Prevalence and Molecular Characterization of Mycobacterium bovis in Slaughtered Cattle Carcasses in Burkina Faso; West Africa
Abstract
1. Introduction
2. Materials and Methods
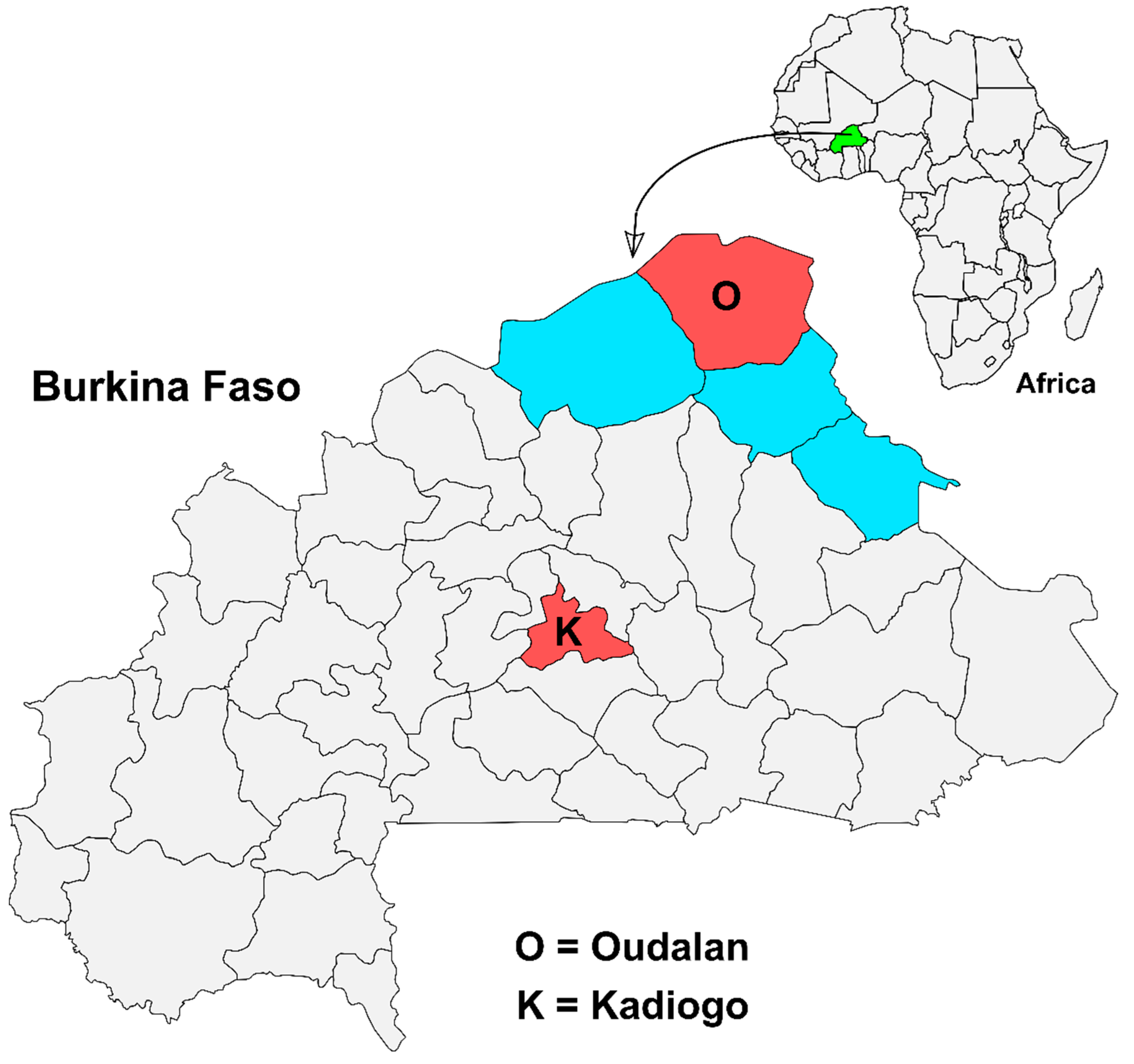
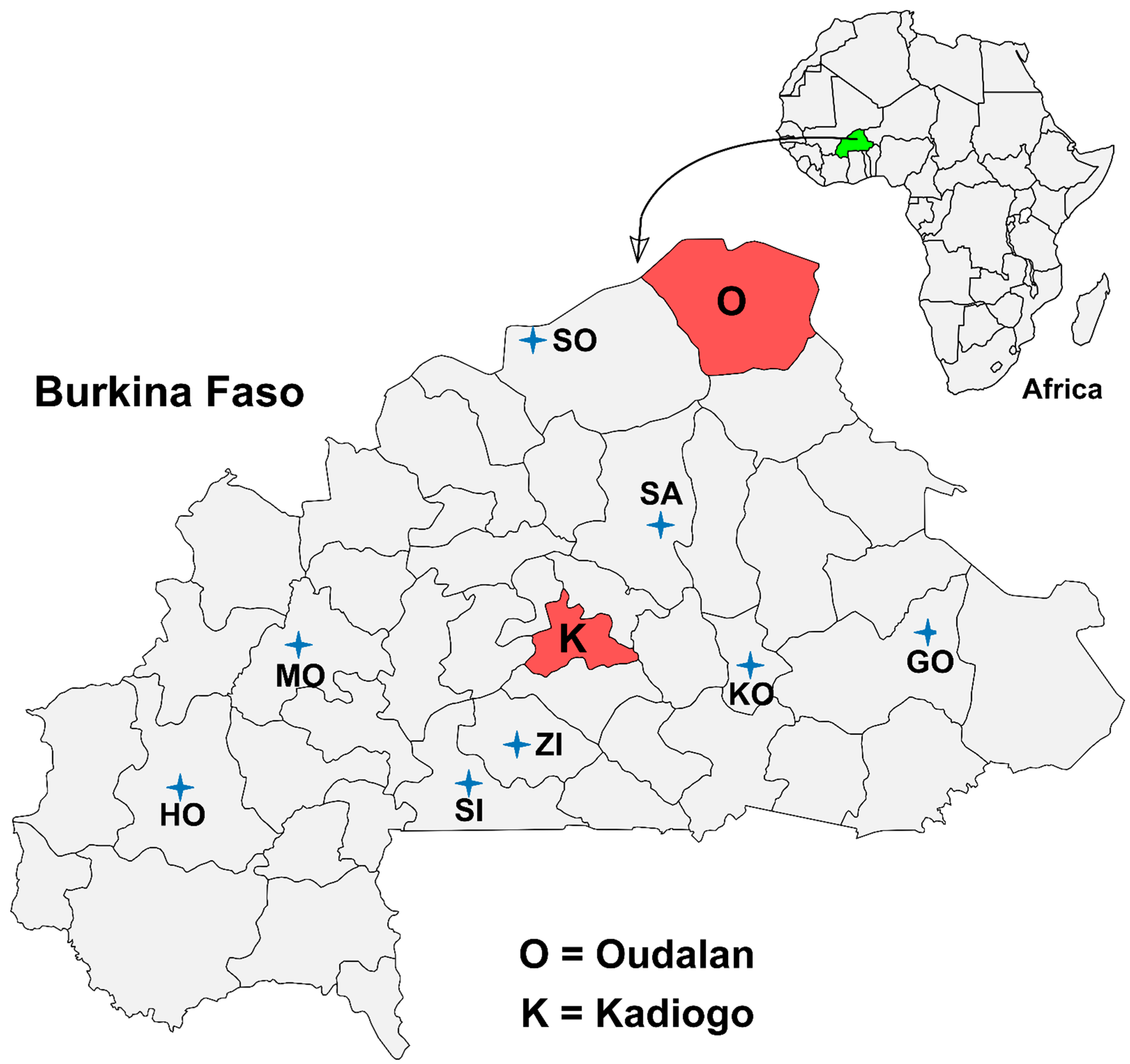
2.1. Study Setting and Design
2.2. Sample Size Calculation and Sampling Strategy
2.3. Antemortem and Postmortem Inspection
2.4. Sample Collection and Transportation
2.5. Laboratory Procedures
2.5.1. Decontamination of the Samples
2.5.2. Smear Microscopy
2.5.3. Mycobacterial Culture
2.5.4. Line Probe Assay
2.5.5. Sanger Sequencing
2.5.6. Spoligotyping
2.5.7. 24-Loci MIRU-VNTR Typing
2.6. Statistical Analysis
3. Results
3.1. Study Population
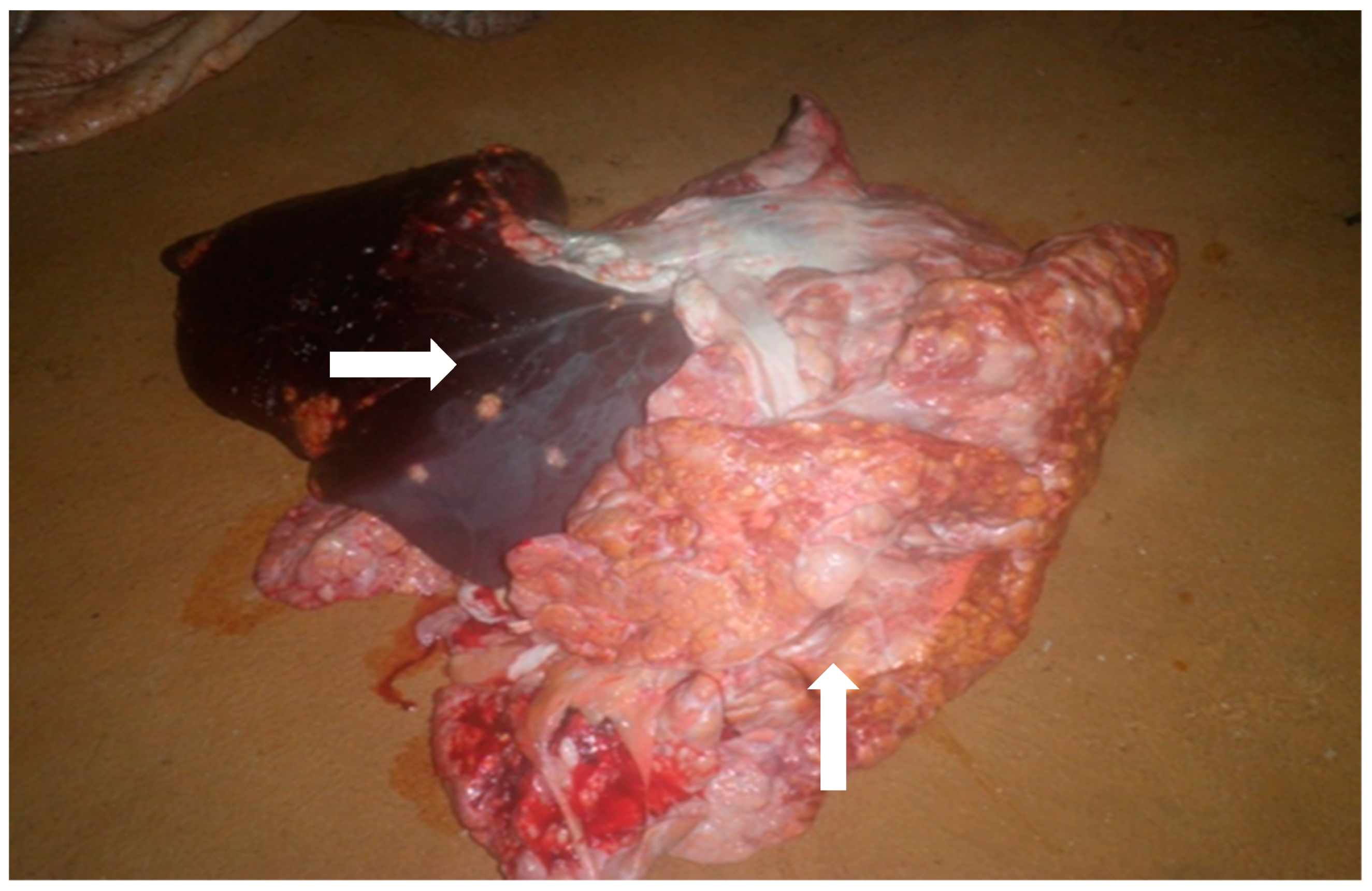
3.2. Prevalence and Anatomical Site of Tuberculous Lesions
3.3. Isolation and Species Identification
3.4. Molecular Typing of the MTBC Strains
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Radostits, O.M.; Gay, C.C.; Blood, D.C.; Hinchelift, K.W. Diseases associated with bacteria—IV—Mycobacterium spp. In Veterinary Medicine: A Text Book of Disease of Cattle, Sheep, Pig, Goat and Horses, 10th ed.; Harcourt Publisher Ltd.: London, UK, 2007; Volume 2, p. 2065. [Google Scholar]
- World Organisation for Animal Health. Bovine Tuberculosis. In Manual of Diagnostic Tests and Vaccines for Terrestrial Animals; The World Organisation for Animal Health (OIE): Paris, France, 2018; p. 17. [Google Scholar]
- World Organisation for Animal Health. Infection with Mycobacterium Tuberculosis Complex. In Terrestrial Animal Health Code; The World Organisation for Animal Health (OIE): Paris, France, 2019; p. 6. [Google Scholar]
- Thoen, C.; Lobue, P.; de Kantor, I. The importance of Mycobacterium bovis as a zoonosis. Vet. Microbiol. 2006, 112, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Thoen, C.O.; James, H.S.; Michael, J.G. Mycobacterium Bovis Infection in Animals and Humans, 2nd ed.; Blackwell Publishing: Oxford, UK, 2006. [Google Scholar]
