The Use of Probiotic Megasphaera elsdenii as a Pre-Harvest Intervention to Reduce Salmonella in Finishing Beef Cattle: An In Vitro Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Culture Preparation
2.3. Sample Preparation
2.4. Microbiological Analyses
2.5. Volatile Fatty Acid and pH Analyses
2.6. Statistical Analyses
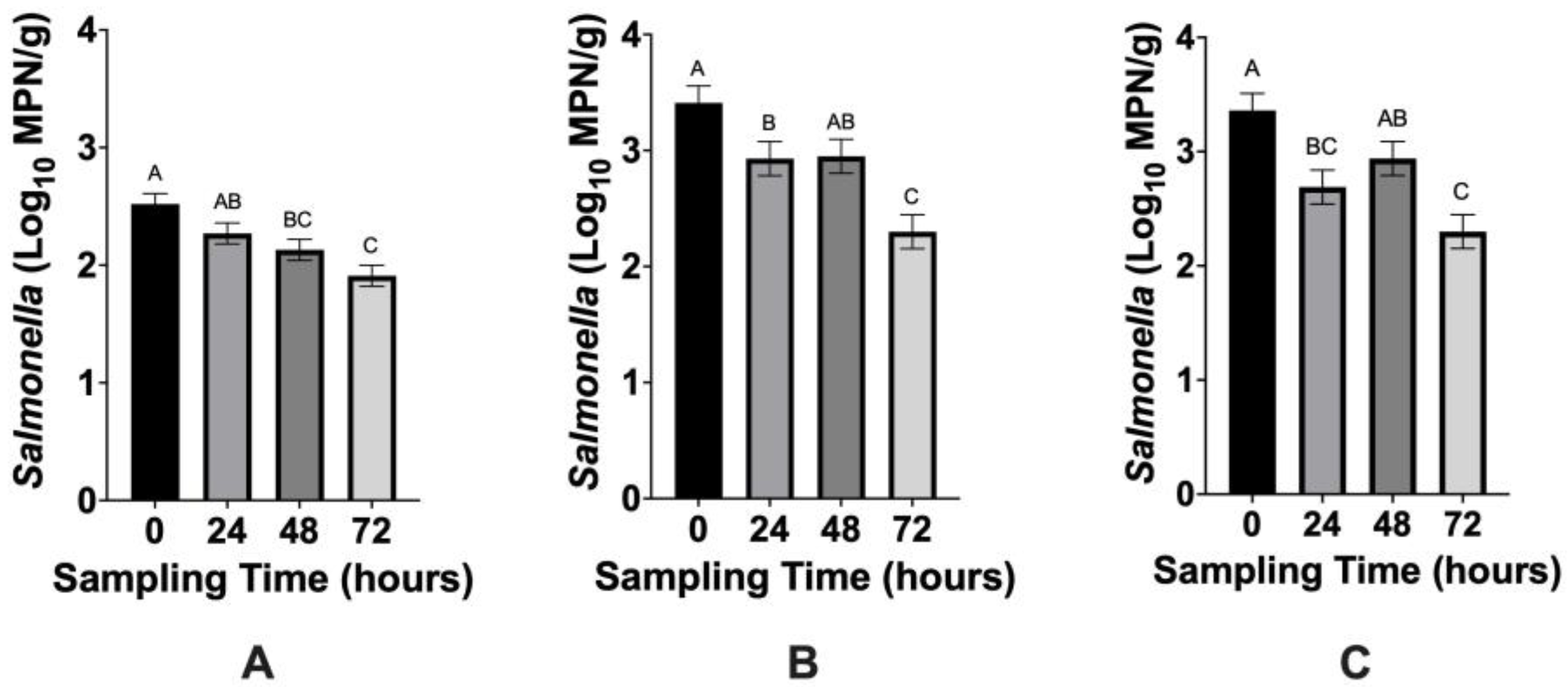
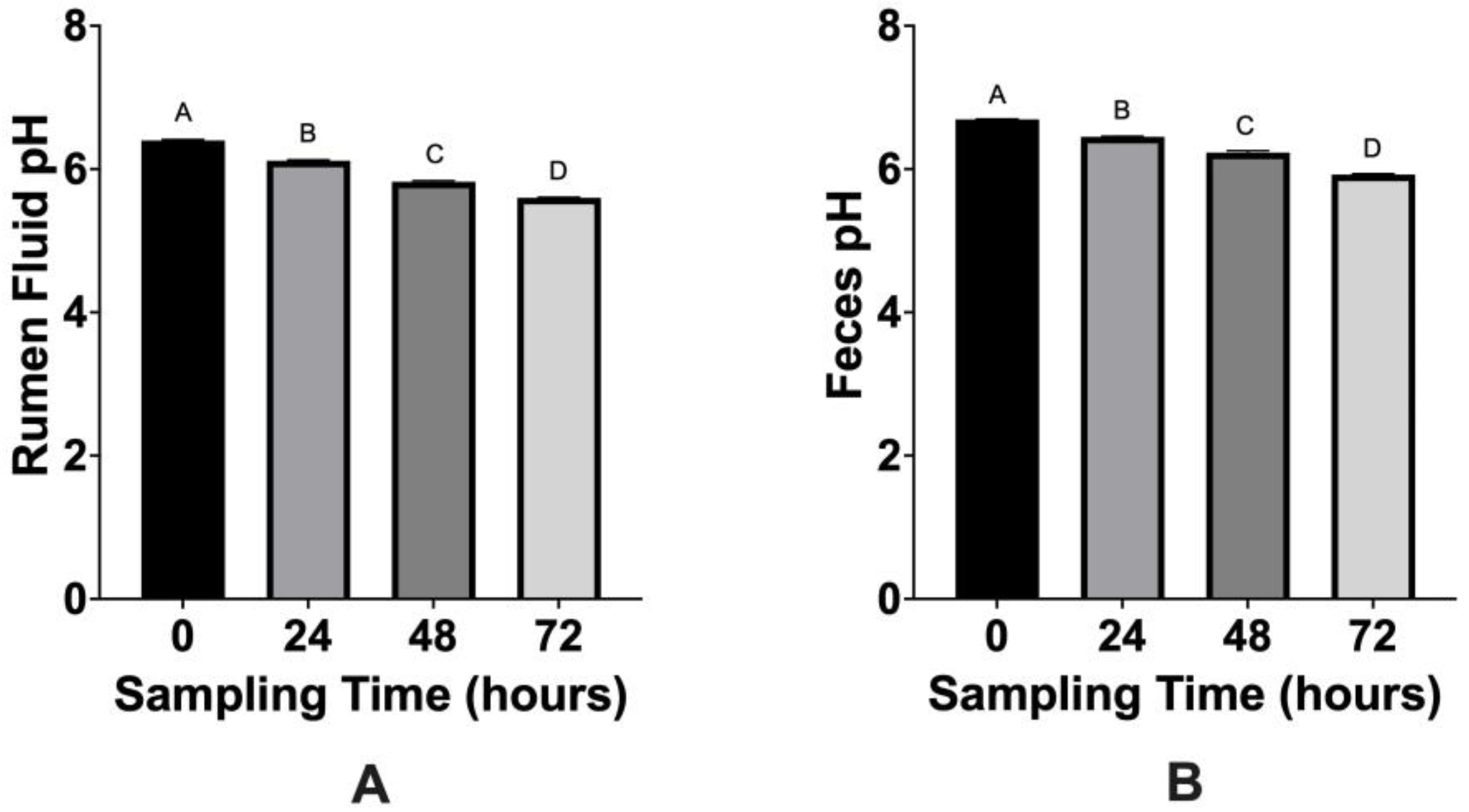
3. Results
3.1. Microbiological Analyses
3.2. VFA and pH Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ohta, N.; Norman, K.N.; Norby, B.; Lawhon, S.D.; Vinasco, J.; den Bakker, H.; Loneragan, G.H.; Scott, H.M. Population dynamics of enteric Salmonella in response to antimicrobial use in beef feedlot cattle. Sci. Rep. 2017, 7, 14310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brüssow, H. Adjuncts and alternatives in the time of antibiotic resistance and in-feed antibiotic bans. Microb. Biotechnol. 2017, 10, 674–677. [Google Scholar] [CrossRef] [PubMed]
- Acuff, G.R.; Dickson, J. Ensuring Safety and Quality in the Production of Beef. Volume 1: Safety; Burleigh Dodds Science Publishsing Limited: Philadelphia, PA, USA, 2017. [Google Scholar]
- McEvoy, J.M.; Doherty, A.M.; Sheridan, J.J.; Blair, I.S.; McDowell, D.A. The prevalence of Salmonella spp. in bovine faecal, rumen and carcass samples at a commercial abattoir. J. Appl. Microbiol. 2003, 94, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Brichta-Harhay, D.M.; Guerini, M.N.; Arthur, T.M.; Bosilevac, J.M.; Kalchayanand, N.; Shackelford, S.D.; Wheeler, T.L.; Koohmaraie, M. Salmonella and Escherichia coli O157:H7 contamination on hides and carcasses of cull cattle presented for slaughter in the United States: An evaluation of prevalence and bacterial loads by immunomagnetic separation and direct plating methods. Appl. Environ. Microbiol. 2008, 74, 6289–6297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fegan, N.; Vanderlinde, P.; Higgs, G.; Desmarchelier, P. A study of the prevalence and enumeration of Salmonella enterica in cattle and on carcasses during processing. J. Food Prot. 2005, 68, 1147–1153. [Google Scholar] [CrossRef] [PubMed]
