Substrate Utilisation and Energy Metabolism in Non-Growing Campylobacter jejuni M1cam
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Growth Conditions
2.2. Biolog Assay
- 1.
- Each well was examined for visual colour change due to the reduction of the Biolog Redox dye as an indication of active respiration.
- 2.
- Absorbance (OD) was measured for each well at 24 h. Absorbance at OD captures the effect of both bacterial OD measurements and the reduced Biolog Redox dye (though the reduced form of the Biolog Redox dye absorbs maximally at 590 nm, it has an absorbance range between 400–750 nm). Therefore, the effect of substrate on bacterial cells was measured using CFU experiments, as described below.
- 3.
- A 1 μL loop was dipped into each well, and subsequently streaked onto 1/4 of an MH agar plate. These plates were incubated under standard microaerophilic conditions (as described in the Materials and Methods Section 2.1) and CFU were counted after 24 h. The maximum number of colonies counted were 200. If the streaks contained ≥200 CFU, streaks were recorded as ‘TNTC’ (Too Numerous to Count).
- 4.
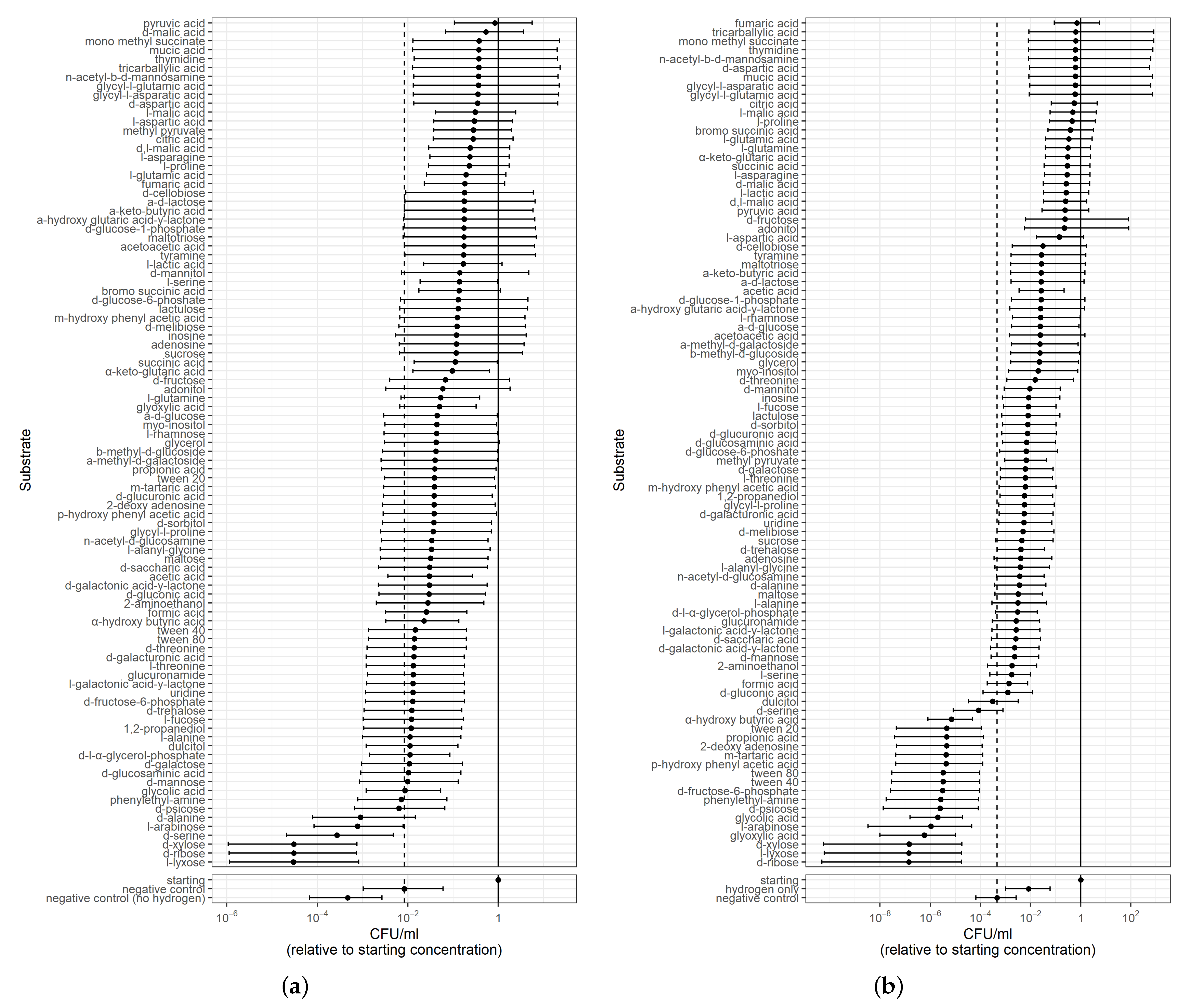
- Finally, CFU were counted using serial dilutions for selected wells. These wells were selected based on a preliminary Biolog assay (examined solely for visual colour change) and GSM analysis. Substrates that indicated respiration based on colour change in our preliminary Biolog assay, and/or supported respiration in the GSM, were chosen. Substrates that didn’t support respiration, both in the preliminary Biolog assay and GSM, such as Tween 80, were also selected as a measure of negative control along with a no-carbon well. The selected wells were: no carbon source (negative control), L-aspartic acid, L-proline, L-glutamic acid, L-asparagine, L-glutamine, L-serine, succinic acid, bromosuccinic acid, L-lactic acid, formic acid, fumaric acid, D,L-malic acid, D-malic acid, L-malic acid, citric acid, acetic acid, pyruvic acid, glycolic acid, glyoxylic acid, -hydroxy butyric acid, -keto-glutaric acid and Tween 80. CFU were counted after 24 h.