- Ameni, G.; Tadesse, K.; Hailu, E.; Deresse, Y.; Medhin, G.; Aseffa, A.; Hewinson, G.; Vordermeier, M.; Berg, S. Transmission of Mycobacterium tuberculosis between farmers and cattle in central Ethiopia. PLoS ONE 2013, 8, e76891. [Google Scholar] [CrossRef] [PubMed]
- Hlokwe, T.M.; Said, H.; Gcebe, N. Mycobacterium tuberculosis infection in cattle from the Eastern Cape Province of South Africa. BMC Vet. Res. 2017, 13, 299. [Google Scholar] [CrossRef]
- Sibhat, B.; Asmare, K.; Demissie, K.; Ayelet, G.; Mamo, G.; Ameni, G. Bovine tuberculosis in Ethiopia: A systematic review and meta-analysis. Prev. Vet. Med. 2017, 147, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Sanou, A.; Tarnagda, Z.; Kanyala, E.; Zingue, D.; Nouctara, M.; Ganame, Z.; Combary, A.; Hien, H.; Dembele, M.; Kabore, A.; et al. Mycobacterium bovis in Burkina Faso: Epidemiologic and genetic links between human and cattle isolates. PLoS Negl. Trop. Dis. 2014, 8, e3142. [Google Scholar] [CrossRef]
- Dibaba, A.B.; Daborn, C.J.; Cadmus, S.; Michel, A. The Current Status of Bovine Tuberculosis in Africa. In Tuberculosis in Animals: An African Perspective; Dibaba, A., Kriek, N., Thoen, C., Eds.; Springer: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Azami, H.Y.; Zinsstag, J. Economics of Bovine Tuberculosis: A One Health Issue. In Bovine Tuberculosis; Chambers, M., Gordon, S., Olea-Popelka, F., Barrow, P., Eds.; CAB International: Wallingford, UK, 2018. [Google Scholar]
- Schiller, I.; Oesch, B.; Vordermeier, H.M.; Palmer, M.V.; Harris, B.N.; Orloski, K.A.; Buddle, B.M.; Thacker, T.C.; Lyashchenko, K.P.; Waters, W.R. Bovine tuberculosis: A review of current and emerging diagnostic techniques in view of their relevance for disease control and eradication. Transbound. Emerg. Dis. 2010, 57, 205–220. [Google Scholar] [CrossRef]
- Carruth, L.; Roess, A.A.; Mekonnen, Y.T.; Melaku, S.K.; Nichter, M.; Salman, M. Zoonotic tuberculosis in Africa: Challenges and ways forward. Lancet 2016, 388, 2460–2461. [Google Scholar] [CrossRef]
