Abstract
Intestinal mucositis is a commonly reported side effect in oncology practice. Probiotics are considered an excellent alternative therapeutic approach to this debilitating condition; however, there are safety questions regarding the viable consumption of probiotics in clinical practice due to the risks of systemic infections, especially in immune-compromised patients. The use of heat-killed or cell-free supernatants derived from probiotic strains has been evaluated to minimize these adverse effects. Thus, this work evaluated the anti-inflammatory properties of paraprobiotics (heat-killed) and postbiotics (cell-free supernatant) of the probiotic Lactobacillus delbrueckii CIDCA 133 strain in a mouse model of 5-Fluorouracil drug-induced mucositis. Administration of paraprobiotics and postbiotics reduced the neutrophil cells infiltrating into the small intestinal mucosa and ameliorated the intestinal epithelium architecture damaged by 5-FU. These ameliorative effects were associated with a downregulation of inflammatory markers (Tlr2, Nfkb1, Il12, Il17a, Il1b, Tnf), and upregulation of immunoregulatory Il10 cytokine and the epithelial barrier markers Ocln, Cldn1, 2, 5, Hp and Muc2. Thus, heat-killed L. delbrueckii CIDCA 133 and supernatants derived from this strain were shown to be effective in reducing 5-FU-induced inflammatory damage, demonstrating them to be an alternative approach to the problems arising from the use of live beneficial microorganisms in clinical practice.
1. Introduction
Mucositis is characterized as a gastrointestinal (GIT) mucosal inflammation that frequently occurs in patients with cancer disease submitted to radiotherapy [1,2] and/or chemotherapy treatments (5-Fluorouracil, irinotecan, oxaliplatin, etc.) [3,4]. This debilitating condition is associated with pain and difficulty eating, leading to malnutrition, increasing the infection risk, alteration in the patient’s clinical status, and therapy delays [5,6,7].
Many strategies have been investigated to prevent and/or treat mucositis [5,8]. According to the pathobiology phases described by Sonis [3], the chemotherapy-induced dysbiotic intestinal microbiota has an essential role in the progression and severity of mucositis [9]. In this context, some probiotic microorganisms (e.g., Lactobacillus sp., Bifidobacterium sp., Saccharomyces sp.) have been intentionally explored as a therapeutic tool against the intestinal mucositis caused by chemotherapy treatment due to their ability to regulate the dysbiotic microbiota and their well-known anti-inflammatory properties, which mainly occur through the inhibition of the nuclear factor kappa B (NF-κB) signaling pathway with subsequent cellular and humoral immunomodulation [10,11,12,13]. Other positive effects attributed to some probiotic strains include the production of beneficial secreted metabolites such as short-chain fatty acids (SCFA) (e.g., acetate, propionate, and butyrate), inhibition of pathogenic bacterial growth by antimicrobial compounds (e.g., acetic and lactic acids and bacteriocins), and reinforcement of the intestinal barrier by mucus production and induced expression of tight junction proteins [14,15]. Probiotics can use these mechanisms in a strain-specific manner to protect and improve the intestinal mucosa healing process altered by chemotherapy.
Although some preclinical studies report the host’s beneficial effects related to “live” probiotics consumption [16,17,18,19,20], there are many questions regarding their safety concerns in clinical practice, especially in premature infants or immune-compromised patients. In these conditions, probiotics can translocate into the blood system and increase the risk of systemic infections [21,22,23,24]. The use of non-viable/inactivated probiotics, known as paraprobiotics (e.g., heat-killed probiotics) or products/molecules secreted or derived from these microorganisms, known as postbiotics (e.g., cell-free supernatant, surface layer proteins, cell lysates), besides minimizing or avoiding these possible adverse effects, has also been evaluated in anti-inflammatory therapies [25,26]. Paraprobiotics or postbiotics are an effective therapeutic alternative to gastrointestinal inflammation since they can also stimulate the host’s immune system to form an anti-inflammatory profile [15,27,28]. However, like probiotics, these biotics’ safety evaluations must also be performed before use for therapeutic purposes [25].
The anti-inflammatory properties and the intestinal barrier function conferred by the potential probiotic bacterium Lactobacillus delbrueckii subsp. lactis CIDCA 133 in a murine model of 5-FU-induced mucositis was previously demonstrated by our research group [29]. Ameliorative effects promoted by this strain were mainly associated with the reduction of inflammatory cells, improvement in mucus-producing goblet cell count, secretory IgA levels and intestinal permeability reduction, modulation of markers involved in NF-κB signaling pathway activation (Tlr2, Nfkb1, Il6, and Il1b), and regulation of tight junction protein expression [29,30]. Additionally, a probiogenomics study of this strain showed that its anti-inflammatory profile could be related to genes encoding secreted and membrane surface proteins able to interact with immune proteins involved in the NF-κB signaling pathway [31]. Regarding its safety concerns, it was reported that CIDCA 133 (107 CFU/mL) did not cause blood hemolysis, mucin degradation, or epithelial damage in healthy mice, evidencing that this strain presents safety levels for probiotic application [32].
Few studies have evaluated the effects of heat-inactivated or cell-free supernatant on intestinal mucositis, mainly studies focused on the Lactobacillus delbrueckii species. The L. delbrueckii CIDCA 133 strain is a potential health-promoting bacterium. However, no beneficial effects have been reported with either the inactivation or extractable/secreted bioactive products derived from this strain. Thus, this work evaluated the anti-inflammatory properties of heat-killed and cell-free supernatants of L. delbrueckii CIDCA 133 in a mouse inflammation model induced by 5-Fluorouracil.
2. Materials and Methods
2.1. Bacterial Culture Conditions
The strain Lactobacillus delbrueckii subsp. lactis CIDCA 133 (culture collection of Center for Research and Development in Food Cryotechnology of the National University of La Plata, Argentina) was grown on de Man, Rogosa and Sharpe (MRS) broth (Kasvi, São José dos Pinhais, Brazil) at 37 °C for 16 h. The strain was deposited at the Bacteria Collection from Environment and Health (CBAS) of the Oswaldo Cruz Foundation (FIOCRUZ) (access number: CBAS 815).
2.2. Lactobacillus delbrueckii CIDCA 133 Preparation (Viable, Heat-Inactivated, and Cell-Free Supernatant)
Viable bacteria were centrifuged (1520× g for 15 min at 4 °C), washed two times with sterile phosphate-buffered saline (PBS) 0.1 M (pH 7.2), and adjusted to a concentration of 109 CFU/mL. The cell-free supernatant (CFS) was collected and buffered to a pH of 7.2 using TRIS 1 M (Sigma-Aldrich, St. Louis, MO, USA), as previously described by Prisciandaro et al. [33]. The CFS (15 mL) was sterilized with a 0.22 μm filter (Kasvi, São José dos Pinhais, Brazil), concentrated with Vivaspin 20 (weight cut-off 10,000 Kilodalton (kDa) (Sartorius, Gottingen, Germany), and stored at −80 °C until administration in mice. The heat-killed CIDCA 133 (109 CFU/mL) was prepared by heat treatment (15 min, 121 °C). The inactivation process and CFS sterilization were confirmed after plating 100 μL of these biotics in MRS agar (Kasvi, São José dos Pinhais, Brazil).
2.3. Animals
Six-week-old conventional BALB/c mice (males) weighing 20–24 g were obtained from the Bioterism Center (CEBIO) at the Federal University of Minas Gerais (UFMG, Belo Horizonte, Brazil). This study was approved by the Local Animal Experimentation Ethics Committee (CEUA-UFMG, Protocol n◦ 112/2020). Mice were housed in ventilated polycarbonate cages under controlled room conditions: 12 h light/dark cycle, temperature 25 ± 2 °C, and ad libitum access to standard chow and water. All procedures were conducted according to Brazilian Association for Laboratory Animal Science (SBCAL) guidelines.
2.4. Experimental Design
Mice were split into six experimental groups (n = six animals per group): negative control (NC), inflamed (MUC), inflamed (5-FU), inflamed and treated with viable CIDCA 133 (LDv), heat-killed inactivated CIDCA 133 (LDi), and CIDCA 133 cell-free supernatant (LDs). Mice received doses of 300 μL containing 109 CFU/mL of CIDCA 133 (LDv and LDi groups) or 300 μL of CIDCA 133 supernatants (LDs group) for 13 days by intragastric gavage. Control groups received 300 μL of PBS 0.1M solution (NC and MUC groups) or MRS broth (5-FU group) by the same route. On the 10th day, mice (MUC, 5-FU, LDv, LDi, and LDs groups) were inflamed intraperitoneally with a single injection of 5-Fluorouracil (Fauldfluor®) (300 mg/kg) (Libbs, São Paulo, Brazil) [29]. Sterile saline solution injection (NaCl 0.9%) (Vetec, Rio de Janeiro, Brazil) (Figure 1) was administered to the negative control group. At the end of the experimental procedure, 72 h after mucositis induction, mice were euthanized by an anesthetic overdose (ketamine 300 mg/kg and xylazine 30 mg/kg solution) (Syntec, Tamboré, Brazil), and afterward, ileum sections were collected for analysis.