2.3. Statistical Modelling
2.3.1. Analysis of Absorbances
2.3.2. Analysis of CFU
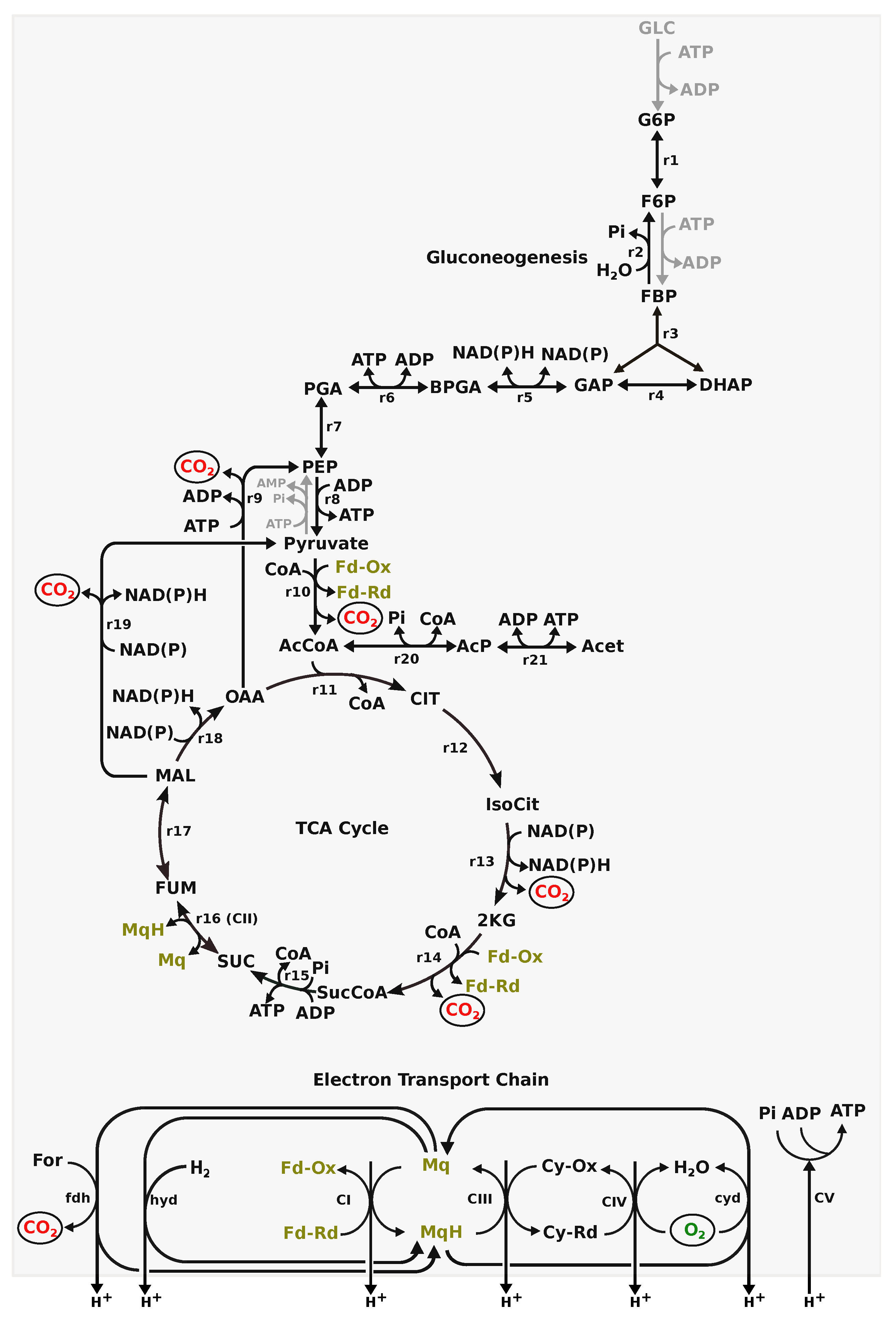
2.4. Genome-Scale Metabolic Model
2.4.1. GSM Analysis
- l.1
- single substrate import in the absence of H: LP in Equation (1) was solved, for the production of 1 unit of ATP (), allowing import of single organic substrate (present in the PM1 plate) and constraining the import of all other substrates to 0. This analysis was performed in the absence of H (i.e., flux through H exchange reaction was set to 0), to represent the experimental condition without H. A feasible solution represents GSM’s ability to produce energy on a given substrate but not necessarily biomass/growth.
- 1.2
- with constraints on the production of biomass: LP, as described above, was solved with an additional constraint, , to define flux through the biomass transporters, as described in [8], as a proxy for growth. A feasible solution represents GSM’s ability to produce biomass or grow on a given substrate.
- 1.3
- in the presence of H alone: LP in Equation (1) was solved, for the production of 1 unit of ATP, allowing import of H while constraining import of all organic substrate to 0 (the experimental condition of the negative control in the H condition).
2.4.2. ATP Demand Variation Analysis
2.4.3. The Cost of ATP Demand
3. Results
3.1. PM1 Analysis
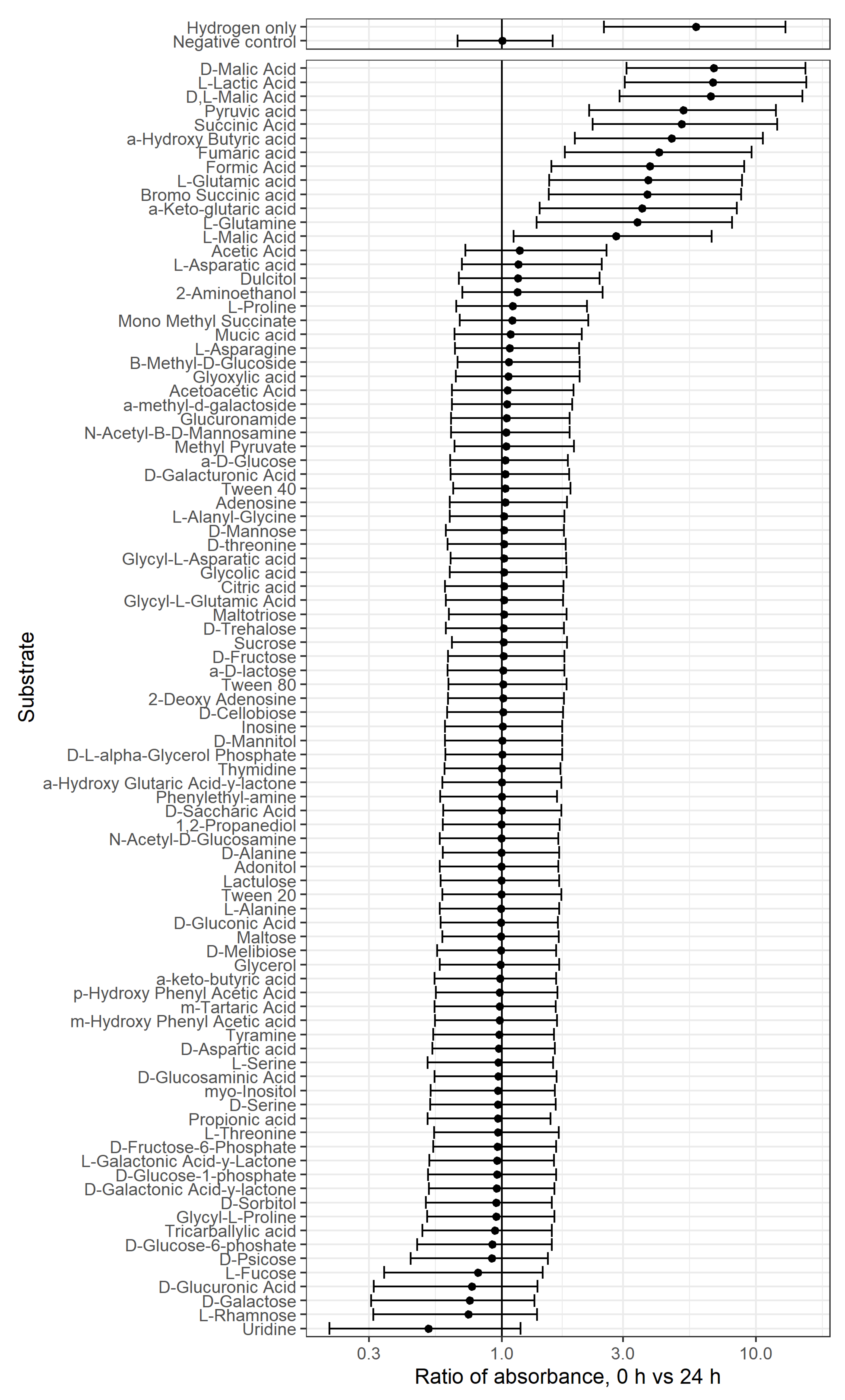
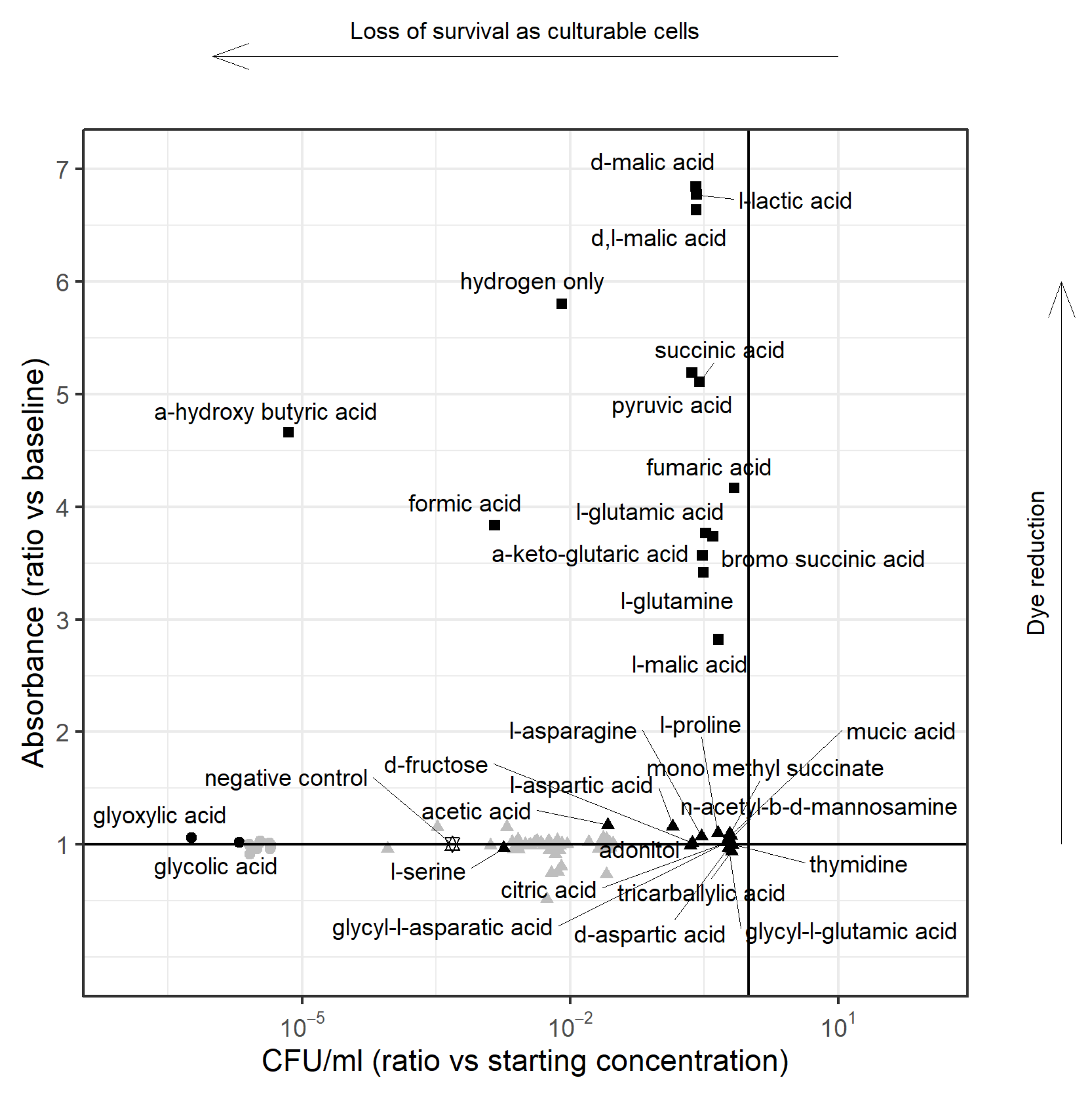
3.1.1. PM1 Analysis in the Presence of Hydrogen
3.1.2. PM1 Analysis for Survival on Single Substrate, including Hydrogen
3.2. GSM Analysis
3.2.1. Substrate Utilisation
3.2.2. Role of H on Energy Metabolism
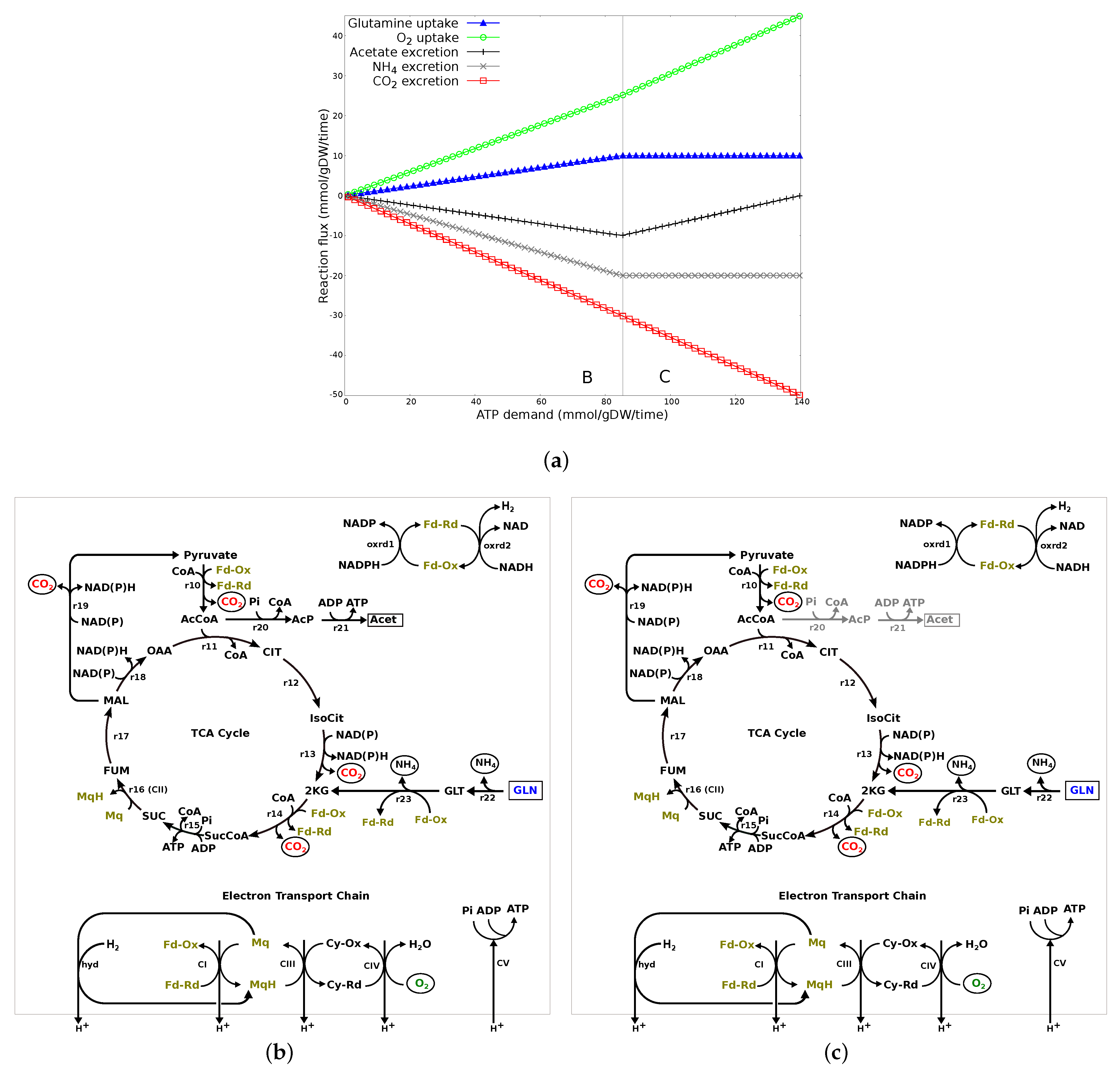
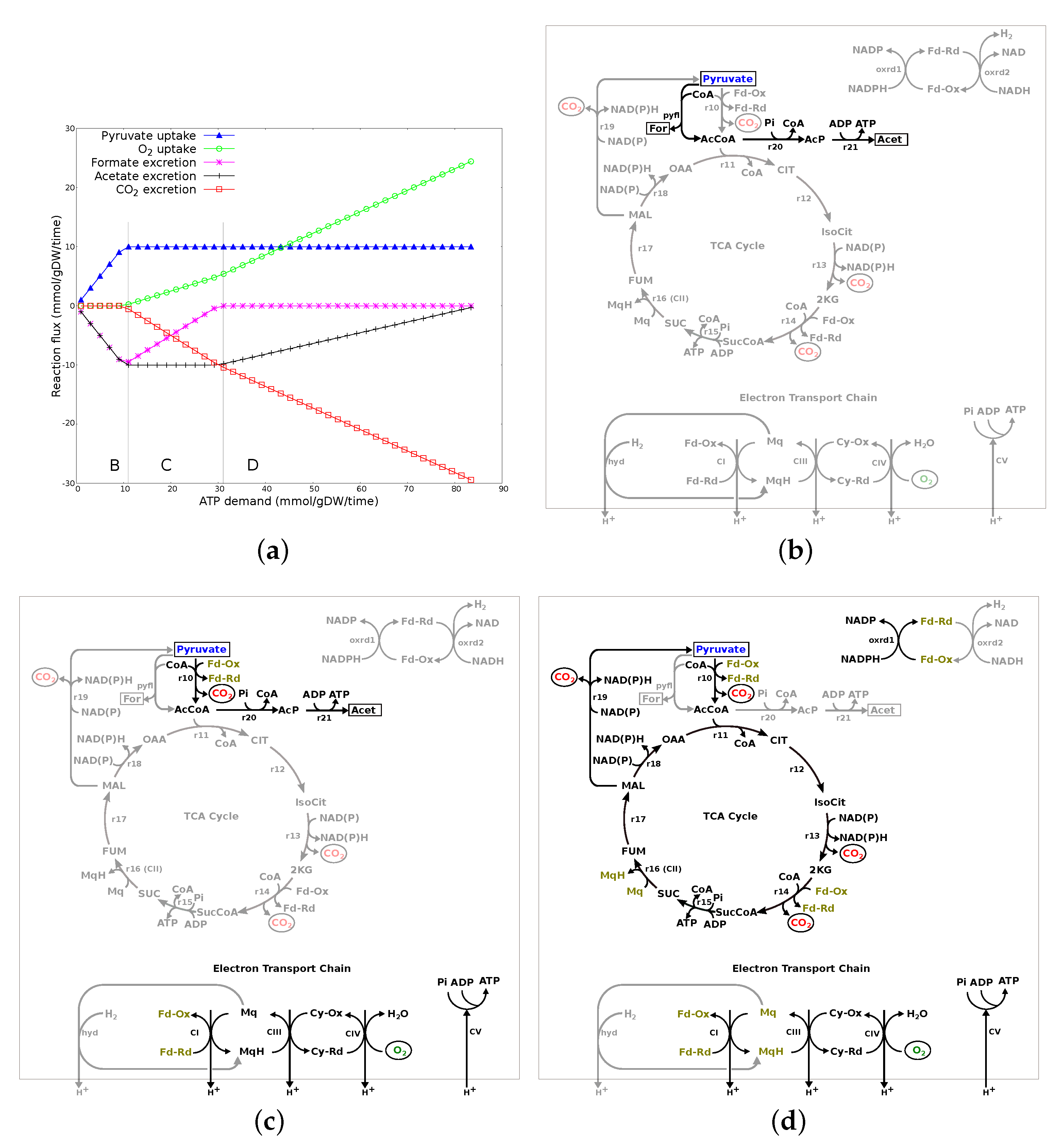
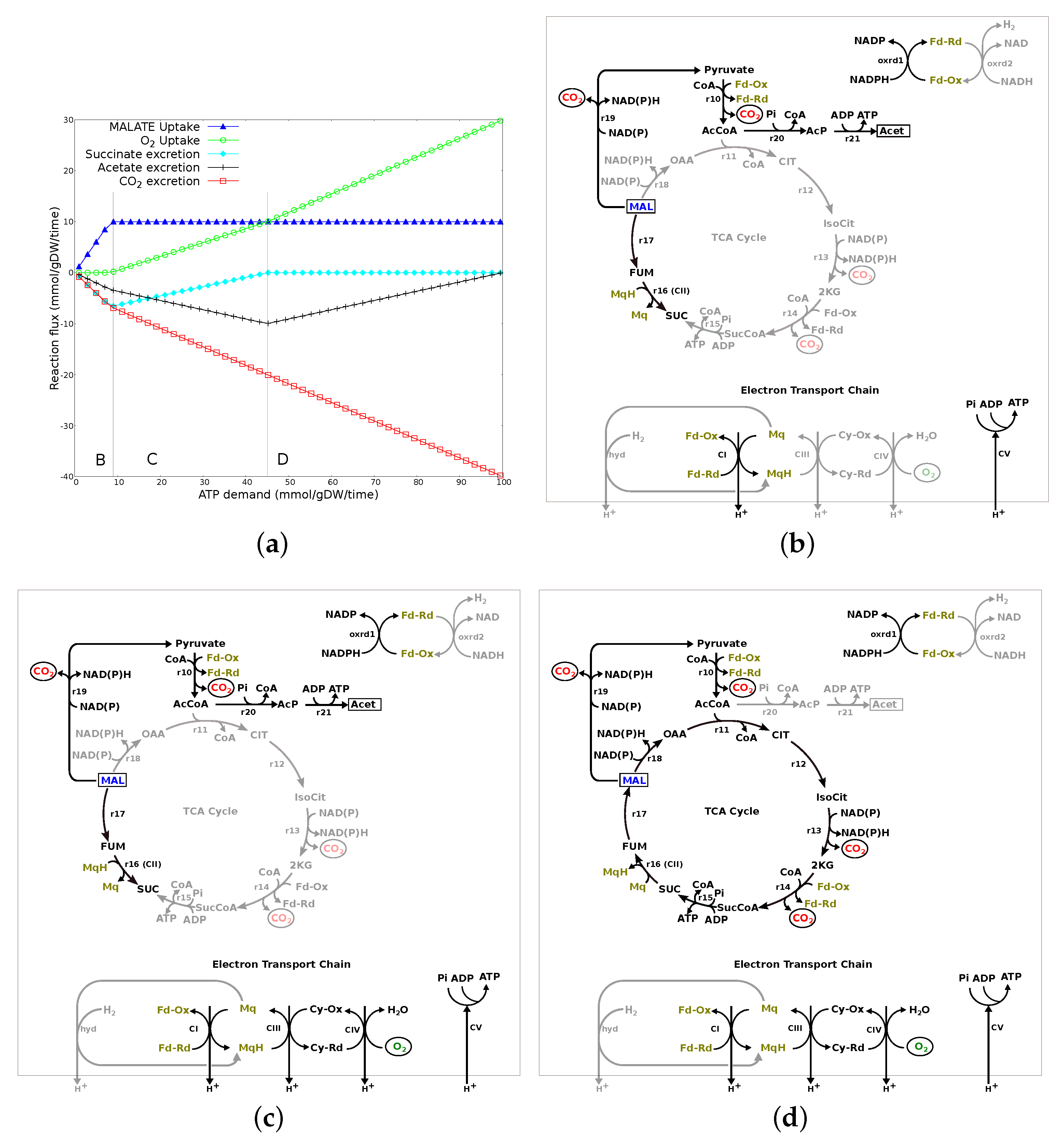
3.2.3. ATP Demand Variation Analysis
3.2.4. Summary of Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Genome-Scale Metabolic Models Overview
Appendix B. GSM Curation and Refinement from Experimental Observation
Appendix C. Experimental Data

References
- Kaakoush, N.O.; Rodríguez, N.C.; Mitchell, H.M.; Man, S.M. Global epidemiology of campylobacter infection. Clin. Microbiol. Rev. 2015, 28, 687–720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Facciolà, A.; Riso, R.; Avventuroso, E.; Visalli, G.; Delia, S.; Laganà, P. Campylobacter: From microbiology to prevention. J. Prev. Med. Hyg. 2017, 58, E79–E92. [Google Scholar] [PubMed]
- Daniel, N.; Casadevall, N.; Sun, P.; Sugden, D.; Aldin, V. The Burden of Foodborne Disease in the UK 2018; The Royal Society Publishing: London, UK, 2020. [Google Scholar]
