Characteristics of Fungal Communities and Internal Mildew Occurrence during the Stages of Planting and Storing of Sunflower Seed in China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material Collection and Sampling Strategy
2.2. Microbial Changes and the Occurrence of Internal Mildew
2.2.1. DNA Extraction and Sequencing
2.2.2. Statistical Analysis
2.3. Identification of Pathogen Causing Mildew
2.3.1. Fungal Isolation and Molecular Identification
2.3.2. Pathogenicity Identification
2.4. The Conditions Required for Internal Mildewing during Storage
2.5. Irradiation Treatment to Prevent the Occurrence of Internal Mildewing during Storage
2.5.1. γ-ray Irradiation
2.5.2. Post-Irradiation Mildew Incidence Rate in High-Humidity Conditions
2.5.3. Acid and Peroxide Values of the γ-Irradiated Kernels
3. Results
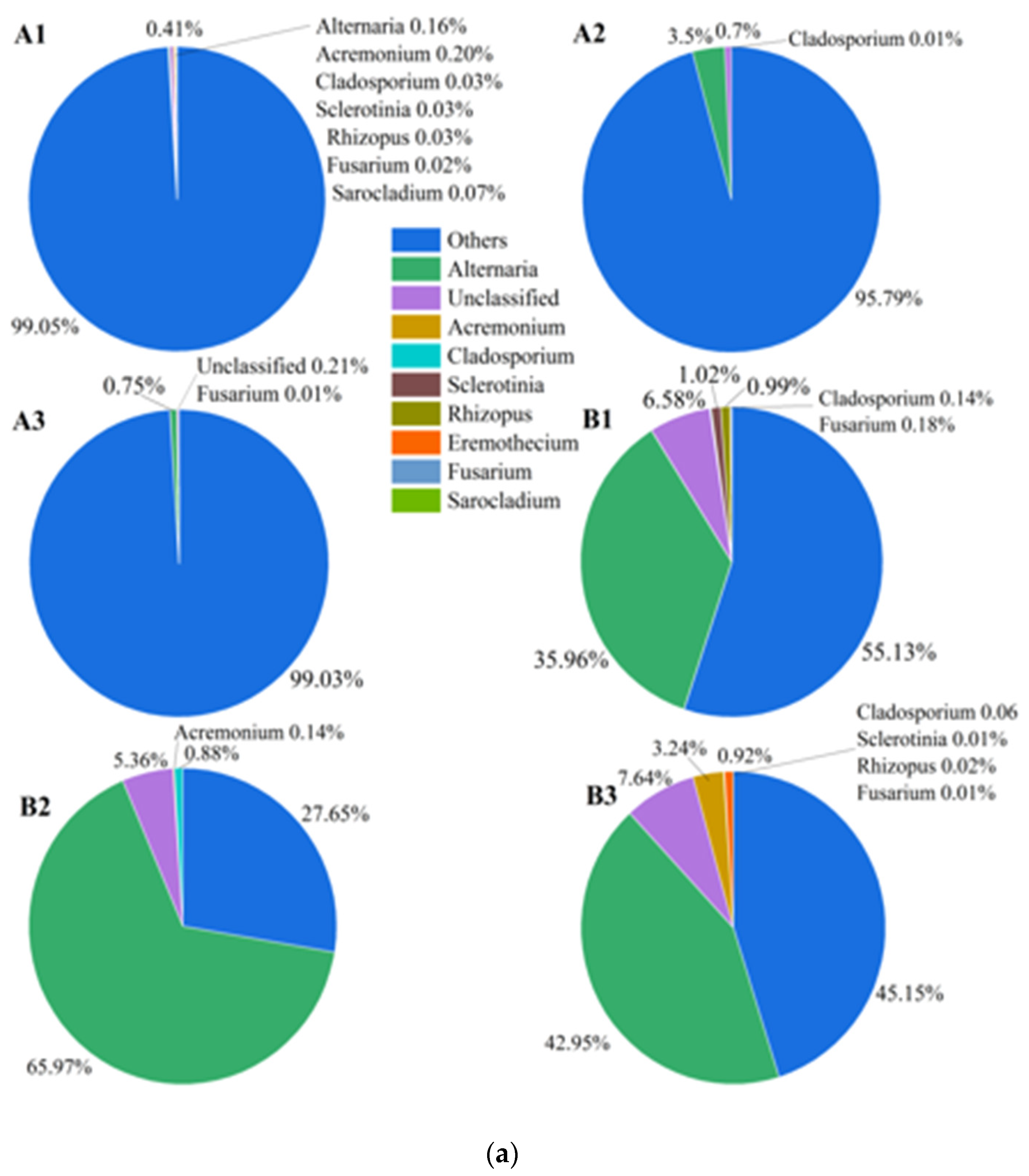
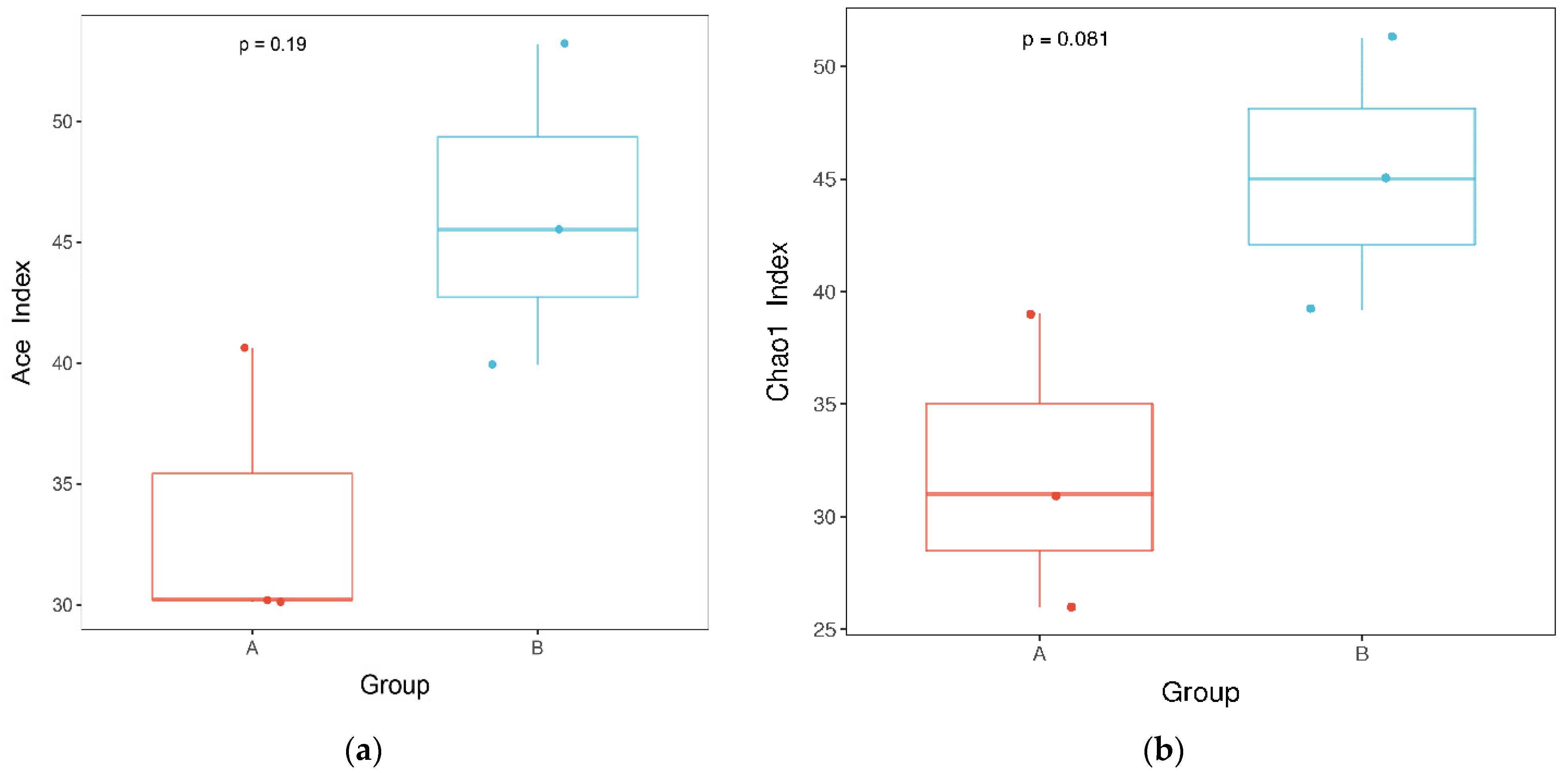
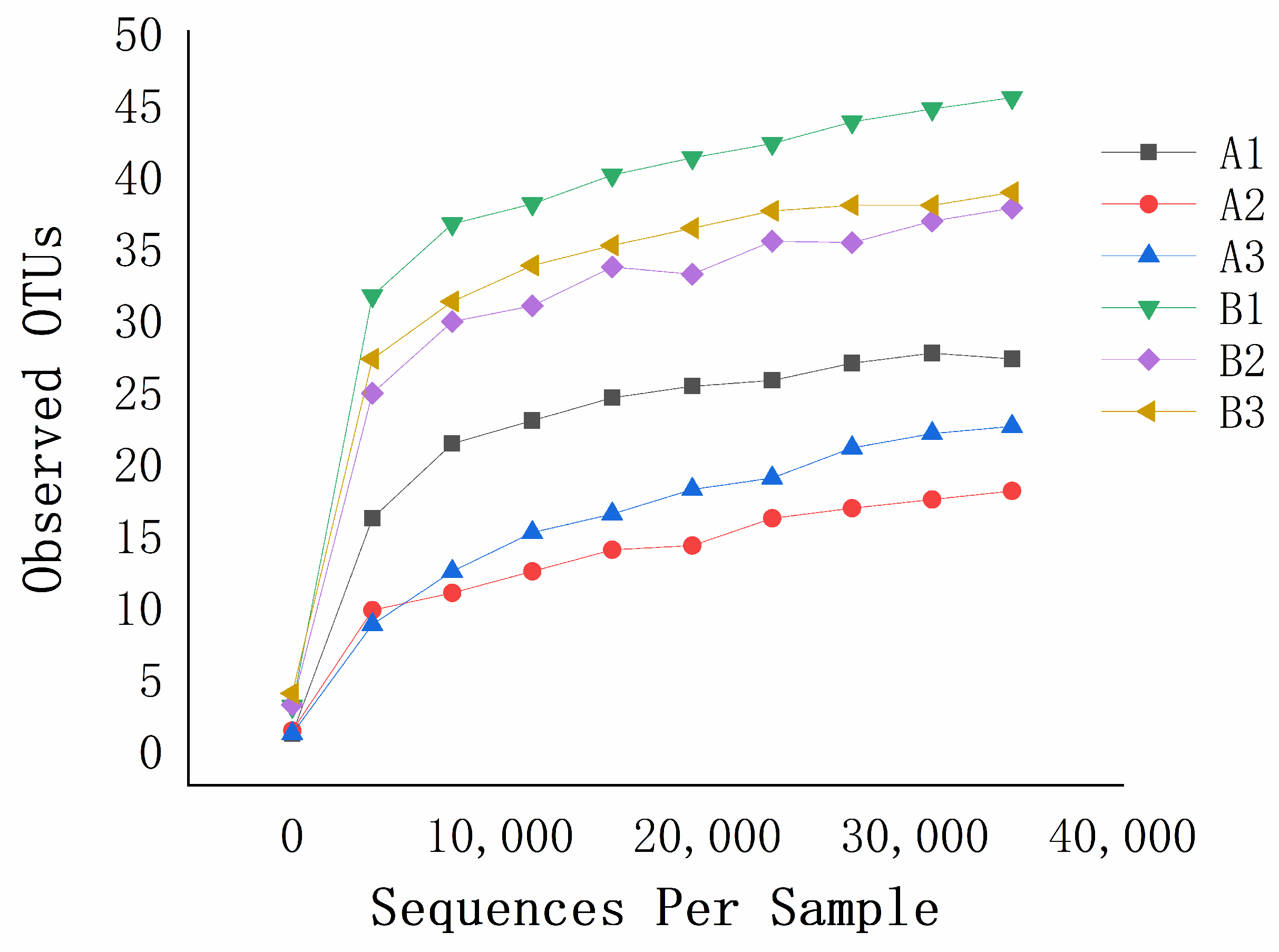
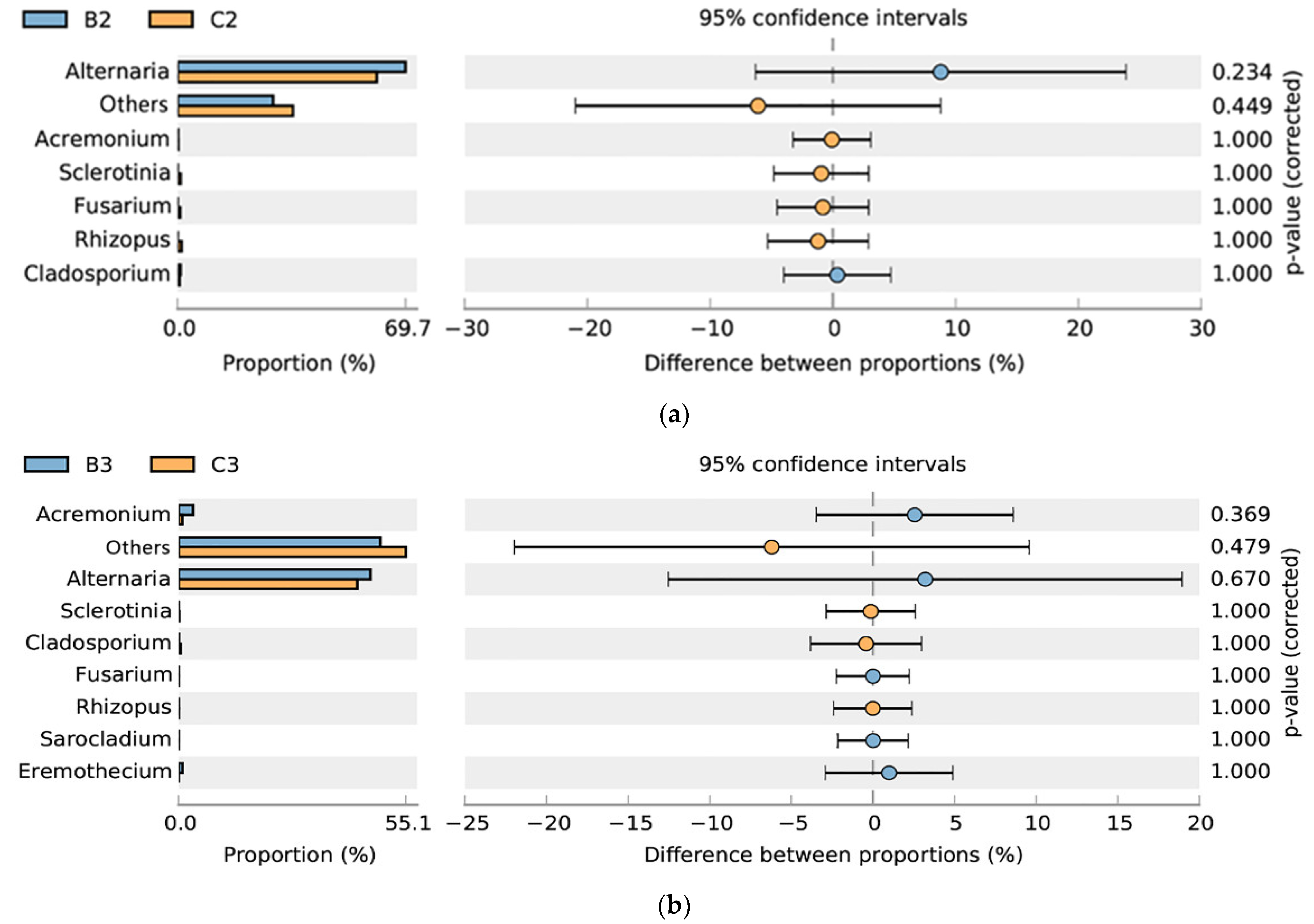
3.1. Effects of Mildew on the Fungal Community of the Sunflower-Seed Kernel