- Webb, H.E.; Brichta-Harhay, D.M.; Brashears, M.M.; Nightingale, K.K.; Arthur, T.M.; Bosilevac, J.M.; Kalchayanand, N.; Schmidt, J.W.; Wang, R.; Granier, S.A.; et al. Salmonella in peripheral lymph nodes of healthy cattle at slaughter. Front. Microbiol. 2017, 8, 2214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wells, J.E.; Kim, M.; Bono, J.L.; Kuehn, L.A.; Benson, A.K. Meat Science and Muscle Biology Symposium: Escherichia coli O157:H7, diet, and fecal microbiome in beef cattle. J. Anim. Sci. 2014, 92, 1345–1355. [Google Scholar] [CrossRef] [PubMed]
- Holland, B.P.; Richards, C.J.; Krehbiel, C.R.; VanOverbeke, D.L.; Wilson, B.K.; Jacob, M.E.; Nagaraja, T.G.; Step, D.L. Feeding wet distillers grains plus solubles with and without a direct-fed microbial to determine performance, carcass characteristics, and fecal shedding of Escherichia coli O157:H7 in feedlot heifers. J. Anim. Sci. 2016, 94, 297–305. [Google Scholar] [CrossRef]
- Zhang, G.; Gilliland, S.E.; Krehbiel, C.R.; Rust, S.R. Bacterial direct-fed microbials in ruminant diets: Performance response and mode of action. J. Anim. Sci. 2003, 81, E120–E132. [Google Scholar] [CrossRef]
- Wilson, B.K.; Krehbiel, C.R. Current and Future Status of Practical Applications: Beef Cattle. In Direct-Fed Microbials and Prebiotics for Animals: Science and Mechanisms of Action; Callaway, T.R., Ricke, S.C., Eds.; Springer: New York, NY, USA, 2012; pp. 137–152. [Google Scholar]
- McAllister, T.A.; Beauchemin, K.A.; Alazzeh, A.Y.; Baah, J.; Teather, R.M.; Stanford, K. Review: The use of direct fed microbials to mitigate pathogens and enhance production in cattle. Can. J. Anim. Sci. 2011, 91, 193–211. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Shen, Y.; Wang, C.; Ding, L.; Zhao, F.; Wang, M.; Fu, J.; Wang, H. Megasphaera elsdenii Lactate Degradation Pattern Shifts in Rumen Acidosis Models. Front. Microbiol. 2019, 10, 162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weimer, P.J.; Moen, G.N. Quantitative analysis of growth and volatile fatty acid production by the anaerobic ruminal bacterium Megasphaera elsdenii T81. Appl. Microbiol. Biotechnol. 