- Humphrey, T.; O’Brien, S.; Madsen, M. Campylobacters as zoonotic pathogens: A food production perspective. Int. J. Food Microbiol. 2007, 117, 237–257. [Google Scholar] [CrossRef] [PubMed]
- Casanova, C.; Schweiger, A.; von Steiger, N.; Droz, S.; Marschall, J.; Diekema, D.J. Campylobacter concisus pseudo-outbreak caused by improved culture conditions. J. Clin. Microbiol. 2015, 53, 660–662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tenover, F.C.; Patton, C.M. Naturally occurring auxotrophs of Campylobacter jejuni and Campylobacter coli. J. Clin. Microbiol. 1987, 25, 1659–1661. [Google Scholar] [CrossRef] [Green Version]
- Alazzam, B.; Bonnassie-Rouxin, S.; Dufour, V.; Ermel, G. MCLMAN, a new minimal medium for Campylobacter jejuni NCTC 11168. Res. Microbiol. 2011, 162, 173–179. [Google Scholar] [CrossRef]
- Tejera, N.; Crossman, L.; Pearson, B.; Stoakes, E.; Nasher, F.; Djeghout, B.; Poolman, M.; Wain, J.; Singh, D. Genome-scale metabolic model driven design of a defined medium for Campylobacter jejuni M1cam. Front. Microbiol. 2020, 11, 1072. [Google Scholar] [CrossRef]
- Bochner, B.; Gadzinski, P.; Panomitros, E. Phenotype MicroArrays for high-throughput phenotypic testing and assay of gene function. Genome Res. 2001, 11, 1246–1255. [Google Scholar] [CrossRef] [Green Version]
- Lempp, M.; Lubrano, P.; Bange, G.; Link, H. Metabolism of non-growing bacteria. Biol. Chem. 2020, 401, 1479–1485. [Google Scholar] [CrossRef]
- Chen, Y.; Nielsen, J. Energy metabolism controls phenotypes by protein efficiency and allocation. Proc. Natl. Acad. Sci. USA 2019, 116, 17592–17597. [Google Scholar] [CrossRef] [Green Version]
- Roszak, D.B.; Colwell, R.R. Survival strategies of bacteria in the natural environment. Microbiol. Rev. 1987, 51, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Hofreuter, D.; Novik, V.; Galán, J.E. Metabolic diversity in Campylobacter jejuni enhances specific tissue colonization. Cell Host Microbe 2008, 4, 425–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hofreuter, D. Defining the metabolic requirements for the growth and colonization capacity of Campylobacter jejuni. Front. Cell Infect. Microbiol. 2014, 4, 137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parkhill, J.; Wren, B.; Mungall, K.; Ketley, J.; Churcher, C.; Basham, D.; Chillingworth, T.; Davies, R.; Feltwell, T.; Holroyd, S.; et al. The genome sequence of the food-borne pathogen Campylobacter jejuni reveals hypervariable sequences. Nature 2000, 403, 665–668. [Google Scholar] [CrossRef] [Green Version]