3.2. Identification of the Pathogen Causing Mildew
3.3. Investigation of the Source of Internal Mildewing during Field-Planting and Storage Stages
3.3.1. Sunflower Seeds Are the Most Vulnerable to Internal Mildew Naturally during Field-Planting
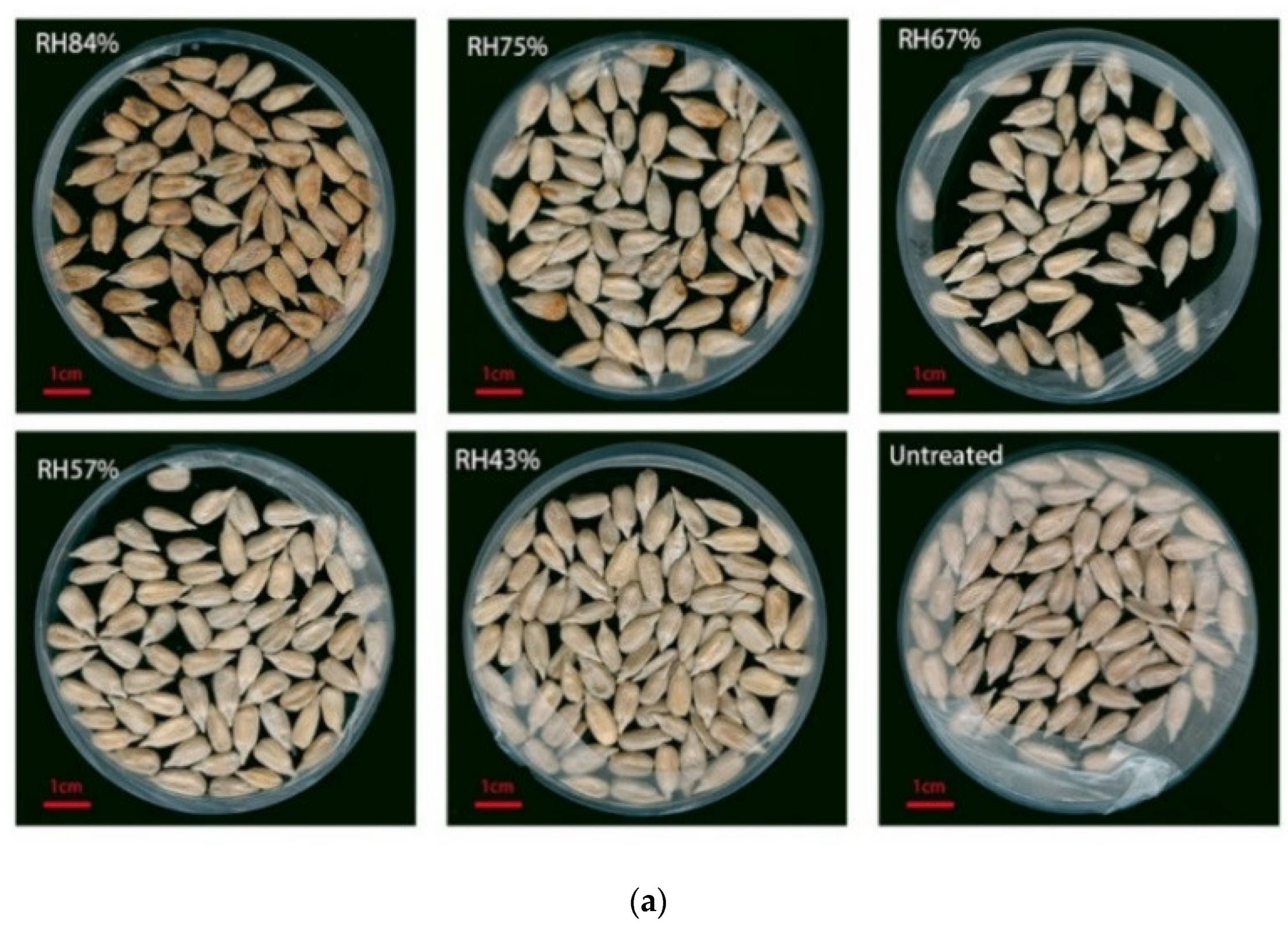
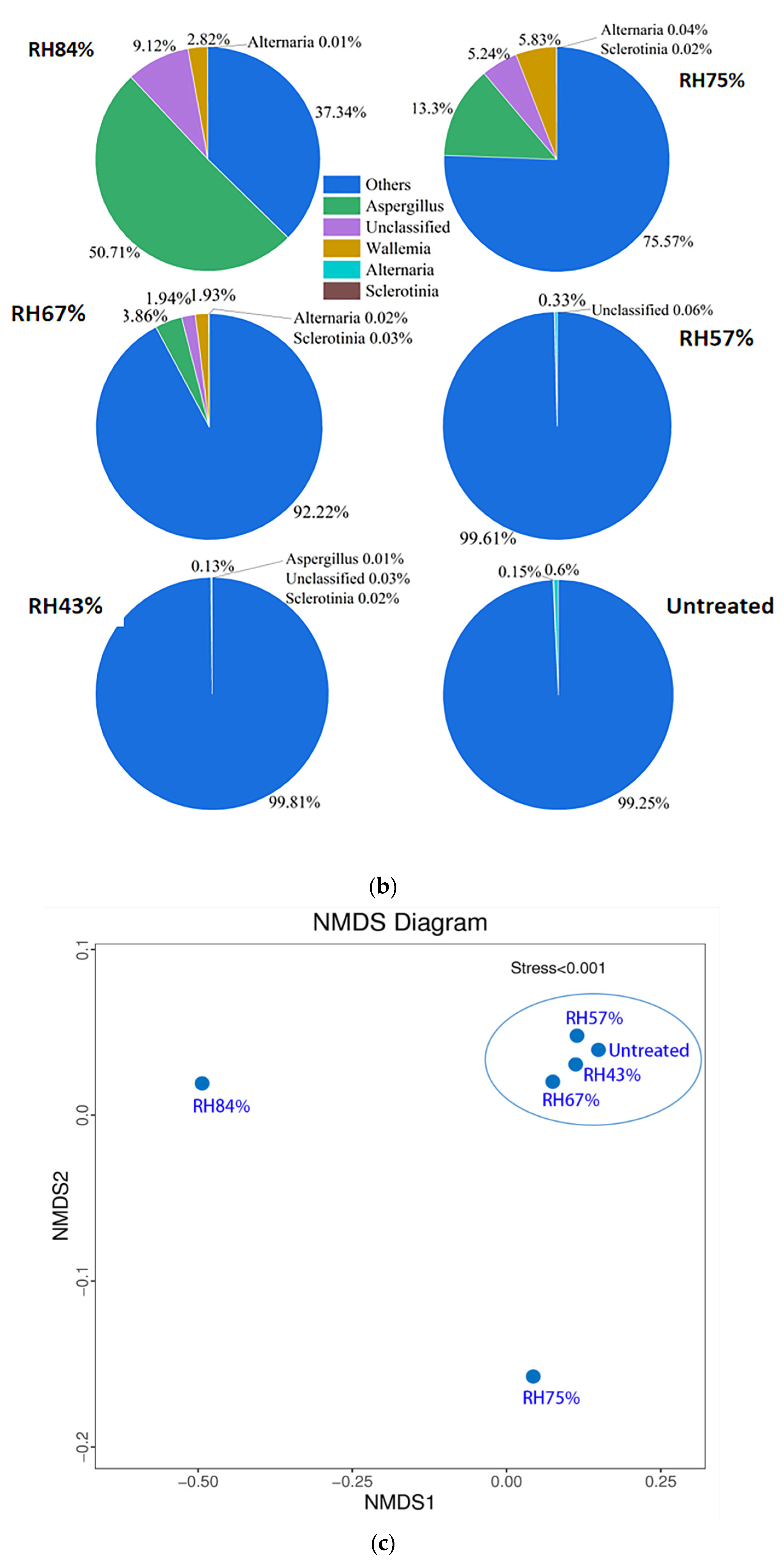
3.3.2. The Conditions for Occurrence of Internal Mildew during Storage
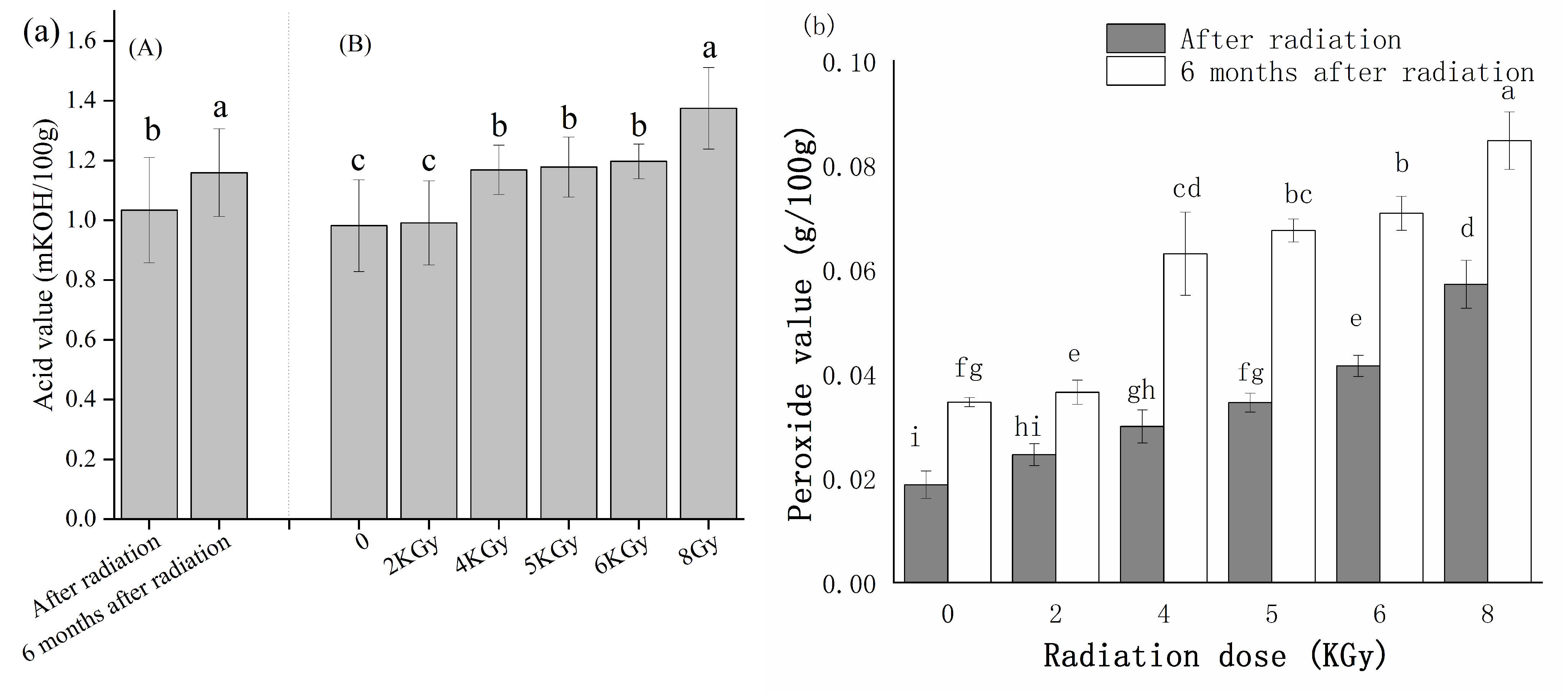
3.3.3. Irradiation Treatment to Prevent the Occurrence of Internal Mildew during Storage under High-Humidity Conditions
4. Discussion
4.1. Alternaria Was the Only Dominant Pathogenic Fungus Causing the Internal Mildewing of Post-Storage Sunflower-Seed Kernels
4.2. Sunflower Seeds Are Most Vulnerable to Internal Mildew during Field-Planting
4.3. A Combination of Field Management Targeting Alternaria and 5 kGy γ-ray Radiation Can Control Internal Mildew
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, R. Current situation and development prospect of sunflower seed oil industry in China. China Oils Fats 2020, 45, 1–3. [Google Scholar]