- World Organisation for Animal Health. Global Tuberculosis Report 2019; World Health Organization: Geneva, Switzerland, 2019; p. 297. [Google Scholar]
- Kerubo, G.; Ndungu, P.; Shuaib, Y.A.; Amukoye, E.; Revathi, G.; Homolka, S.; Kariuki, S.; Merker, M.; Niemann, S. Molecular Epidemiology of Mycobacterium tuberculosis Complex Strains in Urban and Slum Settings of Nairobi, Kenya. Genes 2022, 13, 475. [Google Scholar] [CrossRef]
- Kemal, J.; Sibhat, B.; Abraham, A.; Terefe, Y.; Tulu, K.T.; Welay, K.; Getahun, N. Bovine tuberculosis in eastern Ethiopia: Prevalence, risk factors and its public health importance. BMC Infect. Dis. 2019, 19, 39. [Google Scholar] [CrossRef]
- Soudre, A. Trypanosomosis, Genetic Diversity and Admixture in Cattle Breeds of Burkina Faso. Ph.D. Thesis, Universität für Bodenkultur Wien, Vienna, Austria, 2011. [Google Scholar]
- Thrusfield, M. Veterinary Epidemiology, 3rd ed.; Wiley-Blackwell Science: London, UK, 2007. [Google Scholar]
- Shuaib, Y.A.; Niemann, S.; Khalil, E.A.G.; Schaible, U.E.; Wieler, L.H.; Bakheit, M.A.; Mohamed-Noor, S.E.; Abdalla, M.A.; Richter, E. Mycobacterial infections in carcasses of ruminants slaughtered at the two slaughterhouses of Kassala, Sudan. Rev. Élev. Méd. Vét. Pays Trop. 2018, 70, 131–136. [Google Scholar] [CrossRef]
- Nigo, L.S.; John, B.T.; Lobojo, D.L.; Lita, E.P.; Osman, A.Y.; Shuaib, Y.A. Prevalence and Financial Losses of Cystic Echinococcosis in Slaughtered Goats at Gumbo Slab in Juba County, South Sudan. Parasitologia 2022, 2, 54–62. [Google Scholar] [CrossRef]
- Gaming Labs International. Mycobacteriology Laboratory Manual; WHO: Geneva, Swizerland, 2014. [Google Scholar]
- Shuaib, Y.A.; Khalil, E.A.G.; Schaible, U.E.; Wieler, L.H.; Bakheit, M.A.M.; Mohamed-Noor, S.E.; Abdalla, M.A.; Homolka, S.; Andres, S.; Hillemann, D.; et al. Smear Microscopy for Diagnosis of Pulmonary Tuberculosis in Eastern Sudan. Tuberc. Res. Treat. 2018, 2018, 8038137. [Google Scholar] [CrossRef] [PubMed]
