation Modeling System. Microorganisms. 2022; 10(7):1453. https://doi.org/10.3390/microorganisms10071453
Chicago/Turabian StyleLi, Yunwei, Jingjing Jiang, Liying Zhu, Xin Wang, Weilin Wan, Danhua Wang, and Zhenghong Li. 2022. "Evaluation of Intestinal Microbial Metabolites in Preterm Infants with Different Initial Feeding Methods by In Vitro Fermentation Modeling System" Microorganisms 10, no. 7: 1453. https://doi.org/10.3390/microorganisms10071453
APA StyleLi, Y., Jiang, J., Zhu, L., Wang, X., Wan, W., Wang, D., & Li, Z. (2022). Evaluation of Intestinal Microbial Metabolites in Preterm Infants with Different Initial Feeding Methods by In Vitro Fermentation Modeling System. Microorganisms, 10(7), 1453. https://doi.org/10.3390/microorganisms10071453