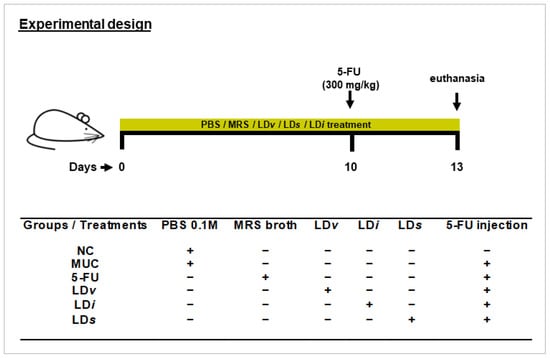
Figure 1.
Experimental design protocol. Mice received gavage with 300 μL of PBS 0.1 M (NC and MUC group), MRS broth (5-FU group), viable CIDCA 133 (LDv group), heat-inactivated CIDCA 133 (LDi group), or cell-free supernatant (LDs) for 13 days. Mice were inflamed with a single dose of 5-Fluorouracil (300 mg/kg) on the 10th day. Symbols indicate (+) presence or (−) absence of treatment.
2.5. Inflammatory Cell Infiltration
The recruitment of neutrophils and eosinophils to intestinal mucosa was performed by myeloperoxidase (MPO) [34] and eosinophil peroxidase (EPO) [35] enzymatic activity assays, respectively. For MPO, ileum tissues (100 mg) were homogenized with 1.9 mL of buffered solution (pH 4.7) (NaCl 0.1 M (LabSynth, Diadema, Brazil), NaH2PO4 0.02 M (LabSynth, Diadema, Brazil), and Na2EDTA 0.015 M (LabSynth, Diadema, Brazil)) and centrifuged (9500× g for 10 min at 4 °C). After a hypotonic process (0.2% NaCl solution plus 1.6% NaCl solution containing glucose 5% (LabSynth, Diadema, Brazil)) and centrifugation (9500× g for 10 min at 4 °C), the pellet was homogenized in a NaH2PO4 (0.05 M) solution (pH 5.4) containing 0.5% hexadecyltrimethylammonium bromide (Sigma-Aldrich, St. Louis, MO, USA) and submitted to lysis process with three cycles of freeze-thaw in liquid nitrogen. After the second centrifugation (9500× g for 15 min at 4 °C), the supernatant was collected for colorimetric assay. Thus, 25 μL of supernatant was added to 25 μL of 3,3,5,5′-Tetramethylbenzidine 1.6 mM (Sigma-Aldrich, St. Louis, MO, USA) previously diluted in dimethyl sulfoxide (Sigma-Aldrich, St. Louis, MO, USA). After adding 100 μL of H2O2 0.5 mM (Vetec, Rio de Janeiro, Brazil), the plates were incubated at 37 °C for 5 min.
For EPO, 100 mg of ileum tissues were homogenized in 1.9 mL of PBS 0.1 M (pH 7.4). After centrifugation (9500× g for 15 min at 4 °C), a hypotonic process (0.2% NaCl solution, plus 1.6% NaCl solution containing glucose 5%) and second centrifugation (9500× g for 15 min at 4 °C), the pellet was homogenized in a PBS 0.1 M solution (pH 7.4) containing 0.5% hexadecyltrimethylammonium bromide and submitted to lysis process with three cycles of freeze-thaw in liquid nitrogen. After another centrifugation (9500× g for 15 min at 4 °C), the supernatant was collected for colorimetric assay. For this purpose, 75 μL of supernatant were added to 75 μL of O-phenylenediamine 1.5 mM (Sigma-Aldrich, St. Louis, MO, USA), diluted in Tris–HCl 0.075 mM (pH 8) plus H2O2 6.6 mM, and incubated at 20 °C for 30 min. Both enzymes’ reactions were stopped by adding 50 μL of H2SO4 1 M (Vetec, Rio de Janeiro, Brazil). The absorbance of the products of the enzymatic assays was measured at 450 nm (MPO) and 492 nm (EPO) on a microplate spectrophotometer (Bio-Rad 450 model, Bio-Rad Laboratories). The results were expressed as MPO or EPO arbitrary units/mg of tissue based on absorbance.
2.6. Gene Expression of Cytokines and Epithelial Barrier Markers
2.6.1. Total RNA Isolation
The profile of cytokines and genetic markers involved with epithelial barrier function was evaluated through gene expression. For this, ileum total RNA isolation was carried out using Pure Link™ RNA Mini Kit (Invitrogen, Carlsbad, CA, USA), according to the recommended protocol. Samples were analyzed using the NanoDrop 2000 spectrophotometer (Thermo Scientific, Waltham, MA, USA) and 1.5 % agarose gel to verify the total RNA concentration and quality. Residual genomic DNA was removed using the Turbo DNA-free™ Kit (Invitrogen, Carlsbad, CA, USA). Complementary DNA was produced with 2 μg of RNA using the High-Capacity cDNA Reverse Transcription kit (Applied Biosystems™, ThermoFisherC, Waltham, MA, USA), according to the recommended protocol.
2.6.2. Quantitative PCR (qPCR)
Quantitative PCR was carried out with the PowerUp™ SYBR® Green Master Mix (ThermoFisher) on the ABI PRISM 7900HT Sequence Detection System (Applied Biosystems™) under the following steps: 95 °C for 10 min, and 40 cycles of 95 °C for 15 s, and 60 °C for 1 min. The Toll-like receptor 2 (Tlr2), nuclear factor-kappa B p105 subunit (Nfkb1), interleukins b (Il1b), 10 (Il10), 12 (Il12), 17-alfa (Il17a), tumor necrosis factor (Tnf), transforming growth factor beta-1 (Tgfb1), mucin 2 (Muc2), claudin 1 (Cldn1), 2 (Cldn2), and 5 (Cldn5), zonulin (Hp), and occludin (Ocln) were used as gene-specific primers (Table 1) [36,37,38,39,40]. Gene expression results were analyzed following the 2−ΔΔCT method using GAPDH (Gapdh) and β-actin (Actb) as endogenous references.

Table 1.
qPCR primer sequences.
2.7. Histological and Morphometric Analysis
The ileum sections were collected for histological analysis, washed with PBS 0.1 M, longitudinally opened, rolled up, and immersed in 10% buffered formaldehyde solution (Neon, Suzano, Brazil) for 24 h until tissue fixation. This material was embedded in paraffin, and sections of 4 μm thickness were stained with hematoxylin and eosin (HE). The histological inflammation score was examined by a pathologist and evaluated according to the Soares et al. [41] method. Histological images were captured by a BX41 optical microscope (Olympus, Tokyo, Japan) with a 20× magnification objective, and the morphometric parameters were evaluated by measuring 20 villi height and 20 crypt depth with the ImageJ 1.51j.8 software (National Institutes of Health, Bethesda, MD, USA).
2.8. Statistical Analysis
The results are presented as the mean ± standard deviation. Data were evaluated by one-way ANOVA followed by Tukey’s post hoc test (parametric data) or by the Kruskal–Wallis test and post-tested by Dunn’s test (nonparametric data). Graphs were generated and data analysis performed using GraphPad Prism 8.0 software (GraphPad Software) and a p-value < 0.05.
3. Results
3.1. Heat-Killed Lactobacillus delbrueckii CIDCA 133 Improved Weight Loss in Chemotherapy-Inflamed Mice
Body weight loss was higher in the MUC (−9.9 ± 0.85%) (Figure 2A) and 5-FU (−7.0 ± 0.68%) (Figure 2D) groups when compared to the NC group (0.00 ± 0.5%) (p < 0.0001) (Figure 2A,D). Treatment with viable (LDv) (−6.6 ± 1.1%) and heat-killed CIDCA 133 (LDi) (−6.5 ± 0.97%) (Figure 2A) improved the weight loss (p < 0.001). However, the cell-free supernatant (LDs) (−5.95 ± 1.51%) had no protective effect on this parameter (p = 0.26) (Figure 2D).
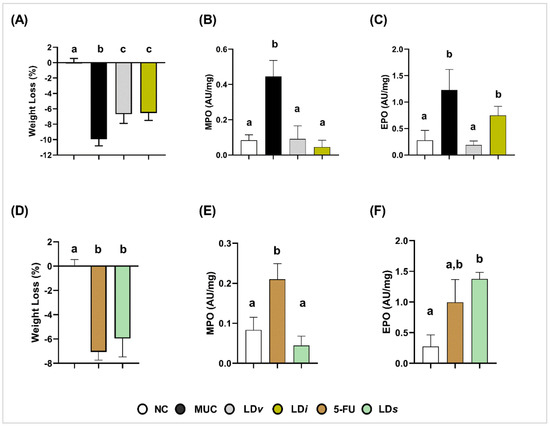
Figure 2.
Protective effect of heat-killed (LDi) and cell-free supernatant (LDs) of L. delbrueckii CIDCA 133 on 5-FU-induced body weight loss and recruitment of polymorphonuclear cells. (A–C) Viable and heat-killed CIDCA 133. (D–F) CIDCA 133 supernatant. Different letters (a, b, c) indicate statistically significant differences (p < 0.05) by ANOVA followed by Tukey’s (A–E) or by Kruskal–Wallis test followed by Dunn’s post hoc test (F).