- Supply, P.; Allix, C.; Lesjean, S.; Cardoso-Oelemann, M.; Rusch-Gerdes, S.; Willery, E.; Savine, E.; de Haas, P.; van Deutekom, H.; Roring, S.; et al. Proposal for standardization of optimized mycobacterial interspersed repetitive unit-variable-number tandem repeat typing of Mycobacterium tuberculosis. J. Clin. Microbiol. 2006, 44, 4498–4510. [Google Scholar] [CrossRef] [PubMed]
- Weniger, T.; Krawczyk, J.; Supply, P.; Niemann, S.; Harmsen, D. MIRU-VNTRplus: A web tool for polyphasic genotyping of Mycobacterium tuberculosis complex bacteria. Nucleic Acids Res. 2010, 38, W326–W331. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sanou, A.; Dicko, A.; Sow, K.R.; Djibougou, A.; Kabore, A.; Diarra, B.; Ouedraogo, A.K.; Zingue, D.; Nouctara, M.; Tarnagda, Z. Epidemiology and microscopic diagnosis of tuberculosis in pigs and small ruminants slaughtered at Bobo-Dioulasso abattoir, Burkina Faso. Onderstepoort J. Vet. Res. 2021, 88, e1–e6. [Google Scholar] [CrossRef]
- Shuaib, Y.A.; Osman, H.M.; Hussein, M.O.; Bakhiet, M.A.; Omer, R.A.; Al-Nahas, A.; Suliman, S.E.; Abdalla, M.A.; Ismail, A.A. Seroprevalence of Babesia bigemina antibodies in cattle in North Kordofan state, the Sudan. ARC J. Anim. Vet. Sci. 2015, 1, 1–11. [Google Scholar]
- Delafosse, A.; Traoré, A.; Koné, A.Z. Isolement de souches de mycobactéries pathogènes chez des bovins abattus à l’abattoir de Bobo-Dioulasso, Burkina Faso. Rev. Élev. Méd. Vét. Pays Trop. 1995, 48, 301–306. [Google Scholar] [CrossRef]
- Zékiba, T.; Estelle, K.; Dezemon, Z.; Satigui, S.; Issaka, Y.; Thérèse, K.; Nicolas, M.; Lassana, S.; Yassa, D.; Mamadou, S.; et al. Prevalence of Tuberculosis spp. species in bovine carcasses in two slaughterhouses of Burkina Faso. Int. J. Microbiol. Immunol. Res. 2014, 2, 092–100. [Google Scholar]