2013, 97, 4075–4081. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.T.; Depenbusch, B.E.; Drouillard, J.S.; Nagaraja, T.G. Dry-rolled or steam-flaked grain-based diets and fecal shedding of Escherichia coli O157 in feedlot cattle. J. Anim. Sci. 2007, 85, 1207–1212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Callaway, T.R. Diet, Escherichia coli O157:H7, and Cattle: A Review After 10 Years. Curr. Issues Mol. Biol. 2009, 11, 67–79. [Google Scholar] [PubMed]
- Page, J.A.; Lubbers, B.; Maher, J.; Ritsch, L.; Gragg, S.E. Investigation into the Efficacy of Bdellovibrio bacteriovorus as a Novel Preharvest Intervention to Control Escherichia coli O157:H7 and Salmonella in Cattle Using an In Vitro Model. J. Food Prot. 2015, 78, 1745–1749. [Google Scholar] [CrossRef] [PubMed]
- Thieszen, J.; Van Bibber, C.L.; Axman, J.E.; Drouillard, J.S. Lactipro (Megasphaera elsdenii) Increases Ruminal pH and Alters Volatile Fatty Acids and Lactate During Transition to an 80% Concentrate Diet. Kans. Agric. Exp. Stn. Res. Rep. 2015, 1, 13. [Google Scholar] [CrossRef] [Green Version]
- Rush, I.G. Rumen Physiology for the Rancher. In Proceedings of the Range Beef Cow Symposium XXI, Casper, WY, USA, 1–3 December 2009. [Google Scholar]
- United States Environmental Protection Agency. Most Probable Number (MPN) Calculator. Available online: https://mostprobablenumbercalculator.epa.gov/mpnForm (accessed on 26 January 2022).
- Sato, H.; Nakajima, J. Fecal ammonia, urea, volatile fatty acid and lactate levels in dairy cows and their pathophysiological significance during diarrhea. Anim. Sci. J. 2005, 76, 595–599. [Google Scholar] [CrossRef]
- Robinson, J.; Smolenski, W.; Greening, R.; Ogilvie, M.; Bell, R.; Barsuhn, K.; Peters, J. Prevention of acute acidosis and enhancement of feed intake in the bovine by Megasphaera elsdenii 407A. J. Anim. Sci 1992, 70, 310. [Google Scholar]