3.2. Heat-Killed and Cell-Free Supernatant of Lactobacillus delbrueckii CIDCA 133 Reduced Levels of Myeloperoxidase Activity
Inflammatory infiltrates of neutrophils and eosinophils in ileum mucosa were assessed by levels of their respective myeloperoxidase (MPO) and eosinophil peroxidase (EPO) enzymatic activities. High levels of MPO (MUC: 0.44 ± 0.09 AU/mg, Figure 2B; 5-FU: 0.21 ± 0.03 AU/mg, Figure 2E) and EPO (MUC: 1.23 ± 0.38 AU/mg, Figure 2C; 5-FU: 0.99 ± 0.37 AU/mg, Figure 2F) enzymatic activities were detected in the ileum mucosa of inflamed mice when compared to the control group (MPO: 0.08± 0.03 AU/mg; EPO: 0.27 ± 0.19 AU/mg) (Figure 2B,C,E) (p < 0.0001). Treatment with viable CIDCA 133 (LDv) reduced the level activity of both enzymes (MPO: 0.09± 0.07 AU/mg, p < 0.0001; EPO: 0.18 ± 0.07 AU/mg, p < 0.0001) (Figure 2B,C). Positive effects of heat-killed (LDi) (0.09 ± 0.07 AU/mg, p < 0.0001) and cell-free supernatant (LDs) (0.04 ± 0.02 AU/mg, p < 0.0001) were only observed against MPO enzyme levels (Figure 2B,E). No protective effect of heat-killed (LDi) (0.74 ± 0.17 AU/mg, p = 0.06) (Figure 2C) and cell-free supernatant of CIDCA 133 (LDs) (1.37 ± 0.10 AU/mg, p = 0.7130) (Figure 2F) was observed with respect to EPO levels.
3.3. Heat-Killed and Cell-Free Supernatant of Lactobacillus delbrueckii CIDCA 133 Modulated the Gene Expression of Inflammatory Cytokines
The gene expression of pro-inflammatory (Il1b, Il12, Il17a, and Tnf) and anti-inflammatory (Il10 and Tgfb1) cytokines was performed to evaluate the immunoregulatory effects of heat-killed and cell-free supernatant of L. delbrueckii CIDCA 133. Compared to the control group, mice in the MUC group presented an upregulation in the gene expression of Tlr2 (p < 0.001) (Figure 3A), Nfkb1 (p < 0.001) (Figure 3B), Il12 (p < 0.01) (Figure 3C), Il17a (p < 0.001) (Figure 3D), Tnf (p < 0.01) (Figure 3E), Il1b (p < 0.01) (Figure 3F), and Tgfb1 (p < 0.05) (Figure 3H), and a downregulation of the immunoregulatory cytokine Il10 (p < 0.0001) (Figure 3G).
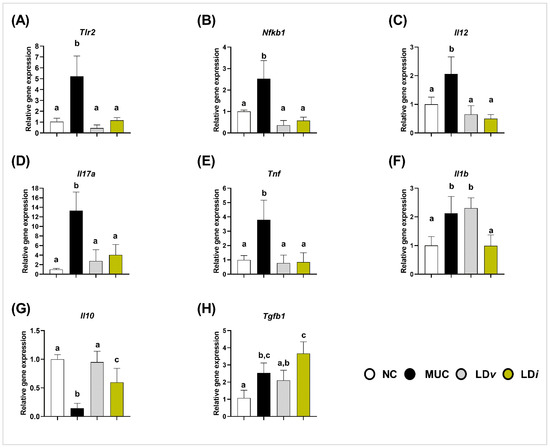
Figure 3.
Heat-killed (LDi) L. delbrueckii CIDCA 133 modulated gene expression of inflammatory cytokines. (A) Tlr2, (B) Nfkb1, (C) Il12, (D) Il17a, (E) Tnf, (F) Il1b, (G) Il10 and (H) Tgfb1. Different letters (a, b, c) indicate statistically significant differences (p < 0.05) by ANOVA followed by Tukey’s post hoc test.
Modulation of these inflammatory markers was observed after treatment with CIDCA 133. Consumption of viable bacteria (LDv) reduced the mRNA expression of Tlr2 (p < 0.0001) (Figure 3A), Nfkb1 (p < 0.0001) (Figure 3B), Il12 (p < 0.001) (Figure 3C), Il17a (p < 0.001) (Figure 3D), Tnf (p < 0.0001) (Figure 3E), and increased Il10 (p < 0.0001) (Figure 3G) when compared to the MUC group. Heat-killed (LDi) treatment downregulated Tlr2 (p < 0.0001) (Figure 3A), Nfkb1 (p < 0.0001) (Figure 3B), Il12 (p < 0.0001) (Figure 3C), Il17a (p < 0.01) (Figure 3D), Tnf (p < 0.0001) (Figure 3E), and Il1b (p < 0.01) (Figure 3F), and upregulated the gene expression of Il10 (p < 0.01) (Figure 3G). No differences were observed between the MUC and LDi groups in Tgfb1 expression (p = 0.112) (Figure 3H).
On the other hand, the 5-FU group exhibited upregulation of Tlr2 (p < 0.0001) (Figure 4A), Nfkb1 (p < 0.001) (Figure 4B), Il17a (p < 0.05) (Figure 4D) and Il1b (p < 0.0001) (Figure 4F), and downregulation of Il10 (p < 0.01) (Figure 4E). Treatment with the cell-free supernatant of CIDCA 133 (LDs) downregulated the gene expression of Tlr2 (p < 0.0001) (Figure 4A), Nfkb1 (p < 0.0001) (Figure 4B), pro-inflammatory cytokines Il12 (p < 0.01) (Figure 4C), and Il17a (p < 0.05) (Figure 4D), and upregulated anti-inflammatory cytokine Il10 (p < 0.01) (Figure 4E) when compared to the 5-FU group. No differences were observed in Il1b, Tnf, and Tgfb1 gene expression between 5-FU and LDs groups (p > 0.05) (Figure 4F–H).
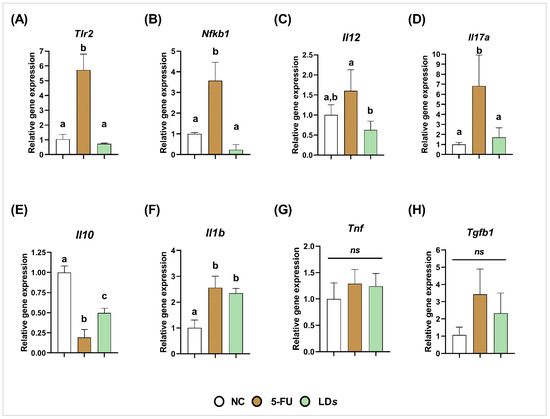
Figure 4.
Cell-free supernatant ((LDs) of L. delbrueckii CIDCA 133 downregulated 5-FU-induced gene expression of pro-inflammatory cytokines. (A) Tlr2, (B) Nfkb1, (C) Il12, (D) Il17a, (E) Il10, (F) Il1b, (G) Tnf and (H) Tgfb1. Different letters (a, b, c) indicate statistically significant differences (p < 0.05) by ANOVA followed by Tukey’s posttest. ns indicates no statistically significant differences.
3.4. Heat-Killed and Cell-Free Supernatant of Lactobacillus delbrueckii CIDCA 133 Regulated Genes Related to Intestinal Epithelial Barrier
The regulatory effect of heat-killed and cell-free supernatant of L. delbrueckii CIDCA 133 on epithelial barrier protection was evaluated through the gene expression of mucin 2 and tight junction proteins (Cldn1, Cldn2, Cldn5, Hp and Ocln). The MUC group exhibited a downregulation in the mRNA expression of Muc2 (p < 0.01) (Figure 5A), Hp (p < 0.0001) (Figure 5B), and Cldn1 (p < 0.0001) (Figure 5D) when compared to the control group. The treatment with viable L. delbrueckii CIDCA 133 (LDv) only upregulated the Cldn5 (p < 0.0001) (Figure 5F) gene expression; however, upregulation of Hp (p < 0.05) (Figure 5B), Ocln (p < 0.01) (Figure 5C), Cldn1 (p < 0.05) (Figure 5D), Cldn2 (p < 0.01) (Figure 5E), and Cldn5 (p < 0.01) (Figure 5F) was observed after heat-killed CIDCA 133 treatment (LDi). No differences were observed in the mRNA expression of Muc2 (Figure 5A) after heat-killed CIDCA 133 treatment and in Hp (Figure 5B), Ocln (Figure 5C), Cldn1 (Figure 5D), and Cldn2 (Figure 5E) after viable CIDCA 133 (LDv) treatment (p > 0.05).
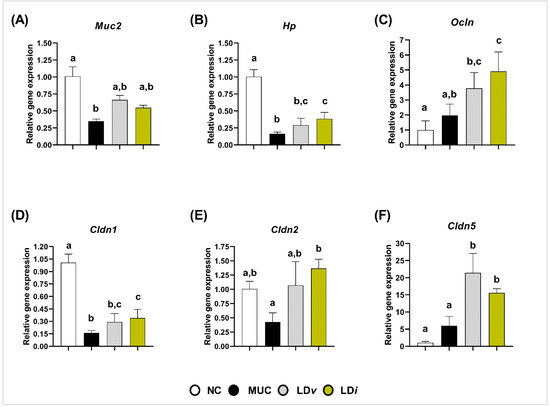
Figure 5.