- Velayudhan, J.; Kelly, D.J. Analysis of gluconeogenic and anaplerotic enzymes in Campylobacter jejuni: An essential role for phosphoenolpyruvate carboxykinase. Microbiology 2002, 148, 685–694. [Google Scholar] [CrossRef] [Green Version]
- Wagley, S.; Newcombe, J.; Laing, E.; Yusuf, E.; Sambles, C.; Studholme, D.; La Ragione, R.; Titball, R.; Champion, O. Differences in carbon source utilisation distinguish Campylobacter jejuni from Campylobacter coli. BMC Microbiol. 2014, 14, 262. [Google Scholar] [CrossRef] [Green Version]
- Stahl, M.; Friis, L.M.; Nothaft, H.; Liu, X.; Li, J.; Szymanski, C.M.; Stintzi, A. L-Fucose utilization provides Campylobacter jejuni with a competitive advantage. Proc. Natl. Acad. Sci. USA 2011, 108, 7194–7199. [Google Scholar] [CrossRef] [Green Version]
- Muraoka, W.T.; Zhang, Q. Phenotypic and genotypic evidence for l-Fucose utilization by Campylobacter jejuni. J. Bacteriol. 2011, 193, 1065–1075. [Google Scholar] [CrossRef] [Green Version]
- Kendall, J.J.; Barrero-Tobon, A.M.; Hendrixson, D.R.; Kelly, D.J. Hemerythrins in the microaerophilic bacterium Campylobacter jejuni help protect key iron-sulphur cluster enzymes from oxidative damage. Environ. Microbiol. 2014, 16, 1105–1121. [Google Scholar] [CrossRef] [Green Version]
- Daucher, J.A.; Krieg, N.R. Pyruvate:ferredoxin oxidoreductase in Campylobacter species. Can. J. Microbiol. 1995, 41, 198–201. [Google Scholar] [CrossRef]
- Kelly, D. The physiology and metabolism of Campylobacter jejuni and Helicobacter pylori. J. Appl. Microbiol. 2001, 90, 16S–24S. [Google Scholar] [CrossRef] [PubMed]
- Kelly, D.J. Complexity and versatility in the physiology and metabolism of Campylobacter jejuni. In Campylobacter, 3rd ed.; American Society of Microbiology: Washington, DC, USA, 2008; pp. 41–61. [Google Scholar]
- Weerakoon, D.R.; Olson, J.W. The Campylobacter jejuni NADH:Ubiquinone oxidoreductase (Complex I) utilizes flavodoxin rather than NADH. J. Bacteriol. 2008, 190, 915–925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jackson, R.J.; Elvers, K.T.; Lee, L.J.; Gidley, M.D.; Wainwright, L.M.; Lightfoot, J.; Park, S.F.; Poole, R.K. Oxygen reactivity of both respiratory oxidases in Campylobacter jejuni: The cydAB genes encode a cyanide-resistant, low-affinity oxidase that is not of the cytochrome bd type. J. Bacteriol. 2007, 189, 1604–1615. [Google Scholar] [CrossRef] [Green Version]
- Pittman, M.; Kelly, D. Electron transport through nitrate and nitrite reductases in Campylobacter jejuni. Biochem. Soc. Trans. 2005, 33, 190–192. [Google Scholar] [CrossRef] [PubMed]