- Bolton, D.J.; Kelly, S.; Lenahan, M.; Fanning, S. In Vitro Studies on the Effect of pH and Volatile Fatty Acid Concentration, as Influenced by Diet, on the Survival of Inoculated Nonacid- and Acid-Adapted Salmonella in Bovine Rumen Fluid and Feces. Foodborne Pathog. Dis. 2011, 8, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Jami, E.; Mizrahi, I. Composition and Similarity of Bovine Rumen Microbiota across Individual Animals. PLoS ONE 2012, 7, e33306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Target Concentrations of M. elsdenii (CFU/g) |
|---|
| Control: 10 mL anaerobic diluent without M. elsdenii = 0 |
| 1.0 × 105 |
| 2.5 × 105 |
| 5.0 × 105 |
| 5.0 × 108 |
| Time Point (Hours) | Megasphaera elsdenii Target Concentration (CFU/g) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0.0 | 1.0 × 105 | 2.5 × 105 | 5.0 × 105 | 5.0 × 108 | ||||||
| Salmonella Log MPN/g | SEM | Salmonella Log MPN/g | SEM | Salmonella Log MPN/g | SEM | Salmonella Log MPN/g | SEM | Salmonella Log MPN/g | SEM | |
| 0 | 1.52 A,a | 0.01479 | 1.31 A,b | 0.01479 | 1.46 A,a,c | 0.01479 | 1.44 A,c | 0.01479 | 1.63 A,d | 0.01479 |
| 24 | 1.54 A,a | 0.01688 | 1.18 B,b | 0.01688 | 1.37 B,c | 0.01688 | 1.05 B,d | 0.01688 | 1.44 B,c | 0.01688 |
| 48 | 1.37 A,a | 0.09226 | 1.37 A,B,a | 0.09226 | 1.43 A,B,a | 0.09226 | 1.22 A,B,a | 0.09226 | 1.40 A,B,a | 0.09226 |
| 72 | 0.76 B,a | 0.0765 | 0.65 C,a | 0.0765 | 0.87 C,a | 0.0765 | 0.67 C,a | 0.0765 | 0.63 C,a | 0.0765 |
| Time Point (Hours) | Megasphaera elsdenii Target Concentration (CFU/g) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0.0 | 1.0 × 105 | 2.5 × 105 | 5.0 × 105 | 5.0 × 108 | ||||||
| Log MPN/g | SEM | Log MPN/g | SEM | Log MPN/g | SEM | Log MPN/g | SEM | Log MPN/g | SEM | |
| 0 | 4.36 A,a | 0.06504 | 4.31 A,a | 0.06504 | 4.44 A,a | 0.06504 | 4.32 A,a | 0.06504 | 4.31 A,a | 0.06504 |
| 24 | 3.62 B,a,b | 0.02313 | 3.54 B,b | 0.02313 | 3.67 B,a | 0.02313 | 3.64 B,a | 0.02313 | 4.05 B,c | 0.02313 |
| 48 | 3.54 B,a | 0.03557 | 3.37 C,b | 0.03557 | 2.54 C,c | 0.03557 | 3.31 C,b,d | 0.03557 | 3.18 C,d | 0.03557 |
| 72 | 3.31 B,a | 0.1186 | 2.44 D,b | 0.1186 | 2.52 C,b | 0.1186 | 2.68 D,b | 0.1186 | 2.87 C,a,b | 0.1186 |