Heat-killed (LDi) L. delbrueckii CIDCA 133 modulated mRNA expression of markers involved in epithelial barrier function. (A) mucin 2, (B) zonulin, (C) occludin (D) claudin 1, (E) claudin 2 (F) claudin 5. Different letters (a, b, c) indicate statistically significant differences (p < 0.05) by ANOVA followed by Tukey’s posttest (B–D,F) or by Kruskal–Wallis test followed by Dunn’s post hoc test (A,E).
The 5-FU group also exhibited a downregulation in the mRNA expression of Muc2 (p < 0.01) (Figure 6A), Hp (p < 0.0001) (Figure 6B), and Cldn1 (p < 0.0001) (Figure 6D), but upregulated expression of Cldn5 (p < 0.05) (Figure 6F) when compared to the control group. However, after treatment with cell-free supernatant of CIDCA 133 (LDs), an upregulation of mRNA expression of Muc2 (p < 0.01) (Figure 6A), Hp (p < 0.05) (Figure 6B), Ocln (p < 0.01) (Figure 6C), Cldn1 (p < 0.05) (Figure 6D), Cldn2 (p < 0.01) (Figure 6E), and Cldn5 (p < 0.01) (Figure 6F) was observed.
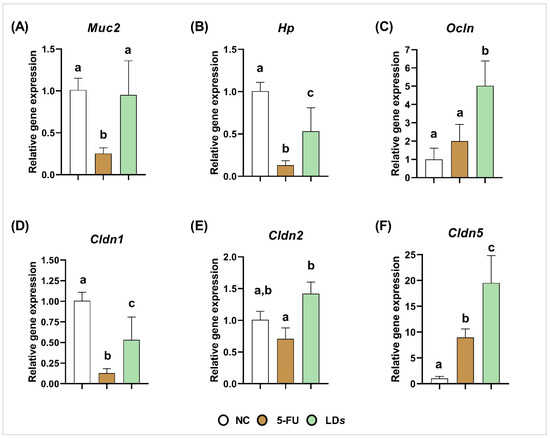
Figure 6.
Cell-free supernatant (LDs) of L. delbrueckii CIDCA 133 modulated mRNA expression of markers involved in epithelial barrier function. (A) mucin 2, (B) zonulin, (C) occludin (D) claudin 1, (E) claudin 2 (F) claudin 5. Different letters (a, b, c) indicate statistically significant differences (p < 0.05) by ANOVA followed by Tukey’s posttest (A–D,F) or by Kruskal–Wallis test followed by Dunn’s post hoc test (E).
3.5. Heat-Killed and Cell-Free Supernatant of Lactobacillus delbrueckii CIDCA 133 Improved Epithelium Intestinal Architecture
Histopathological analysis demonstrated that mice inflamed with 5-Fluorouracil chemotherapy presented intestinal architecture alterations characterized by intense inflammatory cell infiltration into the lamina propria and villi, degeneration of goblet cells, villus shortening, and crypt necrosis when compared to the control group (Figure 7A), corroborating the histopathological score (Figure 7B,C) and morphometric analysis (Figure 7D–I).
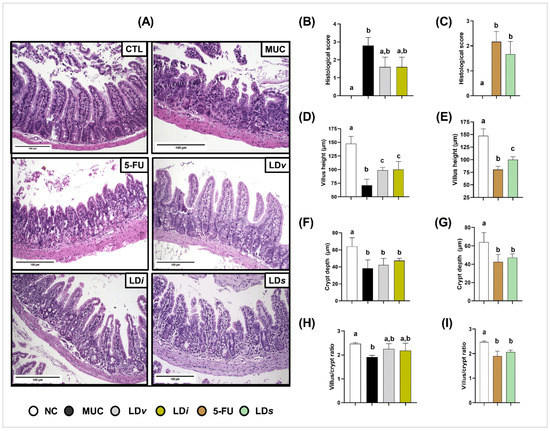
Figure 7.
Heat-killed (LDi) and cell-free supernatant (LDs) of L. delbrueckii CIDCA 133 ameliorated intestinal architecture damage induced by 5-FU administration. (A) Ileum mucosa histopathology images (objective: ×20, scale 100 µm); (B,F) histopathological score; (C,G) villus height; (D,H) crypt depth; and (E,I) villus height to crypt depth ratio, respectively. Different letters (a, b, c) indicate statistically significant differences (p < 0.05) by Kruskal–Wallis test followed by Dunn’s post hoc test (B,F) or ANOVA followed by Tukey’s post hoc test (C–E,G–I).
Treatment with viable (LDv), heat-killed (LDi) (Figure 7D), and cell-free supernatant (LDs) (Figure 7E) of CIDCA 133 had a protective effect on villus shortening and inflammatory cell infiltration into the lamina propria and villus, improving the epithelium architecture of the ileum section (Figure 7A). After consumption of these biotic agents, no beneficial effects were observed in the mice´s histopathological score, crypt depth, or villus/crypt ratio (Figure 7B,C,F–I).
4. Discussion
The anti-inflammatory properties of viable L. delbrueckii CIDCA 133 have been previously reported [29,30,31]. This study evaluated the anti-inflammatory properties of the bacterial inactivation or secreted bioactive products derived from this strain in a 5-Fluorouracil drug-induced mouse intestinal inflammation model.
The mucosal destruction by chemotherapy leads to lower absorption of nutrients, influencing body weight loss [3,41,42]. Our results showed no protective effect against weight loss with the cell-free supernatant of L. delbrueckii CIDCA 133. This lack of protection was possibly due to the removal of small molecules derived from CIDCA 133 after supernatant concentration with Vivaspin 20, thus reducing their interaction with intestinal epithelial cells to provide beneficial properties to the host. These data are similar to the findings of Prisciandaro et al. [33], demonstrating that Escherichia coli Nissle 1917 and Limosilactobacillus fermentum BR11 supernatant did not improve weight loss in mice with intestinal mucositis [33]. On the other hand, the consumption of heat-killed L. delbrueckii CIDCA 133 ameliorated the weight loss in BALB/c mice after 5-FU administration, similarly to viable bacteria, as previously reported by De Jesus et al. [29], and also corroborating with Trindade et al. [43], who reported that heat-killed Lacticaseibacillus rhamnosus CGMCC1.3724 strain influenced weight recovery of 5-FU inflamed mice.
The intestinal architecture alteration resulting from the inflammatory process is one of the most critical features in the pathobiology of chemotherapy-induced mucositis [44,45]. Our results showed that the heat-killed and cell-free supernatants of L. delbrueckii CIDCA 133 improved the ileum epithelium architecture destroyed by 5-FU administration. This protective property can be linked to the ability of these biotics to attenuate villus shortening, upregulate the immunoregulatory cytokine Il10 at the mRNA level, and reduce the inflammatory markers (e.g., Tlr2, Nfkb1, pro-inflammatory cytokines Il1b, Il12, Il17a, Tnf, and neutrophil infiltrates). These findings are supported by other studies in which administration either of inactivated Lacticaseibacillus rhamnosus CGMCC1.3724 [43], Lactiplantibacillus plantarum Zhang-LL [28], and VSL#3 therapy composed of Bifidobacterium breve, B. longum, B. infantis, L. plantarum, Lb. bulgaricus, L. casei, L. acidophilus, and Streptococcus salivarius [46], or secreted products derived from beneficial microorganisms, such as supernatant of mulberry leaf extract fermented by L. acidophilus A4 [47] and supernatant of Faecalibacterium prausnitzii [27], ameliorated the intestinal inflammatory process through modulation of inflammatory markers, such as myeloperoxidase activity levels, Tregs CD4+ Foxp3+ cells, pro-inflammatory cytokines IL1β, IFNγ, IL17A, TNFα, and anti-inflammatory IL10 and IL4 cytokines, thus restoring the intestinal homeostasis.
Interestingly, our results show that the transcripts levels of immunoregulatory cytokine TGFβ1 were upregulated after 5-FU administration and maintained after viable and heat-inactivated CIDCA 133 administration. Furthermore, we only observed a reduction in eosinophil inflammatory infiltrate with viable CIDCA 133 treatment. The protective effect on this parameter was not observed with the paraprobiotic and postbiotic forms of CIDCA 133.
TGFβ1 is an abundant cytokine in the intestinal mucosa with pleiotropic effects. The anti-inflammatory or pro-inflammatory property of this immunoregulatory factor occurs through cellular and environmentally dependent contexts [48,49]. Increased TGFβ levels were observed in the 5-FU-induced inflammation model [50,51,52]. On the other hand, IL10 cytokine levels were reduced [30,53,54,55]. Evidence shows that in the absence/reduction of IL10 cytokine in the intestinal inflammation context, levels of TGFβ1 are increased [19,56]. Thus, we suggest that, due to the reduction of IL10 transcript levels, paraprobiotics and postbiotics of CIDCA 133 maintain the mRNA level of the TGFβ1 cytokine as a compensatory anti-inflammatory mechanism to control tissue damage and restore gut homeostasis disrupted by 5-Fluorouracil chemotherapy.
Regarding infiltrating eosinophils, these cells reside in the intestine, in both normal and inflammatory states [57]. Pieces of evidence show that eosinophil activation and migration can be regulated by microbiota metabolites (e.g., SCFA) [58]. Thus, we believed that SCFA production and microbiota interaction might be a mechanism used by CIDCA 133 in its beneficial effect, suggesting that its viability is necessary to reduce 5-FU-induced eosinophil infiltration in the ileum mucosa.