- Jeswal, P.; Kumar, D. Mycobiota and mycotoxins in sunflower seeds in pre- and post-harvest condition from bihar state. Int. J. Environ. Eng. Sci. Technol. Res. 2013, 1, 328–339. [Google Scholar]
- GB/T 11764-2008; Sunflower Seed, Part 3, Moldy Kernel. China Standard Press: Beijing, China, 2008.
- Nyandieka, H.S.; Nyamogoba, H.D.; Nyamwange, C.I. Distribution of aflatoxins and micro-organisms in peanut and sunflower seed products and their potential health hazards. Pak. J. Med. Res. 2014, 53, 67–70. [Google Scholar]
- Wild, C.P.; Gong, Y.Y. Mycotoxins and human disease: A largely ignored global health issue. Carcinogenesis 2019, 31, 71–82. [Google Scholar] [CrossRef]
- Hussein, H.S.; Brasel, J.M. Toxicity, metabolism, and impact of mycotoxins on humans and animals. Toxicology 2001, 167, 101–134. [Google Scholar] [CrossRef]
- Raß, M.; Schein, C.; Matthäus, B. Virgin sunflower oil. Eur. J. Lipid Sci. Technol. 2008, 110, 618–624. [Google Scholar] [CrossRef]
- Lazaro, E.; Benjamin, Y.; Robert, M. The effects of dehulling on physicochemical properties of seed oil and cake quality of sunflower. Tanzan. J. Agric. Sci. 2014, 13, 41–47. [Google Scholar]
- Scott, P.M.; Zhao, W.; Feng, S. Alternaria toxins alternariol and alternariol monomethyl ether in grain foods in Canada. Mycotoxin Res. 2012, 28, 261–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banu, N.; Muthumary, J.P. Mycobiota of sunflower seeds and samples collected from vegetable oil refinery located in Tamilnadu, India. Mycol. Prog. 2005, 4, 195–204. [Google Scholar] [CrossRef]
- Mushtaq, M.; Hashmi, M.H. Seed-borne mycoflora of sunflower (Helianthus annuus L.). Pak. J. Bot. 2005, 37, 451–457. [Google Scholar]
- Lan, W.; Chen, Q.; Jiang, H. Preliminary study on seed-borne fungi of sunflower seeds. Seed 2009, 28, 45–48. [Google Scholar]
- Skrinjar, M.; Petrovic, Z. Sunflower seed for human consumption as a substrate for the growth of mycopopulations. Acta Period. Technol. 2012, 43, 115–121. [Google Scholar] [CrossRef]