- Weingarten, R.A.; Taveirne, M.E.; Olson, J.W. The dual-functioning fumarate reductase is the sole succinate:quinone reductase in Campylobacter jejuni and is required for full host colonization. J. Bacteriol. 2009, 191, 5293–5300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van der Stel, A.X.; Wösten, M.M.S.M. Regulation of respiratory pathways in Campylobacterota: A Review. Front. Microbiol. 2019, 10, 1719. [Google Scholar] [CrossRef] [Green Version]
- Hoffmant, P.S.; Goodman, T.G. Respiratory physiology and energy conservation efficiency of Campylobacter jejuni. J. Bacteriol. 1982, 150, 319–326. [Google Scholar] [CrossRef] [Green Version]
- Kassem, I.I.; Candelero-Rueda, R.A.; Esseili, K.A.; Rajashekara, G. Formate simultaneously reduces oxidase activity and enhances respiration in Campylobacter jejuni. Sci. Rep. 2017, 7, 40117. [Google Scholar] [CrossRef] [Green Version]
- Stahl, M.; Butcher, J.; Stintzi, A. Nutrient acquisition and metabolism by Campylobacter jejuni. Front. Cell. Infect. Microbiol. 2012, 2, 5. [Google Scholar] [CrossRef] [Green Version]
- Van der Stel, A.X.; Boogerd, F.C.; Huynh, S.; Parker, C.T.; van Dijk, L.; van Putten, J.P.M.; Wösten, M.M.S.M. Generation of the membrane potential and its impact on the motility, ATP production and growth in Campylobacter jejuni. Mol. Microbiol. 2017, 105, 637–651. [Google Scholar] [CrossRef] [Green Version]
- Alwazeer, D. Reducing atmosphere packaging technique for extending the shelf-life of food products. J. Inst. Sci. Technol. 2019, 9, 2117–2123. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Wang, J.; Kuang, Y.; Shen, W. Packaging with hydrogen gas modified atmosphere can extend chicken egg storage. J. Sci. Food Agric. 2022, 102, 976–983. [Google Scholar] [CrossRef] [PubMed]
- Friis, C.; Wassenaar, T.M.; Javed, M.A.; Snipen, L.; Lagesen, K.; Hallin, P.F.; Newell, D.G.; Toszeghy, M.; Ridley, A.; Manning, G.; et al. Genomic characterization of Campylobacter jejuni strain M1. PLoS ONE 2010, 5, e12253. [Google Scholar] [CrossRef] [Green Version]
- Bürkner, P.C. Bayesian item response modeling in R with brms and Stan. J. Stat. Softw. 2021, 100, 1–54. [Google Scholar] [CrossRef]
- Lenth, R.V. Emmeans: Estimated marginal means, aka least-squares means. R Package Version 2022, 1, 30. [Google Scholar]
- Harder, D.; Stolz, J.; Casagrande, F.; Obrdlik, P.; Weitz, D.; Fotiadis, D.; Daniel, H. DtpB (YhiP) and DtpA (TppB, YdgR) are prototypical proton dependent peptide transporters of Escherichia coli. FEBS J. 2008, 275, 3290–3298. [Google Scholar] [CrossRef]
- Li, X.; Zhuo, W.; Yu, J.; Ge, J.; Gu, J.; Feng, Y.; Yang, M.; Wang, L.; Wang, N. Structure of the nucleotide-binding domain of a dipeptide ABC transporter reveals a novel iron–sulfur cluster-binding domain. Acta Crystallogr. Sect. D 2013, 69, 256–265. [Google Scholar] [CrossRef]