The anti-inflammatory profile of heat-inactivated and cell-free supernatant of L. delbrueckii CIDCA 133 was, at least in part, similar to that of its viable form. We believe that these results can be linked to extracellular molecules and cell surface-associated components (e.g., LtaS, SLAP, HtrA, MucBP, and PrtB) identified in the strain genome [31] since these genetic factors interacted the most with human immune protein receptors associated with NF-κB signaling pathway regulation, including NFKB1 protein. These molecular markers were also associated as possibly genetic factors of viable CIDCA 133 involved with its anti-inflammatory properties through the upregulation of immunoregulatory Tgfb1 and Il10 cytokines and downregulation of Nfkb1 gene expression in vivo [30,31].
Cell surface-associated components or extracellular molecules (e.g., surface layer proteins, lipoteichoic acids, exopolysaccharides) can stimulate the immune system of the host through their interaction with intestinal epithelial cells via specific receptors such as Toll-like (TLRs) and NOD receptors (NLRs), thus regulating the balance between the anti-inflammatory and pro-inflammatory responses induced by different signaling pathways, such as NF-κB and MAPK [9,59]. Immunoregulation promoted by these bacterial factors has been previously reported by Chandhni et al. [60], who showed that the extractable surface proteins derived from Lactiplantibacillus plantarum MTCC 5690, Lactobacillus acidophilus NCFM and Limosilactobacillus fermentum MTCC 5689 ameliorated DSS or TNBS-induced acute intestinal inflammation by increasing immunoregulatory IL10 cytokine and decreasing leukocyte infiltration, TNFα, and IFNγ pro-inflammatory markers [60]. Similar findings were also reported by Deutsch et al. [61] when demonstrating that surface proteins, such as SlpB and SlpE, are the biological agents responsible for the anti-inflammatory properties of Propionibacterium freudenreichii strains. These proteins increased the production of the immunoregulatory cytokine IL10 and modulated pro-inflammatory cytokine TNFα, IFNγ, and IL6 responses of human PBMCs cells [61]. Anti-inflammatory properties of extracellular polysaccharides of L. delbrueckii TUA4408L were also reported. These bacterial components attenuated enterotoxigenic Escherichia coli-induced inflammatory response in porcine intestinal epitheliocytes by regulating TLR2/4 receptors and MAPK or NF-κB pathway activation and decreasing IL6, IL8, and monocyte chemoattractant protein-1 (MCP-1) levels [59].
It is also interesting to note that, despite the literature demonstrating an anti-inflammatory profile attributed to the probiotic-derived immune molecular effectors, there is evidence that some of these bioactive factors, such as the B7 peptides derived from the probiotic Bifidobacterium longum, can exacerbate the intestinal inflammatory process by increasing pro-inflammatory markers and immune cell activation (e.g., circulating antigen-presenting cells) [62]. Thus, these findings show that bioactive bacterial factors can elicit differential immune mechanisms depending on the context of the progression stage (early or late) of the inflammation [62]. In this context, despite surface-exposed components or secreted bioactive molecules appearing to be essential for leading the anti-inflammatory process in the host by CIDCA 133, further studies should be conducted to characterize the cytotoxic/pro-inflammatory profiles of these CIDCA 133-derived molecular components or metabolites, especially in severe inflammation models.
The 5-FU chemotherapy-induced intestinal inflammation also leads to loss of epithelial barrier integrity via the depletion of tight junction proteins (TJs), reduction in goblet cell numbers, and increased intestinal permeability [37,45,53]. TJs proteins are part of an interconnected network of adhesion complexes that act as selective barriers between internal and external cellular environments, thus controlling the passage of pathogens and other foreign molecules [63,64]. The reduction in mRNA expression of mucin 2 and tight junction proteins zonulin, claudin 1, and claudin 2 observed in the inflamed mice was attenuated by both heat-killed and cell-free supernatants of L. delbrueckii CIDCA 133. These results are consistent with data obtained by De Jesus et al. [29] and Barroso et al. [30], who demonstrated that viable L. delbrueckii CIDCA 133 reduced intestinal permeability and goblet cell degeneration and upregulated the gene expression of tight junction proteins (Cldn1, Fr11, Hp) altered by 5-FU chemotherapy, thus being essential to reduce the translocation of toxins and pathogenic bacteria, and consequently the inflammation amplification process in the intestinal mucosa [45].
The improvement in levels of mucins and tight junction proteins after treatment with heat-inactivated beneficial microorganisms and their supernatants has been previously reported. For example, mulberry leaf extract supernatant fermented by L. acidophilus upregulated the gene expression of Muc2 and Muc5AC in 5-FU-inflamed mice [47]. Furthermore, secreted factors of E. coli Nissle 1917 attenuated the epithelial barrier disruption induced by enteropathogenic E. coli in Caco-2 cells by enhancing the gene expression of tight junction proteins zonulin-1, claudin-14, and claudin-2 [65]. Trindade et al. [43] also reported that paraprobiotics L. rhamnosus CGMCC1.3724 reduced intestinal permeability induced by 5-FU and enhanced Muc2 gene expression. Promising results were also reported with inactivated B. longum K2-21-4, which modulated the gene expression of claudin-1, zonulin-1, and occludin disrupted by lipopolysaccharide (LPS) in colon epithelial cells [66].
Claudins are the essential transmembrane proteins participating in complex strand networks that regulate the paracellular permeability and maintain the intestinal mucosal barrier function [63,67,68]. The discrepancies in these proteins’ expressions may be related to their different interactions with other membrane compartments [63]. Surprisingly, our results showed that 5-FU administration increased the gene expression of claudin-5, contrary to other studies [9,30,37,45,53,69,70]. One possible explanation for this outcome is that this protein was upregulated as a compensatory response due to decreased expression of other tight junction proteins caused by 5-FU. A similar outcome was observed by Li et al. [45], who demonstrated that 5-FU enhanced the expression levels of tight junction ZO-1 and adhesion molecules such as JAM-A while reducing colonic occludin levels. Another piece of evidence indicates that upregulation of claudin-5 can be a mechanism to accelerate intestinal epithelial differentiation to maintain epithelial homeostasis [71].
Based on our results and the pathobiology of mucositis proposed by Sonis [3], we believe that a proposed mechanism used for paraprobiotics CIDCA 133 and its postbiotics to reduce intestinal mucosal inflammation and reinforce the epithelial barrier occurs due to surface-associated components or secreted molecules able to interact with intestinal epithelial cells via specific pattern recognition receptors (PRRs), mainly TLR receptors. This process would control the dysbiotic intestinal microbiota and stimulate goblet cells to secrete mucin, reinforcing the epithelial barrier. On the other hand, the activation of these epithelial cell receptors by these biotics would induce intestinal immune cells to produce the immunoregulatory IL10 cytokine to control activation of the NF-κB signaling pathway and, consequently, regulate the balance between anti-inflammatory and pro-inflammatory immune responses. This process would reduce the migration of inflammatory cells to the mucosa, with subsequent reduction of oxidative stress generated by these cells. All the above processes would decrease tissue damage, reduce the disruption of the tight junction proteins, improve villus length due to migration of enterocytes produced by stem cells in crypts, and, thus, contribute to intestinal architecture recovery and homeostasis (Figure 8). However, we emphasize that more studies should be carried out to better elucidate the immunological, molecular, and cellular mechanisms of action of paraprobiotics and postbiotics from CIDCA 133 in intestinal mucositis and other inflammation models, including knock-out gene mouse models, proteomics, SCFA analysis, microbiota regulation, evaluation of cytokine levels, and activation of immune regulatory cells.
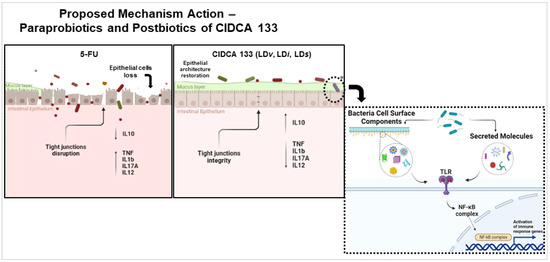
Figure 8.
Proposed mechanism of action of paraprobiotics and postbiotics of L. delbrueckii CIDCA 133 in intestinal mucositis induced by 5-FU chemotherapy.
5. Conclusions
Altogether, our results demonstrated that heat-killed and cell-free supernatants of the Lactobacillus delbrueckii CIDCA 133 strain protected the intestinal mucosa from epithelial damage caused by the 5-FU drug. These ameliorative effects were detectable morphologically and had a profile similar to that of the viable form of CIDCA 133. Modulation of inflammatory parameters and epithelial barrier dysfunction was the primary mechanism used by these biotics to protect the intestinal mucosa from epithelial destruction caused by the 5-FU drug, suggesting them to be an attractive alternative therapeutic approach against intestinal damage induced by chemotherapy and to the problems arising from the use of live beneficial microorganisms in clinical practice.
Author Contributions
Conceptualization, V.L.B., L.C.L.D.J., P.M-A., M.M.D. and V.A.; methodology, V.L.B., L.C.L.D.J., L.M.T., F.L.A.B., L.J.d.S.F., A.d.S.F., J.G.L., M.F.A. and E.F.; formal analysis, V.L.B., L.C.L.D.J., E.F; data curation, V.L.B. and L.C.L.D.J.; writing—original draft preparation, V.L.B., L.C.L.D.J. and J.G.L.; writing—review and editing, L.C.L.D.J., P.M.-A., M.M.D., L.C.J.A. and V.A.; funding acquisition, V.A.; supervision, V.A; All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), grant number 312045/2020-4.