- Handelsman, J. Metagenomics: Application of genomics to uncultured microorganisms. Microbiol. Mol. Biol. Rev. 2004, 68, 671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paul, N. Chapter 13, The infested and contaminated parts of the seeds. In Seed Pathology, 1st ed.; China Agriculture Press: Beijing, China, 1987; p. 284. [Google Scholar]
- Raut, J.G. Location of Alternaria helianthi in sunflower seed and its transmission from seed to plant. Indian Phytopathol. 1985, 38, 522. [Google Scholar]
- GB 5491-85; Sampling Method of Grain and Oilseed, Part 3, Sampling Method. China Standard Press: Beijing, China, 1985.
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [Green Version]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Yang, Q.; Yang, S. Application of near infrared spectroscopy to detect mould contamination in tobacco. J. Near Infrared Spectrosc. 2015, 23, 391–400. [Google Scholar] [CrossRef]
- Paul, N. Chapter 7, Storage fungi. In Seed Pathology, 1st ed.; China Agriculture Press: Beijing, China, 1987; p. 217. [Google Scholar]
- Baselga, A.; Orme, C. Betapart: An R package for the study of beta diversity. Methods Ecol. Evol. 2012, 3, 808–812. [Google Scholar] [CrossRef]
- Parks, D.H.; Tyson, G.W.; Hugenholtz, P.; Beiko, R.G. STAMP: Statistical analysis of taxonomic and functional profiles. Bioinformatics 2014, 30, 3123–3124. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, N.H.; Song, Z.; Bates, S.T. FUNGuild: An open annotation tool for parsing fungal community datasets by ecological guild. Fungal Ecol. 2016, 20, 241–248. [Google Scholar] [CrossRef]
- Altschul, S.F. Basic local alignment search tool (BLAST). J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Hao, G.; Xin, L.; Liang, Q. Saturated salt solution humidity point (2)-data source and salt solution selection. Sens. World 1999, 12, 10–14. [Google Scholar]
- International Seed Testing Association (ISTA). International Seed Inspection Regulations; China Agriculture Press: Beijing, China, 1999; p. 30. [Google Scholar]
- GB/T 5009.37-2003; Analytical Method of Hygienic Standard of Edible Vegetable Oil, Part 4, Physical and Chemical Test. China Standard Press: Beijing, China, 2003.