- Yahyaoui-Azami, H.; Aboukhassib, H.; Bouslikhane, M.; Berrada, J.; Rami, S.; Reinhard, M.; Gagneux, S.; Feldmann, J.; Borrell, S.; Zinsstag, J. Molecular characterization of bovine tuberculosis strains in two slaughterhouses in Morocco. BMC Vet. Res. 2017, 13, 272. [Google Scholar] [CrossRef]
- Shitaye, J.E.; Tsegaye, W.; Pavlik, I. Bovine tuberculosis infection in animal and human populations in Ethiopia: A review. Vet. Med. 2007, 52, 317–332. [Google Scholar] [CrossRef]
- Nalapa, D.P.; Muwonge, A.; Kankya, C.; Olea-Popelka, F. Prevalence of tuberculous lesion in cattle slaughtered in Mubende district, Uganda. BMC Vet. Res. 2017, 13, 73. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A. Bovine tuberculosis at the human-livestock-wildlife interface and its control through one health approach in the Ethiopian Somali Pastoralists: A review. One Health 2020, 9, 100113. [Google Scholar] [CrossRef] [PubMed]
- Ayad, A.; Bensıd, A.; Benabdelhak, A.C.; Aıt-yahıa, F.; Dergal, N.B. First Report on Tuberculosis Based on Slaughterhouse Data in Bejaia Province, Algeria: A Retrospective 10-Year Survey. Kocatepe Vet. J. 2020, 13, 118–124. [Google Scholar] [CrossRef]
- Sidibé, S.; Dicko, N.A.; Fané, A.; Doumbia, R.M.; Sidibé, C.K.; Kanté, S.; Mangané, O.; Konaté, B.; Koné, A.Z.; Maïga, M.S.; et al. Tuberculose bovine au Mali: Résultats d’une enquête épidémiologique dans les élevages laitiers de la zone périurbaine du district de Bamako. Rev. Élev. Méd. Vét. Pays Trop. 2003, 56, 115–120. [Google Scholar] [CrossRef]
- Traoré, A.; Tamboura, H.; Bayala, B.; Rouamba, D.; Yaméogo, N.; Sanou, M. Prévalence globale des pathologies majeures liées à la production laitière bovine en système d’élevage intra- urbain à Hamdallaye (Ouagadougou). Biotechnol. Agron. Soc. Environ. 2004, 8, 3–8. [Google Scholar]
- Biffa, D.; Bogale, A.; Skjerve, E. Diagnostic efficiency of abattoir meat inspection service in Ethiopia to detect carcasses infected with Mycobacterium bovis: Implications for public health. BMC Public Health 2010, 10, 462. [Google Scholar] [CrossRef]