Institutional Review Board Statement
The animal study protocol was approved by the Local Animal Experimentation Ethics Committee of Federal University of Minas Gerais (CEUA-UFMG, Protocol n◦ 112/2020).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available by contacting the corresponding author.
Acknowledgments
We would like to acknowledge the CIDCA 133 center (Center for Research and Development in Food Cryotechnology) and Pablo F. Pérez of the National University of La Plata, Argentine, for bacterial strain supply.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Maria, O.M.; Eliopoulos, N.; Muanza, T. Radiation-Induced Oral Mucositis. Front. Oncol. 2017, 7, 89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, L.; Li, W.; Chen, L.; Su, Q.; Wang, Y.; Guo, Z.; Lu, Y.; Liu, B.; Qin, S. Radiation-Induced Intestinal Damage: Latest Molecular and Clinical Developments. Futur. Oncol. 2019, 15, 4105–4118. [Google Scholar] [CrossRef] [PubMed]
- Sonis, S.T. The Pathobiology of Mucositis. Nat. Rev. Cancer 2004, 4, 277–284. [Google Scholar] [CrossRef]
- Bowen, J.; Al-Dasooqi, N.; Bossi, P.; Wardill, H.; Van Sebille, Y.; Al-Azri, A.; Bateman, E.; Correa, M.E.; Raber-Durlacher, J.; Kandwal, A.; et al. The Pathogenesis of Mucositis: Updated Perspectives and Emerging Targets. Support. Care Cancer 2019, 27, 4023–4033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peterson, D.E.; Jones, J.B.; Petit, R.G. Randomized, Placebo-Controlled Trial of Saforis for Prevention and Treatment of Oral Mucositis in Breast Cancer Patients Receiving Anthracycline-Based Chemotherapy. Cancer 2007, 109, 322–331. [Google Scholar] [CrossRef]
- Elad, S.; Cheng, K.K.F.; Lalla, R.V.; Yarom, N.; Hong, C.; Logan, R.M.; Bowen, J.; Gibson, R.; Saunders, D.P.; Zadik, Y.; et al. MASCC/ISOO Clinical Practice Guidelines for the Management of Mucositis Secondary to Cancer Therapy. Cancer 2020, 126, 4423–4431. [Google Scholar] [CrossRef]
- Rosenthal, D.I. Consequences of Mucositis-Induced Treatment Breaks and Dose Reductions on Head and Neck Cancer Treatment Outcomes. J. Support. Oncol. 2007, 5, 23–31. [Google Scholar]
- Dahlgren, D.; Sjöblom, M.; Hellström, P.M.; Lennernäs, H. Chemotherapeutics-Induced Intestinal Mucositis: Pathophysiology and Potential Treatment Strategies. Front. Pharmacol. 2021, 12, 681417. [Google Scholar] [CrossRef]
- Van Vliet, M.J.; Harmsen, H.J.M.; de Bont, E.S.J.M.; Tissing, W.J.E. The Role of Intestinal Microbiota in the Development and Severity of Chemotherapy-Induced Mucositis. PLoS Pathog. 2010, 6, e1000879. [Google Scholar] [CrossRef] [Green Version]
- Ashaolu, T.J.; Fernández-Tomé, S. Gut Mucosal and Adipose Tissues as Health Targets of the Immunomodulatory Mechanisms of Probiotics. Trends Food Sci. Technol. 2021, 112, 764–779. [Google Scholar] [CrossRef]
- Santos Rocha, C.; Lakhdari, O.; Blottière, H.M.; Blugeon, S.; Sokol, H.; Bermu’dez-Humara’n, L.G.; Azevedo, V.; Miyoshi, A.; Doré, J.; Langella, P.; et al. Anti-Inflammatory Properties of Dairy Lactobacilli. Inflamm. Bowel Dis. 2012, 18, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Petrof, E.O.; Kojima, K.; Ropeleski, M.J.; Musch, M.W.; Tao, Y.; De Simone, C.; Chang, E.B. Probiotics Inhibit Nuclear Factor-ΚB and Induce Heat Shock Proteins in Colonic Epithelial Cells through Proteasome Inhibition. Gastroenterology 2004, 127, 1474–1487. [Google Scholar] [CrossRef] [PubMed]
- Justino, P.F.C.; Franco, A.X.; Pontier-Bres, R.; Monteiro, C.E.S.; Barbosa, A.L.R.; Souza, M.H.L.P.; Czerucka, D.; Soares, P.M.G. Modulation of 5-Fluorouracil Activation of Toll-like/MyD88/NF-ΚB/MAPK Pathway by Saccharomyces boulardii CNCM I-745 Probiotic. Cytokine 2020, 125, 154791. [Google Scholar] [CrossRef] [PubMed]
- Plaza-Diaz, J.; Ruiz-Ojeda, F.J.; Gil-Campos, M.; Gil, A. Mechanisms of Action of Probiotics. Adv. Nutr. 2019, 10, S49–S66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batista, V.L.; da Silva, T.F.; de Jesus, L.C.L.; Coelho-Rocha, N.D.; Barroso, F.A.L.; Tavares, L.M.; Azevedo, V.; Mancha-Agresti, P.; Drumond, M.M. Probiotics, Prebiotics, Synbiotics, and Paraprobiotics as a Therapeutic Alternative for Intestinal Mucositis. Front. Microbiol. 2020, 11, 544490. [Google Scholar] [CrossRef]
- Cereda, E.; Caraccia, M.; Caccialanza, R. Probiotics and Mucositis. Curr. Opin. Clin. Nutr. Metab. Care 2018, 21, 399–404. [Google Scholar] [CrossRef]
- Bian, X.; Wu, W.; Yang, L.; Lv, L.; Wang, Q.; Li, Y.; Ye, J.; Fang, D.; Wu, J.; Jiang, X.; et al. Administration of Akkermansia muciniphila Ameliorates Dextran Sulfate Sodium-Induced Ulcerative Colitis in Mice. Front. Microbiol. 2019, 10, 2259. [Google Scholar] [CrossRef] [Green Version]
- Sokol, H.; Pigneur, B.; Watterlot, L.; Lakhdari, O.; Bermúdez-Humarán, L.G.; Gratadoux, J.-J.; Blugeon, S.; Bridonneau, C.; Furet, J.-P.; Corthier, G.; et al. Faecalibacterium prausnitzii Is an Anti-Inflammatory Commensal Bacterium Identified by Gut Microbiota Analysis of Crohn Disease Patients. Proc. Natl. Acad. Sci. USA 2008, 105, 16731–16736. [Google Scholar] [CrossRef] [Green Version]
- Santos Rocha, C.; Gomes-Santos, A.C.; Garcias Moreira, T.; de Azevedo, M.; Diniz Luerce, T.; Mariadassou, M.; Longaray Delamare, A.P.; Langella, P.; Maguin, E.; Azevedo, V.; et al. Local and Systemic Immune Mechanisms Underlying the Anti-Colitis Effects of the Dairy Bacterium Lactobacillus delbrueckii. PLoS ONE 2014, 9, e85923. [Google Scholar] [CrossRef]
- Quintanilha, M.F.; Miranda, V.C.; Souza, R.O.; Gallotti, B.; Cruz, C.; Santos, E.A.; Alvarez-Leite, J.I.; Jesus, L.C.L.; Azevedo, V.; Trindade, L.M.; et al. Bifidobacterium longum Subsp. longum 51A Attenuates Intestinal Injury against Irinotecan-Induced Mucositis in Mice. Life Sci. 2022, 289, 120243. [Google Scholar] [CrossRef]
- Koyama, S.; Fujita, H.; Shimosato, T.; Kamijo, A.; Ishiyama, Y.; Yamamoto, E.; Ishii, Y.; Hattori, Y.; Hagihara, M.; Yamazaki, E.; et al. Septicemia from Lactobacillus rhamnosus GG, from a Probiotic Enriched Yogurt, in a Patient with Autologous Stem Cell Transplantation. Probiotics Antimicrob. Proteins 2019, 11, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Yelin, I.; Flett, K.B.; Merakou, C.; Mehrotra, P.; Stam, J.; Snesrud, E.; Hinkle, M.; Lesho, E.; McGann, P.; McAdam, A.J.; et al. Genomic and Epidemiological Evidence of Bacterial Transmission from Probiotic Capsule to Blood in ICU Patients. Nat. Med. 2019, 25, 1728–1732. [Google Scholar] [CrossRef] [PubMed]
- Pasala, S.; Singer, L.; Arshad, T.; Roach, K. Lactobacillus Endocarditis in a Healthy Patient with Probiotic Use. IDCases 2020, 22, e00915. [Google Scholar] [CrossRef]
- D’Agostin, M.; Squillaci, D.; Lazzerini, M.; Barbi, E.; Wijers, L.; Da Lozzo, P. Invasive Infections Associated with the Use of Probiotics in Children: A Systematic Review. Children 2021, 8, 924. [Google Scholar] [CrossRef]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef] [PubMed]