| Time Point (Hours) | Megasphaera elsdenii Target Concentration (CFU/g) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0.0 | 1.0 × 105 | 2.5 × 105 | 5.0 × 105 | 5.0 × 108 | ||||||
| Log MPN/g | SEM | Log MPN/g | SEM | Log MPN/g | SEM | Log MPN/g | SEM | Log MPN/g | SEM | |
| 0 | 3.97 A,a | 0.6618 | 4.00 A,B,a | 0.6618 | 4.10 A,a | 0.6618 | 3.93 A,B,a | 0.6618 | 3.57 A,a | 0.6618 |
| 24 | 3.67 A,a | 0.3022 | 3.70 A,a | 0.3022 | 3.27 A,a | 0.3022 | 3.23 A,B,a | 0.3022 | 3.80 A,a | 0.3022 |
| 48 | 3.53 A,a | 0.04092 | 3.40 A,a,b | 0.04092 | 2.50 A,d | 0.04092 | 3.30 A,b,c | 0.04092 | 3.20 A,c | 0.04092 |
| 72 | 3.30 A,a | 0.1287 | 2.43 B,b | 0.1287 | 2.50 A,b | 0.1287 | 2.67 B,b | 0.1287 | 2.90 A,a,b | 0.1287 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Habib, K.; Drouillard, J.; de Aguiar Veloso, V.; Huynh, G.; Trinetta, V.; Gragg, S.E. The Use of Probiotic Megasphaera elsdenii as a Pre-Harvest Intervention to Reduce Salmonella in Finishing Beef Cattle: An In Vitro Model. Microorganisms 2022, 10, 1400. https://doi.org/10.3390/microorganisms10071400
Habib K, Drouillard J, de Aguiar Veloso V, Huynh G, Trinetta V, Gragg SE. The Use of Probiotic Megasphaera elsdenii as a Pre-Harvest Intervention to Reduce Salmonella in Finishing Beef Cattle: An In Vitro Model. Microorganisms. 2022; 10(7):1400. https://doi.org/10.3390/microorganisms10071400
Chicago/Turabian StyleHabib, Kellen, James Drouillard, Vanessa de Aguiar Veloso, Grace Huynh, Valentina Trinetta, and Sara E. Gragg. 2022. "The Use of Probiotic Megasphaera elsdenii as a Pre-Harvest Intervention to Reduce Salmonella in Finishing Beef Cattle: An In Vitro Model" Microorganisms 10, no. 7: 1400. https://doi.org/10.3390/microorganisms10071400
APA StyleHabib, K., Drouillard, J., de Aguiar Veloso, V., Huynh, G., Trinetta, V., & Gragg, S. E. (2022). The Use of Probiotic Megasphaera elsdenii as a Pre-Harvest Intervention to Reduce Salmonella in Finishing Beef Cattle: An In Vitro Model. Microorganisms, 10(7), 1400. https://doi.org/10.3390/microorganisms10071400