- NY/T 902-2004; Green Food Melon Seed, Part 4, Physical and Chemical Indicators. China Standard Press: Beijing, China, 2005.
- Kononenko, G.P.; Ustyuzhanina, M.I. The problem of safe sunflower (Helianthus annuus L.) use for food and fodder purposes. Agric. Biol. 2018, 53, 485–498. [Google Scholar] [CrossRef]
- Huang, B. The hazards of eating moldy food. Food Health 1995, 2, 30. [Google Scholar]
- Chen, Q.; Wang, H.; Liang, Y. Fungal composition and diversity analysis of healthy and rotten tobacco leaves after curing. Acta Agric. Zhejiangensis 2020, 32, 1019–1028. [Google Scholar]
- Zhou, J.; Cheng, Y.; Yu, L. Characteristics of fungal communities and the sources of mold contamination in mildewed tobacco leaves stored under different climatic conditions. Appl. Microbiol. Biotechnol. 2022, 106, 131–144. [Google Scholar] [CrossRef]
- Welty, R.E.; Lucas, G.B. Fungi isolated from flue-cured tobacco at time of sale and after storage. Appl. Microbiol. Biotechnol. 1969, 17, 360–365. [Google Scholar] [CrossRef]
- Marin, S. Alternaria in food products. Curr. Opin. Food Sci. 2016, 11, 1–9. [Google Scholar]
- Herr, L.J. Alternaria helianthi on sunflower in Ohio. Plant Dis. 1982, 66, 509–512. [Google Scholar] [CrossRef]
- Aichinger, G.; Favero, G.D. Alternaria toxins-Still emerging? Compr. Rev. Food Sci. Food Saf. 2021, 20, 4390–4406. [Google Scholar] [CrossRef] [PubMed]
- Arcella, D.; Eskola, M. Dietary exposure assessment to Alternaria toxins in the European population. EFSA J. 2016, 14, 4654. [Google Scholar]
- T/CNFIA 005.10-2019; Quality Grade of Nuts and Seeds, Part 10, Dried Raw Sunflower and Melon Seed. China Food Industry Association: Beijing, China, 2019.
- Krishnappa, M. Location of Alternaria species in sunflower seeds. Plant Dis. Res. 1990, 5, 203–204. [Google Scholar]
- Liu, J.; Deng, J.; Zhang, K.; Wu, H.; Yang, C. Pod mildew on soybeans can mitigate the damage to the seed arising from field mold at harvest time. J. Agric. Food Chem. 2016, 64, 9135–9142. [Google Scholar] [CrossRef]
- Nguyen, J.L.; Schwartz, J.; Dockery, D.W. The relationship between indoor and outdoor temperature, apparent temperature, relative humidity, and absolute humidity. Indoor Air 2014, 24, 103–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robertson, J.A.; Thomas, J.K. Chemical and microbial changes in dehulled confectionery sunflower kernels during storage under controlled conditions. J. Food Sci. Technol. 1976, 39, 18–23. [Google Scholar] [CrossRef]
- Avenot, H.F.; Michailides, T.J. Detection of isolates of Alternaria with multiple-resistance to fludioxonil, cyprodinil, boscalid and pyraclostrobin in California pistachio orchards. Crop Prot. 2015, 78, 214–221. [Google Scholar] [CrossRef]