- Teame, T.; Wang, A.; Xie, M.; Zhang, Z.; Yang, Y.; Ding, Q.; Gao, C.; Olsen, R.E.; Ran, C.; Zhou, Z. Paraprobiotics and Postbiotics of Probiotic Lactobacilli, Their Positive Effects on the Host and Action Mechanisms: A Review. Front. Nutr. 2020, 7, 570344. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Xu, H.; Xu, J.; Guo, X.; Zhao, H.; Chen, Y.; Zhou, Y.; Nie, Y.F. prausnitzii and Its Supernatant Increase SCFAs-Producing Bacteria to Restore Gut Dysbiosis in TNBS-Induced Colitis. AMB Express 2021, 11, 33. [Google Scholar] [CrossRef]
- Jin, J.; Wu, S.; Xie, Y.; Liu, H.; Gao, X.; Zhang, H. Live and Heat-Killed Cells of Lactobacillus plantarum Zhang-LL Ease Symptoms of Chronic Ulcerative Colitis Induced by Dextran Sulfate Sodium in Rats. J. Funct. Foods 2020, 71, 103994. [Google Scholar] [CrossRef]
- De Jesus, L.C.L.; Drumond, M.M.; de Carvalho, A.; Santos, S.S.; Martins, F.S.; Ferreira, Ê.; Fernandes, R.S.; de Barros, A.L.B.; do Carmo, F.L.R.; Perez, P.F.; et al. Protective Effect of Lactobacillus delbrueckii Subsp. lactis CIDCA 133 in a Model of 5 Fluorouracil-Induced Intestinal Mucositis. J. Funct. Foods 2019, 53, 197–207. [Google Scholar] [CrossRef]
- Barroso, F.A.L.; de Jesus, L.C.L.; da Silva, T.F.; Batista, V.L.; Laguna, J.; Coelho-Rocha, N.D.; Vital, K.D.; Fernandes, S.O.A.; Cardoso, V.N.; Ferreira, E.; et al. Lactobacillus delbrueckii CIDCA 133 Ameliorates Chemotherapy-Induced Mucositis by Modulating Epithelial Barrier and TLR2/4/Myd88/NF-ΚB Signaling Pathway. Front. Microbiol. 2022, 13, 858036. [Google Scholar] [CrossRef]
- De Jesus, L.C.L.; Drumond, M.M.; Aburjaile, F.F.; de Jesus Sousa, T.; Coelho-Rocha, N.D.; Profeta, R.; Brenig, B.; Mancha-Agresti, P.; Azevedo, V. Probiogenomics of Lactobacillus delbrueckii Subsp. lactis CIDCA 133: In Silico, In Vitro, and In Vivo Approaches. Microorganisms 2021, 9, 829. [Google Scholar] [CrossRef] [PubMed]
- De Jesus, L.C.L.; de Jesus Sousa, T.; Coelho-Rocha, N.D.; Profeta, R.; Barroso, F.A.L.; Drumond, M.M.; Mancha-Agresti, P.; Ferreira, Ê.; Brenig, B.; Aburjaile, F.F.; et al. Safety Evaluation of Lactobacillus delbrueckii Subsp. lactis CIDCA 133: A Health-Promoting Bacteria. Probiotics Antimicrob. Proteins 2021, in press. [Google Scholar] [CrossRef] [PubMed]
- Prisciandaro, L.D.; Geier, M.S.; Butler, R.N.; Cummins, A.G.; Howarth, G.S. Probiotic Factors Partially Improve Parameters of 5-Fluorouracil-Induced Intestinal Mucositis in Rats. Cancer Biol. Ther. 2011, 11, 671–677. [Google Scholar] [CrossRef] [Green Version]
- Souza, D.G.; Cara, D.C.; Cassali, G.D.; Coutinho, S.F.; Silveira, M.R.; Andrade, S.P.; Poole, S.P.; Teixeira, M.M. Effects of the PAF Receptor Antagonist UK74505 on Local and Remote Reperfusion Injuries Following Ischaemia of the Superior Mesenteric Artery in the Rat. Br. J. Pharmacol. 2000, 131, 1800–1808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strath, M.; Warren, D.J.; Sanderson, C.J. Detection of Eosinophils Using an Eosinophil Peroxidase Assay. Its Use as an Assay for Eosinophil Differentiation Factors. J. Immunol. Methods 1985, 83, 209–215. [Google Scholar] [CrossRef]
- Giulietti, A.; Overbergh, L.; Valckx, D.; Decallonne, B.; Bouillon, R.; Mathieu, C. An Overview of Real-Time Quantitative PCR: Applications to Quantify Cytokine Gene Expression. Methods 2001, 25, 386–401. [Google Scholar] [CrossRef] [Green Version]
- Song, M.-K.; Park, M.-Y.; Sung, M.-K. 5-Fluorouracil-Induced Changes of Intestinal Integrity Biomarkers in BALB/C Mice. J. Cancer Prev. 2013, 18, 322–329. [Google Scholar] [CrossRef] [Green Version]
- Volynets, V.; Rings, A.; Bárdos, G.; Ostaff, M.J.; Wehkamp, J.; Bischoff, S.C. Intestinal Barrier Analysis by Assessment of Mucins, Tight Junctions, and α-Defensins in Healthy C57BL/6J and BALB/CJ Mice. Tissue Barriers 2016, 4, e1208468. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.-W.; Lee, H.-C.; Li, L.-H.; Chiang Chiau, J.-S.; Wang, T.-E.; Chuang, W.-H.; Chen, M.-J.; Wang, H.-Y.; Shih, S.-C.; Liu, C.-Y.; et al. Fecal Microbiota Transplantation Prevents Intestinal Injury, Upregulation of Toll-Like Receptors, and 5-Fluorouracil/Oxaliplatin-Induced Toxicity in Colorectal Cancer. Int. J. Mol. Sci. 2020, 21, 386. [Google Scholar] [CrossRef] [Green Version]
- Zheng, L.; Zhang, Y.-L.; Dai, Y.-C.; Chen, X.; Chen, D.-L.; Dai, Y.-T.; Tang, Z.-P. Jianpi Qingchang Decoction Alleviates Ulcerative Colitis by Inhibiting Nuclear Factor-ΚB Activation. World J. Gastroenterol. 2017, 23, 1180. [Google Scholar] [CrossRef]
- Soares, P.M.G.; Mota, J.M.S.C.; Gomes, A.S.; Oliveira, R.B.; Assreuy, A.M.S.; Brito, G.A.C.; Santos, A.A.; Ribeiro, R.A.; Souza, M.H.L.P. Gastrointestinal Dysmotility in 5-Fluorouracil-Induced Intestinal Mucositis Outlasts Inflammatory Process Resolution. Cancer Chemother. Pharmacol. 2008, 63, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Gan, Y.; Ai, G.; Wu, J.; Luo, H.; Chen, L.; Huang, Q.; Wu, X.; Xu, N.; Li, M.; Su, Z.; et al. Patchouli Oil Ameliorates 5-Fluorouracil-Induced Intestinal Mucositis in Rats via Protecting Intestinal Barrier and Regulating Water Transport. J. Ethnopharmacol. 2020, 250, 112519. [Google Scholar] [CrossRef] [PubMed]
- Trindade, L.M.; Torres, L.; Matos, I.D.; Miranda, V.C.; de Jesus, L.C.L.; Cavalcante, G.; de Souza Oliveira, J.J.; Cassali, G.D.; Mancha-Agresti, P.; de Carvalho Azevedo, V.A.; et al. Paraprobiotic Lacticaseibacillus rhamnosus Protects Intestinal Damage in an Experimental Murine Model of Mucositis. Probiotics Antimicrob. Proteins 2021, in press. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-T.; Ho, T.-Y.; Lin, H.; Liang, J.-A.; Huang, H.-C.; Li, C.-C.; Lo, H.-Y.; Wu, S.-L.; Huang, Y.-F.; Hsiang, C.-Y. 5-Fluorouracil Induced Intestinal Mucositis via Nuclear Factor-ΚB Activation by Transcriptomic Analysis and In Vivo Bioluminescence Imaging. PLoS ONE 2012, 7, e31808. [Google Scholar] [CrossRef] [Green Version]
- Li, H.-L.; Lu, L.; Wang, X.-S.; Qin, L.-Y.; Wang, P.; Qiu, S.-P.; Wu, H.; Huang, F.; Zhang, B.-B.; Shi, H.-L.; et al. Alteration of Gut Microbiota and Inflammatory Cytokine/Chemokine Profiles in 5-Fluorouracil Induced Intestinal Mucositis. Front. Cell. Infect. Microbiol. 2017, 7, 455. [Google Scholar] [CrossRef]
- Sang, L.-X.; Chang, B.; Dai, C.; Gao, N.; Liu, W.-X.; Jiang, M. Heat-Killed VSL#3 Ameliorates Dextran Sulfate Sodium (DSS)-Induced Acute Experimental Colitis in Rats. Int. J. Mol. Sci. 2013, 15, 15–28. [Google Scholar] [CrossRef]
- Oh, N.S.; Lee, J.Y.; Lee, J.M.; Lee, K.W.; Kim, Y. Mulberry Leaf Extract Fermented with Lactobacillus acidophilus A4 Ameliorates 5-Fluorouracil-Induced Intestinal Mucositis in Rats. Lett. Appl. Microbiol. 2017, 64, 459–468. [Google Scholar] [CrossRef]
- Li, M.O.; Wan, Y.Y.; Sanjabi, S.; Robertson, A.-K.L.; Flavell, R.A. Transforming Growth Factor-β Regulation of Immune Responses. Annu. Rev. Immunol. 2006, 24, 99–146. [Google Scholar] [CrossRef]