- ASEAN Football Federation. Tuberculosis in Cattle Interim Manual for the Veterinarian; Division of Veterinary Services, Agriculture, Foresty, and Fisheries: Petaling Jaya, Malaysia; South Africa, 2016; p. 72. [Google Scholar]
- Risco, D.; Serrano, E.; Fernandez-Llario, P.; Cuesta, J.M.; Goncalves, P.; Garcia-Jimenez, W.L.; Martinez, R.; Cerrato, R.; Velarde, R.; Gomez, L.; et al. Severity of bovine tuberculosis is associated with co-infection with common pathogens in wild boar. PLoS ONE 2014, 9, e110123. [Google Scholar] [CrossRef]
- Serrano, M.; Sevilla, I.A.; Fuertes, M.; Geijo, M.; Risalde, M.A.; Ruiz-Fons, J.F.; Gortazar, C.; Juste, R.A.; Dominguez, L.; Elguezabal, N.; et al. Different lesion distribution in calves orally or intratracheally challenged with Mycobacterium bovis: Implications for diagnosis. Vet. Res. 2018, 49, 74. [Google Scholar] [CrossRef]
- Delafosse, A.; Goutard, F.; Thebaud, E. Epidémiologie de la tuberculose et de la brucellose des bovins en zone périurbaine d’Abéché (Tchad). Rev. Élev. Méd. Vét. Pays Trop. 2002, 55, 5–13. [Google Scholar] [CrossRef]
- D’Auria, G.; Torrents, E.; Luquin, M.; Comas, I.; Julian, E. Draft Genome Sequence of Mycobacterium brumae ATCC 51384. Genome Announc. 2016, 4, e00237-16. [Google Scholar] [CrossRef]
- Muller, B.; Hilty, M.; Berg, S.; Garcia-Pelayo, M.C.; Dale, J.; Boschiroli, M.L.; Cadmus, S.; Ngandolo, B.N.; Godreuil, S.; Diguimbaye-Djaibe, C.; et al. African 1, an epidemiologically important clonal complex of Mycobacterium bovis dominant in Mali, Nigeria, Cameroon, and Chad. J. Bacteriol. 2009, 191, 1951–1960. [Google Scholar] [CrossRef] [PubMed]
- Muller, B.; Steiner, B.; Bonfoh, B.; Fane, A.; Smith, N.H.; Zinsstag, J. Molecular characterisation of Mycobacterium bovis isolated from cattle slaughtered at the Bamako abattoir in Mali. BMC Vet. Res. 2008, 4, 26. [Google Scholar] [CrossRef] [PubMed]