- Rychlik, M.; Lepper, H.; Weidner, C. Asam S: Risk evaluation of the Alternaria mycotoxin tenuazonic acid in foods for adults and infants and subsequent risk management. Food Control 2016, 68, 181–185. [Google Scholar] [CrossRef]
- Da Motta, S.; Valente Soares, L.M. Survey of Brazilian tomato products for alternariol, alternariol monomethyl ether, tenuazonic acid and cyclopiazonic acid. Food Addit. Contam. 2001, 18, 630–634. [Google Scholar] [CrossRef]
- Darfour, B.; Ofori, H. Gamma irradiation and drying method: The effects on kola nut powder. Radiat. Phys. Chem. 2021, 185, 109489. [Google Scholar] [CrossRef]
- Song, W.J.; Kim, Y.H.; Kang, D.H. Effect of gamma irradiation on inactivation of Escherichia coli O157:H7, Salmonella Typhimurium and Listeria monocytogenes on pistachios. Lett. Appl. Microbiol. 2018, 68, 96–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]


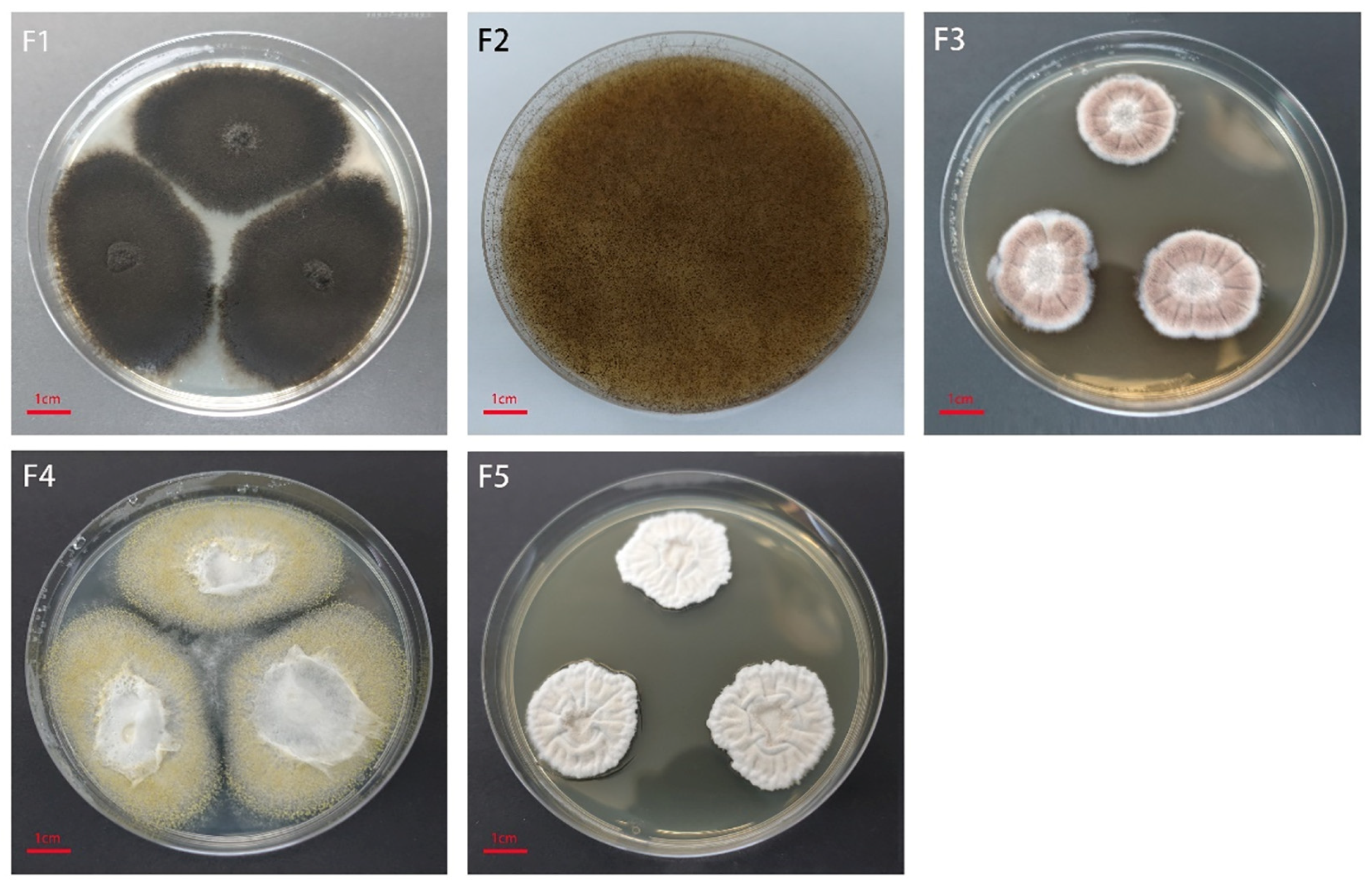



| No. | Taxon | Percent Identity | Accession |
|---|---|---|---|
| F1 | Alternaria sp. | 99.45% | MK649978.1 |
| F2 | Rhizopus stolonifer | 100% | MK722197.1 |
| F3 | Aspergillus sydowii | 99.45% | MH625698.1 |
| F4 | Aspergillus flavus | 99.65% | MW757217.1 |
| F5 | Aspergillus niveus | 100% | MK590295.1 |
| Equilibrated RH (%) | Moisture Content (%) | Appearance |
|---|---|---|
| 84 | 8.81% | Wrinkled, dark-brown spots |
| 75 | 7.72% | Yellow spots |
| 67 | 5.6% | Normal |
| 57 | 5.07% | Normal |
| 43 | 3.98% | Normal |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Yang, Y.; Xu, Z.; Wang, Q.; Liu, B.; Wu, Y. Characteristics of Fungal Communities and Internal Mildew Occurrence during the Stages of Planting and Storing of Sunflower Seed in China. Microorganisms 2022, 10, 1434. https://doi.org/10.3390/microorganisms10071434
Liu J, Yang Y, Xu Z, Wang Q, Liu B, Wu Y. Characteristics of Fungal Communities and Internal Mildew Occurrence during the Stages of Planting and Storing of Sunflower Seed in China. Microorganisms. 2022; 10(7):1434. https://doi.org/10.3390/microorganisms10071434
Chicago/Turabian StyleLiu, Jie, Yang Yang, Zhuopin Xu, Qi Wang, Binmei Liu, and Yuejin Wu. 2022. "Characteristics of Fungal Communities and Internal Mildew Occurrence during the Stages of Planting and Storing of Sunflower Seed in China" Microorganisms 10, no. 7: 1434. https://doi.org/10.3390/microorganisms10071434
APA StyleLiu, J., Yang, Y., Xu, Z., Wang, Q., Liu, B., & Wu, Y. (2022). Characteristics of Fungal Communities and Internal Mildew Occurrence during the Stages of Planting and Storing of Sunflower Seed in China. Microorganisms, 10(7), 1434. https://doi.org/10.3390/microorganisms10071434