- Konkel, J.E.; Chen, W. Balancing Acts: The Role of TGF-β in the Mucosal Immune System. Trends Mol. Med. 2011, 17, 668–676. [Google Scholar] [CrossRef] [Green Version]
- Al-Khrashi, L.A.; Badr, A.M.; AL-Amin, M.A.; Mahran, Y.F. Thymol Ameliorates 5-fluorouracil-induced Intestinal Mucositis: Evidence of Down-regulatory Effect on TGF-β/MAPK Pathways through NF-κB. J. Biochem. Mol. Toxicol. 2022, 36, e22932. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, J.H.; Moon, W.; Park, J.; Park, S.J.; Song, G.A.; Han, S.H.; Lee, J.H. Rebamipide Attenuates 5-Fluorouracil-Induced Small Intestinal Mucositis in a Mouse Model. Biol. Pharm. Bull. 2015, 38, 179–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbosa, M.M.; de Araújo, A.A.; de Araújo Júnior, R.F.; Guerra, G.C.B.; de Castro Brito, G.A.; Leitão, R.C.; Ribeiro, S.B.; de Aragão Tavares, E.; Vasconcelos, R.C.; Garcia, V.B.; et al. Telmisartan Modulates the Oral Mucositis Induced by 5-Fluorouracil in Hamsters. Front. Physiol. 2018, 9, 1204. [Google Scholar] [CrossRef] [PubMed]
- Barroso, F.A.L.; de Jesus, L.C.L.; de Castro, C.P.; Batista, V.L.; Ferreira, Ê.; Fernandes, R.S.; de Barros, A.L.B.; Leclerq, S.Y.; Azevedo, V.; Mancha-Agresti, P.; et al. Intake of Lactobacillus delbrueckii (PExu:Hsp65) Prevents the Inflammation and the Disorganization of the Intestinal Mucosa in a Mouse Model of Mucositis. Microorganisms 2021, 9, 107. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Gan, Y.; Li, M.; Chen, L.; Liang, J.; Zhuo, J.; Luo, H.; Xu, N.; Wu, X.; Wu, Q.; et al. Patchouli Alcohol Attenuates 5-Fluorouracil-Induced Intestinal Mucositis via TLR2/MyD88/NF-KB Pathway and Regulation of Microbiota. Biomed. Pharmacother. 2020, 124, 109883. [Google Scholar] [CrossRef]
- Zhang, L.; Jin, Y.; Peng, J.; Chen, W.; Lisha, L.; Lin, J. Qingjie Fuzheng Granule Attenuates 5-Fluorouracil-Induced Intestinal Mucosal Damage. Biomed. Pharmacother. 2019, 118, 109223. [Google Scholar] [CrossRef]
- Gomes-Santos, A.C.; de Oliveira, R.P.; Moreira, T.G.; Castro-Junior, A.B.; Horta, B.C.; Lemos, L.; de Almeida, L.A.; Rezende, R.M.; Cara, D.C.; Oliveira, S.C.; et al. Hsp65-Producing Lactococcus lactis Prevents Inflammatory Intestinal Disease in Mice by IL-10- and TLR2-Dependent Pathways. Front. Immunol. 2017, 8, 30. [Google Scholar] [CrossRef] [Green Version]
- Rosenberg, H.F.; Masterson, J.C.; Furuta, G.T. Eosinophils, Probiotics, and the Microbiome. J. Leukoc. Biol. 2016, 100, 881–888. [Google Scholar] [CrossRef]
- Theiler, A.; Bärnthaler, T.; Platzer, W.; Richtig, G.; Peinhaupt, M.; Rittchen, S.; Kargl, J.; Ulven, T.; Marsh, L.M.; Marsche, G.; et al. Butyrate Ameliorates Allergic Airway Inflammation by Limiting Eosinophil Trafficking and Survival. J. Allergy Clin. Immunol. 2019, 144, 764–776. [Google Scholar] [CrossRef] [Green Version]
- Wachi, S.; Kanmani, P.; Tomosada, Y.; Kobayashi, H.; Yuri, T.; Egusa, S.; Shimazu, T.; Suda, Y.; Aso, H.; Sugawara, M.; et al. Lactobacillus delbrueckii TUA4408L and Its Extracellular Polysaccharides Attenuate Enterotoxigenic Escherichia coli- Induced Inflammatory Response in Porcine Intestinal Epitheliocytes via Toll-like Receptor-2 and 4. Mol. Nutr. Food Res. 2014, 58, 2080–2093. [Google Scholar] [CrossRef]
- Chandhni, P.R.; Pradhan, D.; Sowmya, K.; Gupta, S.; Kadyan, S.; Choudhary, R.; Gupta, A.; Gulati, G.; Mallappa, R.H.; Kaushik, J.K.; et al. Ameliorative Effect of Surface Proteins of Probiotic Lactobacilli in Colitis Mouse Models. Front. Microbiol. 2021, 12, 679773. [Google Scholar] [CrossRef]
- Deutsch, S.-M.; Mariadassou, M.; Nicolas, P.; Parayre, S.; Le Guellec, R.; Chuat, V.; Peton, V.; Le Maréchal, C.; Burati, J.; Loux, V.; et al. Identification of Proteins Involved in the Anti-Inflammatory Properties of Propionibacterium freudenreichii by Means of a Multi-Strain Study. Sci. Rep. 2017, 7, 46409. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Tomé, S.; Marin, A.C.; Ortega Moreno, L.; Baldan-Martin, M.; Mora-Gutiérrez, I.; Lanas-Gimeno, A.; Moreno-Monteagudo, J.A.; Santander, C.; Sánchez, B.; Chaparro, M.; et al. Immunomodulatory Effect of Gut Microbiota-Derived Bioactive Peptides on Human Immune System from Healthy Controls and Patients with Inflammatory Bowel Disease. Nutrients 2019, 11, 2605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zihni, C.; Mills, C.; Matter, K.; Balda, M.S. Tight Junctions: From Simple Barriers to Multifunctional Molecular Gates. Nat. Rev. Mol. Cell Biol. 2016, 17, 564–580. [Google Scholar] [CrossRef] [PubMed]
- Chelakkot, C.; Ghim, J.; Ryu, S.H. Mechanisms Regulating Intestinal Barrier Integrity and Its Pathological Implications. Exp. Mol. Med. 2018, 50, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Alvarez, C.-S.; Giménez, R.; Cañas, M.-A.; Vera, R.; Díaz-Garrido, N.; Badia, J.; Baldomà, L. Extracellular Vesicles and Soluble Factors Secreted by Escherichia coli Nissle 1917 and ECOR63 Protect against Enteropathogenic E. coli-Induced Intestinal Epithelial Barrier Dysfunction. BMC Microbiol. 2019, 19, 166. [Google Scholar] [CrossRef] [Green Version]
- Guan, J.; Liu, F.; Zhao, S.; Evivie, S.E.; Shi, J.; Li, N.; Zhao, L.; Yue, Y.; Xie, Q.; Huo, G.; et al. Effect of Bifidobacterium longum Subsp. longum on the Proliferative and Tight-Junction Activities of Human Fetal Colon Epithelial Cells. J. Funct. Foods 2021, 86, 104715. [Google Scholar] [CrossRef]
- Tsukita, S.; Furuse, M. Occludin and Claudins in Tight-Junction Strands: Leading or Supporting Players? Trends Cell Biol. 1999, 9, 268–273. [Google Scholar] [CrossRef]
- Findley, M.K.; Koval, M. Regulation and Roles for Claudin-Family Tight Junction Proteins. IUBMB Life 2009, 61, 431–437. [Google Scholar] [CrossRef] [Green Version]
- Yue, X.; Wen, S.; Long-kun, D.; Man, Y.; Chang, S.; Min, Z.; Shuang-yu, L.; Xin, Q.; Jie, M.; Liang, W. Three Important Short-Chain Fatty Acids (SCFAs) Attenuate the Inflammatory Response Induced by 5-FU and Maintain the Integrity of Intestinal Mucosal Tight Junction. BMC Immunol. 2022, 23, 19. [Google Scholar] [CrossRef]
- Carvalho, P.L.A.; Andrade, M.E.R.; Trindade, L.M.; Leocádio, P.C.L.; Alvarez-Leite, J.I.; dos Reis, D.C.; Cassali, G.D.; de Sales Souza e Melo, É.L.; dos Santos Martins, F.; Fernandes, S.O.A.; et al. Prophylactic and Therapeutic Supplementation Using Fructo-Oligosaccharide Improves the Intestinal Homeostasis after Mucositis Induced by 5- Fluorouracil. Biomed. Pharmacother. 2021, 133, 111012. [Google Scholar] [CrossRef]
- Dahan, S.; Rabinowitz, K.M.; Martin, A.P.; Berin, M.C.; Unkeless, J.C.; Mayer, L. Notch-1 Signaling Regulates Intestinal Epithelial Barrier Function, Through Interaction With CD4+ T Cells, in Mice and Humans. Gastroenterology 2011, 140, 550–559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).