- Koro Koro, F.; Ngatchou, A.F.; Portal, J.L.; Gutierrez, C.; Etoa, F.X.; Eyangoh, S.I. The genetic population structure of Mycobacterium bovis strains isolated from cattle slaughtered at the Yaoundé and Douala abattoirs in Cameroon. Rev. Sci. Tech. 2015, 34, 1001–1010. [Google Scholar] [CrossRef] [PubMed]
- Perillo, L.; Cascone, G.; Antoci, F.; Piccione, G.; Giannetto, C.; Salonia, R.; Salina, F.; Giudice, E.; Monteverde, V.; Licitra, F. Prevalence of Infectious Diseases on Dairy Farms Classified on The Basis of Their Biosecurity Score. J. Vet. Res. 2022, 66, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Abbate, J.M.; Arfuso, F.; Iaria, C.; Arestia, G.; Lanteri, G. Prevalence of Bovine Tuberculosis in Slaughtered Cattle in Sicily, Southern Italy. Animals 2020, 10, 1473. [Google Scholar] [CrossRef]

| All, n = 58 | |||
|---|---|---|---|
| Characteristics | Number | Percentage % | IC 95% |
| Lesion’s localization | |||
| generalized to the entire carcass | 2 | 3.5 | [0.4–12.1%] |
| Localized in one or a few organs | 56 | 96.5 | [87.9–99.6%] |
| Age | |||
| Young (less than 2 years) | 1 | 1.8 | [0.0–9.6%] |
| Young adult (between 2 and 6 years | 15 | 26.8 | [15.8–40.3%] |
| Adult (over 6 year) | 40 | 71.4 | [57.8–82.7%] |
| Sex | |||
| Male | 33 | 58.9 | [45.0–71.9%] |
| Female | 23 | 41.1 | [28.1–55.0%] |
| Slaughterhouse | |||
| Ougadougou | 45 | 77.6% | n.a. |
| Oudalan | 13 | 22.4% | n.a. |
| Organ(s) with bTB Lesion | Number | % |
|---|---|---|
| Liver | 6 | 10.3 |
| Lungs | 2 | 3.4 |
| Lungs and thoracic LNs | 12 | 20.7 |
| Lungs, liver, and thoracic and hepatic LNs | 2 | 3.4 |
| Visceral LNs | 33 | 56.9 |
| Liver and hepatic LNs | 1 | 1.7 |
| Whole body (miliary bTB lesions) | 2 | 3.4 |
| Total | 58 | 100 |
| Mycobacterium Species | Number | % |
|---|---|---|
| M. bovis | 20 | 44.4 |
| M. bovis and M. fortuitum | 3 | 6.7 |
| M. bovis and M. elephantis | 3 | 6.7 |
| M. fortuitum | 4 | 8.9 |
| M. elephantis | 3 | 6.7 |
| M. brumae | 2 | 4.4 |
| M. avium | 1 | 2.2 |