- Pletzer, D.; Lafon, C.; Braun, Y.; Köhler, T.; Page, M.G.P.; Mourez, M.; Weingart, H. High-throughput screening of dipeptide utilization mediated by the ABC Transporter DppBCDF and its substrate-binding proteins DppA1-A5 in Pseudomonas aeruginosa. PLoS ONE 2014, 9, e111311. [Google Scholar] [CrossRef] [Green Version]
- Holzhütter, H.G. The principle of flux minimization and its application to estimate stationary fluxes in metabolic networks. Eur. J. Biochem. 2004, 271, 2905–2922. [Google Scholar] [CrossRef]
- Holzhütter, H.G. The generalized flux-minimization method and its application to metabolic networks affected by enzyme deficiencies. Biosystems 2006, 83, 98–107. [Google Scholar] [CrossRef]
- Singh, D.; Carlson, R.P.; Fell, D.A.; Poolman, M.G. Modelling metabolism of the diatom Phaeodactylum tricornutum. Biochem. Soc. Trans. 2015, 43, 1182–1186. [Google Scholar] [CrossRef] [Green Version]
- Poolman, M.G.; Kundu, S.; Shaw, R.; Fell, D.A. Responses to light intensity in a genome-scale model of rice metabolism. Plant Physiol. 2013, 162, 1060–1072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poolman, M.G. ScrumPy: Metabolic modelling with Python. IEE Proc. Syst. Biol. 2006, 153, 375–378. [Google Scholar] [CrossRef] [PubMed]
- Hinton , A., Jr. Growth of Campylobacter in media supplemented with organic acids. J. Food Prot. 2006, 69, 34–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Birk, T.; Grønlund, A.C.; Christensen, B.B.; Knøchel, S.; Lohse, K.; Rosenquist, H. Effect of organic acids and marination ingredients on the survival of Campylobacter jejuni on meat. J. Food Prot. 2010, 73, 258–265. [Google Scholar] [CrossRef]
- González-Fandos, E.; Maya, N. Efficacy of malic acid against Campylobacter jejuni attached to chicken skin during refrigerated storage. J. Food Process. Preserv. 2016, 40, 593–600. [Google Scholar] [CrossRef]
- González-Fandos, E.; Martinez-Laorden, A.; Perez-Arnedo, I. Effect of decontamination treatments on Campylobacter jejuni in chicken. Foods 2020, 9, 1453. [Google Scholar] [CrossRef]
- Bai, Y.; Ding, X.; Zhao, Q.; Sun, H.; Li, T.; Li, Z.; Wang, H.; Zhang, L.; Zhang, C.; Xu, S. Development of an organic acid compound disinfectant to control food-borne pathogens and its application in chicken slaughterhouses. Poult. Sci. 2022, 101, 101842. [Google Scholar] [CrossRef]
- Berridge, M.; Herst, P.; Tan, A. Tetrazolium dyes as tools in cell biology: New insights into their cellular reduction. Biotechnol. Annu. Rev. 2005, 11, 127–152. [Google Scholar] [CrossRef]
- Carbonero, F.; Benefiel, A.; Gaskins, R. Contributions of the microbial hydrogen economy to colonic homeostasis. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 504–518. [Google Scholar] [CrossRef]
- Ge, L.; Qi, J.; Shao, B.; Ruan, Z.; Ren, Y.; Sui, S.; Wu, X.; Sun, X.; Liu, S.; Li, S.; et al. Microbial hydrogen economy alleviates colitis by reprogramming colonocyte metabolism and reinforcing intestinal barrier. Gut Microbes 2022, 14, 2013764. [Google Scholar] [CrossRef] [PubMed]
- Weerakoon, D.R.; Borden, N.J.; Goodson, C.M.; Grimes, J.; Olson, J.W. The role of respiratory donor enzymes in Campylobacter jejuni host colonization and physiology. Microb. Pathog. 2009, 47, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Guccione, E.; Kendall, J.; Hitchcock, A.; Garg, N.; White, M.; Mulholland, F.; Poole, R.; Kelly, D. Transcriptome and proteome dynamics in chemostat culture reveal how Campylobacter jejuni modulates metabolism, stress responses and virulence factors upon changes in oxygen availability. Environ. Microbiol. 2017, 19, 4326–4348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.; Hua, Q. Applications of genome-scale metabolic models in biotechnology and systems medicine. Front. Physiol. 2016, 6, 413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Metris, A.; Reuter, M.; Gaskin, D.; Baranyi, J.; van Vliet, A. In vivo and in silico determination of essential genes of Campylobacter jejuni. BMC Genom. 2011, 12, 535. [Google Scholar] [CrossRef] [Green Version]
- Gu, C.; Kim, G.; Kim, W.; Kim, T.Y.; Lee, S.Y. Current status and applications of genome-scale metabolic models. Genome Biol. 2019, 20, 121. [Google Scholar] [CrossRef] [Green Version]
- Sertbas, M.; Ulgen, K.O. Genome-scale metabolic modeling for unraveling molecular mechanisms of high threat pathogens. Front. Cell Dev. Biol. 2020, 8, 566702. [Google Scholar] [CrossRef]
- Poolman, M.G.; Miguet, L.; Sweetlove, L.J.; Fell, D.A. A genome-scale metabolic model of Arabidopsis and some of its properties. Plant Physiol. 2009, 151, 1570–1581. [Google Scholar] [CrossRef] [Green Version]
- Yeow, M.; Liu, F.; Ma, R.; Williams, T.; Riordan, S.; Zhang, L. Analyses of energy metabolism and stress defence provide insights into Campylobacter concisus growth and pathogenicity. Gut Pathog. 2020, 12, 13. [Google Scholar] [CrossRef]

| Substrates Utilised | Substrate-Level Phosphorylation via Acetate Kinase | Anaerobic Respiration via Fumarate Reductase | Aerobic Respiration | TCA Cycle | Nutrient Cost (Substrate Uptake/ATP) | O2 Uptake/ATP | Enzymatic Cost (Objective Value/ATP) |
|---|---|---|---|---|---|---|---|