| M. asiaticum | 1 | 2.2 |
| M. terrae complex | 1 | 2.2 |
| MTBC and M. Kubicae | 1 | 2.2 |
| Unknown NTM and M. novocastrense | 1 | 2.2 |
| Unknown NTM | 5 | 11.1 |
| Total | 45 | 100 |
| MLVA 15-9 Code | Number |
|---|---|
| 7-7 | 10 |
| 319-7 | 1 |
| 14114-7 | 1 |
| 14116-7 | 2 |
| 14117-7 | 2 |
| 14119-7 | 1 |
| 14121-23 | 1 |
| 14123-5 | 1 |
| 14124-2 | 2 |
| 14125-7 | 1 |
| 14122-421 | 1 |
| 14118-711 | 1 |
| 14120-1141 | 1 |
| ?-7 * | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanyala, E.; Shuaib, Y.A.; Schwarz, N.G.; Andres, S.; Richter, E.; Sawadogo, B.; Sawadogo, M.; Germaine, M.; Lassina, O.; Poppert, S.; et al. Prevalence and Molecular Characterization of Mycobacterium bovis in Slaughtered Cattle Carcasses in Burkina Faso; West Africa. Microorganisms 2022, 10, 1378. https://doi.org/10.3390/microorganisms10071378
Kanyala E, Shuaib YA, Schwarz NG, Andres S, Richter E, Sawadogo B, Sawadogo M, Germaine M, Lassina O, Poppert S, et al. Prevalence and Molecular Characterization of Mycobacterium bovis in Slaughtered Cattle Carcasses in Burkina Faso; West Africa. Microorganisms. 2022; 10(7):1378. https://doi.org/10.3390/microorganisms10071378
Chicago/Turabian StyleKanyala, Estelle, Yassir Adam Shuaib, Norbert Georg Schwarz, Sönke Andres, Elvira Richter, Bernard Sawadogo, Mamadou Sawadogo, Minoungou Germaine, Ouattara Lassina, Sven Poppert, and et al. 2022. "Prevalence and Molecular Characterization of Mycobacterium bovis in Slaughtered Cattle Carcasses in Burkina Faso; West Africa" Microorganisms 10, no. 7: 1378. https://doi.org/10.3390/microorganisms10071378
APA StyleKanyala, E., Shuaib, Y. A., Schwarz, N. G., Andres, S., Richter, E., Sawadogo, B., Sawadogo, M., Germaine, M., Lassina, O., Poppert, S., & Frickmann, H. (2022). Prevalence and Molecular Characterization of Mycobacterium bovis in Slaughtered Cattle Carcasses in Burkina Faso; West Africa. Microorganisms, 10(7), 1378. https://doi.org/10.3390/microorganisms10071378