| Hydrogen | × | × | ✓ | × | NA | 0.33 | 38.33 |
| Formate | × | × | ✓ | × | 0.5 | 0.25 | 30.75 |
| Acetate | × | × | ✓ | ✓ | 0.36 | 0.36 | 43.09 |
| Succinate | ✓ | × | ✓ | ✓ | 0.37–0.73 | 0.27–0.32 | 32.45–37.21 |
| Fumarate | ✓ | ✓ | ✓ | ✓ | 0.4–4.8 | 0.0–0.3 | 12.0–35.57 |
| Malate | ✓ | ✓ | ✓ | ✓ | 0.4–4.8 | 0.0–0.3 | 12.0–35.57 |
| Lactate | ✓ | × | ✓ | ✓ | 0.32–0.75 | 0.25–0.32 | 29.75–36.76 |
| -keto-glutarate | ✓ | × | ✓ | ✓ | 0.37–2.0 | 0.2–0.3 | 24.6–34.67 |
| Citrate | ✓ | × | ✓ | ✓ | 0.4–1.5 | 0.25–0.3 | 31.25–35.49 |
| Pyruvate | ✓ | × | ✓ | ✓ | 0.36–3.0 | 0.0–0.29 | 9.0–34.63 |
| Glycerol | ✓ | ✓ | ✓ | ✓ | 0.25–3.0 | 0.0–0.29 | 20.0–34.6 |
| Proline | ✓ | × | ✓ | ✓ | 0.29–0.42 | 0.29–0.31 | 33.96–36.45 |
| Glutamate | ✓ | × | ✓ | ✓ | 0.36–0.59 | 0.29–0.32 | 34.88–37.67 |
| Glutamine | ✓ | × | ✓ | ✓ | 0.36–0.59 | 0.29–0.32 | 35.0–37.74 |
| L-aspartate | ✓ | ✓ | ✓ | ✓ | 0.45–8.0 | 0.0–0.33 | 18.67–39.43 |
| D-aspartate | ✓ | ✓ | ✓ | ✓ | 0.4–4.8 | 0.0–0.3 | 15.2–35.67 |
| Asparagine | ✓ | × | ✓ | ✓ | 0.45–1.14 | 0.29–0.33 | 36.57–39.54 |
| Serine | ✓ | × | ✓ | ✓ | 0.36–3.0 | 0.0–0.29 | 10.0–34.75 |
| D-Alanine | ✓ | × | ✓ | ✓ | 0.3–1.2 | 0.2–0.3 | 27.4–35.47 |
| L-Alanine | ✓ | × | ✓ | ✓ | 0.3–0.67 | 0.22–0.3 | 27.78–35.57 |
| ALA-GLY | ✓ | × | ✓ | ✓ | 0.33–2.5 | 0.0–0.3 | 13.5–35.96 |
| GLY-ASP | ✓ | ✓ | ✓ | ✓ | 0.4–3.0 | 0.0–0.3 | 13.67–36.02 |
| GLY-GLT | ✓ | × | ✓ | ✓ | 0.35–7.0 | 0.0–0.3 | 20.0–35.65 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stoakes, E.; Savva, G.M.; Coates, R.; Tejera, N.; Poolman, M.G.; Grant, A.J.; Wain, J.; Singh, D. Substrate Utilisation and Energy Metabolism in Non-Growing Campylobacter jejuni M1cam. Microorganisms 2022, 10, 1355. https://doi.org/10.3390/microorganisms10071355
Stoakes E, Savva GM, Coates R, Tejera N, Poolman MG, Grant AJ, Wain J, Singh D. Substrate Utilisation and Energy Metabolism in Non-Growing Campylobacter jejuni M1cam. Microorganisms. 2022; 10(7):1355. https://doi.org/10.3390/microorganisms10071355
Chicago/Turabian StyleStoakes, Emily, George M. Savva, Ruby Coates, Noemi Tejera, Mark G. Poolman, Andrew J. Grant, John Wain, and Dipali Singh. 2022. "Substrate Utilisation and Energy Metabolism in Non-Growing Campylobacter jejuni M1cam" Microorganisms 10, no. 7: 1355. https://doi.org/10.3390/microorganisms10071355
APA StyleStoakes, E., Savva, G. M., Coates, R., Tejera, N., Poolman, M. G., Grant, A. J., Wain, J., & Singh, D. (2022). Substrate Utilisation and Energy Metabolism in Non-Growing Campylobacter jejuni M1cam. Microorganisms, 10(7), 1355. https://doi.org/10.3390/microorganisms10071355








